Open Access Article
Open Access ArticleThe excellent biocompatibility and negligible immune response of the titanium heterometallic MOF MUV-10†
Isabel Abánades
Lázaro
 *a,
Jose M.
Rodrigo-Muñoz
b,
Beatriz
Sastre
b,
María Romero
Ángel
*a,
Jose M.
Rodrigo-Muñoz
b,
Beatriz
Sastre
b,
María Romero
Ángel
 a,
Carlos
Martí-Gastaldo
a,
Carlos
Martí-Gastaldo
 a and
Victoria
del Pozo
*b
a and
Victoria
del Pozo
*b
aInstituto de Ciencia Molecular (ICMol), Universitat de València, Catedrático José Beltrán Martínez no. 2, Paterna 46980, Valencia, Spain. E-mail: Isabel.abanades@uv.es
bDepartment of Immunology, Instituto de Investigación Sanitaria Fundación Jiménez Díaz, Universidad Autónoma de Madrid (IIS-FJD, UAM), and CIBER de Enfermedades Respiratorias (CIBERES), Madrid 28029, Spain. E-mail: VPozo@fjd.es
First published on 16th July 2021
Abstract
The Ti–Ca heterometallic MOF MUV-10 exhibits good dispersibility in phosphate buffer and low phosphate-induced degradation in comparison to other MOF systems. It induces no cytotoxicity towards cells of the immune system and no inmune response, making it an attractive candidate for biomedical applications and demonstrating its safe use for other applications.
Metal–organic frameworks – porous hybrid structures composed of metal clusters linked by multitopic organic ligands1,2 – have garnered a tremendous amount of interest over the past 20 years due to their intrinsic properties such as high porosity and unlimited chemical and structural diversity, which have made them attractive for a variety of applications related to porous and/or functional materials, including drug delivery, antibacterial coatings, gas storage and separation, water pollutant removal, magnetism, heterogeneous catalysis and photocatalysis among others. The variety of choice of metal and linker allows the design of biocompatible MOFs, which together with their tunable size, high loading capacity, easy surface modification and structural stability has positioned them as attractive alternatives for nanoscale and transdermal drug delivery systems (DDSs).
The first use of iron-based MOFs for healthcare applications was reported in 2006,3 and since then a tremendous amount of work has been developed towards their bioapplication.4–7 Due to their biocompatibility, iron-based MOFs are probably the most widely explored MOF materials to date. Zirconium MOFs have recently acquired attention as DDSs6 as Zr is also biocompatible and its hard Lewis acid–base coordination characteristics generally result in higher chemical stability than iron-based MOFs, making them more amenable to surface modification8 and resulting in higher physiological stability, a must for drug delivery applications.6
Titanium-based MOFs – which are photoactive and often have superior structural and chemical stability compared to other MOFs9,10 such as Zr and Fe-based MOFs – are emerging in the literature.11,12 Titanium has low cytotoxicity (better than any other transition metal ion apart from Fe),5 and it is abundant. However, examples of Ti-based MOFs as DDSs are surprisingly scarce in the literature,13–16 and despite their widely explored photocatalytic applications,11,12 which offer the possibility for use in photodynamic therapy,15,16 reports of their biocompatibility are difficult to find.
Assessing the biocompatibility of MOFs and the immune response towards them is imperative not only for their bio-application, but also for any safe industrial application that involves contact with living beings or the environment. For example, MOFs could not be safely used for water treatment or surface coatings if they were not biocompatible or if they generated an immune response. However, reports of biocompatibility or immune response in studies not related to biomedical applications are uncommon.
Herein, we report the remarkable biocompatibility and negligible immune response of Ti MUV-10 heterometallic MOFs of different sizes (1700 to 28 nm), demonstrating their safe industrial application and high potential as a DDSs.
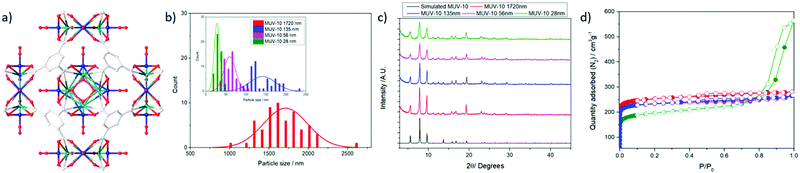
MUV-10, represented in Fig. 1a, is a highly-stable heterometallic Ti(IV)-MOF built from the assembly of tetranuclear TiIV2CaII2(μ3-O)2(H2O)4(RCO2)8 clusters connected to eight neighbouring clusters by eight benzene tricarboxylate (BTC) linkers forming a cubic structure with the unit formula [Ti3Ca3(μ3-O)3(μ2-C6H3(CO2)3)4(OH2)6].17 This microporous framework, with a surface area close to 1000 m2 g−1 and a micropore size of ca. 1 nm,18 is amenable to defect engineering through the introduction of modulators, which also results in a decrease in particle size to the nanometre range due to modulator capping19 and allows simple functionalisation. Additionally, the attachment of modulators as defect-compensating ligands indicates that defect drug loading protocols could be applied to the MUV-10 family.20–22
To study how MOF size affects the biocompatibility, internalisation and reactive oxygen species (ROS) production of J744 macrophages and peripheral blood mononuclear cells (PBMCs) isolated from the blood of human donors, as well as the effect on complement cascade activation that is indicative of an immune response,23 we synthesised a series of MUV-10 MOFs of different sizes (1700, 135, 56 and 28 nm) (Fig. 1b) by tuning the linker![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal ratio and the reaction mixture pH through modulation using acetic or hydrochloric acid (see ESI† Section S.2 for detailed synthetic conditions).
metal ratio and the reaction mixture pH through modulation using acetic or hydrochloric acid (see ESI† Section S.2 for detailed synthetic conditions).
All the MOFs synthesized were highly crystalline and phase pure fine powders (see ESI† Section S.4 for detailed characterization information), as shown in their PXRD profiles (Fig. 1c); their reflection signals broadened with decreasing particle size, which was determined using scanning electron microscopy (SEM). Full characterisation (ESI,† Section S.4) – including FT-IR spectroscopy, thermogravimetric analysis (TGA) and N2 adsorption/desorption isotherms – confirmed the samples were thermally stable (Fig. S6, ESI†) and porous (Fig. 1d). Gravimetric analysis of the thermal decomposition profiles showed the samples were defective (see ESI† Section S.4)24 with ca. 5–8 mol% missing linkers for samples synthesized using an excess of linker (1720, 56 and 28 nm), whereas the sample synthesised using a depleted amount of linker (136 nm) had significantly higher defect promotion, with ∼25 mol% missing linkers. The BET surface areas ranged from ca. 1000 to ca. 750 m2 g−1, with the decrease being related to a reduction of the particle size to ca. 28 nm.25 This was also observed in the increase of external surface area and mesopore percent. The total pore volumes (P/P0 0.9) ranged from 0.40 to 0.50 cm3 g−1, with the smallest sample displaying hysteresis loops characteristic of inter-particle spacing (total pore volume at P/P0 1 = 0.65 cm3 g−1). The appearance of slightly bigger pores compared to the pristine MOF is possibly a consequence of their defective nature (Fig. S8 and S9, ESI†).
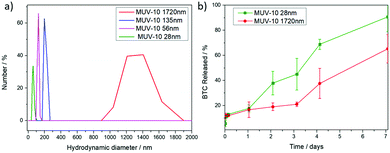
Dynamic light scattering (DLS) measurements were performed in phosphate buffered saline (PBS) 10×, where MOFs are known to suffer higher aggregation than that in water.6 The MOFs were well-dispersed in PBS 10× without a protein spike and displayed hydrodynamic diameters close to the particle sizes determined using SEM, with the smaller samples (56 and 28 nm) displaying minor aggregation and hydrodynamic diameters twice the particle size (Fig. 2a and Fig. S11–S14, ESI†). Good colloidal dispersion is intrinsic for drug delivery applications, but this is often not the case for MOFs and surface coatings are employed to overcome this drawback.6 MOFs are known to form a protein corona under physiological conditions (i.e. cell culture media and blood current) that prevents them from aggregating26 and spiking PBS with bovine serum albumin has been shown to result in drastic improvements in their colloidal dispersion,27 which indicates that MUV-10 may not suffer significant aggregation in cell culture media despite having unmodified surfaces.
The stability of the samples in PBS 10× (pH = 7.4) was investigated using UV–vis spectroscopic determination of linker release (benzene tricarboxylate) for the largest (1700 nm) and smallest samples (28 nm). A small burst of degradation was observed for the largest particle size (∼13%) after 15 minutes, with similar release rates over the first hour to one day (ca. 20%) and a slightly faster release rate for the smaller sample, which degraded significantly after 7 days (∼90% for the smallest and 65% for the largest sample, respectively) (Fig. 2b). However, their degradation under these simulated physiological conditions is remarkably slower than for other MOF systems currently being investigated as DDSs, such as UiO-66 (Zr)6 and MIL-101 (Fe),28 which release ∼80% of their linkers after a few hours and Zr-fumarate MOFs with a ∼60% linker release after 8 hours under comparable degradation conditions.29 The degradation kinetics are comparable with MIL-100 (Fe), which releases ca. 58% of its linker after 7days, although its phosphate-induced degradation was performed using phosphate solutions 10 times less concentrated than in our experiments.30 These results are of higher importance if we consider that our degradation experiments are performed without a dialysis bag, which prolongs the release profiles by adding the molecule dialysis time. This is in agreement with the increased bonding strength between metal ions and ligands, where a smaller ratio of Ti4+ ions results in stronger metal–O bonds than that in analogous Zr4+ MOFs.10
These results also indicate that MOFs composed of tridentate ligands might have low phosphate-induced degradation compared to those composed of bidentate ligands, given that more metal–carboxylate bonds have to be displaced.
To monitor the effect of MUV-10 particle size on its internalisation by cells of the immune system, we postsynthetically loaded the MOFs with calcein, a carboxylate-containing fluorescent molecule that cannot efficiently cross the cell membrane due to its hydrophilicity,31 and so it is an excellent probe for the cellular internalisation of MOFs.32 Calcein was loaded in high amounts from ca. 9.5 to 33.7 w/w%, the loading increased with reducing particle size, which is possibly due to the greater external surface![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk ratio. Characterisation results (see ESI† Section S.5) suggest that calcein is attached to the metal clusters through its carboxylate units, which is in agreement with reports of calcein-loaded Zr-based MOFs.6,32 These results show that MUV-10 is an excellent candidate for the loading of drugs or biomolecules. Using calcein as a model drug, we measured its release in PBS 10×, which showed that MUV-10 is a good drug delivery system, releasing the full amount of loaded calcein after 2 days, with a small burst release of ca. 30% during the first hour despite not using a dialysis bag. MUV-10 of 28 nm released ca. 40% after one day of exposure whereas MUV-10 of 1720 nm released ca. 75% of its loaded calcein (Fig. S36, ESI†), while many bare MOFs previously reported release their full cargo after a few hours in PBS.5,6
bulk ratio. Characterisation results (see ESI† Section S.5) suggest that calcein is attached to the metal clusters through its carboxylate units, which is in agreement with reports of calcein-loaded Zr-based MOFs.6,32 These results show that MUV-10 is an excellent candidate for the loading of drugs or biomolecules. Using calcein as a model drug, we measured its release in PBS 10×, which showed that MUV-10 is a good drug delivery system, releasing the full amount of loaded calcein after 2 days, with a small burst release of ca. 30% during the first hour despite not using a dialysis bag. MUV-10 of 28 nm released ca. 40% after one day of exposure whereas MUV-10 of 1720 nm released ca. 75% of its loaded calcein (Fig. S36, ESI†), while many bare MOFs previously reported release their full cargo after a few hours in PBS.5,6
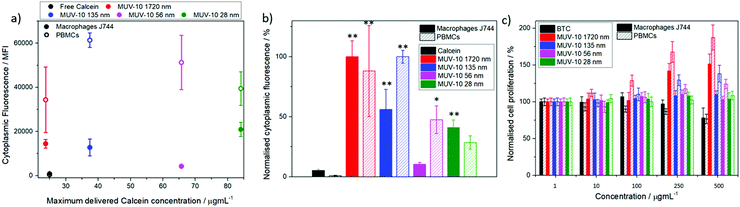
Fluorescence assisted cell sorting (FACS) was used to monitor the cellular internalisation of the calcein@MOFs by cells of the immune system, such as macrophages and PBMCs (see ESI† Section S.3 for detailed in vitro protocols). A drastic increase in their cytoplasmic fluorescence was observed after incubation with 250 μg mL−1 of calcein@MOFs over two hours in comparison to free calcein, with cellular internalisation being higher for PBMCs (see ESI† Section S.6). Given the different calcein loadings of the samples, as represented in Fig. 3a, the mean fluorescence intensity was normalised by the maximum amount of calcein delivered by each sample to determine which particle size demonstrated the highest internalisation (Fig. 3b). Our results indicate that the largest particle sizes were highly internalised both by J744 macrophages and PBM cells, whereas the smallest particles (56 and 28 nm) were significantly less internalised. This is of great importance if we take into account that several reports show opposite trends for the internalisation by cancer cells,29 indicating that smaller particle sizes more easily escape uptake by cells of the immune system while enhancing their internalisation by cancer cells. This is because cells of the immune system typically internalise particles through phagocytosis and recognise more significantly particles of larger sizes, while cancer cells internalise particles through endocytosis, where membrane permeation plays an important role and hence smaller particles are internalised in a more efficient manner.33 Importantly, the MOFs are able to deliver calcein with a more than ca. 20-fold increase to macrophage and PBM cells. Once proven that the MOFs are internalised by cells of the immune system, we assessed the effect on their proliferation using the MTT assay. J744 macrophages and PBMCs were incubated with the MOFs for 24 and 72 hours (see ESI† Section S.3 for protocols and S.7 for tabulated results of independent experiments). The results show excellent biocompatibility, with a cell proliferation of over 100% in all cases, even upon incubation with high concentrations of MOFs (up to 500 μg mL−1), whereas incubation with 500 μg mL−1 of free BTC results in a decrease in J744 macrophage and PBM cell viability to ca. 80% after 72 hours (Fig. 3c).
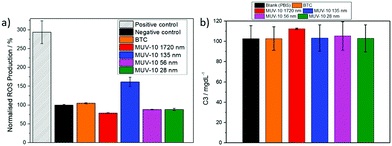
The reactive oxygen species (ROS) production was studied to assess if MUV-10 induces oxidative stress in macrophages (Fig. 4a). The cells were incubated with 250 μg mL−1 of the different MOFs for 2 hours. The 156 nm MOF induced mild generation of ROS (ca. 150%) in J744 cells whereas all the other MOFs did not induce any ROS generation, which is in contrast with literature reports showing up to 200% ROS production upon incubation with similar concentrations of UiO-66 (Zr).21 The ROS production of the 156 nm MOF could be attributed to its higher defectivity (ca. 23.5% missing linkers) compared to the other MOFs (ca. 5–8% missing linkers), which often lowers the energy of the unoccupied d orbitals and increases the likelihood of charge transfer.34
The activation of the complement cascade (C3 and C4)–a part of the immune system that eliminates foreign pathogens, often resulting in activation of phagocytic cells and inflammation23–was investigated following the incubating of blood plasma from five different human donors with the MOFs. Fig. 4b shows the averaged data for the C3 concentrations of the five donors after incubation with 250 μg mL−1 of the MOFs (see S.7, ESI† for the C4 complement), together with a control in which the blood plasma was incubated with a PBS solution (see S.7, ESI† for the C3 and C4 values of each individual donor) that showed no complement cascade activation upon treatment with each of the MOFs.
Taken together, the improved physiological stability towards phosphate-induced degradation, dispersion in PBS and immune system compatibility compared to iron- and zirconium-based MOFs suggests that MUV-10 is an excellent candidate for further investigation as a drug delivery device, with fine control over particle size and functionality made possible through the introduction of modulators. Additionally, this study demonstrates the safe use of MUV-10 for other applications given its excellent biocompatibility.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Horizon 2020 research and innovation programme under the Marie Skłodowska grant agreement No 837804 (DefTiMOFs, MSCA-IF-2018). I. A. L. thanks the European Union's Horizon 2020 research and innovation programme for receipt of the MSCA Individual Fellowship and The University of Valencia for research facilities. Connor JR Wells is acknowledged for proofreading this manusript.References
- A. Carné-Sánchez, I. Imaz, K. C. Stylianou and D. Maspoch, Chem. – Eur. J., 2014, 20, 5192–5201 CrossRef PubMed.
- M. Safaei, M. M. Foroughi, N. Ebrahimpoor, S. Jahani, A. Omidi and M. Khatami, TrAC, Trends Anal. Chem., 2019, 118, 401–425 CrossRef CAS.
- P. Horcajada, C. Serre, M. Vallet-Regí, M. Sebban, F. Taulelle and G. Férey, Angew. Chem., Int. Ed., 2006, 45, 5974–5978 CrossRef CAS PubMed.
- M. Giménez-Marqués, T. Hidalgo, C. Serre and P. Horcajada, Coord. Chem. Rev., 2016, 307, 342–360 CrossRef.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS PubMed.
- I. A. Lázaro and R. S. Forgan, Coord. Chem. Rev., 2019, 380, 230–259 CrossRef.
- J. W. M. Osterrieth and D. Fairen-Jimenez, Biotechnol. J., 2021, 16, 2000005 CrossRef CAS PubMed.
- R. J. Marshall and R. S. Forgan, Eur. J. Inorg. Chem., 2016, 4310–4331 CrossRef CAS.
- H. Assi, G. Mouchaham, N. Steunou, T. Devic and C. Serre, Chem. Soc. Rev., 2017, 46, 3431–3452 RSC.
- S. Yuan, J.-S. Qin, C. T. Lollar and H.-C. Zhou, ACS Cent. Sci., 2018, 4, 440–450 CrossRef CAS PubMed.
- X. Chen, X. Peng, L. Jiang, X. Yuan, H. Yu, H. Wang, J. Zhang and Q. Xia, Chem. Eng. J., 2020, 395, 125080 CrossRef CAS.
- J. Zhu, P.-Z. Li, W. Guo, Y. Zhao and R. Zou, Coord. Chem. Rev., 2018, 359, 80–101 CrossRef CAS.
- J.-L. Song, Z.-Q. Huang, J. Mao, W.-J. Chen, B. Wang, F.-W. Yang, S.-H. Liu, H.-J. Zhang, L.-P. Qiu and J.-H. Chen, Chem. Eng. J., 2020, 396, 125246 CrossRef CAS.
- Y. Xie, X. Liu, X. Ma, Y. Duan, Y. Yao and Q. Cai, ACS Appl. Mater. Interfaces, 2018, 10, 13325–13332 CrossRef CAS PubMed.
- A. Rengaraj, P. Puthiaraj, N.-S. Heo, H. Lee, S. K. Hwang, S. Kwon, W.-S. Ahn and Y.-S. Huh, Colloids Surf., B, 2017, 160, 1–10 CrossRef CAS PubMed.
- G. Lan, K. Ni, S. S. Veroneau, X. Feng, G. T. Nash, T. Luo, Z. Xu and W. Lin, J. Am. Chem. Soc., 2019, 141, 4204–4208 CrossRef CAS PubMed.
- J. Castells-Gil, N. M. Padial, N. Almora-Barrios, J. Albero, A. R. Ruiz-Salvador, J. González-Platas, H. García and C. Martí-Gastaldo, Angew. Chem., Int. Ed., 2018, 57, 8453–8457 CrossRef CAS PubMed.
- N. M. Padial, B. Lerma-Berlanga, N. Almora-Barrios, J. Castells-Gil, I. da Silva, M. de la Mata, S. I. Molina, J. Hernández-Saz, A. E. Platero-Prats, S. Tatay and C. Marti-Gastaldo, J. Am. Chem. Soc., 2020, 142, 6638–6648 CrossRef CAS PubMed.
- I. A. Lázaro, N. Almora-Barrios, S. Tatay and C. Martí-Gastaldo, Chem. Sci., 2020, 12, 2586–2593 RSC.
- I. A. Lázaro, S. A. Lázaro and R. S. Forgan, Chem. Commun., 2018, 54, 2792–2795 RSC.
- I. A. Lázaro, S. Haddad, J. M. Rodrigo-Muñoz, C. Orellana-Tavra, V. del Pozo, D. Fairen-Jimenez and R. S. Forgan, ACS Appl. Mater. Interfaces, 2018, 10, 5255–5268 CrossRef PubMed.
- I. A. Lázaro, C. J. R. Wells and R. S. Forgan, Angew. Chem., Int. Ed., 2020, 59, 5211–5217 CrossRef PubMed.
- M. A. Dobrovolskaia and S. E. McNeil, Nat. Nanotechnol., 2007, 2, 469–478 CrossRef CAS PubMed.
- I. A. Lázaro, Eur. J. Inorg. Chem., 2020, 4284–4294 CrossRef.
- M. Taddei, K. C. Dümbgen, J. A. van Bokhoven and M. Ranocchiari, Chem. Commun., 2016, 52, 6411–6414 RSC.
- C. Orellana-Tavra, R. J. Marshall, E. F. Baxter, I. A. Lázaro, A. Tao, A. K. Cheetham, R. S. Forgan and D. Fairen-Jimenez, J. Mater. Chem. B, 2016, 4, 7697–7707 RSC.
- E. Bellido, T. Hidalgo, M. V. Lozano, M. Guillevic, R. Simón-Vázquez, M. J. Santander-Ortega, Á. González-Fernández, C. Serre, M. J. Alonso and P. Horcajada, Adv. Healthcare Mater., 2015, 4, 1246–1257 CrossRef CAS PubMed.
- K. M. L. Taylor-Pashow, J. D. Rocca, Z. Xie, S. Tran and W. Lin, J. Am. Chem. Soc., 2009, 131, 14261–14263 CrossRef CAS PubMed.
- I. A. Lázaro, S. Haddad, J. M. Rodrigo-Muñoz, R. J. Marshall, B. Sastre, V. del Pozo, D. Fairen-Jimenez and R. S. Forgan, ACS Appl. Mater. Interfaces, 2018, 10, 31146–31157 CrossRef PubMed.
- P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J.-S. Chang, Y. K. Hwang, V. Marsaud, P.-N. Bories, L. Cynober, S. Gil, G. Férey, P. Couvreur and R. Gref, Nat. Mater., 2010, 9, 172–178 CrossRef CAS PubMed.
- B. Maherani, E. Arab-Tehrany, A. Kheirolomoom, D. Geny and M. Linder, Biochimie, 2013, 95, 2018–2033 CrossRef CAS PubMed.
- C. Orellana-Tavra, S. Haddad, R. J. Marshall, I. A. Lázaro, G. Boix, I. Imaz, D. Maspoch, R. S. Forgan and D. Fairen-Jimenez, ACS Appl. Mater. Interfaces, 2017, 9, 35516–35525 CrossRef CAS PubMed.
- T.-G. Iversen, T. Skotland and K. Sandvig, Nano Today, 2011, 6, 176–185 CrossRef CAS.
- A. D. Vos, K. Hendrickx, P. V. D. Voort, V. V. Speybroeck and K. Lejaeghere, Chem. Mater., 2017, 29, 3006–3019 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: MOF synthesis, analysis and in vitro studies. See DOI: 10.1039/d1tb00981h |
| This journal is © The Royal Society of Chemistry 2021 |