Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thermal crystallization of amorphous calcium phosphate combined with citrate and fluoride doping: a novel route to produce hydroxyapatite bioceramics†
Lorenzo
Degli Esposti
 *a,
Smilja
Markovic
*a,
Smilja
Markovic
 b,
Nenad
Ignjatovic
b,
Nenad
Ignjatovic
 b,
Silvia
Panseri
a,
Monica
Montesi
a,
Alessio
Adamiano
a,
Marco
Fosca
c,
Julietta V.
Rau
b,
Silvia
Panseri
a,
Monica
Montesi
a,
Alessio
Adamiano
a,
Marco
Fosca
c,
Julietta V.
Rau
 cd,
Vuk
Uskoković
cd,
Vuk
Uskoković
 e and
Michele
Iafisco
e and
Michele
Iafisco
 *a
*a
aInstitute of Science and Technology for Ceramics (ISTEC), National Research Council (CNR), Via Granarolo 64, 48018 Faenza, Italy. E-mail: lorenzo.degliesposti@istec.cnr.it; michele.iafisco@istec.cnr.it
bInstitute of Technical Sciences of the Serbian Academy of Science and Arts, Knez Mihailova 35/IV, P. O. Box 377, 11000 Belgrade, Serbia
cInstitute of Structure of Matter (ISM), National Research Council (CNR), Via del Fosso del Cavaliere 100, 00133 Rome, Italy
dSechenov First Moscow State Medical University, Institute of Pharmacy, Department of Analytical, Physical and Colloid Chemistry, Trubetskaya 8, Build. 2, 119991 Moscow, Russia
eTardigradeNano LLC, Irvine, CA 92604, USA
First published on 26th April 2021
Abstract
Amorphous calcium phosphate (ACP) is a material of high interest for dentistry, orthopedics, and other biomedical sectors. Being intrinsically metastable, the process of transformation of ACP into a crystalline phase upon heating is of high relevance for the development of innovative bioceramics. Here we have first studied the thermal behavior of a citrate-stabilized ACP (Cit-ACP) also doped with fluoride ions (Cit-FACP) prepared at three different nominal Cit/Ca ratios (i.e. 4, 2, 1) by differential thermal analysis. Next, the physico-chemical features of the crystalline products as well as the in vitro cell response to the materials were investigated. A citrate and fluoride free ACP sample was also tested as the blank. We have found that the activation energy of crystallization of Cit-(F)ACP samples is lower in comparison to the blank ACP and this is influenced by the nominal Cit/Ca molar ratio. Interestingly, we have discovered that the thermal treatment of Cit-(F)ACP at 800 °C yields hydroxyapatite (HA) or fluorapatite (FHA) as the main products differently from blank ACP that, like most of the ACPs reported in the literature, yields β-tricalcium phosphate. This was attributed to the Ca/P ratio of Cit-(F)ACP, which is similar to HA. A study of the crystalline products has revealed that all the (F)HA samples were non-cytotoxic, and retained carbonate ions in the crystal structure despite the heat treatment that should have induced decarbonation. The morphology of the products is influenced by the nominal Cit/Ca ratio and the presence of fluoride, ranging from spherical nanoparticles to micrometric hexagonal rods. Overall, our results prove that the thermal crystallization of Cit-(F)ACP is markedly different from classic ACP based materials and the thermal treatment of Cit-(F)ACP represents an attractive route for producing pure bioactive HA ceramics.
Introduction
Amorphous calcium phosphate (ACP) nanoparticles are one of the most interesting and promising classes of nanomaterials for biomedical application.1,2 The structural unit of ACP first proposed by Betts and Posner3,4 and later confirmed by high resolution imaging techniques,5 is a spherical cluster with a diameter of ca. 9.5 Å with the structure of Ca9(PO4)6. Betts and Posner suggested that ACP particles are composed of random aggregates of these so-called Posner's clusters, where water molecules, corresponding to ca. 20 wt%, “glue” the clusters to yield the Ca9(PO4)6·nH2O (where n is between 3 and 4.5) stoichiometry.6 The Ca/P molar ratio of Posner's clusters is 1.5, but there are reports of several ACPs with different Ca/P molar ratios, which are believed to possess different local arrangements of atoms.1,7 ACP can also incorporate other ions as substituents for Ca2+ and PO43− ions, which in turn influence the Ca/P molar ratio.8,9 Interestingly, the spatial arrangement of Ca2+ and PO43− ions in the Posner's cluster is very similar to some regions of hydroxyapatite (HA, Ca10(PO4)6(OH)2), octacalcium phosphate (OCP, Ca8H2(PO4)6·5H2O), and tricalcium phosphate (TCP, Ca3(PO4)2) unit cells, for which reason the transformation of ACP into all these crystalline phases is thermodynamically favored.10,11 ACP is intrinsically metastable and given sufficient time, temperature and/or humidity, it will crystallize into one of the aforementioned calcium phosphate phases. Indeed, ACP is of high biological relevance because it is the precursor of biogenic HA of bones and teeth.12–15 It was demonstrated by Robinson et al.16 and Beniash et al.17 that the mineralization of tooth enamel occurs through the deposition of spherical ACP nanoparticles into chains that crystallize to HA, and a similar mechanism was also proposed for bone formation.18Apart from their biological relevance, ACP nanoparticles were extensively studied as remineralizing agents in dentistry and as biomaterials for bone repair.1,2 They are used, for example, in remineralizing toothpastes, coatings for prostheses, self-setting injectable cements, and hybrid composites.1,2,15 All these applications rely on the capacity of ACP to crystallize and form HA in the presence of water, similarly to the biogenic formation of HA. Therefore, the transformation of ACP into one or more crystalline phases is an intriguing process and is of great relevance for any ACP-related applications. The most studied crystallization process for ACP is the water-mediated one;19 however, heating in vacuum or air can also induce crystallization.8,20 Upon heating, several transitions occur in ACP, starting from the irreversible loss of structural water and concluding with the transformation to a crystalline phase between 500 and 800 °C. The thermally induced crystallization of ACP usually produces the α- or β-polymorph of TCP.1,2 This process is mainly controlled by a solid-state lattice reordering mechanism, where the Ca9(PO4)6 stoichiometry of ACP leads to the formation of the crystal phase with the same stoichiometry (i.e. TCP).6,21 ACP based materials with different compositions or Ca/P molar ratios were found to produce mixtures of TCP with other calcium phosphate phases (e.g. HA, α- + β-TCP mixtures, and calcium pyrophosphates, ordered in decreasing Ca/P ratio).2,6,8,20,22–30 Apart from a few reports that will be discussed further,8,25,31–34 the typical thermal crystallization of ACP does not yield a pure crystalline HA phase.
Several methods have been reported for preparing ACP, with the most common ones being wet precipitation in aqueous and non-aqueous solvents, quenching of molten calcium phosphates, high-energy mechanochemical synthesis, and sol–gel synthesis.1,2 The main disadvantage of ACP is its instability, which makes its handling and preservation challenging and is a hindrance to its wide-scale application. Indeed, ACP rapidly crystallizes, not only in wet conditions, but also in the dry state.19 Therefore, several additives and ions were studied to stabilize ACP.35,36 The strong interaction of citrate with calcium phosphates was extensively studied in the past.37 Recently we have developed a stable carbonate-doped ACP nanomaterial functionalized with citrate ions (Cit-ACP) that can also be doped with fluoride ions (Cit-FACP).38 We have discovered that citrate successfully stabilizes ACP nanoparticles, making their amorphous structure steady for years in the dry state under atmospheric conditions.38 Furthermore, we have found out that citrate allows one to tune specific properties of the final material by regulating the specific surface area, and that fluoride ions can be successfully delivered to tooth enamel when this material is used as a remineralizing agent in dentistry.38 Indeed, in vitro experiments have proven that Cit-ACP and Cit-FACP nanoparticles are able to attach onto HA crystals of human enamel and dentin and to form a new mineral phase contiguous to the biogenic one, restoring the demineralized tissue into its native structure.38
The main aim of this work is the study of the thermal behavior of Cit-ACP and Cit-FACP nanoparticles and the evaluation of the crystalline products upon heating. The thermochemical parameters of Cit-ACP and Cit-FACP crystallization have been characterized with the use of differential thermal analysis and modeled kinetically, while the physico-chemical features of the crystalline products as well as the in vitro cell response to the materials were investigated.
Experimental
Materials
Calcium chloride dihydrate (CaCl2·2H2O, ≥99.0% pure), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, ≥97.5% pure), hydrochloric acid (HCl, ≥37.0% pure), dimethyl sulfoxide (DMSO, ≥99.5% pure), sodium citrate tribasic dihydrate (Na3(C6H5O7)·2H2O, ≥99.0% pure, hereafter called Na3(Cit)), sodium phosphate dibasic dihydrate (Na2HPO4·2H2O, ≥99.0% pure), sodium carbonate monohydrate (Na2CO3·2H2O, ≥99.0% pure), sodium fluoride (NaF, ≥99.0% pure), paraformaldehyde (≥95.0% pure), phosphate buffered saline (PBS, 1× solution), and t-octylphenoxypolyethoxyethanol (Triton X-100, laboratory grade) were purchased from Sigma Aldrich (St. Louis, MO, USA). 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI, ≥95.0% pure), fetal bovine serum (FBS), fibroblast growth factor basic (FGFb, ≥95.0% pure), fluorescein-5-isothiocyanate-conjugated phalloidin (FITC-conjugated phalloidin, ≥90.0% pure) and penicillin–streptomycin (1000 U mL−1) were purchased from Invitrogen (Waltham, MA, USA). Minimum essential medium α (αMEM) was purchased from Gibco (Gaithersburg, MD, USA). All the solutions were prepared with ultrapure water (18.2 MΩ cm, 25 °C, Arium© pro, Sartorius).Sample preparation
Cit-ACP samples were synthesized as reported by Iafisco et al.38 Cit-ACP was prepared by mixing at room temperature two aqueous solutions, consisting of (i) 100 mL of 100 mM CaCl2 + X mM Na3(Cit) (where X = 100 for Cit-ACP-1, 200 for Cit-ACP-2, and 400 for Cit-ACP-4) and (ii) 100 mL of 120 mM Na2HPO4 + 200 mM Na2CO3. After mixing, the pH was brought to 8.5 with concentrated HCl and after precipitation the particles were collected by centrifugation (7000 rpm, 5 min, 4 °C) and repeatedly washed with ultrapure water. Finally, the materials were freeze-dried overnight. Cit-FACP samples were prepared with the same procedure of Cit-ACPs, but with the addition of 50 mM NaF to solution (ii). Below in the text, Cit-ACP and Cit-FACP will be defined together as Cit-(F)ACP.Citrate-free ACP (thereafter called blank-ACP) used as the reference material was synthesized by slightly modifying the wet chemical precipitation reported by Onuma et al.39 Blank-ACP was prepared by mixing at 4 °C two solutions, consisting of (i) 100 mL of 20 mM CaCl2 and (ii) 100 mL of 20 mM Na2HPO4, and the precipitate was washed three times by centrifugation (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm, 4 min, 4 °C), the first two with cold water and the last with a solution of ethanol–water (70
500 rpm, 4 min, 4 °C), the first two with cold water and the last with a solution of ethanol–water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v), and then freeze-dried.
30 v/v), and then freeze-dried.
Thermal treatment of bulk powders
The amorphous samples were calcined above their crystallization temperature to obtain and then characterize their crystallization products. About 400 mg of powdered samples were put in an alumina crucible and heated in a static oven (KL40/12, Nannetti, Faenza, Italy) from room temperature to 800 °C at a heating rate of 100 °C h−1, and then left to spontaneously cool down. Afterwards, the obtained powders were manually ground to uniform granularity.Chemical, morphological, and structural characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) alloy with a Polaron E5100 sputter-coater (Polaron Equipment, Watford, Hertfordshire, UK). Micrographs were acquired in secondary electron acquisition mode using an acceleration voltage of 3 kV.
20) alloy with a Polaron E5100 sputter-coater (Polaron Equipment, Watford, Hertfordshire, UK). Micrographs were acquired in secondary electron acquisition mode using an acceleration voltage of 3 kV.
Thermal data analysis
The Kissinger kinetic model was employed to calculate the activation energy (Ea) of the crystallization reaction. Specifically, in the model it is assumed that the crystallization rate coincides with the exothermic reaction peak in DTA, and thus the Kissinger model correlates the heating rate (β, K min−1) with the peak temperature (Tp) of crystallization, allowing Ea to be calculated from eqn (1), where R is the gas constant: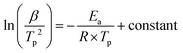 | (1) |
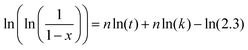 | (2) |
Biological characterization of calcined powder samples
Results and discussion
Structural and compositional characterization of the thermally untreated amorphous samples
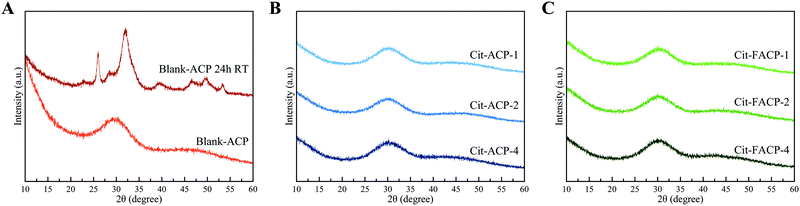
Cit-(F)ACP samples were prepared at three nominal Cit/Ca molar ratios, i.e., Cit/Ca = 1, 2 and 4, corresponding to Cit-(F)ACP-1, Cit-(F)ACP-2 and Cit-(F)ACP-4, respectively. All Cit-(F)ACP samples are amorphous, as previously demonstrated by the presence of only one broad reflection centered at ca. 30° 2θ in the PXRD patterns.38 Blank-ACP displays a similar pattern (Fig. 1A), corroborating its amorphous state. The long-term stability of dry Cit-(F)ACP samples as amorphous materials shown in our previous work38 and ascribed to the presence of citrate ions was further confirmed since the PXRD patterns of the samples collected after four years of storage at room temperature (Fig. 1B and C) are identical to those immediately after preparation. In contrast, blank-ACP powder converts spontaneously into crystalline HA in a matter of hours under ambient conditions (Fig. 1A).2Chemical composition and SSABET of the samples are reported in ref. 38 and for the sake of clarity also in Table 1. All Cit-(F)ACP samples have comparable amounts of calcium, phosphate, citrate, and carbonate ions, and Cit-FACP samples also contain fluoride ions. Blank-ACP does not contain citrate and has a low amount of carbonate, the latter of which is due to atmospheric CO2 dissolved in the solvent during precipitation. The Ca/P molar ratio of Cit-(F)ACP samples is remarkably higher than the Ca/P molar ratio of blank-ACP, and higher than the common value of 1.50 due to the presence of carbonate ions substituting for phosphates. This is supported by previous studies that have shown that the Ca/P ratio of ACP can be increased by incorporating high amounts of carbonate ions engaging in PO4 → CO3 substitution.8,34 The SSABET of the samples is in agreement with their nanometric nature, and the values reported for Cit-(F)ACP are remarkably higher in comparison to blank-ACP. For instance, Cit-(F)ACP samples have SSABET values that range from 200 up to 330 m2 g−1, depending on the nominal Cit/Ca ratio, while the SSABET value of blank-ACP sample is 151 m2 g−1. This latter value is similar to the SSABET of other ACPs reported in the literature, which are typically between 80 and 160 m2 g−1.43,44 The nominal Cit/Ca molar ratio does not influence the stability of Cit-(F)ACP in the dry state nor their structural and compositional features, but the SSABET changes significantly when the nominal Cit/Ca ratio was modified, independently from fluoride doping.38 In particular, the two values are inversely proportional, where the SSABET increased when the nominal Cit/Ca ratio decreased. Previous studies have proven that citrate ions adsorb on the surface of ACP-forming nuclei,45,46 minimizing their size and leading to the high values of SSABET. Therefore, the observed inverse proportionality between SSABET and the Cit/Ca ratio appears counterintuitive if the sole effect of citrate molecules on the dispersion of nuclei and crystallites in the system is presumed, meaning that other microstructural effects must be at work. The decrease of SSABET at higher Cit/Ca ratios could be attributed to an increment of the nucleation lag time with a consequent decrease of porosity. Another option is that the aggregation of ACP particles is responsible for the changes in SSABET, in which case citrate molecules could exhibit the concentration-dependent effect on particle–particle interaction. Indeed, surface additives are usually thought to hinder the surface processes at both low and high concentrations, but they also hamper the ionic diffusion only at high concentrations.47 If a similar scenario applies to the role of citrate in the formation of Cit-(F)ACP particles, it could be possible that citrate slowed down the ionic diffusion at high Cit/Ca ratios, e.g., Cit/Ca = 4, in comparison with Cit/Ca = 1, thus increasing the nucleation lag time,48 which would correspond to the reduction of effective supersaturation and the growth of more fused and larger particles with a lower SSA.
| Sample | Caa (wt%) | Pa (wt%) | Fb (wt%) | Citratec (wt%) | Carbonatec (wt%) | Ca/P (mol) | SSABETd (m2 g−1) |
|---|---|---|---|---|---|---|---|
| a Quantified by ICP-OES. b Quantified by ISEF. c Quantified by TGA. d Calculated from BET N2 adsorption. e From Iafisco et al.38 | |||||||
| Cit-ACP-4 | 29.9 ± 0.7e | 13.6 ± 0.3e | — | 1.9 ± 0.2e | 3.7 ± 0.4e | 1.70 ± 0.02e | 200 ± 20e |
| Cit-ACP-2 | 29.1 ± 1.0e | 13.2 ± 0.3e | — | 2.2 ± 0.2e | 3.8 ± 0.4e | 1.70 ± 0.02e | 287 ± 29e |
| Cit-ACP-1 | 28.0 ± 0.6e | 12.7 ± 0.2e | — | 1.8 ± 0.2e | 3.2 ± 0.3e | 1.70 ± 0.04e | 309 ± 31e |
| Cit-FACP-4 | 31.4 ± 0.4e | 13.6 ± 0.2e | 1.00 ± 0.10e | 1.5 ± 0.2e | 3.8 ± 0.4e | 1.78 ± 0.01e | 213 ± 21e |
| Cit-FACP-2 | 32.1 ± 0.5e | 13.1 ± 0.2e | 1.10 ± 0.10e | 2.0 ± 0.2e | 3.4 ± 0.3e | 1.89 ± 0.01e | 328 ± 33e |
| Cit-FACP-1 | 31.9 ± 0.8e | 13.1 ± 0.3e | 1.30 ± 0.10e | 2.4 ± 0.2e | 3.1 ± 0.3e | 1.88 ± 0.01e | 293 ± 29e |
| Blank-ACP | 28.8 ± 1.1 | 17.2 ± 0.7 | — | — | 0.8 ± 0.1 | 1.29 ± 0.01 | 151 ± 15 |
Analysis of the thermal behavior of the amorphous samples
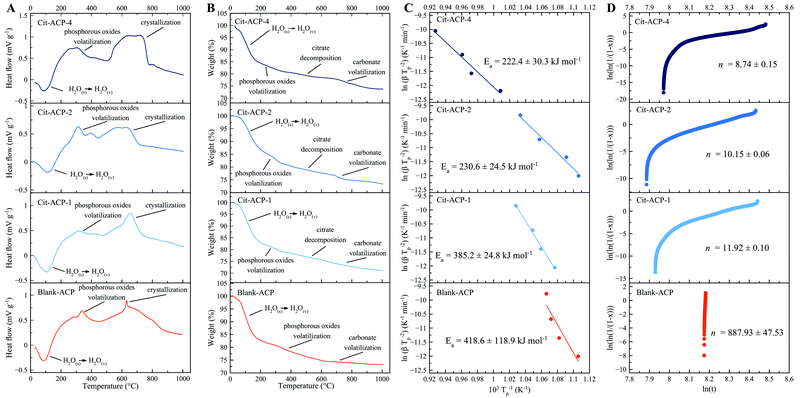
Thermally induced crystallization of Cit-(F)ACP was studied by DTA. In Fig. 2A, DTA curves of Cit-ACP samples together with blank-ACP are presented, while the corresponding TGA curves are reported in Fig. 2B. Cit-ACP and blank-ACP samples undergo three different thermal phenomena during heating. First, an endothermic event occurs at around 100 °C in parallel with a significant mass loss in the 30–200 °C range, which can be attributed to the vaporization of surface-bound and structural water, as is typical for ACP materials.2 The area and the position of this peak is similar in all the samples, indicating a similar hydration degree and bonding strength of water molecules. Second, an exothermic peak occurs at approximately 320 °C, with a corresponding small mass loss in the 200–400 °C range. This peak can be attributed to the heat-induced formation of volatile phosphorous oxides from phosphate and hydrogen phosphate groups.6 Third, the thermally induced crystallization is identified by an exothermic peak in the 650–700 °C range, which is paired with two small mass losses in the 400–600 °C and 600–800 °C ranges due to the decomposition of adsorbed citrate ions and to the volatilization of carbonate ions, respectively. In the case of the blank-ACP sample, the 400–600 °C weight loss is not present, while the 600–800 °C mass loss is small, further confirming their attribution as citrate and carbonate decompositions, respectively. The crystallization exotherm progressively becomes more diffuse and spread over broader temperature ranges with an increase in the nominal Cit/Ca molar ratio (Cit-ACP-4 > Cit-ACP-1 > blank-ACP), indicating a greater polydispersity of physical states occupied by the amorphous units upon crystallization. Furthermore, the area of the crystallization peak increases as the nominal Cit/Ca ratio increases, indicating that the enthalpic gain during the phase transformation increases with the nominal content of citrate. Simultaneously, for all samples the crystallization peaks exhibit a shift to higher temperatures with an increase in the heating rate (Fig. S1A, ESI†). The rate of this shift was used to calculate the crystallization activation energies (Ea) using the Kissinger kinetic model.The Kissinger curves show good fits (Fig. 2C), with the Ea being the highest for the blank-ACP sample (420 kJ mol−1) and the lowest for Cit-ACP-4 (220 kJ mol−1), while Cit-ACP-2 and Cit-ACP-1 take on intermediate values (230 and 385 kJ mol−1, respectively). The Ea of blank-ACP is similar to the values previously reported in the literature for other ACP-based materials.2,31 In detail, other works have estimated values of Ea at 450 kJ mol−1,31 435 kJ mol−1,29 and about 500 kJ mol−1.49 Considering that the Kissinger model assumes that the concentration of nuclei remains constant throughout the crystallization process, the good fitting to the model indirectly confirms that the heat-activated crystallization follows a “martensitic” solid-state lattice reordering mechanism.50 In addition, for Cit-ACP samples, there is a direct proportionality between the Ea and the SSABET values of the samples, which is a strong argument against the thermal crystallization of Cit-ACP as a surface-driven process and in favor of its proceeding through the internal lattice rearrangements. On the contrary, crystallization of Cit-ACP in aqueous physiological conditions was previously proved to be a surface-driven process regulated by the exchange of the ions of the Cit-ACP surface with those present in solution.45 As can be seen in Fig. 2C and 4A, the Ea is not only notably lower in every Cit-ACP sample than in blank-ACP, but it also gets progressively lower as the nominal Cit/Ca ratio increases.
In addition, the Avrami exponent, n, was calculated from Johnson–Mehl–Avrami–Kolmogorov curves (Fig. 2D). This exponent is indicative of the mechanism of reaction, and it is inversely proportional to the nominal Cit/Ca ratio (Fig. 4B). This correlation may indicate that in Cit-ACP samples, especially for Cit-ACP-4, the crystallization process is less surface-controlled and more diffusion-controlled, which would agree with internal lattice rearrangement as the mechanism of crystallization. Another indication inferable from this correlation is that citrate lowers the degrees of freedom associated with the crystallization reaction, thus making it more streamlined. This correlation is also in agreement with the reduction of the Ea with the increase of nominal Cit/Ca ratio, which further suggests that the thermally induced crystallization becomes more spontaneous and, thus, more reliant on diffusion than on more complex kinetic, surface-related processes.
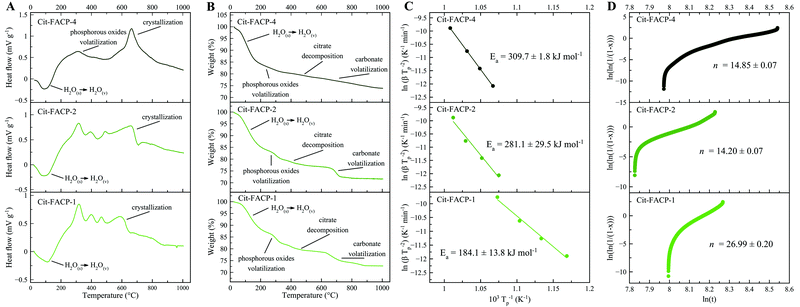
In Fig. 3A and B, the DTA and TGA curves for Cit-FACP samples are reported, which are similar to the curves for Cit-ACP samples. However, Cit-FACP-1 and Cit-FACP-2 samples present multiple exothermic peaks in the region between 300 and 600 °C, and the TGA diagrams corroborate this by showing a more step-like weight loss in this temperature range. This indicates a different behavior of Cit-FACP samples in comparison to Cit-ACP ones.
Furthermore, for Cit-FACP samples, the crystallization exotherm becomes more diffuse and spread over broader temperature ranges with an increase in the nominal Cit/Ca molar ratio. The Kissinger curves calculated from the shift of the crystallization peak (Fig. S1B, ESI†) show good fits (Fig. 3C), and the Ea values (310 kJ mol−1, 280 kJ mol−1, 185 kJ mol−1 for CitFACP-4, Cit-FACP-2, and Cit-FACP-1, respectively) are directly correlated with the Cit/Ca ratio but are not clearly correlated with SSABET, in contrast to the trend observed for Cit-ACP samples (Fig. 2D and 4A).
Therefore, the relationship between Cit/Ca ratios and the Ea is more complex in fluoride-doped Cit-FACP samples. This is somewhat expected because fluoride is not only a component that affects the crystallization rate and mechanism,51 but also the one that gets completely integrated inside the crystal lattice, unlike the citrate molecules. It is likely that for Cit-FACP samples the crystallization process is more surface-controlled than for Cit-ACP samples and the factors that influence crystallization Ea are therefore more complex. This hypothesis is also suggested by the higher Avrami exponents of Cit-FACP samples in comparison to Cit-ACP ones, which suggests a more surface-controlled process (Fig. 4B). Similarly to Cit-ACP, the Avrami exponent of Cit-FACP samples also decreases with the Cit/Ca ratio (Fig. 3E and 4B).
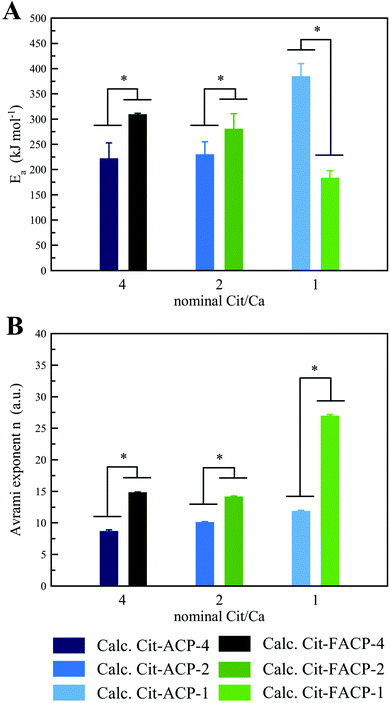 | ||
| Fig. 4 Comparison between the (A) Ea and (B) Avrami exponent of crystallization of Cit-ACP and Cit-FACP samples at different nominal Cit/Ca ratios (*p ≤ 0.04). | ||
The transformation from amorphous to crystalline phase of Cit-(F)ACP samples was investigated by EDXRD. The EDXRD patterns of Cit-(F)ACP-4 and Cit-(F)ACP-1 stored at RT and heated at 400 °C and 700 °C are reported in Fig. 5. The patterns of the samples at 400 °C present small and broad diffraction peaks. Cit-ACP-1 and Cit-FACP-1 present a peak at 32° that is more defined in comparison to Cit-ACP-4 and Cit-FACP-4, where the latter two resemble more the pristine materials. This difference agrees with DTA data, where samples with a nominal Cit/Ca ratio of 1 have a sharper crystallization peak in comparison to the sample with a Cit/Ca of 4, for which the complete crystallization occurred over a larger temperature range. The patterns at 700 °C show a poorly crystalline product for all the samples, which was indexed as HA (PDF card file 00-009-0432). Overall, this result suggests that all Cit-(F)ACP materials crystallize into HA with heating.
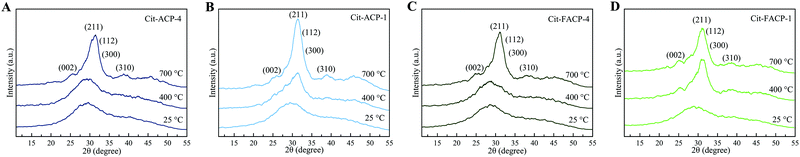 | ||
| Fig. 5 EDXRD patterns of (A) Cit-ACP-4, (B) Cit-ACP-1, (C) Cit-FACP-4, and (D) Cit-FACP-1 at 25 °C, 400 °C, and 700 °C. | ||
Chemical, morphological, and biological characterization of crystalline materials obtained by the thermal treatment of the amorphous samples
Based on the above-reported findings, we next studied the crystallization products obtained by calcining the Cit-(F)ACP and blank-ACP powders. A calcination temperature of 800 °C was used, which is above the crystallization exotherm of all the samples.The PXRD patterns of the calcined Cit-(F)ACP samples (Fig. 6) show that HA is the main product for all the samples. On the other hand, the PXRD pattern of calcined blank-ACP shows that there is no HA in the product, and all the peaks are attributed to γ-calcium pyrophosphate (γ-Ca2P2O7) and to β-TCP. This mixture of crystal phases was previously reported for other calcined ACP samples.26 Based on the broadness and the intensity of HA peaks, all the samples have a comparable crystallinity. Calcined Cit-ACP-4 is composed of pure HA, while the other Cit-(F)ACP samples present additional peaks at 37.4° and 53.9°, which are indexed as calcium oxide (CaO, PDF card file 00-037-1497). Semi-quantitative phase analysis through Rietveld refinement was performed (Table 2), showing that the CaO content is limited and remains below 1 wt% and 5 wt% for calcined Cit-ACP and Cit-FACP samples, respectively. Taking into account that Cit-FACP samples have a Ca/P molar ratio significantly higher than the Ca/P ratio of stoichiometric HA (i.e., 1.67), it is likely that the excess in calcium reacts with atmospheric oxygen during thermal crystallization to form CaO, as occurs during the calcination of non-stoichiometric HA.52,53
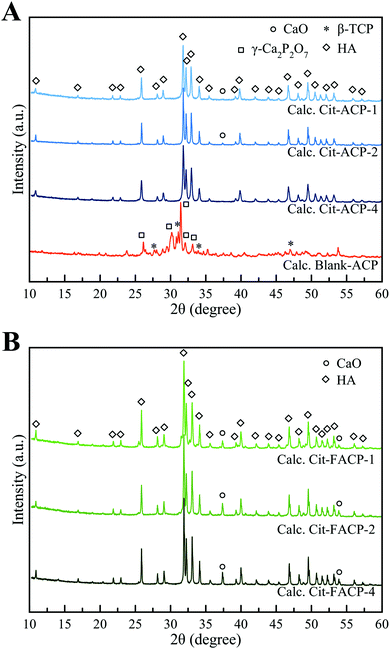 | ||
| Fig. 6 PXRD patterns of calcined (A) blank-ACP, Cit-ACP-4, Cit-ACP-2, Cit-ACP-1, and (B) Cit-FACP-4, Cit-FACP-2, and Cit-FACP-1. | ||
| Sample | Composition | HA a–b cell axes (Å) | HA c cell axis (Å) | |
|---|---|---|---|---|
| HA (wt%) | CaO (wt%) | |||
| Calc. Cit-ACP-4 | 100.0 ± 0.0 | 0.0 ± 0.0 | 9.416 ± 0.005 | 6.890 ± 0.005 |
| Calc. Cit-ACP-2 | 99.0 ± 0.1 | 1.0 ± 0.1 | 9.420 ± 0.005 | 6.887 ± 0.005 |
| Calc. Cit-ACP-1 | 99.7 ± 0.1 | 0.3 ± 0.1 | 9.421 ± 0.005 | 6.884 ± 0.005 |
| Calc. Cit-FACP-4 | 95.6 ± 0.1 | 4.4 ± 0.1 | 9.385 ± 0.005 | 6.888 ± 0.005 |
| Calc. Cit-FACP-2 | 95.4 ± 0.1 | 4.6 ± 0.1 | 9.384 ± 0.005 | 6.886 ± 0.005 |
| Calc. Cit-FACP-1 | 97.9 ± 0.1 | 2.1 ± 0.1 | 9.386 ± 0.005 | 6.888 ± 0.005 |
The HA unit cell parameters of calcined samples are reported in Table 2. The a-cell parameter of Cit-FACP samples is ca. 0.3 Å shorter than that of Cit-ACP samples, while the c-unit cell parameter is similar for both sets of materials. This difference indicates that the main phase of calcined Cit-FACP samples is fluorapatite (FHA, Ca10(PO4)6F2), since it is well reported in the literature that FHA possesses a slightly shorter a-cell axis than HA due to a shorter Ca2+–F− distance than that of Ca2+–OH−.54–56
The FT-IR spectra of calcined Cit-(F)ACP samples (Fig. 7) are in agreement with the PXRD patterns. Indeed, all Cit-(F)ACP samples present the typical bands of an apatitic phase. The main peaks correspond to the apatitic vibrational bands of phosphate groups: the symmetric stretching band ν1PO4 at ca. 960 cm−1, the triply degenerated antisymmetric stretching band ν3PO4 at ca. 1025 cm−1 with a visible shoulder at 1090 cm−1, and the triply degenerated bending band ν4PO4 at ca. 560 and 600 cm−1 with a shoulder at 575 cm−1.57 On the other hand, the calcined blank-ACP sample displays a completely different spectrum, confirming its structural difference in comparison to Cit-(F)ACP samples. This spectrum presents several bands that can be associated with the bending and the stretching of PO4 groups at 450–650 cm−1 and 950–1150 cm−1, respectively, which in turn are composed of several sub-bands that are due to the superposition of γ-calcium pyrophosphate and β-TCP vibrational modes. It can be observed that for all the samples, except for Cit-ACP-4, the ν3PO4 band is particularly broad. This has been described by Antonakos et al.57 as an elongation of P–O bond lengths caused by the thermal decarbonation of crystalline HA, and was observed on carbonate-doped HA from natural sources calcined at high temperatures.57,58 The broadening did not occur for Cit-ACP-4 because in this case the calcination temperature of 800 °C was not enough to alter the structure upon decarbonation.
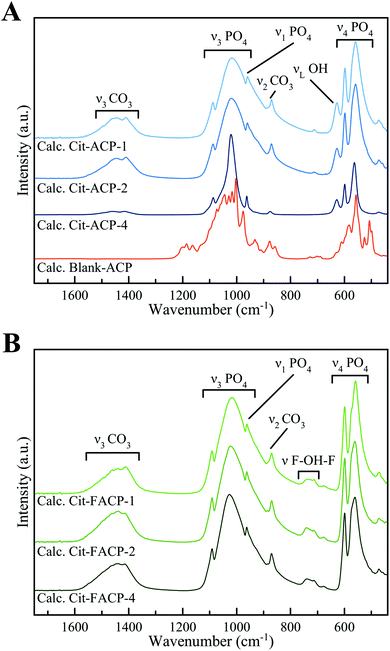 | ||
| Fig. 7 FT-IR spectra of calcined (A) blank-ACP, Cit-ACP-4, Cit-ACP-2, Cit-ACP-1, and (B) Cit-FACP-4, Cit-FACP-2, and Cit-FACP-1. | ||
Only crystalline Cit-ACP samples present the additional band at 628 cm−1, which corresponds to the librational motion of apatitic hydroxyl ions (νLOH).59 The absence of this band in crystalline Cit-FACP samples is further evidence of the formation of FHA, since this phase does not have a significant content of hydroxyl ions, which have been replaced by F− ions.55,60 Moreover, the calcined Cit-FACP samples possess a small band at ca. 720–740 cm−1, which is due to the vibrational motion of the few OH− ions trapped between F− ions in FHA (νF-OH-F).61
Interestingly, the calcined Cit-(F)ACP samples present bands at ca. 870 cm−1 and in the region between 1350–1550 cm−1 that can be attributed to the ν2CO3 and ν3CO3 vibrations of the carbonate ion in an apatitic environment. This finding indicates that the calcination product is a carbonate-doped (F)HA. In particular, for all the samples, the bands peak at 870, 1411 and 1445 cm−1, which is characteristic for a B-type carbonate substitution, where CO32− substitutes PO43− ions.62 As expected, none of the samples presented the ν3CO3 band at ca. 1550–1600 cm−1, indicating that citrate ions decomposed during heating, as shown by the results of the DTA analysis. The relative intensity of the ν3CO3 band in respect to the ν3PO4 band (ICO3/IPO4) is comparable for all the samples except for Cit-ACP-4, which is notably weaker. This indicates a lower carbonate content, as confirmed by the TGA data (see below).
The chemical composition of the crystalline samples is reported in Table 3. For all samples, the relative contents of Ca, P, and F are higher than those of their untreated counterparts, while the Ca/P ratio does not change. This confirms that upon calcination, the water and citrate molecules were removed without altering the chemical composition of the materials. In addition, the presence of fluoride in the Cit-FACP samples confirms the formation of FHA.
| Sample | Caa (wt%) | Pa (wt%) | Fb (wt%) | Citratec (wt%) | Carbonatec (wt%) | Ca/P (mol) |
|---|---|---|---|---|---|---|
| a Quantified by ICP-OES. b Quantified by ISEF. c Quantified by TGA. | ||||||
| Calc. Cit-ACP-4 | 36.3 ± 1.4 | 16.6 ± 0.5 | — | — | 0.4 ± 0.1 | 1.69 ± 0.01 |
| Calc. Cit-ACP-2 | 39.3 ± 1.9 | 17.9 ± 0.9 | — | — | 2.5 ± 0.3 | 1.70 ± 0.02 |
| Calc. Cit-ACP-1 | 36.8 ± 1.4 | 17.1 ± 0.6 | — | — | 2.1 ± 0.2 | 1.66 ± 0.01 |
| Calc. Cit-FACP-4 | 37.6 ± 0.7 | 16.0 ± 0.2 | 2.0 ± 0.2 | — | 2.3 ± 0.2 | 1.82 ± 0.01 |
| Calc. Cit-FACP-2 | 38.3 ± 0.5 | 16.3 ± 0.3 | 2.5 ± 0.1 | — | 1.7 ± 0.2 | 1.82 ± 0.02 |
| Calc. Cit-FACP-1 | 36.9 ± 0.7 | 16.2 ± 0.4 | 2.1 ± 0.4 | — | 2.9 ± 0.3 | 1.76 ± 0.01 |
| Calc. blank-ACP | 35.7 ± 0.5 | 21.0 ± 0.3 | — | — | — | 1.31 ± 0.01 |
From these data, it is established that Cit-(F)ACP crystallizes into (F)HA without forming TCP. As noted earlier, upon heating, conventional ACP transforms into TCP because the thermal crystallization is controlled by a solid-state lattice reordering mechanism, and the basic ACP unit has a Ca/P ratio identical to TCP.6,21 In this regard, the thermal crystallization of ACP into pure HA does occur, but based on literature reports, this has been achieved only in few specific conditions, explicitly in the cases of ACPs produced by plasma-spraying of HA,31,32 by sol–gel synthesis,25,33 or by wet precipitation in the presence of strong carbonate doping with a carbonate content higher than 4 wt%.8,34 In the case of plasma-sprayed ACP, HA formation was thought to be derived from direct nucleation of the crystalline phase during the plasma spheroidization process,31 meaning that the crystal phase selectivity was due to a seeding effect. In the other cases, HA was achieved only when the ACP has a Ca/P molar ratio identical to stoichiometric HA (1.67).8,25,33,34 When the Ca/P ratio of ACP deviated from 1.67, a mixture of HA and TCP was obtained. Considering that Cit-ACP samples have a Ca/P ratio of 1.70, it is likely that in our case the formation of HA was due to the same effect.
Here, it must be taken into account that the production of ACP with the Ca/P ratio of 1.67 is not straightforward because this kind of ratio cannot be achieved by simple wet precipitation – the product inevitably has a Ca/P ratio of 1.506 – and more complex procedures are required, such as doping with high amounts of carbonate ions, or sol–gel syntheses. In our case, the simultaneous presence of citrate and carbonate ions in Cit-(F)ACP is an alternative method to achieve a Ca/P ratio of 1.67 and, therefore, to produce (F)HA by calcination.
Another peculiar trait of Cit-(F)ACP is that with calcination, the carbonate content decreases from ca. 3–4 wt% to ca. 2–3 wt%. This incomplete sublimation of the structural carbonates is an interesting finding, taking into account that the calcination temperature was within the thermal range in which decarbonation usually occurs (600–1000 °C).63 As suggested by the results of the FT-IR analysis, not all of the carbonates were lost with calcination, which represents a remarkable result that will be investigated in more detail in our future studies. In fact, the obtainment of carbonate-doped ceramic HA is a notable achievement because carbonate-doped HA is more bioactive and resorbable than pure HA.64 To the best of our knowledge, the currently used methods to sinter HA while preserving carbonate ions do not involve conventional heating procedures, such as sintering under CO2 flow63 or using spark plasma sintering.9 The crystalline Cit-ACP-4 sample has a notably lower content of carbonate in comparison with the other samples, and its TGA curve is more complex and presents multiple thermal events in the range of 600–1000 °C (Fig. S3, ESI†). It is likely that Cit-ACP-4 does not only contain carbonate ions but also carbon, as proven by its darker color (Fig. S4, ESI†). The incorporation of a carbonaceous phase into the particles is due to the incomplete oxidation of citrate and carbonates during calcination, which may have been caused by the lower SSABET of the Cit-ACP-4 sample. In this respect, the possibility of forming bioceramic/graphitic composite materials is an interesting concept emerging from these findings and will be investigated in more detail in the future.
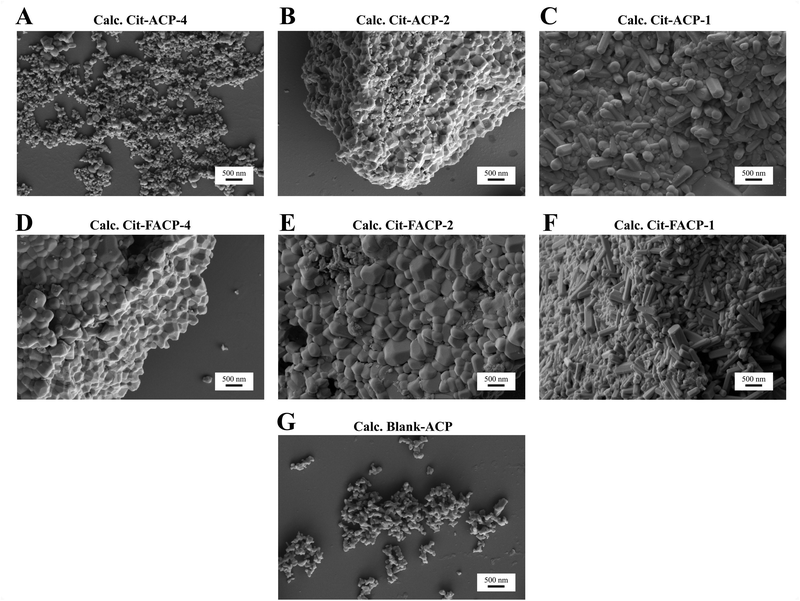
The morphology of the calcined samples was analyzed using FEG-SEM. In Fig. 8 and in Table 4 the representative micrographs as well as the typical particle morphologies and dimensions for each sample are reported. Cit-ACP-4 (Fig. 8A) is composed of spherical particles with a morphology similar to the pristine material,38 and has a low degree of particle sintering. Apart from some bigger grains, the particle size is between 60 and 120 nm in diameter. On the contrary, Cit-ACP-2 particles (Fig. 8B) are bigger and partially sintered, with dimensions between 200 and 400 nm in diameter. Cit-ACP-1 particles (Fig. 8C) are not only bigger than the other Cit-ACP samples, but also present a different morphology, comprising hexagonal rods, with diameters in the 150–250 nm range and lengths that span from 400 nm to 1 μm. Clearly, with the reduction of the Cit/Ca ratio, the crystal growth becomes less hindered in the direction of assuming the hexagonal symmetries of HA.
| Sample | Main particle morphology | Average dimensions (nm) |
|---|---|---|
| Calc. Cit-ACP-4 | Spherical | 60–120 |
| Calc. Cit-ACP-2 | Squared | 200–400 |
| Calc. Cit-ACP-1 | Hexagonal rod | 400–1000 (length) |
| 200–350 (width) | ||
| Calc. Cit-FACP-4 | Squared | 300–500 |
| Calc. Cit-FACP-2 | Squared | 350–600 |
| Calc. Cit-FACP-1 | Hexagonal rod | 300–1000 (length) |
| 150–250 (width) | ||
| Calc. blank-ACP | Irregular | 100–150 |
The morphology of the Cit-FACP-4 and Cit-FACP-2 (Fig. 8D and E) particles is similar to Cit-ACP-2, with fused grains having diameters between 300 and 500 nm for Cit-FACP-4 and between 350 and 600 nm for Cit-FACP-2. Cit-FACP-1 particles (Fig. 8F) are similar to Cit-ACP-1 particles, since both take the form of hexagonal rods with a diameter in the 200–350 nm range and lengths that range from 300 nm to 1 μm. The crystalline rods of Cit-ACP-1 and Cit-FACP-1 are very similar to calcined HA particles reported in the literature, where the morphology reflects the hexagonal crystalline system of HA.65,66 Finally, the blank-ACP sample (Fig. 8G) is composed of small and irregular particles that are partially fused together and have diameters in the range of 100–150 nm.
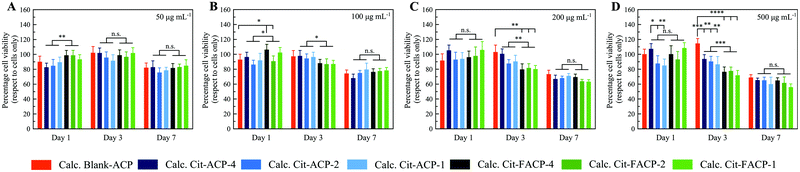
To assess the absence of toxicity of the crystalline samples, an in vitro study was performed evaluating the viability, proliferation, and morphology of MSCs in contact with them. A quantitative comparative analysis of all the calcined samples at different time points is presented in Fig. 9, showing the percentage of viable cells relative to the cell only control. MTT assay is one of the most frequently used methods for evaluating cell proliferation and cytotoxicity and is based on the reduction of the tetrazolium salt by mitochondrial dehydrogenases of actively growing cells. This assay quantifies the metabolic activity of the cells and is an indirect measure of cell viability, and thus of potential cytotoxic effects exerted by the materials.67,68 All the samples show a slight time- and dose-dependent cytotoxicity, including calcined blank-ACP, which is known to be a non-cytotoxic material.69 In fact, the percentage of live cells at the higher particle concentrations (i.e., 200 and 500 μg mL−1) decreased at day 7 compared to the cells only, without significant differences among the samples. The decreasing cell viability observed at day 7 can be ascribable to the effects of the long-term culture in a limited culture area with the presence of a relatively concentrated material. The ANOVA variance analysis, used to compare the effect of the two groups of samples (Cit-ACP vs. Cit-FACP), showed that at day 3, the Cit-FACP group significantly decreased the cell viability at 100, 200 and 500 μg mL−1 (p-value < 0.05 or less). This specific cellular response for this set of samples is probably induced by the presence of higher quantities of CaO rapidly converting to Ca(OH)2, which is a material known to be cytotoxic due to its basification effect on the media.70
Looking at the differences between the cells grown for 3 days with the higher doses of the materials (200 and 500 μg mL−1), it can be concluded that all the Cit-FACP samples induce a slight, statistically significant decrease of cell viability compared to Blank-ACP (p-value < 0.01 or less). In addition, the Cit-ACP group at 500 μg mL−1 showed a slight statistical increase of toxicity compared to calcined blank-ACP (p-value < 0.01 or less), but with a lower effect compared to that of the Cit-FACP group. Finally, it is possible to observe a well-defined trend relative to the effect of Cit-ACP-4 on cell viability. In fact, starting from the concentration of 100 μg mL−1, Cit-ACP-4 seems to exert an inductive effect compared to the other Cit-ACP samples at days 1 and 3 of culture, and this trend becomes statistically different when the cells were grown for 1 day with the dose of 500 μg mL−1. As shown in Fig. 9, the viability of cells treated with Cit-ACP-4 is statistically different when compared with the viability of Cit-ACP-2 and Cit-ACP-1 treatment, with p-values less than 0.05. This result agrees with the compositional data, as Cit-ACP-4 is composed of pure HA, without any traces of CaO.
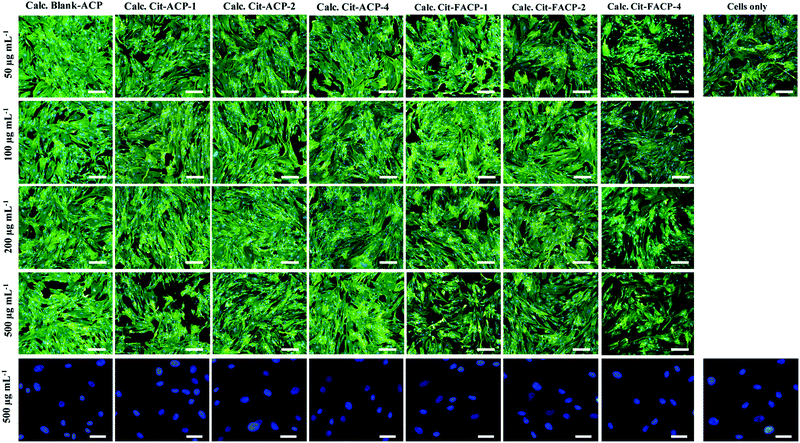
The morphological evaluation of MSCs grown on the samples after 48 h confirms that only a weak level of dose- and time-dependent cytotoxicity is exerted. Fig. 10 demonstrates that the presence of any of the samples at any of the concentrations tested does not compromise the cytoplasmic and nuclear morphology. The cells are well-spread on the culture plastic surface, showing the typical cytoplasmic elongations without the presence of morphological cell damage markers. Overall, all the biological characterizations prove that all the calcined samples have a good biocompatibility toward MSCs and do not induce any significant cytotoxic responses.
Conclusions
In this work we have studied the thermal crystallization of Cit-(F)ACP samples and citrate-free blank-ACP with calorimetric and other analytical techniques and we have characterized the obtained crystalline products. The influence of the nominal Cit/Ca ratio of the precursors and of fluoride doping were analyzed. We have demonstrated that the thermal crystallization of Cit-ACP is remarkably different from the standard ACP materials (i.e., blank-ACP). First, the crystallization is more favored in Cit-(F)ACP, as these samples have a lower crystallization Ea in comparison to that of blank-ACP. In addition, Ea of crystallization is influenced by the nominal Cit/Ca molar ratio. Citrate mainly regulates the surface area of the materials; however, the relationship between SSABET and Ea is complex, as in Cit-ACP samples there is a direct correlation between the SSABET and Ea, whereas in Cit-FACP there is no clear correlation. Considering the Avrami exponents, it is likely that in the Cit-ACP samples the process is more diffusion-controlled and dependent on microstructural effects, while in the Cit-FACP samples the process is more complex and surface-dependent. Secondly, we have discovered that the heat treatment of Cit-(F)ACP at 800 °C yields (F)HA as the main product instead of TCP and calcium pyrophosphate, which are instead produced by the thermal treatment of blank-ACP. The formation of (F)HA is induced by the Ca/P ratio of Cit-(F)ACP samples, which is similar to the Ca/P ratio of HA and different from the ratio of TCP. In the case of the Cit-ACP samples, HA was the only product, while for Cit-FACP samples a small amount of CaO formed alongside FHA. The study of the crystalline products has evinced that (F)HA retains carbonate ions in the crystal structure despite the heat treatment at 800 °C that should have induced decarbonation. Furthermore, the morphology of the calcined products was influenced by the nominal Cit/Ca ratio and by fluoride doping, ranging from rounded nanoparticles to micrometric hexagonal rods. In addition, all the samples were biocompatible and non-cytotoxic, with minimal reductions in the viability of MSCs evident only at the highest concentrations tested (≥200 μg mL−1) and for prolonged incubation times (>7 days). Overall, we have previously demonstrated that Cit-(F)ACP is an excellent biomaterial for dental remineralization due to its capability to attach to dental surfaces and to crystallize into (F)HA in the oral environment. In this work we have discovered that the thermal crystallization of Cit-(F)ACP is a complex physical process, highly dependent on the presence of foreign additives (citrate and fluoride) and leading to interesting products different from the ones reported in the literature. We envisaged that thermal crystallization of Cit-(F)ACP nanoparticles could be a simple and straightforward method to produce advanced bioactive (F)HA based ceramics for different medical applications such as generation of coating for metallic prosthesis and dense or porous three-dimensional scaffolds. Indeed, the preservation of carbonate content, the limited grain growth and the possibility to maintain foreign ions in the structure are interesting properties of HA ceramics that are not easily achievable and up to date require special unconventional treatments such as sintering under CO2 flow, spark plasma sintering, or cold pressing.9,63,71Author contributions
L. Degli Esposti: conceptualization, investigation, visualization, writing – original draft, writing – review & editing; S. Markovic: investigation, writing – review & editing; N. Ignjatovic: investigation, writing – review & editing; S. Panseri: investigation, writing – review & editing; M. Montesi: investigation, writing – review & editing; A. Adamiano: investigation, writing – review & editing; M. Fosca: investigation, writing – review & editing; J. V. Rau: investigation, writing – review & editing; V. Uskoković: conceptualization, investigation, formal analysis, supervision, writing – original draft, writing – review & editing; M. Iafisco: conceptualization, supervision, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Italian Ministry of Health (Bando Ricerca Finalizzata 2016, no. GR-2016-02364704).References
- C. Combes and C. Rey, Acta Biomater., 2010, 6, 3362–3378 CrossRef CAS PubMed.
- S. V. Dorozhkin, Acta Biomater., 2010, 6, 4457–4475 CrossRef CAS PubMed.
- F. Betts and A. S. Posner, Mater. Res. Bull., 1974, 9, 353–360 CrossRef CAS.
- A. S. Posner, F. Betts and N. C. Blumenthal, Prog. Cryst. Growth Charact., 1980, 3, 49–64 CrossRef CAS.
- L. Wang, S. Li, E. Ruiz-Agudo, C. V. Putnis and A. Putnis, CrystEngComm, 2012, 14, 6252–6256 RSC.
- E. Eanes, Calcif. Tissue Res., 1970, 5, 133–145 CrossRef CAS PubMed.
- S. V. Dorozhkin, Prog. Biomater., 2016, 5, 9–70 CrossRef CAS PubMed.
- Y. Li, F. Kong and W. Weng, J. Biomed. Mater. Res., Part B, 2009, 89, 508–517 CrossRef PubMed.
- C. Ortali, I. Julien, M. Vandenhende, C. Drouet and E. Champion, J. Eur. Ceram. Soc., 2018, 38, 2098–2109 CrossRef CAS.
- A. S. Posner and F. Betts, Acc. Chem. Res., 1975, 8, 273–281 CrossRef CAS.
- V. Uskokovic, Cryst. Growth Des., 2019, 19, 4340–4357 CrossRef CAS.
- J. Zhao, Y. Liu, W.-B. Sun and H. Zhang, Chem. Cent. J., 2011, 5, 1–7 CrossRef PubMed.
- M. Hannig and C. Hannig, Nat. Nanotechnol., 2010, 5, 565–569 CrossRef CAS PubMed.
- M. A. S. Melo, S. F. F. Guedes, H. H. K. Xu and L. K. A. Rodrigues, Trends Biotechnol., 2013, 31, 459–467 CrossRef CAS PubMed.
- J. Zhao, Y. Liu, W.-B. Sun and X. Yang, J. Dent. Sci., 2012, 7, 316–323 CrossRef.
- C. Robinson, R. C. Shore, S. R. Wood, S. J. Brookes, D. A. M. Smith, J. T. Wright, S. Connell and J. Kirkham, Connect. Tissue Res., 2003, 44, 65–71 CrossRef CAS PubMed.
- E. Beniash, R. A. Metzler, R. S. Lam and P. Gilbert, J. Struct. Biol., 2009, 166, 133–143 CrossRef CAS PubMed.
- J. Mahamid, B. Aichmayer, E. Shimoni, R. Ziblat, C. Li, S. Siegel, O. Paris, P. Fratzl, S. Weiner and L. Addadi, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 6316–6321 CrossRef CAS PubMed.
- A. L. Boskey and A. S. Posner, J. Phys. Chem., 1973, 77, 2313–2317 CrossRef CAS.
- S. Liu, W. Weng, Z. Li, L. Pan, K. Cheng, C. Song, P. Du, G. Shen and G. Han, J. Mater. Sci.: Mater. Med., 2009, 20, 359 CrossRef CAS PubMed.
- E. Eanes and A. Posner, Calcif. Tissue Res., 1968, 2, 38–48 CrossRef CAS PubMed.
- N. Döbelin, T. J. Brunner, W. J. Stark, M. Eggimann, M. Fisch and M. Bohner, Calcif. Tissue Res., 1968,(2), 38–48 Search PubMed.
- R. Kumar, P. Cheang and K. Khor, Acta Mater., 2004, 52, 1171–1181 CrossRef CAS.
- P. Layrolle and A. Lebugle, Chem. Mater., 1994, 6, 1996–2004 CrossRef CAS.
- P. Layrolle, A. Ito and T. Tateishi, J. Am. Ceram. Soc., 1998, 81, 1421–1428 CrossRef CAS.
- Y. Li, W. Weng and K. C. Tam, Acta Biomater., 2007, 3, 251–254 CrossRef CAS PubMed.
- M. Maciejewski, T. J. Brunner, S. F. Loher, W. J. Stark and A. Baiker, Thermochim. Acta, 2008, 468, 75–80 CrossRef CAS.
- S. Somrani, C. Rey and M. Jemal, J. Mater. Chem., 2003, 13, 888–892 RSC.
- V. Uskoković, S. Marković, L. Veselinović, S. Škapin, N. Ignjatović and D. P. Uskoković, Phys. Chem. Chem. Phys., 2018, 20, 29221–29235 RSC.
- L. Sinusaite, A. Kareiva and A. Zarkov, Cryst. Growth Des., 2021, 21(2), 1242–1248 CrossRef CAS.
- C. Feng, K. Khor, S. Kweh and P. Cheang, Mater. Lett., 2000, 46, 229–233 CrossRef CAS.
- K. A. Gross, V. Gross and C. C. Berndt, J. Am. Ceram. Soc., 1998, 81, 106–112 CrossRef CAS.
- P. Layrolle and A. Lebugle, Chem. Mater., 1996, 8, 134–144 CrossRef CAS.
- Y. Li, D. Li and W. Weng, Int. J. Appl. Ceram. Technol., 2008, 5, 442–448 CrossRef CAS.
- E. C. Reynolds, Spec. Care Dentist., 1998, 18, 8–16 CrossRef CAS PubMed.
- X. Yang, B. Xie, L. Wang, Y. Qin, Z. J. Henneman and G. H. Nancollas, CrystEngComm, 2011, 13, 1153–1158 RSC.
- C. Berg and H.-G. Tiselius, Urol. Res., 1989, 17, 167–172 CrossRef CAS PubMed.
- M. Iafisco, L. Degli Esposti, G. B. Ramírez-Rodríguez, F. Carella, J. Gómez-Morales, A. C. Ionescu, E. Brambilla, A. Tampieri and J. M. Delgado-López, Sci. Rep., 2018, 8, 17016 CrossRef PubMed.
- K. Onuma and M. Iijima, Sci. Rep., 2017, 7, 2711 CrossRef PubMed.
- J. M. Hughes, M. Cameron and K. D. Crowley, Am. Mineral., 1989, 74, 870–876 CAS.
- K. J. Roche and K. T. Stanton, J. Fluorine Chem., 2014, 161, 102–109 CrossRef CAS.
- A. Coelho, Coelho Software, 2012 Search PubMed.
- J. Vecstaudza and J. Locs, J. Alloys Compd., 2017, 700, 215–222 CrossRef CAS.
- R. Sun, M. Åhlén, C.-W. Tai, É. G. Bajnóczi, F. de Kleijne, N. Ferraz, I. Persson, M. Strømme and O. Cheung, Nanomaterials, 2020, 10, 20 CrossRef CAS PubMed.
- K. Chatzipanagis, M. Iafisco, T. Roncal-Herrero, M. Bilton, A. Tampieri, R. Kroger and J. M. Delgado-Lopez, CrystEngComm, 2016, 18, 3170–3173 RSC.
- J. M. Delgado-López, R. Frison, A. Cervellino, J. Gómez-Morales, A. Guagliardi and N. Masciocchi, Adv. Funct. Mater., 2014, 24, 1090–1099 CrossRef.
- V. Uskoković, Rev. J. Chem., 2013, 3, 271–303 CrossRef PubMed.
- V. M. Fokin, J. W. Schmelzer, M. L. Nascimento and E. D. Zanotto, J. Chem. Phys., 2007, 126, 234507 CrossRef PubMed.
- R. Gelli, M. Tonelli, F. Ridi, M. Bonini, H. M. Kwaambwa, A. R. Rennie and P. Baglioni, J. Colloid Interface Sci., 2021, 589, 367–377 CrossRef CAS PubMed.
- H. E. Kissinger, Anal. Chem., 1957, 29, 1702–1706 CrossRef CAS.
- L. M. Rodríguez-Lorenzo, J. N. Hart and K. A. Gross, J. Phys. Chem. B, 2003, 107, 8316–8320 CrossRef.
- K. Haberko, M. M. Bućko, J. Brzezińska-Miecznik, M. Haberko, W. Mozgawa, T. Panz, A. Pyda and J. Zarębski, J. Eur. Ceram. Soc., 2006, 26, 537–542 CrossRef CAS.
- T. Goto and K. Sasaki, Ceram. Int., 2014, 40, 10777–10785 CrossRef CAS.
- L. Jha, S. Best, J. Knowles, I. Rehman, J. D. Santos and W. Bonfield, J. Mater. Sci.: Mater. Med., 1997, 8, 185–191 CrossRef CAS PubMed.
- L. Rodrıguez-Lorenzo, J. Hart and K. Gross, Biomaterials, 2003, 24, 3777–3785 CrossRef.
- L. Degli Esposti, A. Adamiano, A. Tampieri, G. B. Ramirez-Rodriguez, D. Siliqi, C. Giannini, P. Ivanchenko, G. Martra, F.-H. Lin, J. M. Delgado-López and M. Iafisco, Cryst. Growth Des., 2020, 20(5), 3163–3172 CrossRef CAS.
- A. Antonakos, E. Liarokapis and T. Leventouri, Biomaterials, 2007, 28, 3043–3054 CrossRef CAS PubMed.
- E. Tkalčec, J. Popović, S. Orlić, S. Milardović and H. Ivanković, Mater. Sci. Eng., C, 2014, 42, 578–586 CrossRef PubMed.
- S. Koutsopoulos, J. Biomed. Mater. Res., Part B, 2002, 62, 600–612 CrossRef CAS PubMed.
- N. Rameshbabu, T. S. Kumar and K. P. Rao, Bull. Mater. Sci., 2006, 29, 611–615 CrossRef CAS.
- F. Freund and R. M. Knobel, J. Chem. Soc., Dalton Trans., 1977, 1136–1140 RSC.
- J. M. Delgado-López, M. Iafisco, I. Rodríguez, A. Tampieri, M. Prat and J. Gómez-Morales, Acta Biomater., 2012, 8, 3491–3499 CrossRef PubMed.
- Z. Zyman and M. Tkachenko, J. Eur. Ceram. Soc., 2011, 31, 241–248 CrossRef CAS.
- Y. Doi, T. Shibutani, Y. Moriwaki, T. Kajimoto and Y. Iwayama, J. Biomed. Mater. Res., Part B, 1998, 39, 603–610 CrossRef CAS.
- S. Pramanik, A. S. M. Hanif, B. Pingguan-Murphy and N. A. Abu Osman, Materials, 2013, 6, 65–75 CrossRef CAS PubMed.
- P. Ideia, L. Degli Esposti, C. C. Miguel, A. Adamiano, M. Iafisco and P. C. Castilho, Int. J. Appl. Ceram. Technol., 2021, 18, 235–243 CrossRef CAS.
- V. Uskoković and T. A. Desai, Mater. Sci. Eng., C, 2014, 37, 210–222 CrossRef PubMed.
- M. V. Berridge and A. S. Tan, Arch. Biochem. Biophys., 1993, 303, 474–482 CrossRef CAS PubMed.
- H.-S. Ryu, K. S. Hong, J.-K. Lee, D. J. Kim, J. H. Lee, B.-S. Chang, D.-H. Lee, C.-K. Lee and S.-S. Chung, Biomaterials, 2004, 25, 393–401 CrossRef CAS PubMed.
- B. Alliot-Licht, A. Jean and M. Gregoire, Arch. Oral Biol., 1994, 39, 481–489 CrossRef CAS PubMed.
- J. Guo, H. Guo, A. L. Baker, M. T. Lanagan, E. R. Kupp, G. L. Messing and C. A. Randall, Angew. Chem., 2016, 128, 11629–11633 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1tb00601k |
| This journal is © The Royal Society of Chemistry 2021 |