Cyanophenyl spiro[acridine-9,9′-fluorene]s as simple structured hybridized local and charge-transfer-based ultra-deep blue emitters for highly efficient non-doped electroluminescent devices (CIEy ≤ 0.05)†
Taweesak
Sudyoadsuk
a,
Sujinda
Petdee
a,
Chokchai
Kaiyasuan
 a,
Chaiyon
Chaiwai
a,
Praweena
Wongkaew
b,
Supawadee
Namuangruk
a,
Chaiyon
Chaiwai
a,
Praweena
Wongkaew
b,
Supawadee
Namuangruk
 c,
Pongsakorn
Chasing
a and
Vinich
Promarak
c,
Pongsakorn
Chasing
a and
Vinich
Promarak
 *ab
*ab
aDepartment of Materials Science and Engineering, School of Molecular Science and Engineering, Vidyasirimedhi Institute of Science and Technology, Wangchan, Rayong 21210, Thailand. E-mail: vinich.p@vistec.ac.th
bResearch Network of NANOTEC-VISTEC on Nanotechnology for Energy, Vidyasirimedhi Institute of Science and Technology, Wangchan, Rayong 21210, Thailand
cNational Nanotechnology Center (NANOTEC), National Science and Technology Development Agency, Pathum Thani 12120, Thailand
First published on 5th April 2021
Abstract
The hybridized local and charge-transfer (HLCT) excited state is a successful approach to accomplish both high external and internal quantum efficiency. To obtain deep blue emissive HLCT emitters, two cyanophenyl substituted spiro[acridine-9,9′-fluorene] isomers, namely SAFmCN and SAFpCN, were designed and synthesized. The photophysical and density functional theory (DFT) results confirmed that both molecules exhibited HLCT features with strong ultra-deep blue emissions in both solution and film states with emission peaks at 410–433 nm. The non-doped OLED devices showed emissions in high-definition television (HDTV) standard blue color (CIEy ≤ 0.05) with a narrow full width at half maximum (50–56 nm), excellent electroluminescence (EL) performance and a low turn-on voltage of 3.2 V. Particularly, the SAFpCN-based device achieved a maximum luminance efficiency (CE) and maximum external quantum efficiency (EQE) of 6.54 cd A−1 and 4.63%, respectively.
Introduction
Blue light-emitting diodes (OLEDs) play a crucial role in OLED technologies for applications in both full-color displays and solid-state lighting.1 Particularly, efficient and stable deep blue OLEDs with Commission Internationale de I’Eclairage (CIE) coordinates of (0.15, 0.06) are vital for high-definition television (HDTV) displays, which is an upcoming display market for OLEDs.2 Besides, the electroluminescence (EL) performance of deep blue OLEDs has been relatively low compared to those of the green and red ones due to the deep-blue emitter's intrinsic wide-bandgap.3 So far, there have been several useful strategies for developing deep blue-emitting materials for high-efficiency devices.1a,4 Blue OLEDs fabricated with typical fluorescence emitters or triplet–triplet fusion (TTF) emitters usually exhibit good deep blue color quality with more excellent operational stability than blue phosphorescent OLEDs.5 Nonetheless, the efficiency of typical blue fluorescence or TTF emitters is much lower than that of phosphorescence emitters because OLEDs using standard fluorescence or TTF emitters have theoretical limits of maximum internal quantum efficiency (IQE) of 25% and 62.5%, respectively.5,6 Lately, donor–acceptor (D–A) type thermally activated decayed fluorescence (TADF)7 and hybridized local and charge-transfer (HLCT)2a,8 blue-emitting materials have received sizable attention since both emitters can theoretically realize 100% IQE.4a Unfortunately, TADF blue OLEDs need an appropriate host with high triplet energy to reduce aggregation quenching and exciton confinement, and suffer from a sharp efficiency roll-off at high current density due to the long-lived first triplet excited state excitons. Simultaneously, deep-blue TADF emitters remain rare due to the bathochromic shift related to intramolecular charge transfer (CT).9 HLCT-based emitters feature the properties of both a locally-excited (LE) state and a CT state, and the conversion of triplet excitons into singlet excitons via reverse intersystem crossing (RISC), giving rise to high photoluminescence (PL) and high exciton utilization efficiencies (ηs).10 HLCT intrinsically retains short-lived exciton character that can avoid serious efficiency roll-off from long-lived exciton quenching. Based on the HLCT concept, efficient deep blue fluorescence emitters for highly efficient and sable non-doped OLEDs could be achieved since, in HLCT, the LE and CT states are highly hybridized into a new state, relieving largely red-shifted emission from a strong CT state.2a,11 Recently, some D–A molecules with an HLCT excited state have been reported, with an ultra-high ηs of nearly 100% in OLEDs.12 However, to date, OLEDs based on HLCT emitters, exhibiting high EL performance and deep-blue emissions with CIEy ≤ 0.05, have been rarely reported.2a,11a,13 To accomplish such demand, we herein employ a highly rigid, orthogonal spiro configuration spiro[acridine-9,9′-fluorene] (SAF)14 as a donor to couple with either meta- or para-cyanophenyl as an acceptor for forming new D–A type HLCT-based deep blue emitters (SAFmCN and SAFpCN, Fig. 1). Indeed, SAFpCN employed as non-doped emitters notably yielded ultra-deep blue OLEDs with CIEy ≤ 0.05, narrow emission spectra, and high CEmax/EQEmax of 6.54 cd A−1/4.63%.Results and discussion
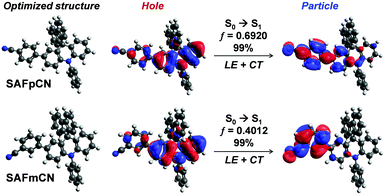
Two cyanophenyl substituted spiro[acridine-9,9′-fluorene] isomers (SAFpCN and SAFmCN) were synthesized by a facile three-step procedure as illustrated in Scheme S1 (ESI†). They were isolated as white solids and structurally confirmed by 1H NMR, 13C NMR, and mass spectroscopy (Fig, S8, ESI†) consistent well with their chemical structures.The optimized structures computed using the B3LYP/6-31G(d,p) function revealed that the fluorene and triphenylamine (TPA) fragments in the SAF unit are nearly perpendicular to each other, showing a sizeable twisting angle of 88° (Fig. 2). This orthogonal geometry is useful for restricting the intermolecular interactions and enhancing the morphological stability.15 As anticipated, the HOMO distribution is mainly located on the electron-donating TPA unit, while the electron-accepting cyanophenyl part dominates the LUMO, signifying a potential bipolar characteristic (Fig, S1, ESI†). The partial orbital overlap between the HOMO and LUMO would help realize high PL and EL efficiency simultaneously as the emitter applied in OLEDs.16 The HOMO–LUMO gaps and energy levels of SAFpCN were narrower and deeper than those of SAFmCN, respectively, due to the para-substitution effect of the strong electron-withdrawing cyano group. To describe the excited-state properties of the two molecules, the natural transition orbitals (NTOs) of the S0 → S1 transition calculated using the TD-B3LYP/6-31G(d,p) method clearly showed the HLCT transition characteristics (Fig. 2). The overlap of the hole and particle distribution on the phenyl ring of the TPA unit suggests a LE-like transition, which is essential for high-efficiency fluorescence.
Meanwhile, the separation of holes and particles leads to a CT transition. Thus, the transition of S0–S1 should be involved in both LE and CT transition. The overlap density between holes and particles is expanded mainly in SAFpCN relative to that of SAFmCN, indicating an enhanced LE component in the S1 state due to the para-cyano's significant contribution substitution. The oscillator strength (f) of S0 → S1 transition of SAFpCN (0.6920) was also calculated to be higher than that of SAFmCN (0.4012), implying that a higher PL could be expected in SAFpCN than in SAFmCN. As evident from the energy landscape plots (Fig. S2, ESI†), in all cases, the energy gaps between the S1 and T2 states (∼0.02 eV) are substantially narrower than that between T2 and T1 states (0.77–1.09 eV), indicating that the RISC rate (kRISC) from the T2 to S1 state should be higher than the internal conversion rate (kICT) from the T2 to T1 states, giving rise to a possibly hot exciton (short-lived exciton) channel for the RISC of the T2 → S1 process according to the El-Sayed rule.17
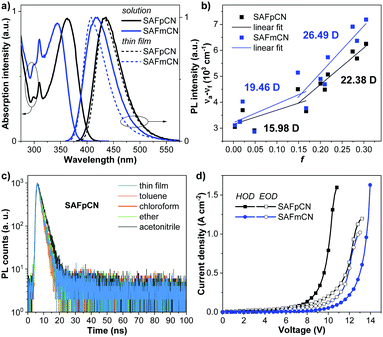
The excited state features of SAFpCN and SAFmCN are further evaluated by characterizing their optical properties. As shown in Fig. 3a, the absorption peak at ∼312 nm may relate to the π–π* transition of TPA, while the longer wavelength absorption peaks at 340–360 nm may stem from the CT transition from D to A. The two compounds in solution and thin film states displayed strong deep blue emissions peaked at 435 nm and 433 nm for SAFpCN, and 418 nm and 410 nm for SAFmCN, respectively. The absolute PL quantum yield (ΦPL) of SAFpCN in toluene was as high as 84%, reflecting an apparent LE state emission character. In comparison, ΦPL of SAFmCN in toluene was only 28%, ascribed to the intrinsic property that the CT state always causes a low PL efficiency.18 A similar trend was observed in the solid film state, and the ΦPL values for SAFpCN and SAFmCN were measured to be 45% and 30%, respectively. Both compounds displayed apparent solvatochromic shifts with the increase of solvent polarity from hexane to acetonitrile, indicating that their emissive states are typical CT characters (Fig. S3 and Table S1, ESI†).8a The PL spectra in low-polarity hexane reveal mild vibronic structures, and then the PL spectra gradually broadened and become structureless with the increase in the solvent polarity. The solvatochromic effect was further analyzed by plotting the Stokes shift (νa − νf) vs. the solvent orientational polarizability (f), according to the Lippert–Mataga equation. As depicted in Fig. 3b, both compounds show two independent slopes of the fitted line suggesting the existence of two different excited-states, whose dipole moments (μe), are estimated to be 22.38 D for SAFpCN and 26.49 D for SAFmCN in high-polarity solvents and 15.98 D for SAFpCN and 19.46 D for SAFmCN in low-polarity solvents, respectively. The large μe values can be considered as a CT-like state, while the small μe values should be assigned to the typical excited-state, which is a LE-like state. These results indicate that the emissive states of SAFpCN and SAFmCN exhibit sufficiently hybridized LE and CT character, consistent with the HLCT state's description.8a The presence of the HLCT state could ensure a relatively high PL efficiency and a large fraction of singlet exciton generation in EL.3 Further time-resolved PL measurements suggested that the PL of both molecules evolves from only one excited state and the HLCT state was supposed to be formed rather than a simple mixture of the LE and CT states, since their PL traces gave single-exponential lifetimes (τ) of 2–5 ns in different solvent polarities and thin films (Fig. 3c).
Thermal property analyses by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) confirmed that both SAFpCN and SAFmCN were thermally stable materials with decomposition temperatures at 5% weight loss (T5d) of as high as 377–387 °C and a glass transition temperatures (Tg) of 94–95 °C (Table 1, Fig. S4, ESI†). Cyclic voltammetry (CV) traces of the two compounds displayed a single reversible oxidation process at a half-wave potential (E1/2) of ∼1.2 V, assigned to the oxidation of the TPA unit. They exhibited good electrochemical stability and reversibility, as their repeated CV scans showed unchanged CV traces (Fig. S5, ESI†). The HOMO/LUMO energy levels in the thin-film determined by photoelectron yield spectroscopy (AC-2) in air (Fig. S6, ESI†) and the optical band gaps (Eoptg) were estimated to be −5.73/−2.69 eV for SAFpCN and −5.64/-2.46 eV for SAFmCN (Table 1). Besides, the results of the hole and electron-only devices disclosed that SAFpCN possessed better hole mobility and charge balance than SAFmCN (Fig. 3d and Table 1). The hole and electron mobilities for SAFpCN and SAFmCN were calculated by combining the Mott–Gurney equation and Frenkel effect19 to be 2.90 × 10−6 and 1.86 × 10−6 cm2 V−1 s−1, and 1.03 × 10−7 and 1.33 × 10−6 cm2 V−1 s−1, respectively.
| Compd | λ abs (log(ε))a (nm, M−1 cm−1) | λ PL sola/filmb (nm)a | Φ PL sola/filmb (%)c | τ sola/filmb (ns)d | T g/Tm/T5de (°C) | E 1/2 vs. Ag/Ag+ (V)f | E optg (eV)g | HOMO/LUMO (eV)h | Hole/electron mobilityi (cm2 V−1 s−1) |
|---|---|---|---|---|---|---|---|---|---|
| a Measured in 2 × 10−5 mol L−1 CHCl3 solution. b Measured in thin-film coated on fused silica substrates. c Absolute PL quantum yield measured using an integrating sphere. d Transient PL decay of solid films. e Determined by DSC and TGA under N2 flow. f Analysed by CV in CH2Cl2 containing 0.1 M n-Bu4NPF6 under argon flow. g Estimated from the absorption onset of the solid film: Eg = 1240/λonset. h HOMO measured by AC-2 of the neat film and LUMO = HOMO + Eg. i Determined by using a hole only device (ITO/NPB 10 nm/Compd 80 nm/NPB 10 nm/Al 100 nm) and electron only device (ITO/TmPyPB 10 nm/Compd 80 nm/TmPyPB 10 nm/LiF 0.5 nm/Al 100 nm). | |||||||||
| SAFpCN | 312(4.27), 360(4.51) | 435/433 | 84/45 | 2/2 | 94/273/387 | 1.12 | 3.04 | −5.73/−2.69 | 2.90 × 10−6/1.86 × 10−6 |
| SAFmCN | 312(4.49), 340(4.56) | 418/410 | 28/33 | 3/3 | 95/288/377 | 1.12 | 3.18 | −5.64/−2.46 | 1.03 × 10−7/1.33 × 10−6 |
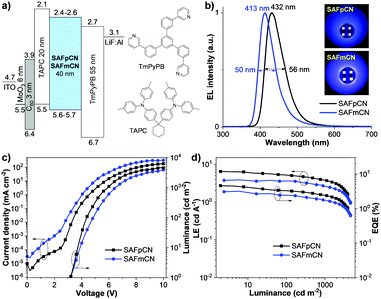
To evaluate the deep blue EL properties of SAFpCN and SAFmCN as the emissive layer (EML), non-doped OLEDs were fabricated with the optimized configuration of ITO/MoO3 (6 nm)/C60 (3 nm)/4,4′-cyclohexylidenebis[N,N-bis(p-tolyl)aniline] (TAPC) (20 nm)/SAFpCN or SAFmCN (40 nm)/1,3,5-tris(3-pyridyl-3-phenyl)benzene (TmPyPB) (55 nm)/LiF (0.5 nm)/Al (150 nm). ITO and LiF/Al were used as the anode and cathode in these devices, respectively, and MoO3/C60 as a hole-injection layer. The high electron mobility of TmPyPB as the electron-transporting and hole-blocking layer and high hole mobility of TAPC as the hole-transporting and exciton blocking layer will possibly regulate the exciton recombination zone width in the EML. In contrast, their suitable energy levels and appropriate band alignments between the layers will facilitate hole/electron accumulation and exciton recombination at the interfaces (Fig. 4a).20 The non-doped device's characteristics and data are shown in Fig. 4 and are listed in Table 2. The SAFpCN- and SAFmCN-based devices exhibited strong deep blue emissions with λmax at 432 and 413 nm, and CIE of (0.153, 0.054) and (0.160, 0.046), respectively. Although the EL spectrum of the device based on SAFpCN exhibited a slight red shift compared with that of SAFmCN, both devices still emitted a deep blue light in the HDTV standard blue colour region (CIE of (0.15, 0.06)).21 Moreover, these OLEDs also showed narrow EL spectra with a full width at half maximum (FWHM) of 50–56 nm (Fig. 4b), proving a superior colour purity of deep blue colour. The EL spectra are consistent with their PL emissions in the film without emission peaks at lower buffer layers' wavelengths. They show stability by increasing the voltage from 3 to 11 V (Fig. S7, ESI†). This indicates pure EML emissions with effective charge recombination in the EML and excimer emission as well as exciplex emissions from TAPC/EML and EML/TmPyPB interfaces being efficiently suppressed. Accordingly, all devices showed a low turn-on voltage (Von) of 3.2 V (Fig. 4c). The device based on SAFpCN achieved excellent EL performance, with a maximum current efficiency (CEmax) of 6.54 cd A−1, an EQEmax of 4.63%, a slight efficiency roll-off (3.89% at 100 cd m2; 3.31% at 1000 cd m2), a maximum luminance (Lmax) of 3900 cd m−2 and a maximum power efficiency (PEmax) of 5.63 lm W−1 (Fig. 4d). The SAFmCN-based device showed somewhat lower EL performance with CEmax/EQEmax of 3.86 cd A−1/3.18%. To the best of our knowledge, the SAFpCN-based device is considered as one of the high EL performance deep blue HLCT-based OLEDs with CIEy ≤ 0.05.8c,21b,22 The high EL efficiency of SAFpCN-based devices could be ascribed to the synergetic effects of HLCT character and high and balanced charge mobilities of the emitter, and the optimized device structure. The possible RISC process via upper excited states of the HLCT-emitter could somewhat boost up the EQE, whereas a well charge-balanced device could contribute to widen the recombination zone in the EML and result in low driving voltage and long lifetime device.23 The ηs values of the non-doped devices of SAFpCN and SAFmCN were calculated to be 51% and 41%,24 respectively, exceeding the theoretical limit of 25% for traditional fluorescent OLEDs.
| EML | V on (V) | λ EL (nm) | FWHM (nm) | L max (cd m−2) | J max (mA cm−2) | EQEmax/CEmax/PEmaxb(%/cd A−1/lm W−1) | CIE (x, y) | η s (%) |
|---|---|---|---|---|---|---|---|---|
| a ITO/MoO3 6 nm/C60 3 nm/TAPC 20 nm/EML 40 nm/TmPyPB 55 nm/LiF 0.5 nm/Al 150 nm. b External quantum efficiency/luminance efficiency/power efficiency. | ||||||||
| SAFpCN | 3.2 | 432 | 56 | 3900 | 180 | 4.63/6.54/5.63 | 0.153, 0.054 | 51 |
| SAFmCN | 3.2 | 413 | 50 | 6420 | 325 | 3.18/3.86/2.50 | 0.160, 0.046 | 41 |
Experimental
Materials and methods
All reagents were purchased from Aldrich and TCI companies and used without further purification. Solvents were dried by using standard procedures.1H-NMR and 13C-NMR spectra were recorded on Bruker Avance-600 MHz spectrometers. High-resolution mass spectra were analyzed using a Bruker microflex MALDI-TOF mass spectrometer. Differential scanning calorimetry (DSC) and thermogravimetric (TGA) analyses were performed using a PerkinElmer DSC-8500 thermal analyzer and a TGA/DSC1 Mettler Toledo with a heating rate of 10 °C min−1 under N2 flow, respectively. UV-visible absorption spectra were recorded on a PerkinElmer UV Lambda 1050 spectrometer. Photoluminescence and lifetime were measured by using a FLS980 Edinburgh fllmer UV Lambda 1050 s. The absolute photoluminescence quantum yield (ΦPL) was determined by using an integrating sphere. Photoelectron spectroscopy (AC-2) was performed on a Riken-Keiki ultraviolet photoelectron spectrometer AC-2 in air. Cyclic voltammetry (CV) measurements were carried out using an Autolab potentiostat PGSTAT 101 equipped with a three-electrode setup (platinum, glassy carbon, and Ag/AgCl). Melting points were measured using a Krüss KSP1N melting point meter and are uncorrected.
The quantum chemical calculations were performed with Gaussian 09 program package using density functional theory (DFT).25 The HOMO and LUMO distributions of the complexes were calculated with the DFT B3LYP/6-31G(d) function.
OLED devices with non-doped configurations of ITO/MoO3 (6 nm)/C60 (3 nm)/4,4′-cyclohexylidenebis[N,N-bis(p-tolyl)aniline] (TAPC) (20 nm)/SAFpCN or SAFmCN (40 nm)/1,3,5-tris(3-pyridyl-3-phenyl)benzene (TmPyPB) (55 nm)/LiF (0.5 nm)/Al (150 nm) were fabricated and characterized as followed. The cleaned and patterned indium tin oxide (ITO) glass substrate (12 Ω sq−1) was treated by UV ozone for 30 min. MoO3 (6 nm) and C60 (3 nm) as the hole injection layer and TAPC (20 nm) as the hole transport layer were deposited by thermal evaporation at an evaporation rate of 1 Å s−1 from low-temperature evaporator sources in a Kurt J. Lasker mini SPECTROS 100 thin film deposition system under a base pressure of 5 × 10−6 bar. A quartz oscillator thickness sensor is used to monitor the film thickness. SAFpCN or SAFmCN as the EML was then deposited on top with 40 nm thickness. The 55 nm thick TmPyPB as the electron transport layer (ETL), 0.5 nm thick LiF, and 150 nm thick aluminum layers were subsequently deposited through a shadow mask on the top of the EML without breaking the vacuum to form an active diode area of 4 mm2. Hole and electron only devices with configurations of ITO/NPB 10 nm/SAFpCN or SAFmCN 80 nm/NPB 10 nm/Al 100 nm and ITO/TmPyPB 10 nm/SAFpCN or SAFmCN 80 nm/TmPyPB 10 nm/LiF 0.5 nm/Al 100 nm were also fabricated, respectively. Current density–voltage–luminance (J–V–L) characteristics were measured simultaneously using a Keithley 2400 source meter and a Hamamatsu Photonics PMA-12 multi-channel analyzer. The absolute external quantum efficiency (EQE) of OLED devices was obtained by using a Hamamatsu Photonics C9920-12 external quantum efficiency measurement system utilizing an integrating sphere. All the measurements were performed under an ambient atmosphere at room temperature.
Synthesis and characterisation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) followed by recrystallisation in a mixture of DCM/methanol to obtain a white solid (0.16 g, 68%). M.p. = 276 °C. 1H NMR (600 MHz, CDCl3) δ 7.82 (d, J = 7.6 Hz, 2H), 7.74 (t, J = 7.8 Hz, 2H), 7.61 (t, J = 7.5 Hz, 1H), 7.53 (d, J = 7.2 Hz, 2H), 7.47 (dd, J = 12.1, 8.0 Hz, 4H), 7.40 (td, J = 7.5, 0.7 Hz, 2H), 7.28 (td, J = 7.5, 0.8 Hz, 2H), 7.23 (d, J = 8.4 Hz, 2H), 7.17 (dd, J = 8.7, 2.2 Hz, 1H), 6.95 (ddd, J = 8.5, 7.3, 1.5 Hz, 1H), 6.64 (d, J = 2.2 Hz, 1H), 6.61 (td, J = 7.5, 0.9 Hz, 1H), 6.46 (d, J = 8.7 Hz, 1H), 6.43 (dd, J = 7.8, 1.4 Hz, 1H), 6.39 (d, J = 7.9 Hz, 1H). 13C NMR (151 MHz, CDCl3) δ 156.37, 145.01, 142.08, 141.04, 140.88, 139.37, 132.47, 131.36, 131.11, 131.01, 128.90, 128.64, 127.99, 127.88, 127.52, 126.69, 126.59, 126.15, 125.90, 125.83, 125.04, 121.32, 120.24, 119.27, 115.46, 115.05, 109.69, 57.00. HRSM MALDI-TOF m/z [M]+ calcd for C38H24N2 508.1939, found 508.4335.
hexane) followed by recrystallisation in a mixture of DCM/methanol to obtain a white solid (0.16 g, 68%). M.p. = 276 °C. 1H NMR (600 MHz, CDCl3) δ 7.82 (d, J = 7.6 Hz, 2H), 7.74 (t, J = 7.8 Hz, 2H), 7.61 (t, J = 7.5 Hz, 1H), 7.53 (d, J = 7.2 Hz, 2H), 7.47 (dd, J = 12.1, 8.0 Hz, 4H), 7.40 (td, J = 7.5, 0.7 Hz, 2H), 7.28 (td, J = 7.5, 0.8 Hz, 2H), 7.23 (d, J = 8.4 Hz, 2H), 7.17 (dd, J = 8.7, 2.2 Hz, 1H), 6.95 (ddd, J = 8.5, 7.3, 1.5 Hz, 1H), 6.64 (d, J = 2.2 Hz, 1H), 6.61 (td, J = 7.5, 0.9 Hz, 1H), 6.46 (d, J = 8.7 Hz, 1H), 6.43 (dd, J = 7.8, 1.4 Hz, 1H), 6.39 (d, J = 7.9 Hz, 1H). 13C NMR (151 MHz, CDCl3) δ 156.37, 145.01, 142.08, 141.04, 140.88, 139.37, 132.47, 131.36, 131.11, 131.01, 128.90, 128.64, 127.99, 127.88, 127.52, 126.69, 126.59, 126.15, 125.90, 125.83, 125.04, 121.32, 120.24, 119.27, 115.46, 115.05, 109.69, 57.00. HRSM MALDI-TOF m/z [M]+ calcd for C38H24N2 508.1939, found 508.4335.
Conclusions
In summary, we have developed a simple design and structure of D–A-based deep blue emitters bearing spiro-acridine as donors and cyanophenyl as acceptors. The materials exhibit HLCT characters with strong ultra-deep blue emissions in both solution and film states with emission peaks at 410–433 nm. The non-doped OLED fabricated with SAFpCN emits deep blue color in the HDTV standard blue region with a narrow full-width at half maximum of 56 nm, CIE of (0.153, 0.054), a brightness of 3900 cd m−2, a CEmax of 6.54 cd A−1, an EQEmax of 4.63% and a singlet exciton utilization efficiency of 51%. These results offer a straightforward approach to design HLCT-based deep blue fluorescent molecules suitable for fabricating efficient and stable non-doped deep blue EL devices.Author contributions
T. S.: investigation, data curation and writing – original draft preparation. S. P., C. K., C. C., P. W., S. N., and P. C.: investigation and data curation. V. P.: supervision, funding acquisition and writing – reviewing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledged the financial support from the Thailand Research Fund (RTA6080005) and NANOTEC, NSTDA, Ministry of Science and Technology, Thailand, through its program of Research Network NANOTEC. Many thanks also go to Vidyasirimedhi Institute of Science and Technology for supporting both Postdoctoral Fellowship (to T. S.) and equipment.Notes and references
- (a) J. H. Lee, C. H. Chen, P. H. Lee, H. Y. Lin, M. K. Leung, T. L. Chiu and C. F. Lin, J. Mater. Chem. C, 2019, 7, 5874 RSC; (b) Y. Im, S. Y. Byun, J. H. Kim, D. R. Lee, C. S. Oh, K. S. Yook and J. Y. Lee, Adv. Funct. Mater., 2017, 27, 1603007 CrossRef.
- (a) C. Fu, S. Luo, Z. Li, X. Ai, Z. Pang, C. Li, K. Chen, L. Zhou, F. Li, Y. Huang and Z. Lu, Chem. Commun., 2019, 55, 6317 RSC; (b) A. K. Pal, S. Krotkus, M. Fontani, C. F. R. Mackenzie, D. B. Cordes, A. M. Z. Slawin, I. D. W. Samuel and E. Zysman-Colman, Adv. Mater., 2018, 30, 1704961 CrossRef PubMed.
- (a) M. Godumala, S. Choi, M. J. Cho and D. H. Choi, J. Mater. Chem. C, 2019, 7, 2172 RSC; (b) D. Wang, C. Cheng, T. Tsuboi and Q. Zhang, CCS Chem., 2020, 2, 1278 CrossRef CAS.
- (a) Z. Xu, B. Z. Tang, Y. Wang and D. Ma, J. Mater. Chem. C, 2020, 8, 2614 RSC; (b) C. Poriel and J. Rault-Berthelot, Adv. Funct. Mater., 2020, 30, 1910040 CrossRef CAS; (c) S. Wang, M. Qiao, Z. Ye, D. Dou, M. Chen, Y. Peng, Y. Shi, X. Yang, L. Cui, J. Li, C. Li, B. Wei and W.-Y. Wong, iScience, 2018, 9, 532 CrossRef CAS PubMed.
- X. Yang, X. Xu and G. Zhou, J. Mater. Chem. C, 2015, 3, 913 RSC.
- (a) K. Klimes, Z. Q. Zhu and J. Li, Adv. Funct. Mater., 2019, 29, 1903068 CrossRef; (b) X. C. Hang, T. Fleetham, E. Turner, J. Brooks and J. Li, Angew. Chem., Int. Ed., 2013, 52, 6753 CrossRef CAS PubMed.
- K. Matsuo and T. Yasuda, Chem. Sci., 2019, 10, 10687 RSC.
- (a) W. Li, Y. Pan, L. Yao, H. Liu, S. Zhang, C. Wang, F. Shen, P. Lu, B. Yang and Y. Ma, Adv. Opt. Mater., 2014, 2, 892 CrossRef CAS; (b) X. Lv, M. Sun, L. Xu, R. Wang, H. Zhou, Y. Pan, S. Zhang, Q. Sun, S. Xue and W. Yang, Chem. Sci., 2020, 11, 5058 RSC; (c) W. C. Chen, Y. Yuan, S. F. Ni, Q. X. Tong, F. L. Wong and C. S. Lee, Chem. Sci., 2017, 8, 3599 RSC; (d) H. Zhang, B. Zhang, Y. Zhang, Z. Xu, H. Wu, P.-A. Yin, Z. Wang, Z. Zhao, D. Ma and B. Z. Tang, Adv. Funct. Mater., 2020, 30, 2002323 CrossRef CAS; (e) H. Zhang, J. Zeng, W. Luo, H. Wu, C. Zeng, K. Zhang, W. Feng, Z. Wang, Z. Zhao and B. Z. Tang, J. Mater. Chem. C, 2019, 7, 6359 RSC; (f) C. Zhou, S. Xiao, M. Wang, W. Jiang, H. Liu, S. Zhang and B. Yang, Front. Chem., 2019, 7, 141 CrossRef CAS PubMed; (g) C. Wang, X.-L. Li, Y. Gao, L. Wang, S. Zhang, L. Zhao, P. Lu, B. Yang, S.-J. Su and Y. Ma, Adv. Opt. Mater., 2017, 5, 1700441 CrossRef; (h) W. Xie, B. Li, X. Cai, M. Li, Z. Qiao, X. Tang, K. Liu, C. Gu, Y. Ma and S.-J. Su, Front. Chem., 2019, 7, 276 CrossRef CAS PubMed.
- (a) M. Kim, J. M. Choi and J. Y. Lee, Chem. Commun., 2016, 52, 10032 RSC; (b) C. Y. Chan, L. S. Cui, J. U. Kim, H. Nakanotani and C. Adachi, Adv. Funct. Mater., 2018, 28, 1706023 CrossRef.
- W. Z. Yuan, X. Bin, G. Chen, Z. He, J. Liu, H. Ma, Q. Peng, B. Wei, Y. Gong, Y. Lu, G. He and Y. Zhang, Adv. Opt. Mater., 2017, 5, 1700466 CrossRef.
- (a) J. Tagare and S. Vaidyanathan, J. Mater. Chem. C, 2018, 6, 10138 RSC; (b) B. Liu, Z. W. Yu, D. He, Z. L. Zhu, J. Zheng, Y. D. Yu, W. F. Xie, Q. X. Tong and C. S. Lee, J. Mater. Chem. C, 2017, 5, 5402 RSC.
- (a) Y. Liu, H. Liu, Q. Bai, C. Du, A. Shang, D. Jiang, X. Tang and P. Lu, ACS Appl. Mater. Interfaces, 2020, 12, 16715 CrossRef CAS PubMed; (b) X. Chen, Z. Yang, W. Li, Z. Mao, J. Zhao, Y. Zhang, Y. C. Wu, S. Jiao, Y. Liu and Z. Chi, ACS Appl. Mater. Interfaces, 2019, 11, 39026 CrossRef CAS PubMed; (c) H. Usta, D. Alimli, R. Ozdemir, S. Dabak, Y. Zorlu, F. Alkan, E. Tekin and A. Can, ACS Appl. Mater. Interfaces, 2019, 11, 44474 CrossRef CAS PubMed; (d) V. Thanikachalam, P. Jeeva and J. Jayabharathi, RSC Adv., 2017, 7, 13604 RSC; (e) Z. Li, N. Xie, Y. Xu, C. Li, X. Mu and Y. Wang, Org. Mater., 2020, 2, 11 CrossRef CAS.
- X. Qiu, Y. Xu, C. Wang, M. Hanif, J. Zhou, C. Zeng, Y. Li, Q. Jiang, R. Zhao, D. Hu and Y. Ma, J. Mater. Chem. C, 2019, 7, 5461 RSC.
- W. Sun, N. Zhou, Y. Xiao, S. Wang and X. Li, Dyes Pigm., 2018, 154, 30 CrossRef CAS.
- T. Liu, H. Sun, C. Fan, D. Ma, C. Zhong and C. Yang, Org. Electron., 2014, 15, 3568 CrossRef CAS.
- F. Liu, Y. Tan, H. Liu, X. Tang, L. Gao, C. Du, J. Min, H. Jin and P. Lu, J. Mater. Chem. C, 2020, 8, 6883 RSC.
- R. Chen, Y. Tang, Y. Wan, T. Chen, C. Zheng, Y. Qi, Y. Cheng and W. Huang, Sci. Rep., 2017, 7, 6225 CrossRef PubMed.
- S. Zhang, L. Yao, Q. Peng, W. Li, Y. Pan, R. Xiao, Y. Gao, C. Gu, Z. Wang, P. Lu, F. Li, S. Su, B. Yang and Y. Ma, Adv. Funct. Mater., 2015, 25, 1755 CrossRef CAS.
- Y. Li, R. G. Clevenger, L. Jin, K. V. Kilway and Z. Peng, J. Mater. Chem. C, 2014, 2, 7180 RSC.
- S. Wu, S. Li, Q. Sun, C. Huang and M. K. Fung, Sci. Rep., 2016, 6, 25821 CrossRef CAS PubMed.
- (a) T. Shan, Y. Liu, X. Tang, Q. Bai, Y. Gao, Z. Gao, J. Li, J. Deng, B. Yang, P. Lu and Y. Ma, ACS Appl. Mater. Interfaces, 2016, 8, 28771 CrossRef CAS PubMed; (b) G. Li, J. Zhao, D. Zhang, J. Zhu, Z. Shi, S. Tao, F. Lu and Q. Tong, New J. Chem., 2017, 41, 5191 RSC.
- (a) W. C. Chen, Y. Yuan, Y. Xiong, A. L. Rogach, Q. X. Tong and C. S. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 26268 CrossRef CAS PubMed; (b) X. Qiu, S. Ying, C. Wang, M. Hanif, Y. Xu, Y. Li, R. Zhao, D. Hu, D. Ma and Y. Ma, J. Mater. Chem. C, 2019, 7, 592 RSC.
- S. W. Culligan, A. C. A. Chen, J. U. Wallace, K. P. Klubek, C. W. Tang and S. H. Chen, Adv. Funct. Mater., 2006, 16, 1481 CrossRef CAS.
- J. Jayabharathi, G. Goperundevi, V. Thanikachalam and S. Panimozhi, ACS Omega, 2019, 4, 15030 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1tc00406a |
| This journal is © The Royal Society of Chemistry 2021 |