Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A TbPc2 sub-monolayer deposit on a titanium dioxide ultrathin film: magnetic, morphological, and chemical insights†
Andrea Luigi
Sorrentino
 ab,
Irene
Cimatti
b,
Giulia
Serrano
ab,
Irene
Cimatti
b,
Giulia
Serrano
 *ab,
Lorenzo
Poggini
*ab,
Lorenzo
Poggini
 *bc,
Brunetto
Cortigiani
b,
Luigi
Malavolti
de,
Edwige
Otero
f,
Philippe
Sainctavit
fg,
Matteo
Mannini
*bc,
Brunetto
Cortigiani
b,
Luigi
Malavolti
de,
Edwige
Otero
f,
Philippe
Sainctavit
fg,
Matteo
Mannini
 b,
Roberta
Sessoli
b,
Roberta
Sessoli
 b and
Andrea
Caneschi
b and
Andrea
Caneschi
 a
a
aDIEF – Department of Industrial Engineering and INSTM Research Unit, University of Florence, Via S. Marta 3, I-50139 Florence, Italy. E-mail: giulia.serrano@unifi.it
bDICUS – Department of Chemistry ‘‘Ugo Schiff’’ and INSTM Research Unit, University of Florence, I-50019 Sesto Fiorentino (FI), Italy
cICCOM-CNR, via Madonna del Piano 10, 50019 Sesto Fiorentino, Italy. E-mail: lpoggini@iccom.cnr.it
dInstitute for Functional Matter and Quantum Technologies, University of Stuttgart, 70569 Stuttgart, Germany
eMax Planck Institute for Solid State Research, 70569 Stuttgart, Germany
fSynchrotron SOLEIL, L’Orme des Merisiers, Saint Aubin, France
gIMPMC, UMR7590 CNRS, Sorbonne Université, MNHN, Paris, France
First published on 10th September 2021
Abstract
Thin inorganic films (i.e., metal oxides) are often used as decoupling layers to optimize the interactions between the magnetic layers of molecules and metallic surfaces. For deposits of single-molecule magnets (SMMs), a decoupling layer can minimise the hybridization of the metallic substrate that is responsible for the quenching of their typical magnetic bistability. Here, we explored the potential of a single layer of titania to be used as a decoupling layer, which could represent an interesting playground for widespread use in many technological applications. We used a TiO2 monolayer with a lepidocrocite-like structure grown on a Ag(100) substrate for the deposition of the terbium(III) bis-phthalocyaninato (TbPc2) complex. A multi-technique approach employing X-ray photoelectron spectroscopy and scanning tunnelling microscopy was used to examine the integrity of a TbPc2 sub-monolayer deposit and to study the molecular adsorption configuration on the TiO2 film. Furthermore, X-ray magnetic circular dichroism was used to investigate the magnetic properties of the TbPc2 sub-monolayer, revealing that the TiO2 film successfully preserves the molecular spin character. X-ray-based magnetic measurements showed that the quantum tunnelling of the magnetization characterizing a bulk of molecules is still present and that the present titania film displays a decoupling effect of comparable efficiency to that of a graphene layer.
Introduction
Molecular spintronics is a growing research field focusing on developing innovative devices for sensing, data storage, and quantum applications by combining the properties of inorganic and organic materials.1,2 In the last few years, single-molecule magnets (SMMs) have aroused significant interest for their use as magnetic building blocks in spintronic micro-devices and nano-devices3–7 due to their low-temperature magnetic bistability8 and unique quantum behaviour.9 Among systems showing the SMM behaviour, the terbium(III) bis-phthalocyaninato (TbPc2) neutral complex, consisting of a TbIII ion coordinated by two phthalocyanine ligands staggered by about 45° with respect to each other,6,7,10–12 is considered as a perfect candidate for testing these perspectives because of its slow magnetization dynamics at liquid helium temperatures.13 Besides, its molecular nature allows the formation of monolayer and sub-monolayer deposits on a solid surface. In bulk, the magnetic behaviour of TbPc2 is dominated by a strong uniaxial anisotropy with the easy axis of magnetization located perpendicular to the Pc rings. The large energy separation between the ground doublet (Jz = ± 6) and the first excited state is responsible for the magnetic bistability as an effective barrier of several hundreds of kelvin opposes the reversal of the magnetization.14 However, when assembled on different substrates, its magnetism can be highly influenced by the substrate.15–19 The planar structure of the double-deckers strongly favours a strong electronic interaction with the surface through the π-electrons of the ligands. In particular, a significant quenching of the magnetic bistability in sub-monolayer films assembled on bare metal surfaces has been reported.17,20–23 In contrast, it has been demonstrated that decoupling layers (e.g., graphene or a MgO thin film) can reduce this effect.19,24–27 Indeed, according to Wäckerlin et al.,27 using a single layer of MgO is not sufficient to efficiently decouple the TbPc2 molecule from a metal substrate. Increasing the MgO layer thickness up to 5 monolayers (ML) improves the magnetic bistability, and TbPc2 features a large magnetic remanence at variance with the butterfly-shaped hysteresis loop observed in the bulk phase.27,28 The mechanisms behind these substantial changes of SMM properties are still unclear. It has been suggested that the low phonon dispersion of the metal oxide could be a key ingredient for the observation of this enhancement.28 Furthermore, the possibility of a charge transfer to/from the substrate can affect the crystal field splitting acting on the terbium(III) ion and induce a slower magnetic relaxation.16To obtain further insights on this topic, we investigated the use of an ultrathin film of titanium dioxide (TiO2) grown as a single layer on the Ag(100) surface as an alternative substrate for TbPc2 SMMs.29 The deposition of atoms and molecules on TiO2 surfaces was already deeply investigated in light of the high surface reactivity of titania surfaces due to the presence of active catalytic sites as surface defects.30,31 In particular, the deposition of metallated phthalocyanine MPc systems on a bulk TiO2 substrate showed that molecules in direct contact with the surface interact strongly and can undergo an oxidation process.31–34 Furthermore, our recent studies on bulk TiO2 single crystals revealed that also the sub-monolayer of TbPc2 molecules deposited on the TiO2(110) rutile surface undergoes a strong interfacial interaction independently of the surface preparation and the presence of surface defects.35
In this work, we studied the molecular organization and the magnetic properties of a sub-monolayer of TbPc2 molecules thermally sublimated on a TiO2 film grown with a lepidocrocite-like structure on Ag(100) (TiO2-L). Using X-ray photoelectron spectroscopy (XPS) and scanning tunnelling microscopy (STM), we evaluated the presence of intact molecules and their organization on the surface. Finally, by using synchrotron-based X-ray absorption spectroscopy, we evaluated the structural and magnetic properties of the sub-monolayer molecular deposit, confirming the lying-down absorption of the molecules and evidencing the persistence at 2.0 K of the SMM behaviour.
Experimental
The Ag(100) single crystal was cleaned by several cycles of Ar+ sputtering (1500 eV) and annealing (770 K for 30 min) in UHV before the TiO2 film growth. Titanium was subsequently deposited by a Ti (99.999%) rod using an electron beam source. The deposition rate was evaluated by XPS and STM measurements. The TiO2 thin film formation was achieved by depositing the metal under an oxygen partial pressure of 2 × 10−6 mbar in consecutive steps according to the procedure reported in ref. 29 and 36. After the film deposition, the sample was annealed up to 770 K for one hour. The epitaxial growth of the TiO2 thin film on Ag(100) was confirmed by low-energy electron diffraction (LEED). The chemical composition and morphology were confirmed by XPS and STM measurements, providing results that are in line with previous reports.29,36 The sublimation of TbPc2 was carried out in a UHV chamber equipped with a home-made Knudsen cell filled with TbPc2 powders. XPS data were acquired using monochromatic Al Kα radiation (hν = 1486.6 eV, SPECS mod. XR-MS focus 600) operating at a power of 100 W (13 kV and 7.7 mA) and a SPECS Phoibos 150 1DLD electron analyser mounted at 54.44° to the X-ray source. The XPS spectra were collected at normal emission with the fixed pass energy set to 40 eV. The spectra were analysed using the CasaXPS software. All the spectra were calibrated at the Ti 2p3/2 signal at 459.3 eV. The background in the spectra was subtracted using a linear background, and the deconvolution of the XPS spectra was carried out as a combination of Gaussian and Lorentzian functions (70/30).35 LEED patterns were acquired using an Omicron NG-LEED setup, and the simulation pattern was obtained using the software LEEDPat42.37STM measurements were carried out using an Omicron VT-STM at room temperature for TiO2 characterization and at 35 K for the characterization of TbPc2 deposits. All the images were acquired using an electrochemically etched W tip. Sample preparation and investigation by using XPS, LEED and STM were performed in situ with the multi-technique platform available in our laboratory. The TbPc2 deposition has thus been repeated at the synchrotron using the UHV preparation chamber present at the DEIMOS beamline in vacuum connection with the XAS end station. The molecular deposition was performed using the same Knudsen cell used for in-house experiments and operating under similar geometrical and physical conditions. The TiO2-L substrate was prepared in Florence (Italy) and transferred to the DEIMOS beamline facility in Paris (France) using an HV suitcase (with a base pressure of about 10−6 mbar).
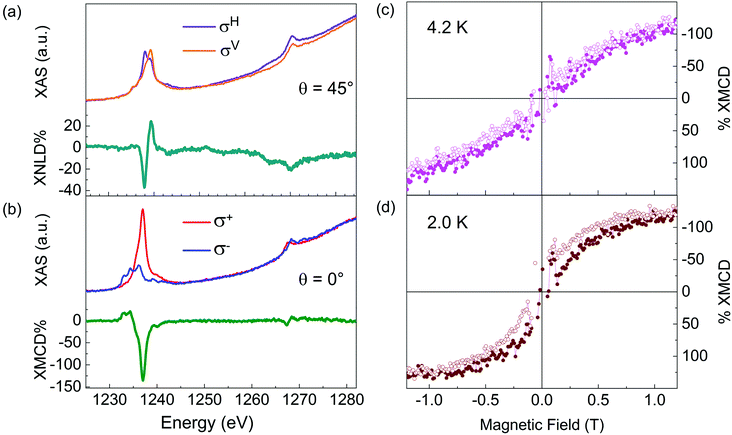
X-Ray absorption spectroscopy (XAS) experiments were performed at the DEIMOS beamline38 (SOLEIL synchrotron, Paris), employing both linear and circular polarization and total electron yield (TEY) detection.39 The investigated temperatures were 4.2 ± 0.2 and 2.0 ± 0.2 K. All the XAS spectra were acquired at B = 3 T at the Tb M4,5 edges and the θ parameter was defined as the angle between the k X-ray propagation vector and the normal n to the surface that always lies in the horizontal plane. The X-ray natural linear dichroism (XNLD) was extracted for θ =45° as the difference between the horizontally (σH) and vertically (σV) polarized light. The XNLD contribution was normalized with respect to the M5 edge maximum of the isotropic spectrum at θ = 45° (1/3σV + 2/3σH) and expressed in percentage (% XNLD).40 XNLD was not measured at θ = 0° since no signal was expected for any orientation of the molecules because, at the macroscopic scale, there is always a cylindrical symmetry in the plane. X-ray magnetic circular dichroism (XMCD) was measured at θ = 0° as the difference between the XAS spectra obtained using circularly polarized light (σ− − σ+), normalized to the M5 edge maximum of (σ+ + σ−)/2 and expressed in percentage (% XMCD). Finally, the magnetic hysteresis curves were obtained by monitoring the field dependence of % XMCD at the Tb M5 edge (1237 eV).
Results and discussion
The TiO2-L film growth was studied by XPS to confirm the expected film stoichiometry29 and the Ti oxidation state, while STM was used to confirm its quality and morphology. The Ti 2p and O 1s XPS core level spectra are presented in Fig. 1a (top panel) and Fig. S1 (ESI†) (top panel), respectively. Concerning the Ti 2p region, the main TiIV component (filled in yellow) was accompanied by a small contribution attributed to TiIII sites (filled in green).41,42 The semi-quantitative analysis of the film (Table S1, ESI†) gave a Ti/O ratio of about 0.4, reflecting a slight excess of oxygen on the surface.29 Additional details about the XPS characterization can be found in the XPS section of the ESI.† The lepidocrocite structure of the achieved deposit was confirmed by a LEED pattern (Fig. 2a), showing the presence of the TiO2-L structure (red rectangles) on the Ag(100) surface (dashed black square) (see Fig. S2 of the ESI† for details about the LEED analysis). The STM analysis (Fig. 2b and Fig. S3a, ESI†) confirmed the presence of TiO2 islands covering about 80% of the Ag(100) surface. TiO2 islands showed a height of about 0.4 nm (Fig. S3b, ESI†), as previously reported for a single layer of TiO2 with a lepidocrocite-like structure, TiO2-L.29,43 TiO2 islands featured alternating bright and dark stripes due to the lattice mismatch between the film and the silver surface lattice (see Fig. 2b).29 The observed TiO2-L corrugation had the expected periodicity of 10 nm and a height modulation of 0.05 nm. The narrow dark stripes are due to a compression of the oxide layer along the short side of the unit cell, which coincides with the Ag(100) lattice parameters, while the wider bright patches correspond to the unstrained film.29 The low roughness of the investigated sample further confirms the sub-monolayer nature of the TiO2 film. In fact, thicker deposits lead to non-uniform Stranski–Krastanov growth on Ag(100).36 As non-ordered 3D islands appear under these conditions, the growth was limited to the first TiO2 layer. A sub-monolayer of TbPc2 (see the inset of Fig. 1b, bottom panel) was sublimated on TiO2-L according to the procedure reported in the Experimental section. Fig. 1b (bottom panel) shows the XPS spectrum of the C 1s core level of the TbPc2 sub-monolayer deposit. The spectrum was fitted using four components. The main one, located at 284.3 eV (filled in grey), was ascribed to the C–C bonds of the phthalocyanine (Pc) ligand, while the second one at 285.5 eV (filled in blue) was ascribed to the C–N bonds of the Pc rings; two satellites (filled in grey and blue) were found at 287.0 eV and 288.1 eV, respectively, which were attributed to C–C and C–N shake-up contributions.16,44 Due to the overlapping between the N 1s and Ag 3d XPS signals and between the Tb 3d and C KLL plus Ag MNN Auger signals, a semi-quantitative elemental analysis of the molecular layer was not affordable. However, the analysis of the C 1s region indicated a TbPc2 deposit weakly interacting with the substrate.16,35,45 This is at variance with previous findings for TbPc2 or phthalocyanine molecules in direct contact with a bulk TiO2 substrate where oxidation of the molecular species was observed.31–35 Such a difference in the substrate reactivity could be an indication of the absence of catalytic sites characteristic of many TiO2 surfaces, such as oxygen vacancies,30,31 or it can be attributed to a different intrinsic reactivity of the TiO2 lepidocrocite structure compared to the rutile phase used in our previous investigation.35,46 We notice that also the Ti 2p and O 1s spectra collected after the TbPc2 deposition (Fig. 1a and Fig. S1 (ESI†), bottom panels) do not reveal significant variations in both the line shape and the semi-quantitative analysis (Table S1, ESI†), thus confirming the innocent role played by the TiO2 layer on the assembled molecules. Fig. 2c shows a low-temperature STM image of TbPc2 molecules covering about 10% of TiO2-L islands on Ag(100). After molecular deposition, the corrugated lepidocrocite-like structure29 is still visible (see the profile in Fig. S4, ESI†), confirming that the TbPc2 absorption did not alter the TiO2 surface. The small-scale STM images of TbPc2 molecules adsorbed on TiO2-L are presented in Fig. 2d and e. TbPc2 molecules show their typical four-lobed features in agreement with previous reports on other surfaces,15,18,23 indicating a lying down configuration (Pc planes parallel to the surface), as observed on metals and metal oxides.18,19,21,23 The STM line profiles (the inset in Fig. 2e) reveal that the TbPc2 molecules have an apparent height of 0.30 ± 0.05 nm and a lateral dimension of 2.5 ± 0.1 nm in agreement with previous reports about single TbPc2 molecules on metals.23,47 It is worth noting that molecules do not pack in regular islands19,27,47,48 but lie isolated with a random distribution on the TiO2-L surface, similar to what was observed on Cu(100)21,23 and for CoPc molecules on TiO2(100).32 On the bare Ag(100) areas, small features attributable to phthalocyanines were observed (Fig. S5, ESI†). Their presence may result from (i) a partial thermal decomposition occurring during the deposition process47,49,50 or (ii) a partial fragmentation of the TbPc2 complex on Ag(100) in the proximity of the reactive TiO2-L step edges.51,52Low-temperature XAS studies were carried out on the TbPc2 sub-monolayer on TiO2-L to evaluate the structural and magnetic properties of the molecular layer. The linear and circularly polarized absorption spectrum was recorded at the Tb M4,5 edge. The XNLD spectrum (Fig. 3a) revealed a strong negative dichroic signal at 1237 eV, confirming the preferential lying-down configuration of the TbPc2 monolayer16,17,19,20,27 observed by STM. The XMCD signal (Fig. 3b) measured at 2 K confirmed the characteristic M5 edge dichroic signal of TbIII ions located at 1237 eV. Dichroism was found to be ca. 135%, in agreement with previous results obtained for the TbPc2 thin film with a similar absorption geometry.16,17,19,20,27 Indeed, the shape of the XMCD spectrum revealed the presence of a saturated TbIII system characterized by J = L + S = 6 total angular momentum.15,20 Finally, hysteresis loops were recorded at the maximum signal of XMCD (see the Experimental section) at 4.2 K (Fig. 3c) and 2.0 K (Fig. 3d). At 4.2 K, a hysteretic behaviour was substantially absent, at variance with the bulk phase,53 while the opening of the hysteresis loop was observed at a lower temperature of 2.0 K. The hysteretic behaviour can be better appreciated by looking at the ΔXMCD(H) value obtained by plotting the difference of the XMCD signal of the up and down branches of the hysteresis loops, ΔXMCD(H) = XMCD(H↑) − XMCD(H ↓) (Fig. S6a, ESI†). This result indicates that the magnetic bistability at 2.0 K is preserved, thus confirming that the TiO2-L film causes an effective decoupling from the metal substrate.15,27 On the other hand, the observed typical butterfly shape of the magnetic hysteresis loop (Fig. 3d) suggests that the quantum tunnelling of magnetization is not suppressed, similarly to what was reported for other bi-dimensional materials such as graphite26 and graphene,19 but at variance with the behaviour observed on MgO films where large magnetic remanence was observed.27,28 However, a direct comparison with the more efficient decoupling performed by MgO layers cannot be made due to the different thicknesses of the films investigated.27,29 Indeed, studies on MgO multilayer films (up to 6 ML) showing an increase of the magnetic remanence of TbPc2 also revealed the suppression of conduction electrons scattering from the metal to the molecule.27 Additionally, we cannot exclude that the partially negative charge of the lepidocrocite-like ultrathin film36,54 might also influence the electronic distribution of a TbPc2 deposit, causing an alteration of the SMM magnetization dynamics from the bulk behaviour.
Conclusions
XPS and STM measurements showed the integrity of TbPc2 molecules after the thermal deposition on TiO2-L. The STM images indicated a high absorption affinity of this SMM with TiO2, resulting in isolated and randomly distributed molecules. Furthermore, the STM images and XNLD spectra indicated a preferential lying-down configuration of the TbP2 molecules on the TiO2-L structure. The XMCD spectrum shows a marked dichroic signal revealing the maintenance of a TbIII system with an easy axis of magnetization perpendicular to the surface. A butterfly-shaped hysteresis loop was observed at 2.0 K without the suppression of the QTM process, similarly to what was previously observed on graphene. Further, local probe-based spectroscopic experiments combined with theoretical modelling could shed further light on the surface interaction and on the magnetism of TbPc2 on TiO2 thin films to clarify the role of the surface in the suppression or persistence of the SMM behaviour.55,56 Our results confirmed that ultra-thin layers of decoupling oxides can be used to engineer the interactions between SMMs and metallic substrates.Author contributions
A. L. S., I. C., G. S., M. M., and B. C. designed the film architecture. A. L. S., I. C., and G. S. prepared the molecular and oxide deposits and performed the in-house experiments. A. L. S., I. C., G. S, L. P., M. M., R. S., A. C., L. M., E. O., and P. S. designed, performed, and discussed the synchrotron experiments. G. S., L. P. and M. M. drafted the manuscript. M. M., R. S., and A. C. supervised the work. All authors have contributed to and approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge SOLEIL for the provision of the synchrotron radiation facilities. The authors thank P. Ohresser and L. Joly for their assistance in using the DEIMOS beamline (proposal 20170926). The European COST Action CA15128 MOLSPIN and the FET Open Femtoterabyte project are acknowledged for financial support. Italian MIUR for Progetto Dipartimenti di Eccellenza 2018-2022 project (ref. B96C1700020008) and Fondazione Cassa di Risparmio di Firenze for SPIN-E2 project (ref. 2020.1634) are also acknowledged for financial support.Notes and references
- S. Sanvito, Chem. Soc. Rev., 2011, 40, 3336–3355 RSC.
- A. Cornia and P. Seneor, Nat. Mater., 2017, 16, 505–506 CrossRef CAS PubMed.
- G. Cucinotta, L. Poggini, A. Pedrini, F. Bertani, N. Cristiani, M. Torelli, P. Graziosi, I. Cimatti, B. Cortigiani, E. Otero, R. Sessoli, M. Mannini, P. Ohresser, P. Sainctavit, A. Dediu, E. Dalcanale, R. Sessoli and M. Mannini, Adv. Funct. Mater., 2017, 27, 1703600 CrossRef.
- M. Urdampilleta, N. V. Nguyen, J. P. Cleuziou, S. Klyatskaya, M. Ruben and W. Wernsdorfer, Int. J. Mol. Sci., 2011, 12, 6656–6667 CrossRef CAS PubMed.
- A. Candini, S. Klyatskaya, M. Ruben, W. Wernsdorfer and M. Affronte, Nano Lett., 2011, 11, 2634–2639 CrossRef CAS PubMed.
- R. Vincent, S. Klyatskaya, M. Ruben, W. Wernsdorfer and F. Balestro, Nature, 2012, 488, 357–360 CrossRef CAS PubMed.
- L. Bogani and W. Wernsdorfer, Nat. Mater., 2008, 7, 179–186 CrossRef CAS PubMed.
- G. Christou, D. Gatteschi, D. N. Hendrickson and R. Sessoli, MRS Bull., 2000, 66–71 CrossRef CAS.
- G. Serrano, L. Poggini, M. Briganti, A. L. Sorrentino, G. Cucinotta, L. Malavolti, B. Cortigiani, E. Otero, P. Sainctavit, S. Loth, F. Parenti, A.-L. L. Barra, A. Vindigni, A. Cornia, F. Totti, M. Mannini and R. Sessoli, Nat. Mater., 2020, 19, 546–551 CrossRef CAS PubMed.
- M. Urdampilleta, S. Klyatskaya, J.-P. Cleuziou, M. Ruben and W. Wernsdorfer, Nat. Mater., 2011, 10, 502–506 CrossRef CAS PubMed.
- S. Thiele, R. Vincent, M. Holzmann, S. Klyatskaya, M. Ruben, F. Balestro and W. Wernsdorfer, Phys. Rev. Lett., 2013, 111, 037203 CrossRef CAS PubMed.
- M. Urdampilleta, S. Klayatskaya, M. Ruben and W. Wernsdorfer, ACS Nano, 2015, 9, 4458–4464 CrossRef CAS PubMed.
- C. Godfrin, A. Ferhat, R. Ballou, S. Klyatskaya, M. Ruben, W. Wernsdorfer and F. Balestro, Phys. Rev. Lett., 2017, 119, 187702 CrossRef CAS PubMed.
- N. Ishikawa, M. Sugita, N. Tanaka, T. Ishikawa, S. Koshihara and Y. Kaizu, Inorg. Chem., 2004, 43, 5498–5500 CrossRef CAS PubMed.
- E. Moreno Pineda, T. Komeda, K. Katoh, M. Yamashita and M. Ruben, Dalton Trans., 2016, 45, 18417–18433 RSC.
- M. Mannini, F. Bertani, C. Tudisco, L. Malavolti, L. Poggini, K. Misztal, D. Menozzi, A. Motta, E. Otero, P. Ohresser, P. Sainctavit, G. G. Condorelli, E. Dalcanale and R. Sessoli, Nat. Commun., 2014, 5, 4582 CrossRef CAS PubMed.
- L. Malavolti, L. Poggini, L. Margheriti, D. Chiappe, P. Graziosi, B. Cortigiani, V. Lanzilotto, F. B. de Mongeot, P. Ohresser, E. Otero, F. Choueikani, P. Sainctavit, I. Bergenti, V. A. Dediu, M. Mannini and R. Sessoli, Chem. Commun., 2013, 49, 11506 RSC.
- G. Serrano, S. Wiespointner-Baumgarthuber, S. Tebi, S. Klyatskaya, M. Ruben, R. Koch and S. Müllegger, J. Phys. Chem. C, 2016, 120, 13581–13586 CrossRef CAS.
- G. Serrano, E. Velez-Fort, I. Cimatti, B. Cortigiani, L. Malavolti, D. Betto, A. Ouerghi, N. B. Brookes, M. Mannini and R. Sessoli, Nanoscale, 2018, 10, 2715–2720 RSC.
- L. Margheriti, D. Chiappe, M. Mannini, P.-E. Car, P. Sainctavit, M.-A. Arrio, F. B. de Mongeot, J. C. Cezar, F. M. Piras, A. Magnani, E. Otero, A. Caneschi and R. Sessoli, Adv. Mater., 2010, 22, 5488–5493 CrossRef CAS PubMed.
- S. Stepanow, J. Honolka, P. Gambardella, L. Vitali, N. Abdurakhmanova, T. Tseng, S. Rauschenbach, S. L. Tait, V. Sessi, S. Klyatskaya, M. Ruben and K. Kern, J. Am. Chem. Soc., 2010, 132, 11900–11901 CrossRef CAS PubMed.
- L. Vitali, S. Fabris, A. M. Conte, S. Brink, M. Ruben, S. Baroni and K. Kern, Nano Lett., 2008, 8, 3364–3368 CrossRef CAS PubMed.
- Z. Deng, S. Rauschenbach, S. Stepanow, S. Klyatskaya, M. Ruben and K. Kern, Phys. Scr., 2015, 90, 098003 CrossRef.
- S. Marocchi, A. Candini, D. Klar, W. Van den Heuvel, H. Huang, F. Troiani, V. Corradini, R. Biagi, V. De Renzi, S. Klyatskaya, K. Kummer, N. B. Brookes, M. Ruben, H. Wende, U. del Pennino, A. Soncini, M. Affronte and V. Bellini, ACS Nano, 2016, 10, 9353–9360 CrossRef CAS PubMed.
- V. Corradini, A. Candini, D. Klar, R. Biagi, V. De Renzi, A. Lodi Rizzini, N. Cavani, U. del Pennino, S. Klyatskaya, M. Ruben, E. Velez-Fort, K. Kummer, N. B. Brookes, P. Gargiani, H. Wende and M. Affronte, Nanoscale, 2018, 10, 277–283 RSC.
- M. Gonidec, R. Biagi, V. Corradini, F. Moro, V. De Renzi, U. del Pennino, D. Summa, L. Muccioli, C. Zannoni, D. B. Amabilino and J. Veciana, J. Am. Chem. Soc., 2011, 133, 6603–6612 CrossRef CAS PubMed.
- C. Wäckerlin, F. Donati, A. Singha, R. Baltic, S. Rusponi, K. Diller, F. Patthey, M. Pivetta, Y. Lan, S. Klyatskaya, M. Ruben, H. Brune and J. Dreiser, Adv. Mater., 2016, 28, 5195–5199 CrossRef PubMed.
- M. Studniarek, C. Wäckerlin, A. Singha, R. Baltic, K. Diller, F. Donati, S. Rusponi, H. Brune, Y. Lan, S. Klyatskaya, M. Ruben, A. P. Seitsonen and J. Dreiser, Adv. Sci., 2019, 6, 1901736 CrossRef CAS PubMed.
- A. Atrei, A. M. Ferrari, D. Szieberth, B. Cortigiani and G. Rovida, Phys. Chem. Chem. Phys., 2010, 12, 11587 RSC.
- S. C. Li, L. N. Chu, X. Q. Gong and U. Diebold, Science, 2010, 328, 882–884 CrossRef CAS PubMed.
- S. Yu, S. Ahmadi, P. Palmgren, F. Hennies, M. Zuleta and M. Göthelid, J. Phys. Chem. C, 2009, 113, 13765–13771 CrossRef CAS.
- N. Ishida and D. Fujita, J. Phys. Chem. C, 2012, 116, 20300–20305 CrossRef CAS.
- P. Palmgren, K. Nilson, S. Yu, F. Hennies, T. Angot, J. M. Layet, G. Le Lay and M. Gothelid, J. Phys. Chem. C, 2008, 112, 5972–5977 CrossRef CAS.
- S. Ahmadi, B. Agnarsson, I. Bidermane, B. M. Wojek, Q. Noël, C. Sun and M. Göthelid, J. Chem. Phys., 2014, 140, 174702 CrossRef PubMed.
- G. Serrano, A. L. Sorrentino, L. Poggini, B. Cortigiani, C. Goletti, R. Sessoli and M. Mannini, Phys. Chem. Chem. Phys., 2021, 23, 12060–12067 RSC.
- A. Atrei, B. Cortigiani and A. M. Ferrari, J. Phys.: Condens. Matter, 2012, 24, 445005 CrossRef PubMed.
- K.E. Hermann (FHI) and M.A. Van Hove (HKBU), Berlin/Hong Kong, 2014.
- P. Ohresser, E. Otero, F. Choueikani, K. Chen, S. Stanescu, F. Deschamps, T. Moreno, F. Polack, B. Lagarde, J.-P. Daguerre, F. Marteau, F. Scheurer, L. Joly, J.-P. Kappler, B. Muller, O. Bunau and P. Sainctavit, Rev. Sci. Instrum., 2014, 85, 013106 CrossRef CAS PubMed.
- R. Nakajima, J. Stöhr and Y. U. Idzerda, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 6421–6429 CrossRef CAS.
- C. Brouder, J. Phys.: Condens. Matter, 1990, 2, 701–738 CrossRef CAS.
- M. J. Jackman, A. G. Thomas and C. Muryn, J. Phys. Chem. C, 2015, 119, 13682–13690 CrossRef CAS.
- A. L. Sorrentino, G. Serrano, L. Poggini, B. Cortigiani, K. E. El-Kelany, M. D’Amore, A. M. Ferrari, A. Atrei, A. Caneschi, R. Sessoli and M. Mannini, J. Phys. Chem. C, 2021, 125, 10621–10630 CrossRef CAS.
- G. T. Harrison, M. C. Spadaro, C. L. Pang, D. C. Grinter, C. M. Yim, P. Luches and G. Thornton, Mater. Sci. Technol., 2016, 32, 203–208 CrossRef CAS.
- Y. Zhang, T. Learmonth, S. Wang, A. Y. Matsuura, J. Downes, L. Plucinski, S. Bernardis, C. O’Donnell and K. E. Smith, J. Mater. Chem., 2007, 17, 1276 RSC.
- A. Pedrini, L. Poggini, C. Tudisco, M. Torelli, A. E. Giuffrida, F. Bertani, I. Cimatti, E. Otero, P. Ohresser, P. Sainctavit, M. Suman, G. G. Condorelli, M. Mannini and E. Dalcanale, Small, 2018, 14, 1702572 CrossRef PubMed.
- W.-K. Li, X.-Q. Gong, G. Lu and A. Selloni, J. Phys. Chem. C, 2008, 112, 6594–6596 CrossRef CAS.
- K. Katoh, Y. Yoshida, M. Yamashita, H. Miyasaka, B. K. Breedlove, T. Kajiwara, S. Takaishi, N. Ishikawa, H. Isshiki, Y. F. Zhang, T. Komeda, M. Yamagishi and J. Takeya, J. Am. Chem. Soc., 2009, 131, 9967–9976 CrossRef CAS PubMed.
- F. Ara, Z. K. Qi, J. Hou, T. Komeda, K. Katoh and M. Yamashita, Dalton Trans., 2016, 45, 16644–16652 RSC.
- M. Toader, M. Knupfer, D. R. T. Zahn and M. Hietschold, J. Am. Chem. Soc., 2011, 133, 40 CrossRef PubMed.
- A. Kumar, K. Banerjee and P. Liljeroth, Nanotechnology, 2017, 28, 082001 CrossRef PubMed.
- H. Fei Wen, M. Miyazaki, Q. Zhang, Y. Adachi, Y. Jun Li and Y. Sugawara, Phys. Chem. Chem. Phys., 2018, 20, 28331 RSC.
- X. Q. Gong and A. Selloni, J. Catal., 2007, 249, 134–139 CrossRef CAS.
- L. Malavolti, M. Mannini, P.-E. Car, G. Campo, F. Pineider and R. Sessoli, J. Mater. Chem. C, 2013, 1, 2935 RSC.
- S. Tosoni and G. Pacchioni, J. Phys. Chem. C, 2020, 124, 20960–20973 CrossRef CAS.
- S. Baumann, W. Paul, T. Choi, C. P. Lutz, A. Ardavan and A. J. Heinrich, Science, 2015, 350, 417–420 CrossRef CAS PubMed.
- W. Paul, K. Yang, S. Baumann, N. Romming, T. Choi, C. P. Lutz and A. J. Heinrich, Nat. Phys., 2017, 13, 403–407 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional XPS data and analysis; LEED images and simulations; STM images. See DOI: 10.1039/d1tc03408a |
| This journal is © The Royal Society of Chemistry 2021 |