Stable down-conversion white light-emitting devices based on highly luminescent copper halides synthesized at room temperature†
Abstract
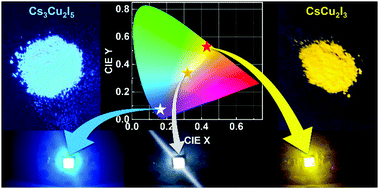
In recent years, the metal-halide perovskites have attracted intensive attention in the field of white light-emitting devices (WLEDs). However, their further commercial applications are severely hampered by the poor stability and lead toxicity of such materials. In this work, we demonstrate two types of lead-free ternary copper halides (blue-emissive Cs3Cu2I5 and yellow-emissive CsCu2I3), which were synthesized by a convenient supersaturated recrystallization method at room temperature. Both materials have particularly good crystallinity and excellent stability against ultraviolet light, heat, and air oxygen/moisture. Temperature-dependent photoluminescence (PL) and time-resolved PL decay measurements confirm that the highly luminescent and largely Stokes-shifted broadband emission of both materials derived from the self-trapped exciton-related recombination. By using a mixture of Cs3Cu2I5 and CsCu2I3 as the down-conversion phosphors, a high-performance WLED is demonstrated with the color coordinates of (0.324, 0.330), a correlated color temperature of 5877 K, and a color rendering index of 88.4. Also, a high luminous efficiency of 54.6 lm W−1 is realized, which is the highest value among the lead-free perovskite-based WLEDs. More importantly, the studied WLED demonstrates excellent operation stability, and almost no emission degradation occurs after continuously working for 100 h in air ambient. The results suggest that such ternary copper halides may be potentially attractive candidates for the fabrication of efficient, stable, and environmentally friendly WLEDs.
- This article is part of the themed collection: Editor’s choice collection: luminescent metal halides


 Please wait while we load your content...
Please wait while we load your content...