A fast charging/discharging all-solid-state lithium ion battery based on PEO-MIL-53(Al)-LiTFSI thin film electrolyte
Kai Zhu,
Yexiang Liu and
Jin Liu*
School of Metallurgy and Environment, Central South University, Changsha, 410083, China. E-mail: jinliu@csu.edu.cn
First published on 6th August 2014
Abstract
Metal–organic framework aluminum 1,4-benzenedicarboxylate (MIL-53(Al)) is used as a filler for a polyethylene oxide (PEO) based thin film electrolyte. With the participation of MIL-53(Al), the ionic conductivity of this electrolyte is increased from 9.66 × 10−4 S cm−1 to 3.39 × 10−3 S cm−1 at 120 °C and the oxidation potential is raised from 4.99 V to 5.10 V. In addition, an all-solid-state LiFePO4/Li button battery based on the electrolyte is fabricated. At 5 C and 120 °C, the battery delivers the discharge capacity of 136.4 mA h g−1 in the initial cycle, 129.2 mA h g−1 in the 300th cycle, and 83.5 mA h g−1 in the 1400th cycle. At 10 C and 120 °C, its discharge capacity is 116.2 mA h g−1 in the initial cycle and 103.5 mA h g−1 in the 110th cycle. The results indicate that this metal–organic framework (MIL-53(Al)) is a novel structural modifier for solid polymer electrolytes in fast charging/discharging lithium ion batteries.
1. Introduction
All-solid-state polymer lithium ion batteries (LIBs) have better safety than conventional LIBs since the flammable liquid electrolyte is replaced by a solid electrolyte.1–5 However, the cycle performance of batteries assembled by such solid polymer electrolytes does not have a strong appeal when batteries are charged/discharged at high current densities. Generally, the fast charging/discharging capability of a battery is determined by subjecting the battery to high charging/discharging rates where about 40% of the charge/discharge state must be obtained within 15 min (about 1.6 C rate).6 This requires the ability of conductible ions in the electrolyte to transport quickly between two electrodes.7 Thus, various studies were focused on the improvement of ionic conductivities of solid polymer electrolytes,8,9 especially on the modification of polyethylene oxide (PEO) based electrolytes.10–18 For example, the ionic conductivity was increased to 2.0 × 10−4 S cm−1 at 80 °C by adding silane-treated Al2O3.16 The LiFePO4/Li battery assembled by this electrolyte showed a discharge capacity of 140 mA h g−1 in the 100th cycle at 0.2 C and 90 °C. In 2013, we reported the PEO based electrolyte with metal–organic-framework-5 (MOF-5) as a filler for the all-solid-state LiFePO4/Li battery.17 The ionic conductivity increased to 7.9 × 10−4 S cm−1 from 3.9 × 10−4 S cm−1 at 75 °C. The battery attained the initial discharge capacity of 151 mA h g−1 and 45% capacity retention in the 100th cycle at 1 C and 80 °C. However, it did not charge/discharge at higher rates. One reason for this would be the strong absorption capacity of MOF-5 for water and small molecules, resulting in instability of the electrolyte. Recently, another MOF, aluminium(III)-1,3,5-benzenetricarboxylate (Al-BTC), was reported as a filler to add to PEO based electrolytes.18 An ionic conductivity of ∼7 × 10−4 S cm−1 at 70 °C was measured. The LiFePO4/Li battery with the electrolyte delivered a specific capacity of about 100 mA h g−1 at 2 C and 70 °C. At 5 C, the battery had a low specific capacity of 40 mA h g−1.MOFs are inorganic–organic hybrid materials that are primarily applied in the fields of drug delivery, optoelectronics, sensing and catalysis.19–22 Aluminum 1,4-benzenedicarboxylate (MIL-53(Al)) is one of the MOFs that is built up of corner-sharing AlO4(OH)2 octahedra.23 It has remarkable water and oxygen stability compared to MOF-5.24 In this study, the PEO based electrolyte was prepared by using MIL-53(Al) as a filler and lithium bis(trifluoro-methanesulfonyl)imide (LiTFSI) as the lithium salt. The synthesized electrolyte's ionic conductivity is 3.39 × 10−3 S cm−1 at 120 °C, which is higher than the 9.66 × 10−4 S cm−1 of that without MIL-53(Al). The LiFePO4/Li battery based on this electrolyte has an average discharge capacity of 103.5 mA h g−1 for 110 cycles at a high rate of 10 C and 120 °C, demonstrating the potential of the modified PEO electrolyte based lithium ion battery in faster charging/discharging applications.
2. Experimental
2.1 Preparation of MIL-53(Al) nanoparticles
Aluminum 1,4-benzenedicarboxylate (MIL-53(Al)) nanoparticles were synthesized under hydrothermal conditions by treating 1,4-benzenedicarboxylic acid (H2BDC, 99%) and aluminum nitrate nonahydrate (Al(NO3)3·9H2O, 98%) with N,N-dimethylformamide (DMF, +99.9%) and deionized water.23 The materials were stirred for 2 h, and then transferred to a Teflon-lined steel autoclave (50 mL). The autoclave was heated in an oil bath at 160 °C for 50 h. The free H2BDC and water in the sample were removed by washing with absolute alcohol and filtering 3 times. The sample was heated in a vacuum at 120 °C for 12 h to obtain a white powder product.2.2 Preparation of thin film electrolytes
The polyethylene oxide (PEO, MW 4 × 106, 99.9%) was thoroughly dried at 50 °C for 12 h, and the lithium bis(trifluoro-methanesulfonyl)imide (LiN(SO2CF3)2, LiTFSI, +99.5%) was dried at 100 °C in a vacuum for 24 h before use. Firstly, LiTFSI was added to acetonitrile (CH3CN, AR grade) and stirred for 2 h. Then, MIL-53(Al) nanoparticles were added to the solution and stirred for 10 min. After that, PEO was dispersed in the solution and stirred for 36 h. The result was a homogenized colloidal solution. Finally, the solution was cast and dried into a thin film at 80 °C for 24 h in an argon-filled glove box, resulting in the formation of a PEO-MIL-53(Al)-LiTFSI thin film electrolyte. The thickness of the solid electrolyte thin film was about 60 μm and the diameter of the solid electrolyte thin film was 20 mm. Using this electrolyte, all-solid-state LiFePO4/Li batteries were fabricated for all evaluations.2.3 Characterization and instruments
The surface morphologies of the MIL-53(Al) nanoparticle and the thin film electrolyte were observed with a scanning electron microscope (SEM, sirion 200). The X-ray diffraction (XRD, RINT-2000, Rigaku) patterns were collected from a Rigaku/TTR-III powder diffractometer equipped with Cu-Kα radiation (λ = 1.5418 Å) at 25 °C. The diffraction pattern was recorded from 5° to 60° with a step size of 10° min−1. The zeta electric potential of the MIL-53(Al) nanoparticles was measured with a Brookhaven Zeta Plus instrument (USA). Thermogravimetric analysis (TGA) was carried out on Perkin-Elmer Pyris-1. The thin film electrolytes were loaded in hermetically sealed aluminum pans, and measurements were carried out at a heating rate of 10 °C min−1 from 48 °C to 500 °C. The mechanical strength of the thin film electrolytes was measured with a Shimadzu TA Q800-1706 instrument with a tensile speed of 1 N min−1.The electrochemical properties of the electrolytes were measured by using a PARSTAT 2273 system (PerkinElmer Instrument, USA). The electrochemical window was determined using blocking stainless steel electrode/electrolyte/Li batteries at a scan rate of 10 mV s−1 from 2.5 V to 6.5 V. The ionic conductivities were determined by AC impedance spectroscopy, which was carried out in the 500 kHz to 10 Hz frequency range. The equation for calculating the conductivity is
 | (1) |
The lithium ion transference number of the electrolyte was tested in a symmetric cell (Li/electrolytes/Li) by using a PARSTAT 2273. The symmetric Li/electrolyte/Li battery was polarized at a small voltage of 10 mV. AC impedance plots of the cell before and after polarization were obtained.
The cycling performance of the all-solid-state LiFePO4/electrolyte/Li batteries was investigated by using the LiFePO4/electrolyte/Li batteries. Button cells (2025) with diameter 20 mm and thickness 2.5 mm were used to fabricate the batteries. The thickness of the LiFePO4 cathode was about 30 μm. Charge/discharge cycles of the batteries were performed on a Land instrument (Wuhan Land Electronic Co., Ltd. China), with the tests using cut-off voltages of 4.0 V (charge) and 2.5 V (discharge). All the batteries were assembled in an argon-filled UNILAB glove box.
3. Results and discussions
3.1 Characteristics of MIL-53(Al) nanoparticles and the PEO-MIL-53(Al)-LiTFSI thin film electrolyte
Fig. 1a shows the XRD pattern of MIL-53(Al), in which the characteristic peaks of the sample are identical to the pattern simulated by the lattice parameters of MIL-53(Al).23 The surface morphologies of MIL-53(Al) and the PEO-MIL-53(Al)-LiTFSI thin film electrolyte were investigated by SEM. Cylindrical nanoparticles in the MIL-53(Al) sample and a uniform surface for the electrolyte were observed in Fig. 1b and c respectively, suggesting that the cylindrical particles would not damage the architecture of the thin film. | ||
| Fig. 1 (a) XRD pattern of MIL-53(Al) and the simulated pattern based on the crystallographic data of MIL-53(Al);23 SEM images of (b) MIL-53(Al) and (c) the PEO-MIL-53(Al)-LiTFSI thin film electrolyte. | ||
3.2 Lithium ionic conductivities of thin film electrolytes
The ionic conductivities of the thin film electrolytes with different EO (ethylene oxide in PEO)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li molar ratios and different MIL-53(Al) concentrations at 30 °C, 60 °C and 80 °C were measured, with the results listed in Table 1. At a fixed MIL-53(Al) concentration of 10 wt%, the electrolyte with the EO
Li molar ratios and different MIL-53(Al) concentrations at 30 °C, 60 °C and 80 °C were measured, with the results listed in Table 1. At a fixed MIL-53(Al) concentration of 10 wt%, the electrolyte with the EO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li ratio of 15
Li ratio of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 had the highest ionic conductivities at all three temperatures compared to the other ratios of 10
1 had the highest ionic conductivities at all three temperatures compared to the other ratios of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20
1, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 25
1 and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. At the constant EO
1. At the constant EO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li ratio of 15
Li ratio of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and various MIL-53(Al) concentrations of 0, 10 and 20 wt%, there were the highest ionic conductivities at 10 wt% MIL-53(Al) and at all three temperatures. Hence, the EO
1 and various MIL-53(Al) concentrations of 0, 10 and 20 wt%, there were the highest ionic conductivities at 10 wt% MIL-53(Al) and at all three temperatures. Hence, the EO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li ratio of 15
Li ratio of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the MIL-53(Al) concentration of 10 wt% were selected as optimized parameters for the preparation of the electrolyte.
1 and the MIL-53(Al) concentration of 10 wt% were selected as optimized parameters for the preparation of the electrolyte.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li ratios
Li ratios
EO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li Li |
X | Ionic conductivities (S cm−1) | ||
|---|---|---|---|---|
| 30 °C | 60 °C | 80 °C | ||
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 | 1.29 × 10−5 | 4.30 × 10−4 | 9.03 × 10−4 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 | 1.62 × 10−5 | 4.48 × 10−4 | 9.71 × 10−4 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 | 3.36 × 10−6 | 8.41 × 10−5 | 1.68 × 10−4 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 | 1.14 × 10−6 | 6.60 × 10−5 | 1.65 × 10−4 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 6.35 × 10−7 | 4.70 × 10−5 | 9.24 × 10−5 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 | 1.13 × 10−6 | 2.63 × 10−5 | 2.41 × 10−4 |
Previous studies found that the ionic conduction behavior is different in the crystalline phase and amorphous phase of the PEO based electrolyte, and the ionic conductivity is higher in the amorphous phase than in the crystalline phase.25 In order to determine the phase transition temperature and find the applicable operating temperature range of the PEO-MIL-53(Al)-LiTFSI electrolyte, ionic conductivities at 40, 45, 50, 55, 60, 65, 70, 80, 100, 120 and 150 °C were measured (see Fig. 2). The ionic conductivity of the electrolyte is 3.39 × 10−3 S cm−1 at 120 °C, 3.5 times greater than that of the PEO-LiTFSI electrolyte without MIL-53(Al) (9.66 × 10−4 S cm−1) at the same EO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li ratio of 15
Li ratio of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Meanwhile, the log
1. Meanwhile, the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) σ shows a linear relation with 1/T at low temperatures, while at high temperatures a nonlinear relation is presented. Thus, by fitting the data with the Arrhenius equation (
σ shows a linear relation with 1/T at low temperatures, while at high temperatures a nonlinear relation is presented. Thus, by fitting the data with the Arrhenius equation (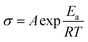 )26 at low temperatures and the Vogel–Tamman–Fulcher (VTF) equation (
)26 at low temperatures and the Vogel–Tamman–Fulcher (VTF) equation ( )27 at high temperatures, the phase transition temperatures of 50.3 °C for the PEO-MIL-53(Al)-LiTFSI electrolyte and 56.9 °C for the PEO-LiTFSI electrolyte are obtained, where σ is the ionic conductivity, A is the pre-exponential factor, Ea is the activation energy for ionic transport, R is the gas constant, k is the Boltzmann constant, B is the pseudo activation energy, T is the test absolute temperature, and T0 is the equilibrium glass transition temperature. There is a decrease of 6.6 °C in the phase transition temperature. This is ascribed to the addition of MIL-53(Al), leading to the higher ionic conductivities of the PEO-MIL-53(Al)-LiTFSI electrolyte. In addition, above the phase transition temperatures, both the electrolytes are amorphous and the ionic conductivities are higher. These results indicate that the ionic conduction behavior is indeed related to the phase structure.
)27 at high temperatures, the phase transition temperatures of 50.3 °C for the PEO-MIL-53(Al)-LiTFSI electrolyte and 56.9 °C for the PEO-LiTFSI electrolyte are obtained, where σ is the ionic conductivity, A is the pre-exponential factor, Ea is the activation energy for ionic transport, R is the gas constant, k is the Boltzmann constant, B is the pseudo activation energy, T is the test absolute temperature, and T0 is the equilibrium glass transition temperature. There is a decrease of 6.6 °C in the phase transition temperature. This is ascribed to the addition of MIL-53(Al), leading to the higher ionic conductivities of the PEO-MIL-53(Al)-LiTFSI electrolyte. In addition, above the phase transition temperatures, both the electrolytes are amorphous and the ionic conductivities are higher. These results indicate that the ionic conduction behavior is indeed related to the phase structure.
 | ||
Fig. 2 Temperature-dependent conductivities for the PEO-10 wt%MIL-53(Al)-LiTFSI electrolyte and the PEO-LiTFSI electrolyte at the same EO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li ratio of 15 Li ratio of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The circles are the experimental data and the lines are the fitted results.28,29 Inset: the AC impedance spectra of the PEO-MIL-53(Al)-LiTFSI electrolyte at selected temperatures of 40, 60, 80, 120 and 150 °C. 1. The circles are the experimental data and the lines are the fitted results.28,29 Inset: the AC impedance spectra of the PEO-MIL-53(Al)-LiTFSI electrolyte at selected temperatures of 40, 60, 80, 120 and 150 °C. | ||
The lithium ion transference number, t+, is another desirable parameter of electrolytes.30,31 The measurement of t+ was carried out by using AC impedance spectroscopy and chronoamperometry (CA). Fig. 3 shows the relation between time and current crossing a symmetric Li/PEO-MIL-53(Al)-LiTFSI/Li battery polarized by a small voltage of 10 mV at 80 °C. The inset is the AC impedance spectra of the same battery before and after the polarization. Then, the t+ can be calculated by the following equation:32
 | (2) |
 | ||
| Fig. 3 Chronoamperometry of the Li/PEO-MIL-53(Al)-LiTFSI/Li cells at a potential of 10 mV at 80 °C. Inset: the AC impedance spectra of the same battery before and after the polarization. | ||
3.3 Lithium ion conduction mechanism for the PEO-MIL-53(Al)-LiTFSI electrolyte
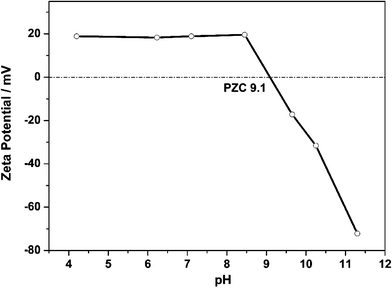
The mechanism for the enhancement of lithium ion transfer properties has been preliminarily explored. The zeta electric potential of the MIL-53(Al) nanoparticles was measured and the result is shown in Fig. 4. The point of zero charge (PZC) for the particles is at pH 9.1. When pH is lower than 9.1, the surface exhibits a Lewis acidic property. Previous studies found that the ionic conductivity and the lithium ion transference number were increased when adding Lewis acidic ceramic nanoparticles.33–36 In this study, the pH of the PEO-MIL-53(Al)-LiTFSI electrolyte is about 7, thus, the particles have strong Lewis acidic properties. Under this condition, the N(SO2CF3)2− anions of the LiTFSI salt may reside on the Lewis acidic surfaces of MIL-53(Al) nanoparticles, while the Li+ ions are released from the salt and approach the ether oxygen atoms in the PEO chains. This interaction not only disturbs the crystallization of PEO, but also increases the lithium salt dissolution. This experiment demonstrates again that the Lewis acidic nanoparticles can improve the lithium ionic conductivity in solid polymer electrolytes.3.4 Electrochemical stability of the electrolytes
The electrochemical stability of the PEO-LiTFSI electrolyte and the PEO-MIL-53(Al)-LiTFSI electrolyte at 80 °C and 120 °C were measured by using linear sweep voltammograms (LSV) in the potential range of 2.5 V to 6.5 V (vs. Li+/Li) (see Fig. 5). The curves represent oxidation decomposition at the potentials of 5.31 V at 80 °C and 5.10 V at 120 °C for the PEO-MIL-53(Al)-LiTFSI electrolyte. For a comparison, the electrochemical stability of the PEO-LiTFSI electrolyte without MIL-53(Al) was investigated and the lower oxidation potentials of 5.15 V at 80 °C and 4.99 V at 120 °C were measured. It is noticed that there is a small current increase of about 0.2 mA at 4.5 V and 120 °C. Here, this small current increase is ignored in the determination of the decomposition potential.3.5 Thermal stability and mechanical characteristics of the electrolytes
The thermal stability of electrolytes is a key factor that determines the safety performance of batteries. Thermogravimetric analysis (TGA) of the PEO-MIL-53(Al)-LiTFSI electrolyte and the PEO-LiTFSI electrolyte were carried out (results are shown in Fig. 6a). By differential processing of the TGA data, the differential thermogravimetric (DTG) curves for the electrolytes are obtained and are presented in Fig. 6b. Similar three-step degradations in the temperature range of 48–500 °C for both electrolytes are observed. In the PEO-MIL-53(Al)-LiTFSI electrolyte, the first degradation beginning from 195 °C corresponds to the decomposition of PEO.37 The second degradation occurring at 375 °C is mainly due to the decomposition of LiTFSI.38 The third is also caused by the decomposition of PEO. The residual at 500 °C (about 6%) for the PEO-MIL-53(Al)-LiTFSI electrolyte is from partially carbonized MIL-53(Al).23 The decomposition peaks in the DTG curve for the PEO-MIL-53(Al)-LiTFSI electrolyte shift right compared to the PEO-LiTFSI electrolyte, with the LiTFSI decomposition temperature 15 degrees higher. The approximate 1.3% weight loss at 48 °C for both electrolytes is due to water absorbed during the sample transfer process. | ||
| Fig. 6 (a) Thermogravimetric analysis of the PEO-LiTFSI and PEO-MIL-53(Al)-LiTFSI electrolytes and (b) DTG curves of the PEO-LiTFSI and PEO-MIL-53(Al)-LiTFSI electrolytes. | ||
In order to characterize the mechanical strength, dynamic mechanical analysis (DMA) of the PEO-MIL-53(Al)-LiTFSI and PEO-LiTFSI electrolytes was performed. From the stress–strain curves in Fig. 7, the stress of the PEO-MIL-53(Al)-LiTFSI electrolyte is obviously higher than the PEO-LiTFSI electrolyte. The enhancement in the thermal stability and mechanical strength can be attributed to the addition of MIL-53(Al) nanoparticles, which act as crossing-linking centers for PEO, thus constructing a robust network. These results further demonstrate that the MIL-53(Al) nanoparticles with Lewis acidic surfaces are beneficial for the improvement of solid polymer electrolyte performance.
3.6 Cycling performance of all-solid-state LiFePO4/PEO-MIL-53(Al)-LiTFSI/Li batteries
Fig. 8 shows the cycling performance of the LiFePO4/PEO-MIL-53(Al)-LiTFSI/Li batteries at 5 C, at 80 °C and 120 °C. For the purpose of retaining high discharge capacities at fast charging/discharging, the batteries were pre-charged/discharged at 1 C for about ten cycles (the data are not included in Fig. 8). The initial discharge capacity at 5 C is 127.1 mA h g−1 at 80 °C and 136.4 mA h g−1 at 120 °C. After 300 cycles, the discharge capacity is 116.0 mA h g−1 at 80 °C and 129.2 mA h g−1 at 120 °C. In the inset of Fig. 8, when the battery ran 610 cycles at 80 °C, the retention ratio of the discharge capacity is 80%; when cycled at 120 °C, the battery has a discharge capacity of 109.3 mA h g−1 in the 625th cycle with fading of 20%. The batteries were cycled for 1400 cycles at the same rate and the same temperatures, still demonstrating discharge capacities of 65.9 mA h g−1 and 82.9 mA h g−1 at 80 °C and 120 °C, and retention ratios of 52.4% and 61.3%, respectively.In order to further evaluate fast charging/discharging performance, the battery was cycled at 10 C and 120 °C (the result is shown in Fig. 9). When cycling at 10 C (about 4–5 min for charging or discharging), the battery has the discharge capacity of 116.2 mA h g−1 in the first cycle and 103.5 mA h g−1 in the 110th cycle with a retention ratio of 90%. At the same temperature and 1 C, another battery can run 530 cycles with the discharge capacity of 129.8 mA h g−1, retaining 90% of the highest discharge capacity (see Fig. 9). Additionally, the inset of Fig. 9 also shows the typical potential vs. time profiles at 1 C, 10 C and 120 °C. Flat voltage plateaus reflecting the insertion/removal of Li+ ions at the LiFePO4 cathode are seen. Even in the 110th cycle at 10 C, the plateaus still exist. Compared to all-solid-state lithium ion batteries with PEO based electrolyte reported,10–18 the LiFePO4/PEO-MIL-53(Al)-LiTFSI/Li battery exhibits better cycling performance at high rates and temperatures.
4. Conclusions
A PEO-MIL-53(Al)-LiTFSI electrolyte was prepared by using the metal–organic framework MIL-53(Al) as a filler for all-solid-state LIBs. The MIL-53(Al) nanoparticles have Lewis acidic surfaces and interact with N(SO2CF3)2− anions that promote the dissolution of LiTFSI, thus increasing the lithium ionic conductivity. The particles also act as crossing-linking centers for PEO, leading to the increase in thermal stability and mechanical strength from the robust network built. The battery with the electrolyte displays fast charging/discharging abilities. Such electrolytes, which may replace both the separator and liquid electrolyte in conventional batteries, can be used directly for fast charging/discharging all-solid-state lithium ion batteries. This is an important step in expanding applications of lithium ion batteries.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 51274239) and Central South University, which is greatly appreciated.References
- H. J. Zhang, S. Kulkarni and S. L. Wunder, J. Phys. Chem. B, 2007, 111, 3583–3590 CrossRef CAS PubMed.
- J. F. M. Oudenhoven, L. Baggetto and P. H. L. Notten, Adv. Energy Mater., 2011, 1, 10–33 CrossRef CAS PubMed.
- S. Lee, M. Schomer, H. Peng, K. A. Page, D. Wilms, H. Frey, C. L. Soles and D. Y. Yoon, Chem. Mater., 2011, 23, 2685–2688 CrossRef CAS.
- M. Patel, M. U. M. Patel and A. J. Bhattacharyya, ChemSusChem, 2010, 3, 1371–1374 CrossRef CAS PubMed.
- J. Cao, L. Wang, X. M. He, M. Fang, J. Gao, J. J. Li, L. F. Deng, H. Chen, G. Y. Tian, J. L. Wang and S. S. Fan, J. Mater. Chem. A, 2013, 1, 5955–5961 CAS.
- United States Advanced Battery Consortium, Electric Vehicle Battery Test Procedures Manual, Revision 2, Southfield, MI, 1996 Search PubMed.
- F. Croce, G. B. Appetecchi, L. Persi and B. Scrosati, Nature, 1998, 394, 456–458 CrossRef CAS PubMed.
- Y. Lin, J. Li, Y. Q. Lai, C. F. Yuan, Y. Cheng and J. Liu, RSC Adv., 2013, 3, 10722–10730 RSC.
- J. Li, Y. Lin, H. H. Yao, C. F. Yuan and J. Liu, ChemSusChem, 2014, 7, 1901–1908 CrossRef CAS PubMed.
- S. T. Ren, H. F. Chang, L. J. He, X. F. Dang, Y. Y. Fang, L. Y. Zhang, H. Y. Li, Y. L. Hu and Y. Lin, J. Appl. Polym. Sci., 2013, 129, 1131–1142 CrossRef CAS PubMed.
- Y. F. Tong, L. Chen, X. H. He and Y. W. Chen, Electrochim. Acta, 2014, 118, 33–40 CrossRef CAS PubMed.
- Y. H. Li, X. L. Wu, J. H. Kim, S. Xin, J. Su, Y. Yan, J. S. Lee and Y. G. Guo, J. Power Sources, 2013, 244, 234–239 CrossRef CAS PubMed.
- J. S. Syzdek, M. B. Armand, P. Falkowski, M. Gizowska, M. Karzowicz, L. Lukaszuk, M. L. Marcinek, A. Zalewska, M. Szafran, C. Masquelier, J. M. Tarascon, W. G. Wieczorek and G. Zukowska, Chem. Mater., 2011, 23, 1785–1797 CrossRef CAS.
- J. L. Schaefer, D. A. Yanga and L. A. Archer, Chem. Mater., 2013, 25, 834–839 CrossRef CAS.
- R. R. Madathingal and S. L. Wunder, Macromolecules, 2011, 44, 2873–2882 CrossRef CAS.
- B. W. Zewde, S. Admassie, J. Zimmermann, C. S. Isfort, B. Scrosati and J. Hassoun, ChemSusChem, 2013, 6, 1400–1405 CrossRef CAS PubMed.
- C. F. Yuan, J. Li, P. F. Han, Y. Q. Lai, Z. A. Zhang and J. Liu, J. Power Sources, 2013, 240, 653–658 CrossRef CAS PubMed.
- C. Gerbaldi, J. R. Nair, M. A. Kulandainathan, R. S. Kumar, C. Ferrara, P. Mustarelli and A. M. Stephan, J. Mater. Chem. A, 2014, 2, 9948–9954 CAS.
- W. J. Rieter, K. M. Pott, K. M. L. Taylor and W. Lin, J. Am. Chem. Soc., 2008, 130, 11584–11585 CrossRef CAS PubMed.
- K. A. White, D. A. Chengelis, K. A. Gogick, J. Stehman, N. L. Rosi and S. Petoud, J. Am. Chem. Soc., 2009, 131, 18069–18071 CrossRef CAS PubMed.
- A. J. Lan, K. H. Li, H. H. Wu, D. H. Olson, T. J. Emge, W. Ki, M. C. Hong and J. Li, Angew. Chem., Int. Ed., 2009, 48, 2334–2338 CrossRef CAS PubMed.
- S. J. Garibay and S. M. Cohen, Chem. Commun., 2010, 46, 7700–7702 RSC.
- T. Loiseau, C. Serre, C. Huguenard, G. Fink, F. Taulelle, M. Henry, T. Bataille and G. Férey, Chem.– Eur. J., 2004, 10, 1373–1382 CrossRef CAS PubMed.
- J. A. Greathouse and M. D. Allendorf, J. Am. Chem. Soc., 2006, 128, 10678–10679 CrossRef CAS PubMed.
- B. Kumar, S. J. Rodrigues and S. Koka, Electrochim. Acta, 2002, 47, 4125–4131 CrossRef CAS.
- Y. H. Li, X. L. Wu, J. H. Kim, S. Xin, J. Su, Y. Yan, J. S. Lee and Y. G. Guo, J. Power Sources, 2013, 244, 234–239 CrossRef CAS PubMed.
- R. S. Bobadea, S. V. Pakade and S. P. Yawale, J. Non-Cryst. Solids, 2009, 355, 2410–2414 CrossRef PubMed.
- F. B. Dias, L. Plomp and J. J. B. Veldhuis, J. Power Sources, 2000, 88, 169–191 CrossRef CAS.
- C. D. Robitaille and D. Fauteux, J. Electrochem. Soc., 1986, 133, 315–325 CrossRef CAS PubMed.
- M. Doyle, T. F. Fuller and J. Newman, Electrochim. Acta, 1994, 39, 2073–2081 CrossRef CAS.
- K. E. Thomas, S. E. Sloop, J. B. Kerr and J. Newman, J. Power Sources, 2000, 89, 132–138 CrossRef CAS.
- J. Evans, C. A. Vincent and P. G. Bruce, Polymer, 1987, 28, 2324–2328 CrossRef CAS.
- F. Croce, L. Persi, B. Scrosati, F. S. Fiory, E. Plichta and M. A. Hendrickson, Electrochim. Acta, 2001, 46, 2457–2461 CrossRef CAS.
- W. Wieczorek, Z. Florjancyk and J. R. Stevens, Electrochim. Acta, 1995, 40, 2251 CrossRef CAS.
- F. Croce, R. Curini, A. Martinelli, L. Persi, F. Ronci and B. Scrosati, J. Phys. Chem., 1999, 103, 10632–10638 CrossRef CAS.
- N. Angulakshmi, K. S. Nahm, J. R. Nair, C. Gerbaldi, R. Bongiovanni, N. Penazzi and A. M. Stephan, Electrochim. Acta, 2013, 90, 179–185 CrossRef CAS PubMed.
- T. Shodai, B. B. Owens, T. Ostsuka and J. Yamaki, J. Electrochem. Soc., 1994, 141, 2978–2981 CrossRef CAS PubMed.
- M. Echeverri, N. Kim and T. Kyu, Macromolecules, 2012, 45, 6068–6077 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |