Nanoparticle-mediated surfactant therapy in patients with severe COVID-19: a perspective
Abstract
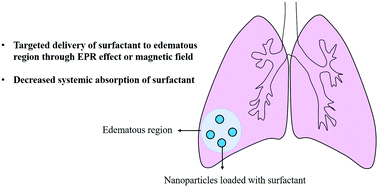
Coronavirus disease 2019 (COVID-19) is an RNA virus-based disease that can be deadly. For critically ill patients, mechanical ventilation is an important life-saving treatment. However, mechanical ventilation shows a trade-off between supporting respiratory function and ventilator-induced lung injury (VILI). Surfactant therapy is a medical administration of exogenous surfactant to supplement or replace deficient or dysfunctional endogenous surfactant. Surfactant therapy can be used to postpone or shorten the use of mechanical ventilation to minimize or avoid VILI, because surfactants can reduce surface tension, improve lung compliance, and enhance oxygenation. In addition, nanotechnology can be applied to improve the therapeutic effect and reduce the adverse effects of surfactants. In this perspective, we discussed how nanoparticles deliver surfactants through intravenous injection and inhalation to the expected lung disease regions where surfactants are mostly needed, and discussed the prospects of nanoparticle-mediated surfactant therapy in the treatment of patients with severe COVID-19.
- This article is part of the themed collection: Journal of Materials Chemistry B Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...