A pyridinium salt with crystalline phase transformation under water vapor and reversible mechanochromic luminescent properties†
Abstract
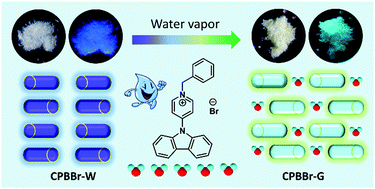
The photophysical properties of an organic aggregate are greatly affected by molecular conformation and intermolecular packing modes. Herein, an organic pyridinium salt was synthesized by quaternization of a pyridine derivative, and the resultant CPBBr dissolved well in both water and polar organic solvents, with bright green emission once doped into cyclodextrin. Under water vapor, the emission color of pristine CPBBr changed from deep blue to green, accompanied by a great enhancement of fluorescence quantum efficiency. Crystal structure analysis indicated that water molecules were involved in the crystal lattice, which promotes crystalline phase transformation from CPBBr-W to CPBBr-G, and greatly inhibits the intramolecular vibration via stabilizing the metastable molecular conformation and strengthening the intermolecular hydrogen bonds. The sharply decreased free volume fraction also demonstrated more compact molecular arrangements in the CPBBr-G crystal. Furthermore, CPBBr exhibited reversible mechanochromic luminescent (MCL) properties, and both the fluorescence efficiency and lifetime greatly increased after mechanical grinding as a result of the formation of an intermolecular charge transfer (CT) complex.
- This article is part of the themed collection: Journal of Materials Chemistry C Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...