SARS-CoV-2 and approaches for a testing and diagnostic strategy
Abstract
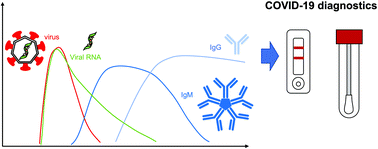
The COVID-19 pandemic has led to an unprecedented global health challenge, creating sudden, massive demands for diagnostic testing, treatment, therapies, and vaccines. In particular, the development of diagnostic assays for SARS-CoV-2 has been pursued as they are needed for quarantine, disease surveillance, and patient treatment. One of the major lessons the pandemic highlighted was the need for fast, cheap, scalable and reliable diagnostic methods, such as paper-based assays. Furthermore, it has previously been suggested that paper-based tests may be more suitable for settings with lower resource availability and may help alleviate some supply chain challenges which arose during the COVID-19 pandemic. Therefore, we explore how such devices may fit in a comprehensive diagnostic strategy and how some of the challenges to the technology, e.g. low sensitivity, may be addressed. We discuss the properties of the SARS-CoV-2 virus itself, the COVID-19 disease pathway, and the immune response. We then describe the different diagnostic strategies that have been pursued, focusing on molecular strategies for viral genetic material, antigen tests, and serological assays, and innovations for improving the diagnostic sensitivity and capabilities. Finally, we discuss pressing issues for the future, and what needs to be addressed for the ongoing pandemic and future outbreaks.
- This article is part of the themed collection: Celebrating International Women’s Day: Women in Materials Science


 Please wait while we load your content...
Please wait while we load your content...