Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Applications of nanoscale metal–organic frameworks as imaging agents in biology and medicine
Fatma
Demir Duman
 and
Ross S.
Forgan
and
Ross S.
Forgan
 *
*
WestCHEM, School of Chemistry, University of Glasgow, University Avenue, Glasgow G12 8QQ, UK. E-mail: ross.forgan@glasgow.ac.uk
First published on 25th March 2021
Abstract
Nanoscale metal–organic frameworks (NMOFs) are an interesting and unique class of hybrid porous materials constructed by the self-assembly of metal ions/clusters with organic linkers. The high storage capacities, facile synthesis, easy surface functionalization, diverse compositions and excellent biocompatibilities of NMOFs have made them promising agents for theranostic applications. By combination of a large variety of metal ions and organic ligands, and incorporation of desired molecular functionalities including imaging modalities and therapeutic molecules, diverse MOF structures with versatile functionalities can be obtained and utilized in biomedical imaging and drug delivery. In recent years, NMOFs have attracted great interest as imaging agents in optical imaging (OI), magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET) and photoacoustic imaging (PAI). Furthermore, the significant porosity of MOFs allows them to be loaded with multiple imaging agents and therapeutics simultaneously and applied for multimodal imaging and therapy as a single entity. In this review, which is intended as an introduction to the use of MOFs in biomedical imaging for a reader entering the subject, we summarize the up-to-date progress of NMOFs as bioimaging agents, giving (i) a broad perspective of the varying imaging techniques that MOFs can enable, (ii) the different routes to manufacturing functionalised MOF nanoparticles and hybrids, and (iii) the integration of imaging with differing therapeutic techniques. The current challenges and perspectives of NMOFs for their further clinical translation are also highlighted and discussed.
1. Introduction
Porous materials are increasingly becoming attractive in biomedical applications due to their excellent intrinsic properties, such as large tunable porosities and fine control of chemical composition.1 Metal–organic frameworks (MOFs), which are also known as porous coordination polymers or porous coordination networks, are a class of porous hybrid solids that are formed by the self-assembly of metal ions or metal ion clusters and organic polydentate bridging linkers.2,3 Owing to their structural and functional tunability, much effort has been made to develop MOFs, particularly nanoscale metal–organic frameworks (NMOFs), over the past two decades to apply them in many areas, including but not limited to gas separation, gas storage,4,5 catalysis,6,7 nonlinear optics,8,9 chemical sensing,10–12 drug delivery13–15 and imaging.16–20NMOFs exhibit various characteristics that make them ideal materials for biomedical applications. Their highly porous structures and versatile functionality allow accommodation of high loadings of therapeutic and imaging agents and their controlled release, as well as protection against enzymatic degradation and self-quenching in living systems.21–24 Compared with conventional nanomaterials such as inorganic zeolites, mesoporous silica, quantum dots, metal nanoparticles, and organic nanocarriers of lipids or polymers, NMOFs typically possess a larger cargo loading capacity, good biocompatibility and ease of functionalisation.25 In the longer term, NMOFs are also intrinsically biodegradable as a result of their relatively labile metal–ligand bonds.26 The effectively infinite combination of metal clusters and organic linkers contributes to their compositional and structural tunability and allows the production of NMOFs with various pore sizes ranging from micropores to macropores, rigid or flexible skeletons, and diverse surface chemistries.27 Furthermore, their mild synthetic conditions enable the design of numerous NMOFs and incorporation of a large variety of molecular functionalities on their inner and outer surfaces, including imaging modalities, therapeutics and targeting ligands.
Since Lin and co-workers first reported the design of NMOFs as potential multimodal contrast enhancing agents for biomedical imaging in 2006,28 an increasing number of studies have used them as imaging agents for optical imaging (OI), magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET) and photoacoustic imaging (PAI).18,28–37 NMOFs offer a potential new class of imaging agents combining the characteristics of both inorganic and organic nanomaterials; the ease of construction of structures with metal ions/clusters or linkers with imaging abilities, or their doping with imaging molecules, makes them highly promising candidates. In comparison to existing imaging agents, NMOFs can offer high loading capacities for cargo molecules, high protection against enzymatic degradation, minimal release in the blood, and efficient delivery to targeted tissues, in contrast to the short blood circulation times and non-specific biodistributions often characteristic of conventional imaging agents.38 For example, NMOF-based MRI contrast agents have shown enhanced relaxivities compared to commercially available Gd contrast agents39 and even other Gd nanoparticulate systems,40,41 while an archetypal Hf MOF showed superior contrast for computed tomographic imaging compared to an I-based agent, even at a lower dose, that could be promising in decreasing the radiation dose to which the patients must be exposed.42 Moreover, NMOFs can be employed as multimodal imaging agents by the combination of multiple imaging functionalities in a single entity, and as image-guided therapy agents by achieving simultaneous imaging and therapy.43–45
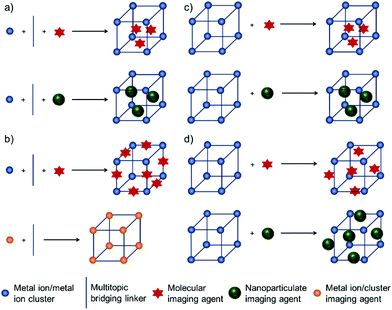
There are four main approaches for the construction of MOFs containing imaging agents (i) encapsulation of the agents within the pores of MOFs during the synthesis, (ii) their incorporation as intrinsic structural components of MOFs, (iii) immersion of already synthesized MOFs in a solution of imaging agents and so loading them inside the pores by size effect, ion-exchange or post-synthetic modification, collectively known as the post-insertion method, and (iv) attachment of imaging agents by covalent conjugation, or electrostatic or hydrophobic interactions, to the surface of MOFs as shown in Scheme 1.46–49
In this review, we will highlight the preparation and applications of NMOFs as imaging and theranostic agents in the biomedical field. Compared to other excellent reviews related to the applications of MOFs in biomedicine,27,50–52 imaging and sensing,38,48,53,54 or theranostics,55,56 this review will solely focus on and discuss the bioimaging applications of NMOFs, exemplifying the different strategies to facilitate mono- and multimodal imaging with select examples, and offering insight into the combination of imaging with other biological applications. This review is not intended to be a comprehensive overview of the field, but an introduction to the varying biomedical imaging techniques that MOFs can be applied to and a discussion of the varying methodologies to engender MOFs with imaging functionality.
2. MOFs as monomodal imaging agents
2.1. Optical imaging
Optical imaging (OI) is an imaging modality that uses light illumination at visible or near-infrared (NIR) wavelengths, which can be detected by optical cameras.57 OI provides real-time visualization of organs, tissues, and cells and monitors the progression of any morphological and biochemical changes non-invasively, allowing intraoperative image-guided surgery. The technique employs neither ionizing radiation, as in computed tomography, nor radioactive compounds, as in positron emission tomography, and it is a rapid and suitable imaging mode for high-throughput screening for both in vitro and in vivo imaging with non-invasion and high signal sensitivity.17 The basis of the technique relies on the absorption of the externally applied photon energy of a certain wavelength by fluorophores, and then its emission as a new photon with energy in the longer wavelengths. However, OI has limitations in terms of quantifying fluorescence intensity in living organisms accurately due to autofluorescence, intrinsic tissue signal attenuation, and the shallow tissue penetration of light. Therefore, many fluorescent materials have been investigated to obtain strong optical imaging agents that can offer deep tissue and intracellular imaging.58Luminescent MOFs have been utilized as effective optical imaging agents in the last two decades due to their attractive properties such as high payloads, tailorable surface chemistry that provides improved pharmacokinetics, and tunable sizes and structures.38 Various MOF structures with luminescent properties for optical biomedical imaging have been developed by combining MOFs with fluorescent dyes, photosensitizers or fluorescent drugs,13,18,29,34,59–72 metal ions/clusters (particularly lanthanides),23,73 or luminescent nanoparticles such as persistent luminescent nanoparticles (PLNPs),74,75 upconversion nanoparticles (UCNPs)76–78 or quantum dots (QDs).79
Fluorescent dyes. Liu et al. have reported the synthesis of phosphorescent MOFs using a phosphorescent ruthenium complex, [Ru(2,2′-bipyridyl-5,5′-dicarboxylic acid)(2,2′-bipyridine)2], as bridging ligand, and Zr(IV) or Zn(II) connecting points, achieving a dye loading of 57.4% and 78.7%, respectively.17 Then, the synthesized nanoparticles were further stabilized with a silica coating, functionalized with poly(ethylene glycol) (PEG) and an anisamide targeting molecule which provided a cancer specific optical imaging of H460 lung cancer cells in vitro. Furthermore, the results showed that zirconium MOFs exhibited higher stability in aqueous conditions compared to zinc MOFs. On the other hand, Ryu et al. size-selectively encapsulated resorufin and rhodamine-6G within nanocrystalline metal–organic frameworks (NMOFs, Fig. 1a), MOF-801, [Zr6O4(OH)4(fumarate)6]n, also known as Zr-fum, in which the linker is fumaric acid, and UiO-67, [Zr6O4(OH)4(BPDC)6]n (BPDC = 4,4′-biphenyldicarboxylate, UiO: Universitetet i Oslo), during their crystal growth and obtained resorufin-in-nMOF-801 (Rs⊂nMOF-801) and rhodamine-6G-in-nUiO-67 (R6G⊂nUiO-67). The Zr-MOF nanoparticles showed exceptional stabilities in biomedical environment, and enhanced photoluminescence properties, such that they could preserve the fluorescence even after 9 days.18 The resulting particles were successfully utilized for reliable and reproducible fluorescence imaging of FL83B human hepatocyte cells and HepG2 human hepatocellular carcinoma cells after being functionalized by galactosylation, to improve the biocompatibility and cellular uptake of MOFs through the high attachment affinity of galactose residues on the nanoparticles to the asialoglycoprotein receptors in the plasma membranes of liver cells (Fig. 1b–e).
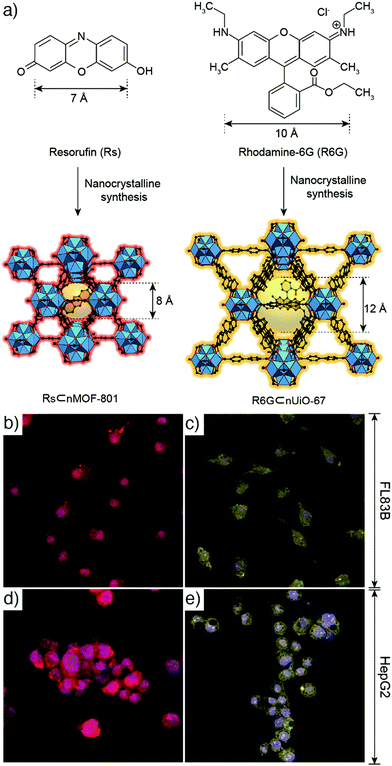 | ||
| Fig. 1 (a) Scheme for the encapsulation of resorufin and rhodamine-6G within NMOFs to produce Rs⊂nMOF-801 and R6G⊂nUiO-67, respectively. Confocal laser scanning microscopic images of (b) and (c) FL83B cells, and (d) and (e) HepG2 cells, after treatment with (b) and (d) galactosylated Rs⊂nMOF-801, and (c) and (e) galactosylated R6G⊂nUiO-67. Reproduced with permission.18 Copyright (2017) American Chemical Society. | ||
In another study, Gao et al. showed encapsulation of rhodamine b (RhB) within a hierarchical-pore metal–organic framework (H-MOF), using a one-step approach that was achieved by assembling MIL-53(Al)-NH2, constructed from [AlO6] octahedral chains connected by 2-aminobenzene-1,4-dicarboxylate (BDC-NH2) linkers, with formula [Al(OH)(BDC-NH2)]n and where MIL stands for Materials of Institute Lavoisier, in an aqueous solution of RhB, which serves as a fluorescent imaging agent.29 The obtained red fluorescence emitting H-MOFs were employed for in vitro imaging of murine gastric cancer 803 (MGC-803) cells and human airway smooth muscle cell (HASMC), and allowed in vivo imaging of athymic nude mice with good stability, biocompatibility and high imaging efficiency, avoiding the interference of autofluorescence. Furthermore, the as-synthesized fluorescent HMOFs were also successfully utilized as a nanocarrier by simultaneously loading the macromolecular drug tetracycline hydrochloride (TCH) and small molecule anticancer drug 5-fluorouracil (5-FU) with high loading efficiency, and delivery into cells. Mao and co-workers reported the fabrication of MOF-based fluorescent probes self-assembled from Zn(II) and imidazole-2-carboxyaldehyde (ICA), namely nanoscale ZIF-90 (formula [Zn(ICA)2]n, where ZIF stands for zeolitic imidazolate framework), to target subcellular mitochondria and visualize mitochondrial adenosine triphosphate (ATP) in live cells.81 Rhodamine (RhB) was encapsulated into ZIF-90 during synthesis, which resulted in its quenching due to the self-quenching effect of RhB (Fig. 2a). In the presence of ATP, the competitive coordination between ATP and the metal node of ZIF-90 led to the decomposition of RhB/ZIF-90 complex and consequently release and fluorescence recovery of RhB, and thus mitochondrial ATP imaging in living cells as shown in Fig. 2b–e.
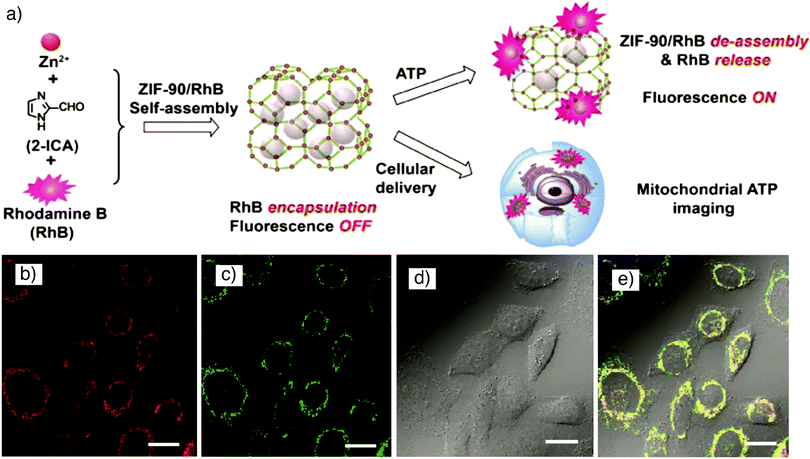 | ||
| Fig. 2 (a) Schematic illustration for the synthesis of RhB/ZIF-90 and host–guest chemistry for fluorescent ATP sensing and mitochondrial ATP imaging in living cells. Confocal laser scanning microscopy images of HeLa cells incubated with (b) RhB/ZIF-90 nanocrystals for 2 h (λex = 559 nm; λem = 570–670 nm), and (c) rhodamine 123 for 15 min (λex = 488 nm; λem = 500–545 nm). (d) Bright-field image of HeLa cells, and (e) overlay of images (b)–(d). Scale bars represent 20 μm. Reproduced with permission.81 Copyright (2017) American Chemical Society. | ||
MOFs were also successfully loaded with 1,3,5,7-tetramethyl-4,4-difluoro-8-bromomethyl-4-bora-3a,4a-diaza-s-indacene (Br-BODIPY) fluorescent dye by postsynthetic modification (PSM) of iron-carboxylate MIL-101(Fe) (trimeric iron(III) octahedral clusters connected with benzene-1,4-dicarboxylate (BDC) linkers of formula [Fe3O(BDC)3(H2O)2X]n, where X is a monocounterion typically OH− or Cl−) and used as optical imaging contrast agents for HT-29 human colon adenocarcinoma cells.59 To increase the stability of the particles and decrease cargo release, the nanosized MIL-101(Fe) particles were further functionalized by a silica coating and conjugated with the cisplatin prodrug (ethoxysuccinato-cisplatin, ESCP) for anticancer therapy. The cytotoxicity studies showed efficient cancer cell killing with the ESCP loaded nanoparticles, but slightly less cytotoxicity than free cisplatin. The functionalization of nanoparticles with a cancer targeting peptide, c(RGDfK), which has high binding affinity toward αvβ3 integrin overexpressed on cancer cells, slightly improved the cytotoxic effect of the nanoparticles providing similar IC50 (the half maximal inhibitory concentration) to that of free cisplatin. Such materials that combine imaging and drug delivery are often termed theranostics, for which MOFs are highly suited.55,56
Similarly, Wuttke et al. reported the encapsulation of fluorescein dye in lipid-coated MOF nanoparticles and their successful cellular uptake.60 In the study, the mesoporous iron(III) carboxylate MIL-100(Fe), which is constructed from octahedral trimers connected by benzene-1,3,5-tricarboxylate (BTC) to give formula [Fe3O(BTC)2(H2O)2X]n with large pore (diameter 2.4–2.9 nm) and window sizes (0.6–0.9 nm), and the mesoporous chromium(III) analogue MIL-101(Cr) with large pore (diameter 2.9–3.4 nm) and window sizes (1.2–1.7 nm), were loaded with fluorescein dye. The dye-loaded MOF nanoparticles were subsequently encapsulated by the formation of a bilayer of lipid DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine) through a controlled solvent-exchange deposition of the lipid molecules onto the MOF surface. The MOF@lipid nanoparticles synergistically combined the properties of liposomes and porous particles, storing the dye molecules inside the MOF pores while the lipid coating prevented the premature release of the dye and improved the colloidal stability of the nanoparticles. In vitro fluorescence microscopy studies showed high accumulation of the fluorescein-loaded nanoparticles with strong emission in T24 human bladder carcinoma cells.
The well-known low-toxic luminescent organic dye calcein (cal) has often been employed as a model drug in order to determine the cell internalization and drug delivery potential of MOF nanoparticles.69,82–84 Calcein is a hydrophilic fluorophore that is unable to cross cell membranes in significant quantities; rather the fluorescent molecule needs a drug delivery system (DDS) to facilitate its transport. In addition, calcein has a self-quenching nature at high concentrations that makes the vesicles carrying high local concentrations of calcein undetectable by fluorescent microscopy. However, upon its release from the carriers and dilution in the cytoplasmic environment, the green fluorescence of calcein can be detected by fluorescence-based techniques.85 Calcein was postsynthetically incorporated into Zr-based MOFs of the UiO topology by Orellana-Tavra et al. to study the endocytosis mechanism of Zr-MOFs through linker functionalization (Fig. 3a).64 In the study, UiO-66 topology MOFs with general formula [Zr6O4(OH)4(BDC)6]n were prepared with different particle sizes and different surface chemistries by substituting the original BDC linker with functionalized or extended linkers, and subsequently loaded with calcein. Fig. 3b schematizes the linkers (L1–L6) used in the study to build the Zr-based MOFs termed as Zr-L1 to Zr-L6, where L1 is BDC; L2–L4 are BDC functionalized with –Br, –NO2, and –NH2, respectively; L5 and L6 are extended linkers naphthalene-2,6-dicarboxylic acid (NDC) and BPDC, respectively. While the particle size does not significantly affect the cellular uptake mechanism in HeLa cells, visualised by intracellular calcein, control of surface chemistry through linker functionalization has a great influence. After 2 h incubation, a high degree of cal@Zr-L2, cal@Zr-L3 and cal@Zr-L4 were localized in cells as seen in Fig. 3c. The endocytosis mechanism can also be probed by the use of selected pharmacological endocytosis inhibitors, which demonstrated minimal particle size effects on uptake of cal@Zr-L1 (Fig. 3d), but also that cal@Zr-L1 (unfunctionalized) and cal@Zr-L3 (–NO2 functionalized) are taken up mostly through clathrin-mediated endocytosis, while cal@Zr-L5 and cal@Zr-L6 are taken up by the caveolae-mediated route, and so may avoid lysosomal degradation, although their total uptake is less than the others (Fig. 3e).
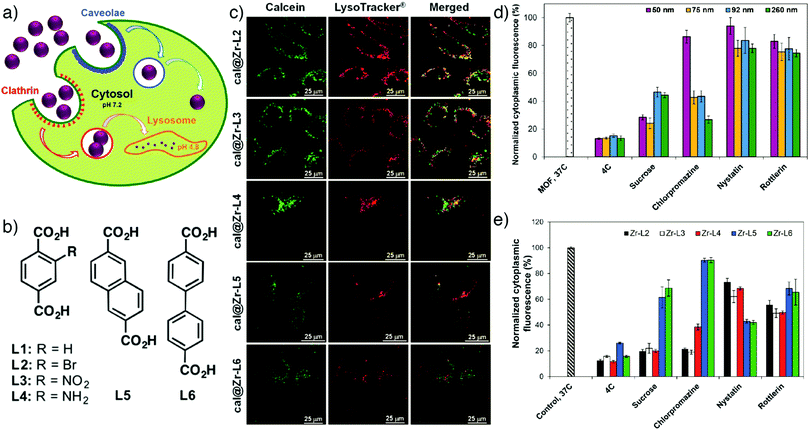 | ||
| Fig. 3 (a) Schematic illustration of different endocytosis mechanisms of Zr-based MOFs. (b) Chemical structures of linkers used to synthesize the Zr-MOFs. (c) Confocal microscopy images of HeLa cells treated with calcein incorporated Zr-based MOFs for 2 h (green fluorescence indicates calcein, red shows lysosome marker LysoTracker-Deep red). Effects of pharmacological endocytosis inhibitors on the uptake of (d) Zr-L1 with different particles sizes, and (e) linker functionalized Zr-based MOFs, as measured by flow cytometry tracking calcein fluorescence. Reproduced with permission.64 Copyright (2017) American Chemical Society. | ||
In another study, Haddad et al. utilized calcein to track the mitochondria targeting efficiencies of UiO-66 nanoparticles loaded with the anticancer drug dichloroacetate (DCA) and functionalized with a triphenylphosphonium (TPP) targeting unit,69 which is a lipophilic cation that accumulates in mitochondria as a consequence of the mitochondrial membrane potential in living cells.65 They reported that MCF-7 human breast cancer cells treated with the targeted MOF system, cal-TPP@(DCA5-UiO-66) demonstrated changes in mitochondrial morphology from elongated and reticular networks (untreated cells, Fig. 4, left) to short, balloon-shaped, fragmented mitochondria (Fig. 4, right) as a result of the DCA toxicity, as observed by structured illumination microscopy. This effect was less active with non-targeted cal@(DCA5-UiO-66) which showed still partially stringy and reticular morphology, as shown by the white arrows in Fig. 4 (centre). The presence of the MOF in the cells could be ascertained by the green fluorescence of the calcein.
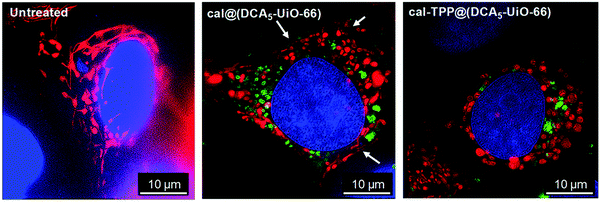 | ||
| Fig. 4 Structured illumination microscopy images of untreated (left), cal@(DCA5-UiO-66) treated (centre), and cal-TPP@(DCA5-UiO-66) (right) treated MCF-7 cells 8 h after incubation. Red fluorescence indicates mitochondria stained with a red fluorescent protein (RFP), blue corresponds to nuclei stained with DRAQ-5™, and green shows MOFs loaded with calcein. White arrows indicate stringy mitochondria. Reproduced with permission.69 Copyright (2020) American Chemical Society. | ||
Photosensitisers. The integration of photosensitizers such as porphyrins, chlorins or indocyanine green (ICG) into MOFs is another approach to obtain MOFs with optical imaging capability. This class of MOFs, named photosensitizer-based MOFs, can act as multifunctional nanoplatforms combining both photothermal imaging and photodynamic therapy (PDT) characteristics. Particularly, porphyrins and their analogues have been widely investigated due to their unique optoelectronic properties and versatility.71,72,80 Furthermore, the integration of porphyrins into MOF structures prevents their easy aggregation and quenching – a consequence of their large hydrophobic planar structures – and retains their optoelectronic properties.45
To obtain porphyrin-based MOFs, two different approaches have primarily been conducted. In the first approach, which results in porphyrin@MOFs, porphyrins are integrated within MOFs as guest molecules through encapsulation into the pores or adsorption on the surface via non-covalent interactions such as van der Waals forces, π–π stacking, or electrostatic interactions, or conjugation through covalent or coordinative bonds.86,87 The second approach uses porphyrins as organic linkers due to their macromolecular heterocyclic structures which can easily be functionalised with groups capable of coordination with metal ions or clusters. This class of MOFs is typically characterized by high porosities that allow accommodation of multiple functional units. Mostly, carboxylate-functionalised porphyrins such as 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin (TCPP) are used as linkers and coordinated with metals to form porphyrinic MOFs. The divalent metal ions such as Zn, Cu, Co and Cd typically coordinate to TCPP to produce 2D MOFs, while tri-and tetravalent Zr, Fe, Hf or Mn metal ions usually form porphyrinic MOFs with 3D structures.87–90 For example, Liu et al. have reported the construction of biocompatible nanoscale zirconium–porphyrin metal–organic framework (NPMOF)-based imaging-guided therapy systems by using a microemulsion strategy and tuning the reaction conditions carefully.71 The NPMOFs were synthesized via a microemulsion template and the auxiliary ligand method using ZrCl4 metal precursor and 5,10,15,20-tetrakis(4-carboxyl)-21H,23H-porphine (TCPP) ligand as well as benzoic acid as the auxiliary ligand, PEG-6000 as capping agent, and cetyltrimethylammonium bromide (CTAB) as surfactant, in DMF at 120 °C for 24 h that resulted in spindle-like particles. The further incubation of the reaction solution under these conditions caused the formation of bulk zirconium–porphyrin metal–organic frameworks with a rod-like shape after 40 h, maintaining the crystal structure of NPMOFs. The obtained MOFs exhibited efficient fluorescent imaging and PDT by achieving high porphyrin loading (59.8%), and high chemotherapy efficacy in tumour tissues through high doxorubicin loading (109% w/w).
Porphyrinic MOFs can also be combined with other functional nanoparticles due to their porosity, to form multifunctional compositions. For example, Zeng et al. showed the fabrication of core–shell gold nanorod@porphyrinic MOFs as multifunctional theranostic nanoparticles for combined photodynamic/photothermal/chemotherapy of tumour.80 The core–shell nanocomposites (Fig. 5a and b) were prepared by the growth of a porphyrinic Zr-MOF, composed of 6-connected Zr6 clusters and TCPP with the formula [Zr6O4(OH)4(H2O)6(OH)6(TCPP)1.5]n, on the surface of functionalized gold nanorods (AuNRs), which acted as seed crystal in the synthesis, and loaded with camptothecin (CPT) anticancer drug for chemotherapy of tumour. The as-synthesized AuNR@MOFs@CPT demonstrated high accumulation in the tumour tissues, exhibiting strong fluorescence intensity arising from porphyrinic MOFs (Fig. 5c–g), and high photothermal activity of AuNRs, evident in the photothermal images of 4T1 tumour-bearing mice irradiated with 808 nm laser after 24 h intravenous injection of AuNR@MOFs@CPT (Fig. 5h and i), therefore achieving high synergistic efficiency of photodynamic, photothermal and chemo-therapies in the tumour.
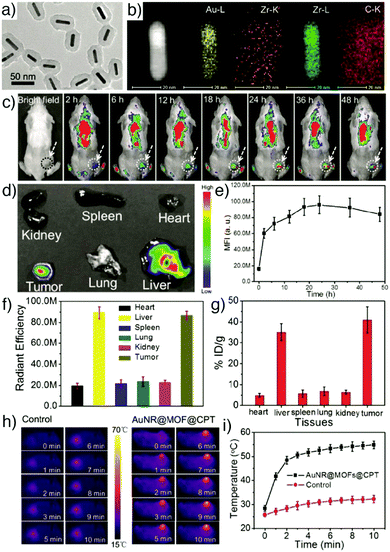 | ||
| Fig. 5 (a) TEM image of AuNR@MOFs showing the core–shell structure. (b) EDX elemental mapping of AuNR@MOFs. (c) Fluorescence images of a 4T1 tumour-bearing mouse at different time points after being injected with AuNR@MOFs@CPT. (d) Ex vivo fluorescence images of major organs and tumour at 24 h post-injection. (e) Time-dependent quantitative fluorescence intensity detected in tumour tissues. (f) Quantitative fluorescence intensity of major organs and tumour. (g) Concentration of gold in major organs and tumour measured by ICP-AES at 24 h post-injection. Irradiation time-dependent (h) in vivo thermal images and (i) temperature change curve of mice after being injected with PBS (control) and AuNR@MOFs@CPT under 808 nm laser irradiation. Reproduced with permission.80 Copyright (2017) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Ju and co-workers showed a different approach for fluorescence cell imaging using photosensitizers.91 The amino-functionalized iron(III) carboxylate MOF, MIL-101(Fe)-NH2 (a derivative of MIL-101(Fe) where the linker is BDC-NH2), was loaded with camptothecin through non-covalent encapsulation, and subsequently functionalized with a folic acid cancer targeting moiety and chlorin e6 (Ce6)-labelled cathepsin B (CaB) substrate peptide to act as the recognition unit, photosensitizer, and signal switch. CaB is a lysosomal cysteine endopeptidase overexpressed in many types of cancer cells. To target CaB and monitor the targeting effect, a CaB-substrate was conjugated with a Ce6 photosensitizer, which resulted in quenching of Ce6 due to the electron transfer from the excited Ce6 to MOFs. Upon incubation of the nanoparticles in cancer cells, Ce6 was released from the CaB-substrate by the cleavage of the substrate via intracellular CaB, and its fluorescence intensity was recovered, providing fluorescence imaging of the cancer cells. In addition to CaB-activatable fluorescence imaging, the nanoparticles demonstrated a significant tumour killing effect by synergistic chemo-photodynamic therapy, which overcomes the multidrug resistance of cancer cells and the low efficiency of PDT due to hypoxia in cancer cells.
To obtain luminescent MOFs using fluorescent salts, Singh et al. synthesized five different fluorescent nanosized salts/NMOFs by reacting 1,5-naphthalenedisulfonic acid (NDS) and Cu(NO3)2·3H2O with a variety of N-heterocyclic ditopic pyridyl based highly fluorescent sensitizers, i.e., 9,10-bis((E)-2-(pyridin-4-yl)vinyl)anthracene (B4PVA), 9,10-bis((E)-2-(pyridin-3-yl)vinyl)anthracene (B3PVA), and 3,3′-(peroxybis(methylene))dipyridine (3PBP) by a diffusion method.70 The resulting fluorescent nanoparticles (FNP) – [NDS2−·B4PVA2+·S] (FNP 1a), [NDS2−·3PBP2+·MeOH·H2O] (FNP 1b), [Cu·NDS·B4PVA] (FNP 1c), [Cu·NDS·B3PVA] (FNP 1d), and [Cu·NDS·3PBP] (FNP 1e) – were of sizes below 100 nm, exhibited supramolecular architectures of the salts/MOFs, and showed high fluorescence arising from pyridylvinyl in the MOF scaffold. The synthesized particles had low cytotoxicity and a clear strong fluorescence signal in the green channel within HeLa and MCF-7 cell lines at the concentration of 10 μg mL−1 (Fig. 6).
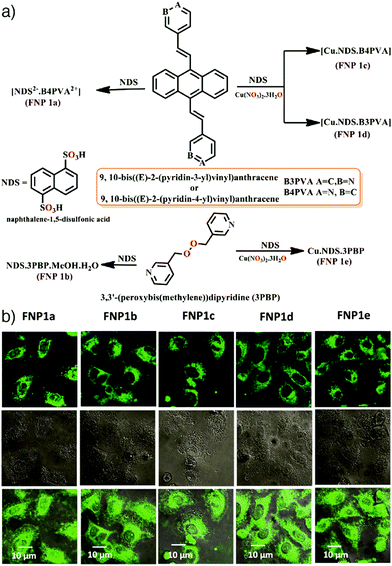 | ||
| Fig. 6 (a) Schematic illustration for the synthesis of five different fluorescent nanosized salts/NMOFs. (b) Confocal laser scanning microscopy images of HeLa cells treated with FNP 1a–1e with a concentration of 10 μg mL−1 for 1 h. Reproduced with permission.70 Copyright (2018) American Chemical Society. | ||
Drugs. Doxorubicin (DOX) is an important chemotherapy agent which inhibits DNA replication by intercalating into the DNA double helix and inducing free radical generation.92,93 However, direct administration of the drug causes undesired side effects by affecting both malignant and non-malignant cells in the body. Therefore, the delivery of the drug has been mostly studied with nanocarriers, and DOX was the first drug to receive clinical approval as a nanoformulation, delivered by a PEGylated liposome.94 In addition to the apoptotic effects, its highly fluorescent nature makes the drug a popular agent to allow nanocarriers to be used as imaging agents as well as therapeutics.95,96 Due to the high drug loading capacity, DOX has been widely studied with MOF structures for both therapy and imaging of tumour tissues.13,34,66–68 For example, Li et al. reported the application of DOX loaded UiO-68-type NMOFs (isoreticular extensions of UiO-66 with terphenyl-based ligands) with a tumour-targeting agent (folic acid, FA) as targeted therapy and imaging system for hepatoma (HepG2) both in vitro and in vivo.66 The NMOFs were prepared as shown in Fig. 7, firstly by the synthesis of Mi-UiO-68 from the maleimide-modified terphenyl ligand, H2L, and ZrCl4 in a DMF solution at 120 °C, followed by their DOX loading to produce DOX@Mi-UiO-68, and finally their decoration with thiolated FA units via a thiol–maleimide Michael-type addition. The obtained DOX@UiO-68-FA exhibited higher antitumour efficacy compared to free DOX and FA-undecorated DOX@Mi-UiO-68, which was confirmed by cell imaging via DOX, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay, and in vivo studies using a HepG2 subcutaneous xenograft murine model (Fig. 7).
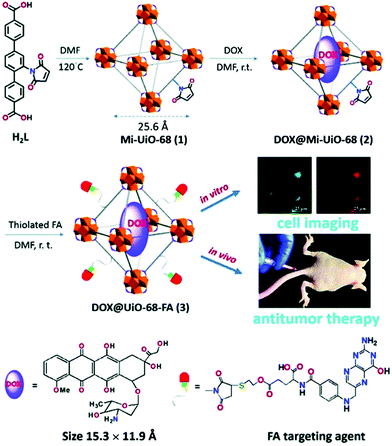 | ||
| Fig. 7 Schematic of the synthesis and application of DOX loaded and FA conjugated Mi-UiO-68 MOFs for targeted cell imaging and antitumour therapy. Reproduced with permission.66 Copyright (2016) The Royal Society of Chemistry. | ||
On the other hand, many lanthanide cations emit in the NIR window of the optical spectrum, which allows deeper tissue imaging than visible light. This is due to the higher tissue penetration of NIR light as a result of lower scattering and absorbing effects of tissues at longer wavelengths, which also provides lower background fluorescence, less signal loss, and thus a higher signal to background ratio.23,104 For example, Gallis et al. have reported the construction of a novel multifunctional MOF materials platform based on rare-earth (Eu, Nd, Yb, Y, and Tb) hexanuclear single metal clusters, as well as tuned compositions of Nd/Yb (Nd0.67/Yb0.33 and Nd0.46/Yb0.54) coordinated by 2,5-dioxybenzene-1,4-dicarboxylate (DOBDC) in the UiO-66 topology with general formula [Ln6(OH)8(DOBDC)5(DOBDC-H2)(H2O)6]n.73 The synthesized MOF materials exhibited both porosity and tunable emission properties over a wide range, from deep red into the second NIR window (∼614–1350 nm) as a function of the metal identity. In addition to this, they met fundamental prerequisites for relevance to biological applications by demonstrating minimal in vitro cytotoxicity and maintaining crystallinity under relevant physiological conditions such as water and phosphate-buffered saline (PBS). The group used EuDOBDC nanoparticles as a proof-of-concept system in this series for in vitro imaging of RAW 264.7 mouse macrophage and HeLa human cervical cancer cells. Their results presented an efficient discrimination between the Eu emission and cell autofluorescence and a long-term conservation of the intrinsic emission in live cells, proving the application of the materials as long-term imaging agents.
Similarly, Foucault-Collet et al. have designed NIR-emitting MOFs using NIR-emitting Yb(III) lanthanide cations and phenylenevinylene dicarboxylate (PVDC) as a sensitizer-ligand for NIR imaging in living cells.23 The sensitizers embedded in the nano-Yb-PVDC-3 MOF structure (Fig. 8a–c), formula [Yb2(PVDC)3(DMF)4]n, served as antennae as a result of the overlap between the excitation spectra of nano-Yb-PVDC-3 MOFs and the absorbance spectra of H2-PVDC, which ensures the energy transfer from the excited sensitizer-ligands to the Yb(III) cations. NIR images demonstrated highly efficient discrimination between the nano-Yb-PVDC-3 MOFs and auto-fluorescence of cells, with emission visible from both the PVDC linker and the Yb(III) ion, providing efficient imaging despite the relatively low quantum yield of the MOFs in water that arises from the energy level of the –OH overtone vibration being so close to that of Yb(III) (Fig. 8d and e).
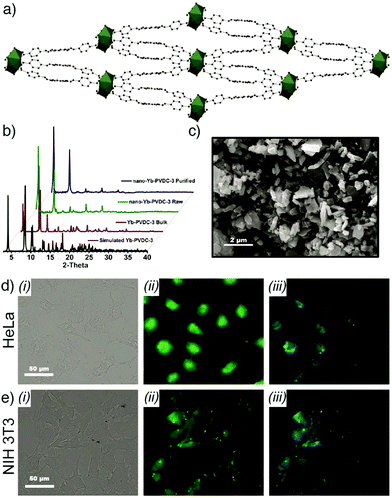 | ||
| Fig. 8 (a) Crystal structure of Yb-PVDC-3. (b) Powder X-ray diffraction (PXRD) patterns of simulated and bulk Yb-PVDC-3 as well as as-synthesised and purified nano-Yb-PVDC-3. (c) SEM image of nano-Yb-PVDC-3. Visible and NIR microscopy images of nanoYb-PVDC-3 in (d) HeLa cells and (e) NIH 3T3 cells: (i) Bright-field, (ii) H2-PVDC emission (λex = 377/50 nm, λem = 445/50 nm), (iii) Yb(III) emission (λex = 377/50 nm, λem = long pass 770 nm) images. Reproduced with permission.23 Copyright (2013) National Academy of Sciences. | ||
The colloidal stability of MOF nanoparticles is a challenging issue to overcome for their in vivo application. Park et al. reported a very facile solution to prepare water-dispersible luminescing MOFs using a simple hand-grinding technique. Tb-MOFs, composed of Tb(III) ions and tripodal carboxylate ligands such as triazine-1,3,5-tribenzoic acid, were stabilised by facile mechanical grinding with a biocompatible and biodegradable surfactant (Pluronic F127), a triblock copolymer composed of two poly(ethylene glycol) blocks at both of the termini of a poly(propylene oxide) block.30 The particles were also employed as drug delivery agents by encapsulating the anticancer drug DOX, and acted as a multifunctional biomaterial, which demonstrated efficient cell imaging and cancer cell killing by drug delivery. In another study, Wang et al. reported multifunctional water-soluble NaLnF4@MOF-Ln (Ln = Y, Tm, Yb, and Eu) nanocomposites with dual-mode luminescence by combining the anti-Stokes luminescence of NaYF4:Tm(III)/Yb(III) nanocrystals with the Stokes luminescence of mesoporous lanthanide MOFs (MOF-Y:Eu(III)), constructed from Ln(NO3)3 (Ln = 95% Y + 5% Eu) and BTC.105 The resulting NaYF4:Tm(III)/Yb(III)@MOF-Y:Eu(III) nanocomposites were coated by polyvinylpyrrolidone (PVP) to promote their cellular uptake and loaded with DOX. The NaLnF4@MOF-Ln nanocomposites exhibited a unique anti-Stokes blue fluorescence and Stokes red fluorescence under laser excitation at a specific wavelength and showed bright blue emission in HeLa cells under a fluorescence microscope with a 980 nm source. DOX release studies revealed the pH-dependence of the drug release from the nanocarriers showing higher release rate in the simulated acidic cancer cell environment.
Persistent luminescent nanoparticles. Among these luminescing structures, persistent luminescent nanoparticles (PLNPs) possess a unique optical phenomenon wherein continuous emission takes place for seconds or hours even after removal of the excitation source.106 Based on these superiorities, Xie and co-workers have recently reported combination of ZIF-8 nanoparticles (tetrahedral zinc(II) ions connected by 2-methylimidazolate (mIm) linkers, formula [Zn(mIm)2]n) with chromium-doped zinc gallogermanate (ZGGO) near-infrared PLNPs. This results in a core–shell multifunctional nanoplatform emitting in the NIR spectral region of the optical spectrum, for elimination of autofluorescence and obtaining deep tissue penetration and low irradiation damage under in vivo conditions.74 ZGGO@ZIF-8 particles were prepared by nucleation crystallization of a ZIF-8 shell on the surface of ZGGO particles. In the study, the PLNPs offered long-term NIR persistent luminescent signals for autofluorescence-free bioimaging with red-light-rechargeable properties while biocompatible ZIF-8 – a well-known pH responsive MOF – provided a shell structure to accommodate the fluorescent anticancer drug DOX (Fig. 9a). The fabricated ZGGO@ZIF-8-DOX multifunctional nanoplatform achieved dual imaging of 4T1 murine breast adenocarcinoma cells by DOX fluorescence and NIR persistent luminescence, and additionally acted as a pH-responsive drug delivery system. The particles showed quite high DOX loading (93.2%) and a drug release profile accelerated in acidic conditions, as well as effective in vivo tumour imaging (Fig. 9b–f) and suppression. Around the same time, Yang and co-workers prepared similar ZGGO@ZIF-8 materials, also by a surface adsorption induced self-assembly of MOFs on the ZGGO PLNPs, and generated comparable results, with enhanced luminescence in the acidic tumour microenvironment, and achieved tumour specific in vitro and in vivo imaging.75 These closely related examples are typical of the incorporation of multiple imaging/therapeutic factors into one MOF-based DDS.
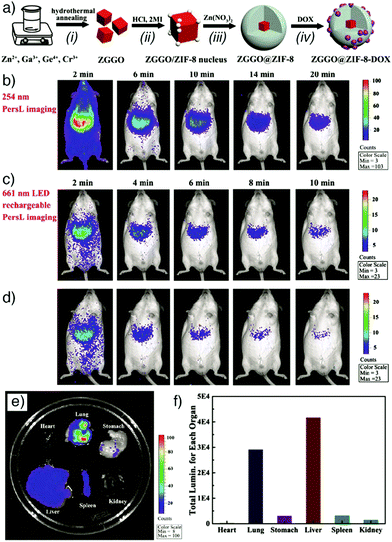 | ||
| Fig. 9 (a) Schematic representations of (i)–(iii) the synthesis of core–shell ZGGO@ZIF-8 multifunctional nanoparticles and (iv) loading with anticancer drug DOX. (b) In vivo persistent luminescence imaging of a mouse, intravenously injected with ZGGO@ZIF-8 (0.2 mL, 1 mg mL−1 in PBS) under 254 nm irradiation for 5 min, (c) after in situ 661 nm LED recharging for 2 min, and (d) after a further interval of 10 min. (e) Ex vivo persistent luminescence imaging after 24 h administration, conducted after 661 nm LED recharging for 2 min and (f) the corresponding total luminescence of major organs in the mouse. Reproduced with permission.74 Copyright (2018) American Chemical Society. | ||
Upconversion nanoparticles. Luminescent UCNPs possess an advantageous optical characteristic in their conversion of long wavelength excitation (usually NIR) to short visible wavelength emission. This feature helps them provide deep light penetration in biological tissues, minimized background auto-fluorescence, narrow emission peaks, and high photostability.76,107 The combination of MOFs with UCNPs can not only enable luminescence imaging but also prevent undesired tissue damage associated with UV excitation. These superiorities have driven researchers to construct upconversion NMOFs as optical imaging and drug delivery agents integrating the distinct advantages of both structures. For example, Chowdhuri et al. prepared upconversion NMOFs constructed by the direct coating of folic acid (FA)-encapsulated ZIF-8 on NaYF4:Yb(III)/Er(III) UCNPs, as depicted in Fig. 10a.76 X-ray diffraction (XRD) studies confirmed the successful combination of NaYF4:Yb(III)/Er(III) UCNPs with ZIF-8, showing characteristic diffraction peaks of both nanoparticles (Fig. 10b), with a very small decrease in the initial optical intensity of UCNPs (Fig. 10c). The prepared UCNP@ZIF-8/FA nanocomposites were subsequently loaded with the anticancer drug 5-FU by adsorption into the MOF nanostructure, which resulted in a high drug loading (685 mg g−1). The upconversion NMOFs functioned efficiently as imaging agents, producing strong fluorescence signals under in vitro conditions with HeLa cells and the FA conjugated UCNP@ZIF-8 showed higher cellular penetration compared to the particles without FA (Fig. 10d). In addition, the as-prepared 5-FU loaded UCNP@ZIF-8/FA nanocomposites showed higher drug release in acidic conditions, suggesting a pH-responsive drug release from the structures, and also induced greater cytotoxicity towards HeLa cells than L929 mouse fibroblast cells due to the higher cellular internalization in HeLa cells though folate receptor (FR)-mediated endocytosis.
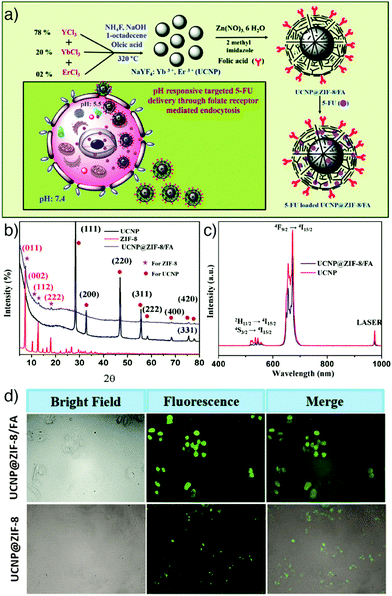 | ||
| Fig. 10 (a) Schematic illustration of the synthetic route to FA encapsulated and 5-FU loaded upconversion MOF composites, 5-FU loaded UCNP@ZIF-8/FA, as a targeted and pH responsive anticancer drug delivery agent. (b) PXRD patterns of the fabricated UCNPs, ZIF-8, and UCNP@ZIF-8/FA. (c) Emission spectra of UCNP and UCNP@ZIF-8/FA (λex = 980 nm). (d) Fluorescence microscopy images of HeLa cells incubated with 5 μg mL−1 of UCNP@ZIF-8/FA (upper) and UCNP@ZIF-8 (lower) for 6 h at 37 °C, with the corresponding bright field and overlay images. Reproduced with permission.76 Copyright (2016) The Royal Society of Chemistry. | ||
The group also reported the fabrication of NaYF4:Yb(III)/Er(III) UCNPs combined with FA encapsulated UiO-66-NH2 NMOFs (a structural analogue of UiO-66, where the linker is BDC-NH2) using a similar synthetic approach and employed them as targeted drug delivery agents loading with DOX anticancer drug.77 The obtained DOX loaded UCNP@UiO-66-NH2/FA nanoparticles improved the therapeutic efficacy of DOX compared to UCNP@UiO-66-NH2 by higher cellular uptake through FR-mediated endocytosis, and also enhanced the fluorescence imaging efficiency in vitro.
In another study, an in situ self-assembly strategy was reported by Yuan et al. to prepare upconversion NMOFs.78 The strategy allowed the coating of positively charged UCNPs with different, negatively charged Zr-MOFs, including UiO-66, UiO-66-NH2, MOF-801, and PCN-223, a 12-connected Zr-porphyrin MOF with formula [Zr6O4(OH)4(TCPP)3]n, through electrostatic interactions. The homogeneous distribution of NaYF4:Yb/Er UCNPs, composed of hexagonal NaYF4 UCNPs, doped with Yb/Er (18/2 mol%), on the MOF surface enabled uniform upconversion luminescence (UCL) on the microscale or single-particle scale. This is promising in a wide range of applications including luminescence-monitored drug delivery, which was shown by DOX release from the nanocomposites, as well as photodynamic therapy, due to the high singlet oxygen generation efficiency of PCN-223@NaYF4:Yb/Er nanocomposites under the excitation of 980 nm light, by the energy transfer from UCNPs to the TCPP linkers of PCN-223.
Quantum dots. Quantum dots (QDs) are semiconductor nanocrystals usually in the size range of 2–10 nm (10–50 atoms), with a structure between bulk and molecular forms, which enables size dependent fluorescence. Narrow photoluminescence bands, broad absorption windows, large molar extinction coefficients, low photobleaching, high quantum yield, and high chemical stability are the major advantages over conventional chromophores.108,109 These remarkable features make them promising optical imaging agents in various application areas, and their combination with MOFs has therefore attracted great attention in recent years.47,79,110,111 In particular, fluorescent carbon nanodots (C-dots) are a novel class of QDs and nanocarbons, displaying strong fluorescence intensity with high physicochemical stability and low toxicity compared with toxic metal-based QDs, which is highly relevant for biomedical use.112 As an example, He et al. integrated these with MOF nanoparticles to obtain C-dot-encapsulated MOF nanocomposites for fluorescence imaging of cancer cells and simultaneous pH-responsive drug delivery.79 The particles were prepared by directly mixing synthesized green fluorescent C-dots with Zn(NO3)2·6H2O and 2-methylimidazole, the precursors to ZIF-8, at room temperature in methanol without stirring for 24 h. The fluorescence intensity and size of the resulting C-dots@ZIF-8 nanoparticles (NPs) were tuned by varying the amount of C-dots and the concentration of the precursors. The NPs were further loaded with 5-FU and employed as pH-responsive anticancer drug delivery vehicles with simultaneous fluorescence imaging of cancer cells (Fig. 11).
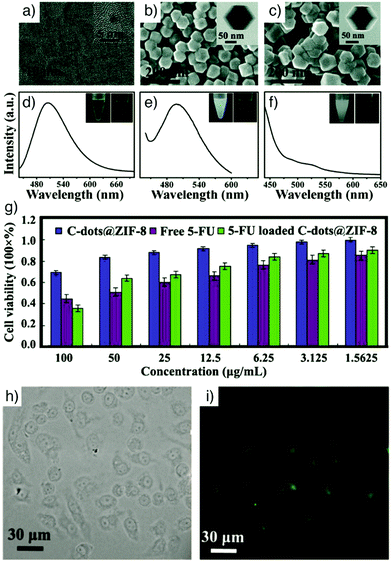 | ||
| Fig. 11 (a) HRTEM image of C-dots (inset shows an HRTEM image of a single C-dot). SEM images of (b) C-dots@ZIF-8 and (c) ZIF-8 (insets represent the corresponding TEM images). Photoluminescence (PL) spectra of (d) bare C-dots, (e) C-dots@ZIF-8, and (f) pure ZIF-8 (λex = 420 nm). Insets are the photographs of the corresponding samples suspended in methanol solution under ambient light (left) and under 365 nm UV light (right). (g) In vitro cytotoxicity of HeLa cells incubated with C-dots@ZIF-8, free 5-FU and 5-FU loaded C-dots@ZIF-8. (h) The differential interference contrast (DIC), and (i) confocal laser scanning microscopy (CLSM) images of HeLa cells incubated with 5-FU loaded C-dots@ZIF-8 for 24 h. Reproduced with permission.79 Copyright (2014) The Royal Society of Chemistry. | ||
Similarly, Chen et al. have incorporated the cationic ruthenium(II) tris(bipyridyl) complex, [Ru(bpy)3]2+, into UiO-67 MOFs for in vitro two-photon fluorescence (TPF) imaging and PDT.119 The theranostic nanoparticles were prepared by soaking the UiO-67 nanoparticles in a DMF solution of [Ru(bpy)3](PF6)2 at 90 °C. Trapping of the [Ru(bpy)3]2+ guest molecules in the MOF structures dramatically improved their quantum yield (Fig. 12a), luminescence lifetime (Fig. 12b), and TPF intensity (Fig. 12c) as a result of the steric confinement effect of MOF pores. The obtained nanoparticles, with an average diameter of ∼92 nm, showed improved stability upon light exposure, which resulted in a strong in vitro cell-killing effect and effective photodynamic therapy towards A549 human lung cancer cells by generating singlet oxygen upon non-invasive light irradiation, while exhibiting good biocompatibility without irradiation (Fig. 12d). Confocal laser scanning microscopy (CLSM) images confirmed that UiO-67-[Ru(bpy)3]2+ NPs were successfully internalized into A549 cells and demonstrated efficient in vitro TPF imaging capability with red fluorescence in the cytoplasm under 880 nm light irradiation (Fig. 12e and f).
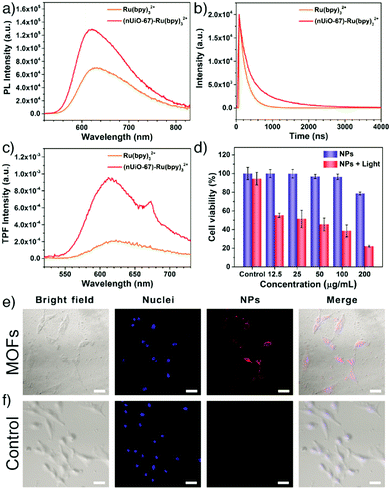 | ||
| Fig. 12 (a) One-photon photoluminescence spectra, (b) time-resolved luminescence decay curves, and (c) two-photon fluorescence (TPF) spectra of Ru(bpy)32+ and the (nUiO-67)-Ru(bpy)32+ NPs. (d) Cell viability of A549 cells with/without light irradiation after being treated with (nUiO-67)-Ru(bpy)32+ NPs at different concentrations (λ > 400 nm, 200 mW cm−2, 10 min). CLSM images of A549 cells (e) treated with (nUiO-67)-Ru(bpy)32+ NPs (upper) and (f) untreated (lower) for 12 h. Blue fluorescence indicates Hoechst 33342 nuclei staining. Red shows nanoparticles (λex = 880 nm, λem = 580–660 nm). The scale bar is 50 μm. Reproduced with permission.119 Copyright (2017) American Chemical Society. | ||
In order to obtain more effective intracellular sensing and a deep tissue imaging agent, Yang et al. also integrated the advantages of two photon excitation with MOFs, obtaining a TP-MOF composite to detect H2S or Zn(II) as model analytes in living tissues, and also to visualize tissues.120 PCN-58, a bis-azidomethyl functionalised analogue of the large-pore Zr MOF UiO-68 comprised of 2′,5′-bis(azidomethyl)-[1,1′:4′,1′′-terphenyl]-4,4′′-dicarboxylate, (TPDC-2CH2N3) coordinated with Zr6 clusters, can be conjugated with target-responsive two-photon organic moieties by Cu(I)-catalysed azide–alkyne cycloaddition (CuAAC) without any cross reactivity towards the MOF structure itself. PCN-58 was reacted with 6-amino-2-(prop-2-yn-1-yl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (alkynyl-BR-NH2) and then the amino group of the structure turned into an azide group by the use of tert-butyl nitrite and trimethylsilyl azide to obtain probe 1 for H2S detection. To achieve probe 2 for Zn(II) detection, PCN-58 was reacted with 2-(bis(pyridin-2-ylmethyl)amino)-N-(1,3-dioxo-2-(prop-2-yn-1-yl)-2,3-dihydro-1H-benzo[de]bisoquinolin-6-yl)acetamide (alkynyl-DL). The constructed TP-MOF probes containing the different two-photon fluorophores were successfully employed in live cells and tissue slices (rat liver and lung tissue), using two-photon microscopy (excited at λ ∼ 820 nm) with a tissue penetration depth of 130 μm to detect H2S and Zn(II), as well as visualize tissues.
Cumulatively, these examples of emissive MOFs and composites highlight the wide range of strategies available to prepare materials for biological imaging. The use of intrinsically luminescent linkers, such as porphyrins, is highly attractive for its simplicity, but many organic fluorophores do not possess highly symmetric structures, which is often a requirement for synthesis of ordered, porous MOFs. Likewise, the coordination chemistry of the lanthanides, which is dominated by steric factors, often results in denser structures with limited porosity. Hence, for combined imaging and drug delivery, postsynthetic incorporation or hybridisation with fluorophores is often the more advantageous approach.
2.2. Magnetic resonance imaging
Magnetic resonance imaging (MRI), founded on the principles of nuclear magnetic resonance (NMR), has become an essential tool for clinical diagnosis over the past few decades. MRI is a non-invasive imaging modality that uses non-ionizing radiation in the radio frequency range and does not employ any radioisotopes, unlike computed tomography and traditional X-rays. It offers harmless tissue imaging with high spatial resolution and tissue contrast, and superb depth of penetration. MRI detects spin reorientation in a magnetic field, and hence provides detailed anatomic images of soft tissues with 3D imaging capability, such as imaging of the brain, heart, and cartilage, as well as detection of tumours and the assessment of therapeutic responses.121,122 MRI signals are produced by the NMR signals of the water protons (1H nuclei) in a specimen.38 However, the intrinsic low signal difference between diseased and normal tissues can be insufficient to detect lesions.To enhance the signal differences between the tissues, MRI contrast agents have been developed to improve the contrast at the region of interest by altering the magnetic fields and accelerating relaxation processes. In the absence of an external magnetic field, the magnetic moments of 1H nuclei are randomly oriented. However, in the external magnetic field of an MRI scanner, the magnetic moments of 1H nuclei are aligned through an applied radio frequency pulse. Upon the removal of radio frequency perturbation, the 1H nuclei undergo a recovery process which usually occurs by two different types of relaxation time: longitudinal relaxation, characterized by the parameter T1 (spin–lattice relaxation time), and transverse relaxation, characterized by the parameter T2 (spin–spin relaxation time).123,124 Depending on the type of the relaxation time, the contrast agents are sorted into two main categories: T1 agents shorten the longitudinal (spin–lattice) relaxation time of the surrounding water protons, and provide a positive contrast and brighter MRI images, whereas T2 agents shorten the transverse (spin–spin) relaxation time of the water protons and create a negative, dark contrast effect.121,123 The efficiency of a contrast agent is determined by the ratio between transverse (r2) and longitudinal (r1) relaxivity values, which are defined as the slope of a plot of 1/T2, and the slope of a plot of 1/T1vs. the concentration, respectively.125 A lower value of r2/r1 (between 1 and 3) indicates T1 type, while higher values (>10) indicate T2 type. Between these values, the materials can show dual contrast characteristics.126
MRI is a powerful imaging modality, however it requires the administration of contrast agents in large doses to enable adequate MRI contrast in target tissues.38 For clinical applications, the most widely used MRI contrast agents are paramagnetic gadolinium (Gd) chelates, which act as T1 contrast agents producing signal enhancement in T1-weighted images.127–129 Small molecule Gd chelates show modest r1 values (∼4–5 mM−1 s−1), so require high doses for an effective contrast. Moreover, the release of free Gd ions from unstable Gd complexes has been associated with nephrogenic systemic fibrosis in renal failure patients and the use of some of the gadolinium based contrast agents was banned in Europe in spring 2007.127,128,130–133 However, the use of the most stable gadolinium-based contrast agents at the lowest possible dose has been mostly commonly accepted as the optimal protocol.131,134
Recently, NMOFs have become a promising alternative to Gd complexes as potential MRI agents, due to their large metal payload, low toxicity, superior stability, and higher relaxivity values, which provide stronger contrast enhancement with lower doses and hence improve diagnostic sensitivity.53 MOF contrast agents can be designed either as T1, T2 or combined T1 and T2 contrast agents by building the structure with MRI active paramagnetic metals as intrinsic components, or combining synthesized MOFs with various MRI contrast agents. Furthermore, they can be simultaneously utilized as drug delivery agents, and directed to a specific area interested in the body by conjugation of various cell targeting ligands.135 Gd-Based MOFs, Mn-based MOFs and Fe-based MOFs have been developed as promising candidates as MRI contrast agents in the literature.28,136–138
Hatakeyama et al. reported the control of size and shape by incorporation of hydrotropes into the reverse microemulsion synthesis of Gd-MOF nanoparticles with a general composition of [Gd(1,4-BDC)1.5(H2O)2]n and [Gd(1,2,4-BTC)(H2O)3]n.22 Hydrotropes are defined as compounds that offer both hydrophobic and hydrophilic features. Specifically, sodium salicylate, used amongst various hydrotropes in the study, exhibited the best size reduction, producing Gd-MOF nanoparticles of an average size of 82 nm with the highest total surface area. The increase in the outer surface areas of the nanoparticles provided enhanced longitudinal relaxivity values (r1) up to 83.9 mM−1 s−1, which is nearly 20 times higher than that of Magnevist (gadopentetate dimeglumine), a clinical positive contrast agent used for comparison in the study. Wang et al. reported the preparation of NMOF-based T1–T2 dual-modal contrast agents for MRI by using a mixture of Eu(III) and Gd(III) as metallic nodes, isophthalic acid (H2IPA) as building blocks, and PVP as a surfactant. The produced spherical Eu,Gd-NMOFs were also coated by a silica layer to improve the particle stability and functionalized with c(RGDyK), a tumour-targeting peptide sequence, which targets integrin αvβ3.19 The Eu,Gd-NMOF@SiO2 nanoparticles showed high longitudinal (38 mM−1 s−1) and transversal (222 mM−1 s−1) relaxivities by inducing signal enhancement on T1- and signal attenuation on T2-weighted images when injected in vivo.
Qin et al. reported the synthesis and comparison of two water-stable Mn- and Gd-based MOFs of N-(4-carboxybenzyl)-(3,5-dicarboxyl)pyridinium bromide (H3CmdcpBr), ([Mn2(Cmdcp)2(H2O)2]·H2O)n (1) and ([Gd(Cmdcp)(H2O)3](NO3)·3H2O)n (2), and showed their applications as strong MRI contrast agents under in vivo conditions.142 The in vitro characterization demonstrated that the Mn-based MOF possessed a slightly higher relaxivity r1 value (17.50 mM−1 s−1) compared with the Gd-based analogue (13.46 mM−1 s−1), and both of them are superior to that of the clinical positive contrast agent, Gd–DTPA (DTPA = diethylene triamine pentaacetate, r1 = 4.87 mM−1 s−1) used as a control in the study (Fig. 13a and b). In vivo MR images of the Mn-based MOF indicated that the nanoparticles have high contrast efficacy over a prolonged timeframe enabling remarkable positive signal enhancement in kidneys (Fig. 13c).
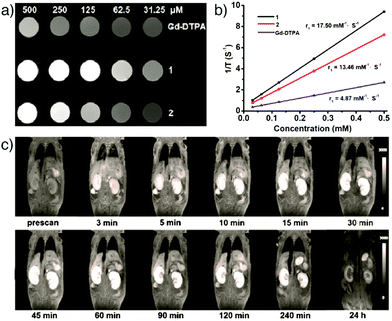 | ||
| Fig. 13 (a) T1-Weighted MR images of ([Mn2(Cmdcp)2(H2O)2]·H2O)n (1) and ([Gd(Cmdcp)(H2O)3](NO3)·3H2O)n (2), as well as the commercial contrast agent Gd–DTPA, at different concentrations in water. (b) The plot of 1/T1vs. the concentrations for each. (c) MR images of a female nude mouse displaying the MR signal intensity in both kidneys at different time points of intravenous post-injection with ([Mn2(Cmdcp)2(H2O)2]·H2O)n. Reproduced with permission.142 Copyright (2017) American Chemical Society. | ||
An alternative and effective strategy to design Fe-based MOFs for MRI is to encapsulate superparamagnetic iron oxide nanoparticles (SPIONs) into MOFs. SPIONs are clinically used MRI contrast agents with an extraordinary ability to shorten T2 relaxation times due to their unique magnetic properties.121,136,145 Zhao et al. reported novel theranostic Fe3O4@UiO-66 core–shell composites, fabricated by in situ growth of a UiO-66 MOF shell on a Fe3O4 core, for drug delivery via UiO-66 and simultaneous MRI by the Fe3O4 nanoparticles (Fig. 14a).136 The decrease in BET surface area (SBET) of UiO-66 MOFs confirmed the interior Fe3O4 core within UiO-66 (Fig. 14b) while the high saturation magnetization (Ms) value showed their superparamagnetic characteristics (Fig. 14c). The core–shell nanoparticles served as excellent T2 contrast agents with a high transverse relaxivity (255.87 mM−1 s−1) and significant darkening effect in HeLa cells at increased concentrations (Fig. 14d), as well as in vivo MRI capability in Kunming mouse and HeLa-tumour bearing mice at increased incubation times (Fig. 14e and f). Furthermore, the particles could be loaded with DOX for simultaneous cancer therapy, and the obtained Fe3O4@UiO-66-DOX particles exhibited high cell mortality and remarkable tumour size inhibition compared with Fe3O4@UiO-66, both in vitro and in vivo.
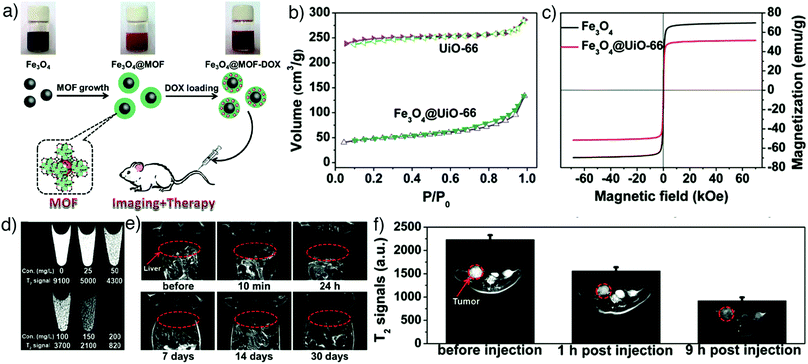 | ||
| Fig. 14 (a) Schematic illustration for the preparation of theranostic Fe3O4@UiO-66 core–shell composites. (b) N2 adsorption–desorption isotherms and (c) magnetization hysteresis curves of UiO-66 and Fe3O4@UiO-66 nanoparticles, confirming effective hybridisation. (d) In vitro T2-weighted MR images of HeLa cells incubated with different concentrations of Fe3O4@UiO-66 composites (0, 25, 50, 100, 150 and 200 mg L−1) for 24 h and enhanced darkening effect by concentration increase. (e) In vivo T2-weighted MR images of a Kunming mouse injected with the composites and the images at different time points (red cycles show the liver region). (f) T2-Weighted MR images of HeLa tumour-bearing mice before and at 1 h and 9 h post-intravenous injection with Fe3O4@UiO-66 (red cycles show the tumour region). Reproduced with permission.136 Copyright (2016) The Royal Society of Chemistry. | ||
In another study, Chowdhuri et al. showed the development of a new magnetic porous system constructed by coating of superparamagnetic Fe3O4 nanoparticle cores by a shell of IRMOF-3, which is constructed from octahedral Zn clusters and BDC-NH2 with formula [Zn4O(BDC-NH2)3]n, for MRI.145 The nanoparticles were further functionalized by conjugation of a cancer targeting ligand, folic acid, and a fluorescent molecule, rhodamine B isothiocyanate, for simultaneous fluorescence imaging. After loading with the anticancer chemotherapy drug paclitaxel, through hydrophobic interactions, the NMOFs showed good therapeutic efficacy in HeLa cells, by high internalization via receptor-mediated endocytosis, and strong T2-weighted MRI contrast due to the high loading amount of MR-active Fe3O4 nanoparticles. These latter examples show that the MOF/nanoparticle hybrid route is an attractive alternative to preparing MOFs with intrinsically magnetically active metals, but the protocol does require additional synthetic steps and additional components, which may complicate large scale manufacture of any clinical MOF-based MRI contrast agents.
2.3. Computed tomography
Computed tomography (CT) is a non-invasive medical imaging technique for 3D visualization of internal structures with high spatial and temporal resolution. CT is based on the attenuation of X-rays, which mainly uses contrast agents with high atomic numbers (high-Z elements) showing high X-ray attenuation ability, such as iodine, gold, barium, bismuth and gadolinium. The attenuation values of most tissues are similar, which makes the differentiation of diseased from normal tissue difficult. However, by using contrast agents containing heavy elements, the desired contrast enhancement can be achieved.33,146,147 Currently available contrast agents in clinical practice are mostly iodine derivatives that may cause severe side effects, like thyroid dysfunction or renal toxicity, due to small amounts of free iodine in the formulation.148,149 In addition, these materials show non-specific distribution and rapid pharmacokinetics, and therefore require administration in high concentrations for enhanced CT imaging resolution.To overcome these limitations, a number of MOF nanoparticles containing high-Z elements have been recently developed and employed as potential CT contrast agents. For example, Lin's group reported the construction of UiO-66 type NMOFs containing high-Z elements (zirconium and hafnium) and showed their applications as CT contrast agents.42,150 Zr and Hf have high atomic numbers of 40 and 72, respectively, making them potential CT contrast agents. In the study, as-synthesized UiO-66(Hf) nanoparticles were further coated with silica and PEG in order to improve biocompatibility, and were employed for in vivo CT imaging of mice, which resulted in higher attenuation in the liver and spleen.42 Zhang et al. developed the concept by the synthesis of an iodine-boron-dipyrromethene (BODIPY)-containing MOF, named UiO-PDT, as a CT imaging agent.33 The nanocrystals were functionalised by exchanging the diiodo-substituted monocarboxyl-functionalized BODIPY (I2-BDP) with BDC linkers at the surfaces of pre-synthesised UiO-66. The obtained UiO-PDT nanocrystals exhibited no obvious severe acute or sub-acute toxicity, even at high injection dosages, and provided good X-ray attenuation – particularly for about 24 h after intravenous administration – as well as high accumulation in the tumour region of Sprague-Dawley rats bearing hepatomas (Fig. 15). In principle, any MOF nanoparticle containing high-Z elements in the linker, metal clusters, or surface functionality could be applied to CT imaging.
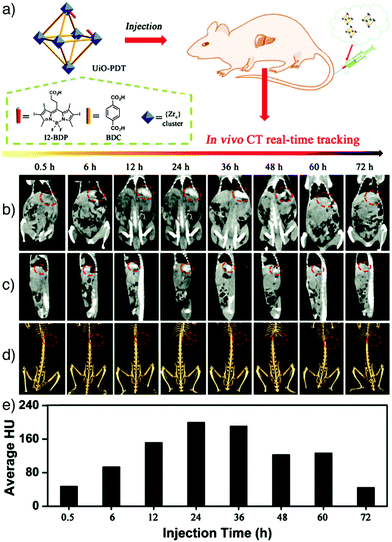 | ||
| Fig. 15 (a) Schematic representation of the construction of UiO-PDT nanostructures as X-ray CT imaging agents. In vivo CT images of a Sprague-Dawley rat at different time points after being injected with UiO-PDT showing the (b) ventral, and (c) the lateral sides of the rat. (d) 3D images revealing the ventral sides of hepatic tumours, highlighted with red circles. (e) The corresponding Hounsfield unit values indicating the quantity of the attenuated signal. Reproduced with permission.33 Copyright (2017) The Royal Society of Chemistry. | ||
2.4. Positron emission tomography
Positron emission tomography (PET) is a commonly used non-invasive imaging modality that diagnoses abnormalities at the cellular/molecular level and provides 3D images with high sensitivity, specificity and limitless depth of penetration.151 PET requires internal administration of a molecule labelled with a positron-emitting radionuclide known as radiotracer (usually at nanomolar concentrations) into the bloodstream of the patient. Various positron-emitting radionuclides, including 18F (t1/2 = 109.8 min), 11C (t1/2 = 20.39 min), 13N (t1/2 = 9.965 min), 15O (t1/2 = 2.037 min), 68Ga (t1/2 = 68 min), 64Cu (t1/2 = 12.7 hours), 89Zr (3.27 days) and 124I (t1/2 = 4.176 days), are used for PET imaging in clinical applications.152,153 The natural decay of the radionuclide comprises emission of a positron from the structure and interaction of this emitted positron with a free or loosely bound electron after it travels only a short distance through the tissue. Ultimately, one positive electron is annihilated with one negative electron, producing two photons which are given off in opposite directions with a 511 keV energy.153 These high-energy gamma-rays are detected with a PET scanner, and the distribution of the radiotracer throughout the patient's body is constructed as a series of 3D images.Based on these advantages, Hong and co-workers reported the application of NMOFs as a radiotracer by developing an intrinsically radioactive UiO-66 NMOF (89Zr–UiO-66) through incorporation of a positron-emitting isotope of zirconium, zirconium-89, during the synthesis.34 The particles were loaded with DOX, and then further surface engineered with pyrene-derived polyethylene glycol (Py–PGA–PEG) and an F3 peptide ligand, to target nucleolin on tumour cells and enhance the cellular uptake and drug delivery of the NMOFs in tumour region (Fig. 16a). The particles showed strong radiochemical and material stability in different biological media, as well as relatively high drug loading capacity (1 mg DOX per mg UiO-66), allowing them to be used as both a therapeutic agent and a fluorescence visualizer. By conjugation of F3 targeting peptides, selective and enhanced uptake of 89Zr–UiO-66 conjugates towards MDA-MB-231 human breast tumour cells was reported via PET images taken at different time points after injection of MDA-MB-231 tumour bearing mice with the nanoparticles (Fig. 16b). The results were further confirmed by ex vivo organ distribution studies obtained by tissue gamma-counting (Fig. 16c) and by ex vivo fluorescence imaging of DOX in tumour-bearing mice.
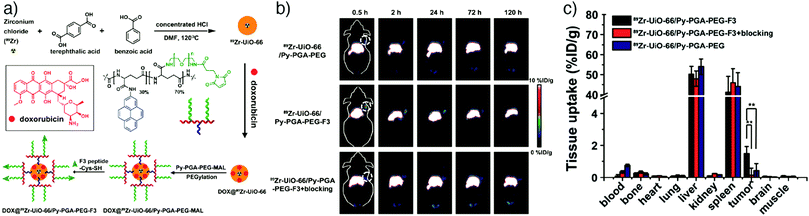 | ||
| Fig. 16 (a) Scheme for the synthesis for 89Zr-UiO-66/Py–PGA–PEG-F3 conjugates. (b) Coronal PET images of MDA-MB-231 tumour bearing mice at different time points after being injected with 89Zr-UiO-66/Py–PGA–PEG-F3, 89Zr-UiO-66/Py–PGA–PEG, and 89Zr–UiO-66/Py–PGA–PEG-F3 with excess F3 peptide for blocking. (c) The corresponding organ distribution at 120 h post-injection obtained by tissue gamma-counting. **P < 0.01. Reproduced with permission.34 Copyright (2017) American Chemical Society. | ||
In another example, Liu's group reported the synthesis and use of 64Cu radiolabelled and DOX loaded amorphous zeolitic imidazolate framework-8 (AZIF-8) to investigate how particle size affects the in vivo distribution of particles and their tumour targeting ability, using an in vivo quantitative tracing strategy based on PET imaging.154 The nanoparticles were synthesized in different sizes by a rapid one-pot aqueous approach. To label the particles with 64Cu, a chelator-free radiolabelling method, which relies on doping of the structure by 64Cu(II) ions in the synthesis, was conducted. The results showed that the intracellular drug release rate increases as the size decreases, but CT imaging shows smaller MOF nanoparticles (60 nm) circulate in the blood longer and achieve over 50% higher tumour accumulation than larger MOFs (130 nm), providing higher antitumour efficacy.
2.5. Photoacoustic imaging
Photoacoustic imaging (PAI), also known as optoacoustic imaging, is a robust, non-invasive and non-ionizing biomedical imaging technique that uses both optical and ultrasonic imaging to visualize anatomical structures and physiological changes in biological tissues. This hybrid imaging modality has received great attention by effectively combining the high optical contrast of optical imaging with the high penetration depth and ultrasonic spatial resolution of ultrasound imaging.155,156 In PAI, tissue is illuminated by short laser pulses, which cause a fast local temperature increase as a result of the absorption of the incident energy and, consequently, formation of a small and localized thermal perturbation. This leads to generation of photoacoustic (PA) pressure waves, which propagate inside the tissue owing to thermal expansion, and these photoacoustic signals can be detected with wideband ultrasound transducers. By measuring the duration and arrival time of the pressure pulses to the transducer, the location and size of the target tissue can be determined.157 In PA imaging, the sensitivity and specificity of the image usually depends on the endogenous contrast. However, the low intensity of internal PA signals in some tissues limits the possibility of obtaining high contrast PA images. Exogenous contrast agents have therefore been used to improve the image quality by PA signal and imaging contrast enhancement. Specifically, carbon nanotubes and noble metal nanoparticles – in particular gold nanoparticles (AuNPs) and gold nanorods (AuNRs) – have been popular in PAI, since they possess strong light absorption ability due to their enhanced surface plasmon resonance.158,159NMOFs have been recently utilized as PAI contrast agents in several studies due to their attractive characteristics such as high porosity, tunable pore size, abundant metal sites and high-guest molecule loading capacity, which allow them to be designed as efficient PA contrast imaging agents as well as therapeutics by simultaneously delivering various drug molecules. For example, Chen and co-workers reported the fabrication of multifunctional MOF nanoparticles for PAI-guided chemo-/photothermal simultaneous tumour therapy by using MIL-100(Fe).20 The Fe MOF nanoparticles were efficiently loaded with curcumin, as a chemotherapeutic agent, and coated with polydopamine (PDA) to achieve photoacoustic contrast and photothermal conversion properties, and enhance the colloidal stability and biocompatibility of the nanoparticles in the systemic circulation. The produced nanoparticles were further functionalized by hyaluronic acid (HA), through the modification of the nanoparticles with HA conjugated PDA, to specifically target CD44 overexpressing tumour cells. The results showed that the nanoparticles accumulated significantly at the tumour site and achieved photoacoustic imaging-guided combinational chemo-/photothermal therapy with high therapeutic efficacy (Fig. 17).
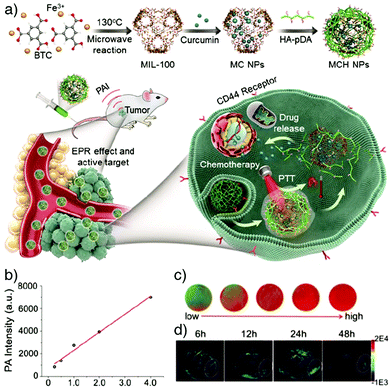 | ||
| Fig. 17 (a) Schematic illustration for the construction and applications of MIL-100(Fe) for photoacoustic imaging-guided chemo-/photothermal combinational tumour therapy. (b) PA intensity of the nanoparticles at different concentrations. (c) Corresponding in vitro PA images. (d) PA images of HeLa tumour-bearing mice at different time points after intravenous injection with the nanoparticles. Reproduced with permission.20 Copyright (2018) American Chemical Society. | ||
Yang et al. reported the synthesis of ZIF-8 derived carbon nanoparticles (ZCNs) using a one-step procedure, to achieve PAI-guided cancer phototherapy and to investigate the effects of the nanoparticle size on phototherapy and PAI contrast ability.35 The ZCNs acted as photothermal agents and photosensitizers by simultaneously generating heat and reactive oxygen species which caused efficient tumour ablation upon NIR laser irradiation, as monitored by in vivo PAI. Furthermore, the results showed that the increase in the nanoparticle size provided enhanced phototherapy effects and PA signal intensity (Fig. 18).
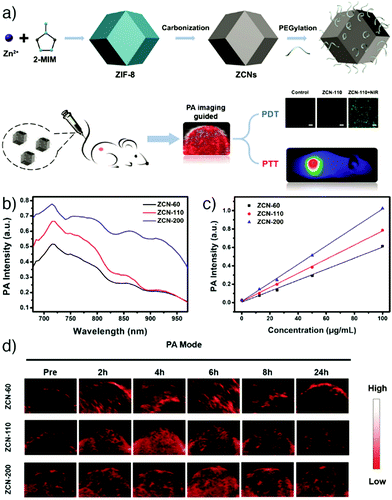 | ||
| Fig. 18 (a) Schematic representation of the synthesis and applications of ZIF-8-derived carbon nanoparticles (ZCNs) for PAI-guided photothermal/photodynamic combined therapy. PA signal intensity of ZCNs in vitro at (b) different excitation wavelengths, and (c) various concentrations. (d) PA imaging of A549 tumour-bearing mice at different times after intravenous injection with ZCNs. The images display the tumour region of the mice. Reproduced with permission.35 Copyright (2018) American Chemical Society. | ||
Similarly, Zhang et al. demonstrated new nanosized metal–organic particles constructed directly using an organic dye, tetrahydroxyanthraquinone (THQ), as linker connected by Cu(II) ions serving as dual-mode therapeutics for PAI-guided photothermal/chemotherapy in the NIR II window (from 1000 to 1350 nm of the optical spectrum).160 After PEGylation, the nanoparticles (termed Cu-THQNPs) achieved excellent biocompatibility and colloidal stability. Their strong absorption in the NIR II window and photo-induced electron transfer (PET) mechanism enabled Cu-THQNPs to act as excellent photothermal agents with a high light-to-heat conversion efficiency (51.3%) at 1064 nm, and also as excellent photoacoustic contrast agents. Moreover, the degradation of the nanoparticles by tumour specific acidic cleavage of the coordination bonds caused the release of Cu(II) into the tumour area, enhancing antitumour activity through catalysing conversion of hydrogen peroxide (H2O2) to hydroxyl radicals (˙OH). In another study, ZIF-8 nanoparticles were combined with AuNRs to obtain PA contrast agents (AuNR@ZIF-8) with high PAI capacity and biocompatibility.161 AuNR@ZIF-8 core–shell nanocomposites were successfully built through a facile step-by-step synthesis method, which involves attaching PVP to the surface of the AuNR core and subsequently growth of ZIF-8 on the AuNR surface. The resulting AuNR@ZIF-8 nanoparticles demonstrated high NIR absorption, good photothermal conversion and PAI efficiency owing to the AuNR core, and easy modification of the MOF surface, as well as high biocompatibility.
Overall, MOFs are being widely investigated as highly promising imaging agents for a number of diverse techniques, primarily as a consequence of their synthetic versatility and the possibility of combining imaging with drug delivery. Imaging functionality can be intrinsic structural components of the MOF or guests bound within pores or on particle surface, and can be incorporated during synthesis or postsynthetically, while MOFs can easily be hybridised with a range of other nanomaterials. It is this versatility that means multiple imaging functionalities can also easily be installed in single particles, leading to multimodal imaging agents.
3. MOFs as multimodal imaging agents
Integration of multiple imaging modalities within a single material is a highly promising approach to overcome the limitations of existing imaging techniques and obtain imaging agents with high stability, biocompatibility, enhanced imaging sensitivity and resolution capacity. The structural and compositional diversity of NMOFs, combined with the wide range of potential functionalization and hybridisation protocols available, means they are excellent candidates for multimodal imaging, in some cases in combination with therapeutic applications.3.1. OI/MRI
Combination of optical and MR imaging features within MOF nanoparticles is one of the most applied strategies to achieve non-invasive bimodal imaging agents with superior contrast capacity. Kundu et al. reported the application of two porous gadolinium(III)-based MOFs, Gd–pDBI-1 and Gd–pDBI-2 (pDBI = 1,4-bis(5-carboxy-1H-benzimidazole-2-yl)benzene) for bimodal imaging (Fig. 19a).162 The change in metal precursor from Gd(NO3)3·6H2O to Gd(OAc)3·6H2O induced the formation of two different MOFs, Gd–pDBI-1 and Gd–pDBI-2, respectively (Fig. 19b), where enhanced porosity was reflected in the higher BET surface area for Gd–pDBI-2 (614 m2 g−1) compared to the negligible N2 adsorption by Gd–pDBI-1 (33 m2 g−1, Fig. 19c). The images recorded by SEM exhibited uniformly shaped spindle-like particles about 1 mm in length and 0.3 mm in width at the centre of Gd–pDBI-2, whereas Gd–pDBI-1 displayed extensively aggregated, 120 nm rod-shaped particles (Fig. 19a). The fluorescent pDBI linker in the structure enabled the application of the MOFs as fluorescent agents – Gd–pDBI-2 exhibited greater luminescence than Gd–pDBI-1 (Fig. 19d) – while the Gd core led them to serve as MRI contrast agents. Confocal microscopy images revealed the bright blue-coloured fluorescence of Gd–pDBI-2 within the cytoplasm of the MCF-7 cells at an excitation wavelength of 405 nm in vitro (Fig. 19e–g). Of the two MOFs, Gd–pDBI-2 exhibited higher longitudinal relaxivity (r1) as an MRI contrast agent (Fig. 19h and i). In vivo MR images conducted on a mouse model confirmed the MRI capabilities of Gd–pDBI-2 particles, generating a partial white contrast on the spleen, kidney, and liver of the animal by using a 0.3 T clinical MRI scanner, as shown in Fig. 19j and k.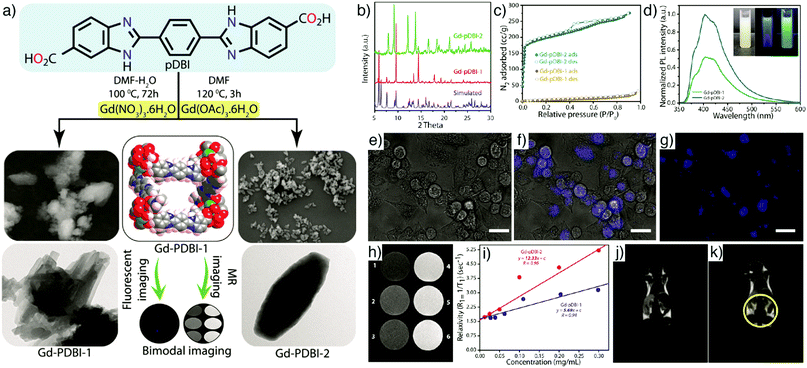 | ||
| Fig. 19 (a) Schematic illustration of the synthesis of Gd–pDBI-1 and Gd–pDBI-2 from the luminescent linker pDBI under different reaction conditions and their applications for simultaneous fluorescence and MR imaging. Left panel displays SEM (top) and TEM (bottom) images of Gd–pDBI-1; right panel displays SEM (top) and TEM (bottom) images of Gd–pDBI-2. (b) PXRD patterns, (c) N2 adsorption isotherms, and (d) PL spectra of Gd–pDBI-1 and Gd–pDBI-2; inset represents aqueous dispersion of Gd–pDBI-2 under visible light (white), 365 nm UV light (blue), and 254 nm UV light (bluish green). Confocal microscopic images of MCF-7 cells incubated with Gd–pDBI-2: (e) bright-field, (f) merged, and (g) fluorescence image (λex = 405 nm). Scale bars 20 μm. (h) T1-Weighted phantom image of Gd–pDBI-2 at different Gd(III) concentrations (1: 12.5 μg mL−1, 2: 25 μg mL−1, 3: 50 μg mL−1, 4: 100 μg mL−1, 5: 200 μg mL−1, 6: 300 μg mL−1). (i) Comparison of longitudinal relaxivity (r1) of Gd–pDBI-1 and Gd–pDBI-2. In vivo MR images of (j) control mouse and (k) mouse injected with Gd–pDBI-2. Reproduced with permission.162 Copyright (2016) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
In another example, Gao et al. reported the synthesis of multifunctional MOFs for fluorescence/magnetic resonance dual-mode imaging and targeted drug delivery.44 In the study, MIL-53(Fe)-NH2 NMOFs, which have attractive properties such as well-defined pores and intense magnetism, were loaded with the anticancer drug 5-FU and decorated with FA for tumour targeting. The nanoparticles were further functionalized by conjugation of a fluorescent reagent, 5-carboxylfluorescein (5-FAM), and employed successfully as MR and optical imaging agents with a satisfactory magnetic performance and strong green fluorescence emission. In addition, the particles achieved higher cellular uptake and inhibition of cell growth in FA-positive MGC-803 cells compared with FA-negative HASMC cells. The group alternatively showed in situ growth of MOFs on the surface of other MOFs as a new strategy to construct dual imaging materials combining MR and optical imaging properties in the same entity.163 Rodlike Eu-MOFs of formula [Eu(BTC)(H2O)]n, which emit red fluorescence, were grown on the surface of spherical MIL-100(Fe) particles that could act as MRI contrast agents, by a simple in situ growth method. MIL-100(Fe) particles were suspended in a DMF/water solution of Eu(NO)3·6H2O and sodium acetate at 60 °C for 72 h, with growth of the Eu-MOF presumably a consequence of partial dissolution at MIL-100(Fe) surfaces and release of BTC linkers. The Fe-MOF/Eu-MOF heterostructures simultaneously exhibited red fluorescence arising from the Eu-MOF, and enhanced T2-weighted MR images from MIL-100(Fe) acting as a contrast agent. The nanocomposites showed strong fluorescence in HASMC human aortic smooth muscle cells and MGC-803 cancer cells, as well as in vivo in athymic nude mice. The Fe-MOF/Eu-MOF heterostructures were further loaded with 5-FU and employed as a therapeutic agent with strong cell growth inhibitory effect in MGC-803 cells.
Li et al. reported the integration of rare-earth-doped UCNPs with MOFs to obtain core–shell UCNP@MOF for luminescent/magnetic dual-mode targeted imaging.164 The particles were prepared by the combination of single NaYF4:Yb,Er UCNP core particles with a MIL-101(Fe)-NH2 NMOF shell, followed by conjugation of poly(ethylene glycol)-2-amino ethyl ether acetic acid (NH2–PEG–COOH) and FA tumour targeting unit, which yielded PEGylated core–shell UCNP@MIL-101(Fe)-NH2@PEG–FA nanostructures. Luminescence from the UCNPs is clearly visible (Fig. 20a) and the Fe-MOF acted as a T2-weighted MRI contrast agent (Fig. 20b). In vitro studies (Fig. 20c) performed on KB cells (FR-positive) and MCF-7 cells (FR-negative), and in vivo studies conducted on KB-tumour bearing mice (Fig. 20d–i), demonstrated that the core–shell nanostructures can simultaneously function as tumour targeted dual-mode UCL/MR imaging agents, providing high luminescence intensity from the UCNP core, and T2-MRI properties as the MIL-101(Fe)-NH2 shell achieves a distinct darkening effect, therefore enabling sensitive imaging with low toxicity and high uptake in FR-positive KB cells.
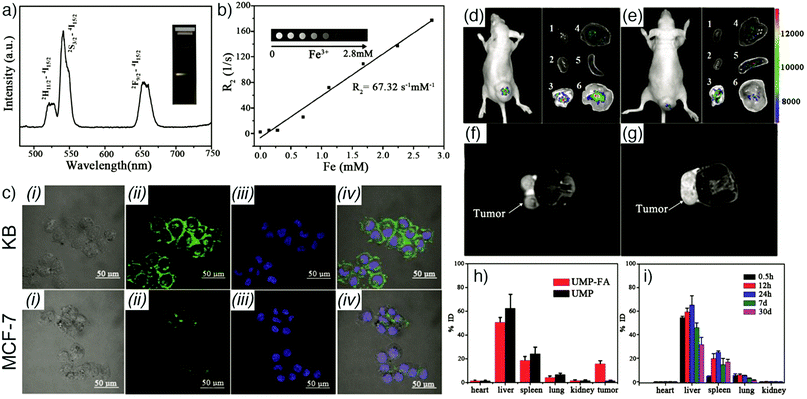 | ||
| Fig. 20 (a) Upconversion emission spectra of UCNP@MIL-101(Fe)-NH2@PEG–FA nanostructures (inset shows UCNP@MIL-101(Fe)-NH2@PEG–FA dispersed in water under 980 nm diode excitation). (b) Relaxation rate r2 (1/T2) versus concentrations of UCNP@MIL-101(Fe)-NH2@PEG–FA at room temperature measured by 3T MRI scanner (inset shows T2-weighted MR images of the nanostructures with various concentrations). (c) CLSM images of KB (top) and MCF-7 (bottom) cells incubated with UCNP@MIL-101(Fe)-NH2@PEG-FA: (i) bright field, (ii) green channel images collected at 500–560 nm, (iii) cell nucleus stained with Hoechst 33342 (blue fluorescence), and (iv) merged images. Dual-modal UCL/MR in vivo imaging of KB tumour-bearing mice and their dissected organs at 24 h post-injection of (d) UCNP@MIL-101(Fe)-NH2@PEG–FA (UMP-FA, targeted) and (e) UCNP@MIL-101(Fe)-NH2@PEG (UMP, without FA, non-targeted). (1) Heart; (2) kidney; (3) lung; (4) liver; (5) spleen; (6) KB tumour. T2-MRI images of KB tumour-bearing mice at 24 h after intravenous injection with (f) UCNP@MIL-101(Fe)-NH2@PEG–FA and (g) UCNP@MIL-101(Fe)-NH2@PEG. (h) Corresponding biodistributions of the two samples. (i) Biodistribution of UCNP@MIL-101(Fe)-NH2@PEG after 0.5 h, 12 h, 24 h, 7 d, and 30 d following intravenous injection. Reproduced with permission.164 Copyright (2015) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
In addition to their T2-MR imaging properties, SPIONs have also attracted great attention for their photothermal conversion efficiency. Zhang et al. combined these characteristics with the photodynamic therapy and fluorescence imaging abilities of a porphyrin MOF structure by constructing core–shell magnetic porphyrin MOF nanocomposites for fluorescence-MR dual-modality imaging-guided photothermal and photodynamic dual-therapy.45 The core–shell nanocomposites were fabricated by the encapsulation of Fe3O4 nanoparticle clusters in a carbon shell, and then in situ self-assembly of a porphyrin MOF (PMOF), constructed from Zr ions and TCPP with the PCN-223 structure, on the surface of these Fe3O4@C nanoparticles. The Fe3O4@C nanoparticles served as T2-weighted MR imaging and PTT agents, while the PMOF enabled simultaneous fluorescence imaging and PDT in the nanocomposite structure. The resulting Fe3O4@C@PMOF nanocomposites demonstrated high biocompatibility and stability, and effective fluorescence and MR dual-modality imaging with high accumulation in the tumour site of tumour-bearing mice. The nanostructures also achieved effective photothermal and photodynamic therapy of tumours without damaging normal tissues through the controllable and local irradiation of the laser.
3.2. MRI/CT
Boyes and co-workers showed utilization of NMOFs as MR and CT bimodal imaging agents by combining [Gd(BDC)1.5(H2O)2]n nanorods, constructed from Gd(III) magnetic centres connected by BDC linkers, and CTAB to prevent the aggregation of the nanoparticles, with Au NPs via poly(acrylic acid) (PAA) bridges. The highly stable Gd-MOF–PAA–Au nanocomposites showed excellent MRI contrast with higher longitudinal relaxivity than the clinically used MRI positive contrast agent Magnevist through Gd(III) in the structure, and strong CT contrast through Au NPs providing a source of high-Z elements. The composites, however, were not tested towards any biological materials.1653.3. MRI/PAI
Chen et al. described a facile approach to synthesize polypyrrole (PPy) composite materials (PPy@MOFs), where a PPY core was surrounded by a MIL-100(Fe) shell loaded with DOX, for magnetic resonance/photoacoustic dual-mode imaging combined with chemo-photothermal cancer therapy.166 PPy NPs are well known for their high photothermal conversion efficiency that allows efficient photothermal cancer therapy, and strong optical contrast for PAI.167–170 To obtain PPy@MOFs, pyrrole was reacted with PVP and FeCl3 in deionized water to generate the PPy core, where PVP was utilized as a stabilizing agent and FeCl3 as a catalyst. Then, trimesic acid was reacted with Fe(III) ions on the PPy surface, which act as reactive sites, for the construction of PPy@MIL-100(Fe), and finally the nanocomposites were loaded with DOX. PPy@MOFs exhibited good biocompatibility and dual-mode PA and MR imaging, as well as excellent photothermal conversion efficiency (∼40%) and NIR/pH-triggered release of DOX, resulting in a synergistic chemo-photothermal therapy of HepG2 human hepatocellular carcinoma cells.3.4. OI/MRI/PAI
MOFs have also been employed as contrast agents for triple-modality OI/MRI/PAI, especially for early diagnosis of tumours. For example, Cai et al. reported NMOF-based multimodal imaging guided photothermal cancer therapy.43 MIL-100(Fe), as a T2-weighted MRI agent, was combined with a photo-responsive NIR emitting organic dye indocyanine green (ICG), after conjugation of the MOFs with hyaluronic acid (HA). The produced nanoparticles (MOF@HA@ICG NPs, Fig. 21a) showed high loading content of ICG (40%), and strong NIR emission intensity and photostability. The in vitro (Fig. 21b–e) and in vivo (Fig. 21f–j) imaging and photothermal toxicity studies exhibited enhanced cellular uptake and tumour accumulation of the HA-functionalized nanoparticles in CD44-positive MCF-7 cells and MCF-7 tumour-bearing mice, providing an effective photothermal toxicity, as well as strong fluorescence, photoacoustic, and magnetic resonance multi-modal imaging capacities compared to MOF@ICG NPs (non-HA-targeted) and free ICG.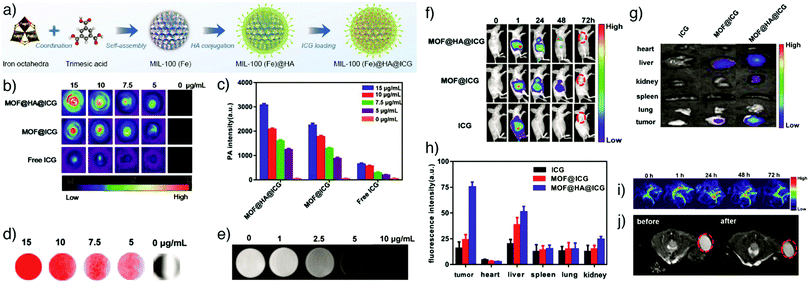 | ||
| Fig. 21 (a) Schematic illustration of the synthesis of MIL-100(Fe) NPs and their functionalization by HA conjugation and ICG loading. Imaging of MCF-7 cells in vitro: (b) PA images with (c) associated PA intensities for free ICQ, ICQ@MIL-100(Fe), and ICQ@HA@MIL-100(Fe), (d) fluorescence images and (e) T2-weighted MR images of MCF-7 cells after incubation with ICQ@HA@MIL-100(Fe). Imaging of MCF-7 tumour-bearing mice in vivo: (f) fluorescence images of mice injected with free ICQ, ICQ@MIL-100(Fe), and ICQ@HA@MIL-100(Fe) solutions, (g) corresponding ex vivo fluorescence images, (h) fluorescence intensities of the organs and tumours, (i) in vivo PA and (j) T2-weighted MR images after MOF@HA@ICG treatment. Reproduced with permission.43 Copyright (2016) American Chemical Society. | ||
Similarly, Yang et al. showed the synthesis of MIL-53(Fe) loaded with a NIR cyanine dye (cypate), which can interact with Fe(III) to construct precursor complexes, for near-infrared fluorescence (NIRF), MR and PA imaging guided PTT/PDT in vivo.171 The cyanine dye in the composition provided NIRF and PA imaging abilities, and acted jointly as a PTT and PDT agent by transforming NIR laser to heat and generating ROS under NIR irradiation, respectively, while MIL-53(Fe) enabled MR imaging. Moreover, incorporation of cypate molecules into the structure increased the extent of defects in the MOF nanoparticles, which enhanced the aqueous solubility and bioavailability of cypate. Further modification of the particles with transferrin, which has high binding affinity for the transferrin receptors overexpressed on cancer cells, improved internalization of the nanoparticles in A549 cells and in tumour regions of A549 tumour-bearing mice with higher NIRF and PA intensity, and more effective tumour ablation was observed compared to untargeted nanoparticles upon a low power laser.
3.5. MRI/CT/PAI
MOF nanoparticles have also been used as triple-modality MRI/CT/PAI contrast agents by fabricating Au@MIL-88A(Fe) nanostructures via controllable growth of a MIL-88A(Fe) shell layer on the surface of AuNRs.172 The core–shell Au@MIL-88(Fe) nanocomposites were further modified by poly(ethylene glycol)–carboxylic acid (PEG–COOH) for higher colloidal stability in vivo. The AuNR core in the structure provided CT enhancement and PAI optical properties simultaneously, while MIL-88(Fe) served as T2-weighted MRI contrast agent. The prepared nanostructures showed low toxicity, and enabled high spatial resolution and effective contrast during in vivo MRI, CT and PAI imaging of glioma. In another study, Yang et al. showed the construction of core–shell and co-doped Mn/Hf-IR825@PDA–PEG NMOPs (NMOP-PEG) through a post-synthesis cation exchange method, and described their application as MRI and PAI contrast agents for bimodal imaging, and as radiosensitizers and photothermal agents for synergistic thermo-radiotherapy.159 The particles were produced by mixing the NMOPs (nanoscale metal–organic particles) containing Mn(II) and IR825, a NIR dye, with Hf(IV), and coating with polydopamine (PDA) and further conjugation with PEG. The Mn(II) ions served as a contrast agent for T1-weighted MR imaging, IR825 provided strong PA contrast and a photothermal effect due to high NIR absorption, Hf(IV) enabled the utilization of the particles as CT contrast agents and for radiation therapy with strong X-ray absorbance, and the further coatings by PDA and PEG endowed them with excellent colloidal stability. The as-synthesized Mn/Hf-IR825@PDA–PEG NMOPs showed efficient tumour retention ability after intravenous injection in a mouse tumour model, which was confirmed by MR/CT and PA images. Additionally, they demonstrated efficient tumour killing efficacy by synergistic PTT and radiation therapy (RT) treatment, which is attributed to the increased oxygenation in the tumour region induced by PTT that repress tumour hypoxia-related RT resistance, and radio-sensitization induced by Hf. Furthermore, the particles were almost completely excreted from the mouse body without causing any dark toxicity within a month, showing them to be biodegradable and multifunctional nanoplatforms for highly integrated clinical functions.4. Conclusions and perspectives
In this review, we have highlighted and discussed the use of MOFs and their composites as promising imaging agents for various imaging modalities in clinical applications. Table 1 summarizes the applications of selected NMOFs as bioimaging agents based on all imaging strategies and examples explained above. For bioimaging, MOFs offer a variety of advantages such as tunable porosity, controlled structure, synthetic versatilities, diverse sizes and morphologies, high surface area, and easy functionalization, which allows one to endow them with multiple functionalities and properties. The wide-ranging choice of both metal ions as coordination centres and organic ligands as linkers further contribute to their compositional and structural diversities. Their porous structures and high surface area allow them to be simultaneously loaded with a wide variety of therapeutic and imaging structures in large amounts, creating MOF-based multimodal imaging-guided theranostic systems. The tunable surface chemistry provides a facile route to modification with various molecules to enhance their biocompatibility, stability in physiological media, and/or the ability to targeting to a specific region in the body. Biodegradability of NMOFs, combined with rapid excretion and short-term body retention, provide a potential pathway to their safe utilization for in vivo applications.| MOFs | Imaging agent | Drug/cargo | Imaging strategy | Further applications | Ref. |
|---|---|---|---|---|---|
| Rs⊂nMOF-801, R6G⊂nUiO-67 | Resorufin, rhodamine-6G | — | OI | Liver cell targeting via galactosylation | 18 |
| MIL-53(Al)-NH2 | RhB | TCH, 5-FU | OI | Drug loading and release | 29 |
| RhB/ZIF-90 | RhB | — | OI | Mitochondrial ATP imaging | 81 |
| MIL-101(Fe) | Br-BODIPY | ESCP | OI | Drug loading and release | 59 |
| Zr-based MOFs | Calcein | α-Cyano-4-hydroxycinnamic acid | OI | Drug delivery, endocytosis | 64 |
| cal-TPP@(DCA5-UiO-66) | Calcein | DCA, TPP | OI | Mitochondria-targeted drug delivery | 69 |
| AuNR@MOFs@CPT | TCPP | CPT, Au NRs | OI | PDT/PTT/chemotherapy | 80 |
| NH2-MIL-101(Fe) | Chlorine e6 | CPT | OI | PDT/chemotherapy, tumour targeting via FA, intracellular CaB targeting via CaB substrate | 91 |
| DOX@Mi-UiO-68-FA | DOX | DOX | OI | Tumour targeting and therapy | 66 |
| FA@Eu:TMU-62-NH2 | Eu | 5-FU | OI | pH-Responsive targeted drug delivery | 103 |
| NaLnF4@MOF-Ln | Ln = Y, Tm, Yb, Eu | DOX | OI (dual-mode luminescence) | pH-Responsive drug delivery | 105 |
| ZGGO@ZIF-8-DOX | ZGGO PLNPs | DOX | OI (persistent luminescence) | pH-Responsive drug delivery | 74 |
| UCNP@ZIF-8/FA | NaYF4:Yb(III), Er(III) | 5-FU | OI (UCL) | pH-Responsive targeted drug delivery | 76 |
| C-dot@ZIF-8 | C-dots | 5-FU | OI | pH-Responsive drug delivery | 79 |
| (nUiO-67)-[Ru(bpy)3]2+ | [Ru(bpy)3]2+ | OI (two-photon fluorescence) | Photodynamic therapy | 119 | |
| Gd-Based MOFs | Gd(III) | Eu(III), Tb(III) | T 1-Weighted MRI | Luminescence imaging | 28 |
| Eu,Gd-NMOF@SiO2 | Gd(III) | Eu(III) | T 1- and T2-weighted MRI | Luminescence imaging, tumour targeting via c(RGDyK) | 19 |
| Mn-IR825@PDA–PEG | Mn(II) | IR825 | T 1-Weighted MRI | Photothermal therapy | 141 |
| Mn- and Gd-based MOFs | Gd(III), Mn(II) | — | T 1-Weighted MRI | — | 142 |
| Iron(III) carboxylate MOFs (MIL-53, MIL-88A, MIL-88Bt, MIL-89, MIL-100 and MIL-101_NH2) | Fe(III) | Busulfan, azidothymidine triphosphate, DOX or cidofovir | T 2-Weighted MRI | Chemotherapy | 31 |
| DOX@ZIF-HA | Fe(III) | DOX | T 1-Weighted MRI | Prostate cancer targeting via HA, chemotherapy | 144 |
| Fe3O4@UiO-66 | Fe3O4 | DOX | T 2-Weighted MRI | Chemotherapy | 136 |
| Fe3O4@IRMOF-3/FA | Fe3O4 | PTX, RITC | T 2-Weighted MRI | Chemotherapy, fluorescence imaging | 145 |
| UiO-PDT | BODIPY | — | CT | — | 33 |
| 89Zr–UiO-66/Py-PGA–PEG-F3 | 89Zr(IV) | DOX, Py–PGA–PEG | PET | Tumour targeting via F3 peptide, chemotherapy, fluorescence imaging | 34 |
| HA–PDA coated MIL-100(Fe) | Fe(III) | Curcumin | PAI | Chemotherapy, PTT, cancer targeting via HA | 20 |
| ZIF-8-derived carbon nanoparticles | Carbon | PAI | PDT, PTT | 35 | |
| Gd–pDBI-1 and Gd–pDBI-2 | Gd(III)/pDBI | — | T 1-Weighted MRI/OI | — | 162 |
| MIL-53(Fe)-NH2-FA-5-FAM/5-FU | Fe(III)/5-FAM | 5-FU | T 2-Weighted MRI/OI | Tumour targeting via FA, chemotherapy | 44 |
| PPy@MIL-100(Fe) | Fe(III)/PPy | DOX | T 2-Weighted MRI/PAI | NIR/pH-triggered drug release, chemotherapy, PTT | 166 |
| MOF@HA@ICG NPs | Fe(III)/ICG | — | T 2-Weighted MRI/OI/PAI | PTT, tumour targeting via HA | 43 |
| Mn/Hf-IR825@PDA-PEG | Mn(II)/IR825/Hf(IV) | — | T 1-Weighted MRI/PAI/CT | Thermo-radiotherapy | 159 |
Beside being conjugated or loaded with active molecules, NMOFs can also be directly constructed from various intrinsically active metal ions or linkers. The use of certain inorganic metal ions with imaging contrast abilities, e.g. Fe(III), Mn(II), Gd(III) and Hf(IV), enables the construction of MOFs as contrast agents for OI, MRI, CT, PET or PAI. Additionally, by using organic linkers with imaging capabilities, such as luminescent porphyrin derivatives, simultaneous imaging and therapy can be achieved. However, there are still many important limitations and challenges that need to be optimized for potential clinical applications of MOFs as imaging contrast agents. Firstly, a systematic in vivo toxicity evaluation, considering the accumulation, retention, and metabolism of any residuals, is essential. This would also require that any MOF proposed as an imaging device could be synthesised with highly reproducible physical properties such as particle size, morphology, and surface chemistry; precision manufacture at this level is not trivial. Secondly, the poor solubility and colloidal stability of MOFs in body fluids significantly limit their in vivo bioimaging applications. For instance, many Zn-carboxylate MOFs exhibit low stability in aqueous conditions due to the labile Zn carboxylate coordination. On the other hand, Zr-based MOFs, which are typically chemically inert, have high affinity towards phosphate ions found in culture medium and phosphate-buffered saline.173 As such, the degradation mechanism and biostability of currently available MOFs requires further studies. However, these problems can potentially be solved by selecting low-toxicity metal ions and organic linkers, and by appropriate functionalization and/or coating of MOFs. Their synthetic, compositional, and functional diversities mean that highly complex systems can be assembled relatively easily, and so it is expected that they will play a significant role in the biomedical field in the near future.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
RSF thanks the Royal Society for a University Research Fellowship and the University of Glasgow for funding. This project received financial support in part from the European Research Council (ERC) under the European Union's Horizon 2020 Programme for Research and Innovation (grant agreement no. 677289, SCoTMOF, ERC-2015-STG).References
- Y. Sun, L. Zheng, Y. Yang, X. Qian, T. Fu, X. Li, Z. Yang, H. Yan, C. Cui and W. Tan, Nano-Micro Lett., 2020, 12, 1–29 CrossRef CAS PubMed.
- X. L. Wang, C. Qin, S. X. Wu, K. Z. Shao, Y. Q. Lan, S. Wang, D. X. Zhu, Z. M. Su and E. B. Wang, Angew. Chem., Int. Ed., 2009, 48, 5291–5295 CrossRef CAS PubMed.
- H.-C. Zhou and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415–5418 RSC.
- A. R. Millward and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 17998–17999 CrossRef CAS PubMed.
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469–472 CrossRef CAS PubMed.
- J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC.
- J. S. Seo, D. Whang, H. Lee, S. Im Jun, J. Oh, Y. J. Jeon and K. Kim, Nature, 2000, 404, 982–986 CrossRef CAS PubMed.
- C. Wang, T. Zhang and W. Lin, Chem. Rev., 2012, 112, 1084–1104 CrossRef CAS PubMed.
- P. Serra-Crespo, M. A. Van Der Veen, E. Gobechiya, K. Houthoofd, Y. Filinchuk, C. E. Kirschhock, J. A. Martens, B. F. Sels, D. E. De Vos and F. Kapteijn, J. Am. Chem. Soc., 2012, 134, 8314–8317 CrossRef CAS PubMed.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- Z. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815–5840 RSC.
- W. Yang, J. Feng, S. Song and H. Zhang, ChemPhysChem, 2012, 13, 2734–2738 CrossRef CAS PubMed.
- H. Zheng, Y. Zhang, L. Liu, W. Wan, P. Guo, A. M. Nyström and X. Zou, J. Am. Chem. Soc., 2016, 138, 962–968 CrossRef CAS PubMed.
- S. Beg, M. Rahman, A. Jain, S. Saini, P. Midoux, C. Pichon, F. J. Ahmad and S. Akhter, Drug Discovery Today, 2017, 22, 625–637 CrossRef CAS PubMed.
- I. Abánades Lázaro, C. J. Wells and R. S. Forgan, Angew. Chem., Int. Ed., 2020, 59, 5211–5217 CrossRef PubMed.
- M. D. Allendorf, C. A. Bauer, R. Bhakta and R. Houk, Chem. Soc. Rev., 2009, 38, 1330–1352 RSC.
- D. Liu, R. C. Huxford and W. Lin, Angew. Chem., Int. Ed., 2011, 50, 3696–3700 CrossRef CAS PubMed.
- U. Ryu, J. Yoo, W. Kwon and K. M. Choi, Inorg. Chem., 2017, 56, 12859–12865 CrossRef CAS PubMed.
- G. D. Wang, H. Chen, W. Tang, D. Lee and J. Xie, Tomography, 2016, 2, 179–187 CrossRef PubMed.
- Y. Zhang, L. Wang, L. Liu, L. Lin, F. Liu, Z. Xie, H. Tian and X. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 41035–41045 CrossRef CAS PubMed.
- K. M. Taylor, W. J. Rieter and W. Lin, J. Am. Chem. Soc., 2008, 130, 14358–14359 CrossRef CAS PubMed.
- W. Hatakeyama, T. J. Sanchez, M. D. Rowe, N. J. Serkova, M. W. Liberatore and S. G. Boyes, ACS Appl. Mater. Interfaces, 2011, 3, 1502–1510 CrossRef CAS PubMed.
- A. Foucault-Collet, K. A. Gogick, K. A. White, S. Villette, A. Pallier, G. Collet, C. Kieda, T. Li, S. J. Geib and N. L. Rosi, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 17199–17204 CrossRef CAS PubMed.
- L. Chen, J.-W. Ye, H.-P. Wang, M. Pan, S.-Y. Yin, Z.-W. Wei, L.-Y. Zhang, K. Wu, Y.-N. Fan and C.-Y. Su, Nat. Commun., 2017, 8, 15985 CrossRef CAS PubMed.
- M. X. Wu and Y. W. Yang, Adv. Mater., 2017, 29, 1606134 CrossRef PubMed.
- A. C. McKinlay, R. E. Morris, P. Horcajada, G. Férey, R. Gref, P. Couvreur and C. Serre, Angew. Chem., Int. Ed., 2010, 49, 6260–6266 CrossRef CAS PubMed.
- J. Yang and Y. W. Yang, Small, 2020, 16, 1906846 CrossRef CAS PubMed.
- W. J. Rieter, K. M. Taylor, H. An, W. Lin and W. Lin, J. Am. Chem. Soc., 2006, 128, 9024–9025 CrossRef CAS PubMed.
- X. Gao, Y. Wang, G. Ji, R. Cui and Z. Liu, CrystEngComm, 2018, 20, 1087–1093 RSC.
- K. M. Park, H. Kim, J. Murray, J. Koo and K. Kim, Supramol. Chem., 2017, 29, 441–445 CrossRef CAS.
- P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette and C. Kreuz, Nat. Mater., 2010, 9, 172–178 CrossRef CAS PubMed.
- Y. Zhang, C. Liu, F. Wang, Z. Liu, J. Ren and X. Qu, Chem. Commun., 2017, 53, 1840–1843 RSC.
- T. Zhang, L. Wang, C. Ma, W. Wang, J. Ding, S. Liu, X. Zhang and Z. Xie, J. Mater. Chem. B, 2017, 5, 2330–2336 RSC.
- D. Chen, D. Yang, C. A. Dougherty, W. Lu, H. Wu, X. He, T. Cai, M. E. Van Dort, B. D. Ross and H. Hong, ACS Nano, 2017, 11, 4315–4327 CrossRef CAS PubMed.
- P. Yang, Y. Tian, Y. Men, R. Guo, H. Peng, Q. Jiang and W. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 42039–42049 CrossRef CAS PubMed.
- J. Della Rocca, D. Liu and W. Lin, Acc. Chem. Res., 2011, 44, 957–968 CrossRef CAS PubMed.
- C. He, D. Liu and W. Lin, Chem. Rev., 2015, 115, 11079–11108 CrossRef CAS PubMed.
- D. Liu, K. Lu, C. Poon and W. Lin, Inorg. Chem., 2014, 53, 1916–1924 CrossRef CAS PubMed.
- Y. Guari, J. Larionova, M. Corti, A. Lascialfari, M. Marinone, G. Poletti, K. Molvinger and C. Guérin, Dalton Trans., 2008, 3658–3660 RSC.
- K. M. Taylor, A. Jin and W. Lin, Angew. Chem., Int. Ed., 2008, 47, 7722–7725 CrossRef CAS PubMed.
- J.-L. Bridot, A.-C. Faure, S. Laurent, C. Rivière, C. Billotey, B. Hiba, M. Janier, V. Josserand, J.-L. Coll, L. Vander Elst, R. Muller, S. Roux, P. Perriat and O. Tillement, J. Am. Chem. Soc., 2007, 129, 5076–5084 CrossRef CAS PubMed.
- K. E. Dekrafft, W. S. Boyle, L. M. Burk, O. Z. Zhou and W. Lin, J. Mater. Chem., 2012, 22, 18139–18144 RSC.
- W. Cai, H. Gao, C. Chu, X. Wang, J. Wang, P. Zhang, G. Lin, W. Li, G. Liu and X. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 2040–2051 CrossRef CAS PubMed.
- X. Gao, M. Zhai, W. Guan, J. Liu, Z. Liu and A. Damirin, ACS Appl. Mater. Interfaces, 2017, 9, 3455–3462 CrossRef CAS PubMed.
- H. Zhang, Y.-H. Li, Y. Chen, M.-M. Wang, X.-S. Wang and X.-B. Yin, Sci. Rep., 2017, 7, 44153 CrossRef PubMed.
- U. Ryu, H. S. Lee, K. S. Park and K. M. Choi, Polyhedron, 2018, 154, 275–294 CrossRef CAS.
- J. Aguilera-Sigalat and D. Bradshaw, Coord. Chem. Rev., 2016, 307, 267–291 CrossRef CAS.
- K. Lu, T. Aung, N. Guo, R. Weichselbaum and W. Lin, Adv. Mater., 2018, 30, 1707634 CrossRef PubMed.
- H. An, M. Li, J. Gao, Z. Zhang, S. Ma and Y. Chen, Coord. Chem. Rev., 2019, 384, 90–106 CrossRef CAS.
- S. Zhang, X. Pei, H. Gao, S. Chen and J. Wang, Chin. Chem. Lett., 2020, 31, 1060–1070 CrossRef CAS.
- S. Keskin and S. Kızılel, Ind. Eng. Chem. Res., 2011, 50, 1799–1812 CrossRef CAS.
- R. Liu, T. Yu, Z. Shi and Z. Wang, Int. J. Nanomed., 2016, 11, 1187–1200 CrossRef CAS.
- S. E. Miller, M. H. Teplensky, P. Z. Moghadam and D. Fairen-Jimenez, Interface Focus, 2016, 6, 20160027 CrossRef.
- J. Zhou, G. Tian, L. Zeng, X. Song and X. W. Bian, Adv. Healthcare Mater., 2018, 7, 1800022 CrossRef.
- J. Yang and Y.-W. Yang, View, 2020, 1, e20 Search PubMed.
- W. Sun, S. Li, G. Tang, Y. Luo, S. Ma, S. Sun, J. Ren, Y. Gong and C. Xie, Int. J. Nanomed., 2019, 14, 10195–10207 CrossRef CAS PubMed.
- S. M. Erturk, C. Johnston, C. Tempany-Afdhal and A. D. Van den Abbeele, in Clinical and Translational Science, ed. D. Robertson and G. H. Williams, Academic Press, San Diego, 2009, pp. 87–104 Search PubMed.
- P. Padmanabhan, A. Kumar, S. Kumar, R. K. Chaudhary and B. Gulyás, Acta Biomater., 2016, 41, 1–16 CrossRef CAS PubMed.
- K. M. Taylor-Pashow, J. Della Rocca, Z. Xie, S. Tran and W. Lin, J. Am. Chem. Soc., 2009, 131, 14261–14263 CrossRef CAS PubMed.
- S. Wuttke, S. Braig, T. Preiß, A. Zimpel, J. Sicklinger, C. Bellomo, J. O. Rädler, A. M. Vollmar and T. Bein, Chem. Commun., 2015, 51, 15752–15755 RSC.
- F. Duan, X. Feng, X. Yang, W. Sun, Y. Jin, H. Liu, K. Ge, Z. Li and J. Zhang, Biomaterials, 2017, 122, 23–33 CrossRef CAS PubMed.
- X. Liu, W. Qi, Y. Wang, R. Su and Z. He, Nanoscale, 2017, 9, 17561–17570 RSC.
- S. S. Nadar and V. K. Rathod, Int. J. Biol. Macromol., 2017, 95, 511–519 CrossRef CAS.
- C. Orellana-Tavra, S. Haddad, R. J. Marshall, I. Abánades Lázaro, G. Boix, I. Imaz, D. Maspoch, R. S. Forgan and D. Fairen-Jimenez, ACS Appl. Mater. Interfaces, 2017, 9, 35516–35525 CrossRef CAS PubMed.
- J. Zielonka, J. Joseph, A. Sikora, M. Hardy, O. Ouari, J. Vasquez-Vivar, G. Cheng, M. Lopez and B. Kalyanaraman, Chem. Rev., 2017, 117, 10043–10120 CrossRef CAS PubMed.
- Y.-A. Li, X.-D. Zhao, H.-P. Yin, G.-J. Chen, S. Yang and Y.-B. Dong, Chem. Commun., 2016, 52, 14113–14116 RSC.
- W. Zhou, L. Wang, F. Li, W. Zhang, W. Huang, F. Huo and H. Xu, Adv. Funct. Mater., 2017, 27, 1605465 CrossRef.
- Y. Song, J. Yang, L. Wang and Z. Xie, ChemMedChem, 2020, 15, 416–419 CrossRef CAS PubMed.
- S. Haddad, I. Abánades Lázaro, M. Fantham, A. Mishra, J. Silvestre-Albero, J. W. Osterrieth, G. S. Kaminski Schierle, C. F. Kaminski, R. S. Forgan and D. Fairen-Jimenez, J. Am. Chem. Soc., 2020, 142, 6661–6674 CrossRef CAS PubMed.
- U. P. Singh, N. Singh, R. Varshney, P. Roy and R. J. Butcher, Cryst. Growth Des., 2018, 18, 2804–2813 CrossRef CAS.
- W. Liu, Y. M. Wang, Y. H. Li, S. J. Cai, X. B. Yin, X. W. He and Y. K. Zhang, Small, 2017, 13, 1603459 CrossRef PubMed.
- H. Chen, J. Wang, D. Shan, J. Chen, S. Zhang and X. Lu, Anal. Chem., 2018, 90, 7056–7063 CrossRef CAS PubMed.
- D. F. Sava Gallis, L. E. Rohwer, M. A. Rodriguez, M. C. Barnhart-Dailey, K. S. Butler, T. S. Luk, J. A. Timlin and K. W. Chapman, ACS Appl. Mater. Interfaces, 2017, 9, 22268–22277 CrossRef CAS PubMed.
- Y. Lv, D. Ding, Y. Zhuang, Y. Feng, J. Shi, H. Zhang, T.-L. Zhou, H. Chen and R.-J. Xie, ACS Appl. Mater. Interfaces, 2018, 11, 1907–1916 CrossRef PubMed.
- H. Zhao, G. Shu, J. Zhu, Y. Fu, Z. Gu and D. Yang, Biomaterials, 2019, 217, 119332 CrossRef CAS PubMed.
- A. R. Chowdhuri, D. Laha, S. Pal, P. Karmakar and S. K. Sahu, Dalton Trans., 2016, 45, 18120–18132 RSC.
- A. R. Chowdhuri, D. Laha, S. Chandra, P. Karmakar and S. K. Sahu, Chem. Eng. J., 2017, 319, 200–211 CrossRef CAS.
- Z. Yuan, L. Zhang, S. Li, W. Zhang, M. Lu, Y. Pan, X. Xie, L. Huang and W. Huang, J. Am. Chem. Soc., 2018, 140, 15507–15515 CrossRef CAS.
- L. He, T. Wang, J. An, X. Li, L. Zhang, L. Li, G. Li, X. Wu, Z. Su and C. Wang, CrystEngComm, 2014, 16, 3259–3263 RSC.
- J. Y. Zeng, M. K. Zhang, M. Y. Peng, D. Gong and X. Z. Zhang, Adv. Funct. Mater., 2018, 28, 1705451 CrossRef.
- J. Deng, K. Wang, M. Wang, P. Yu and L. Mao, J. Am. Chem. Soc., 2017, 139, 5877–5882 CrossRef CAS PubMed.
- C. Orellana-Tavra, R. J. Marshall, E. F. Baxter, I. A. Lázaro, A. Tao, A. K. Cheetham, R. S. Forgan and D. Fairen-Jimenez, J. Mater. Chem. B, 2016, 4, 7697–7707 RSC.
- C. Orellana-Tavra, E. F. Baxter, T. Tian, T. D. Bennett, N. K. Slater, A. K. Cheetham and D. Fairen-Jimenez, Chem. Commun., 2015, 51, 13878–13881 RSC.
- I. Abánades Lázaro, S. Haddad, S. Sacca, C. Orellana-Tavra, D. Fairen-Jimenez and R. S. Forgan, Chem, 2017, 2, 561–578 Search PubMed.
- M. Javadi, W. G. Pitt, C. M. Tracy, J. R. Barrow, B. M. Willardson, J. M. Hartley and N. H. Tsosie, J. Controlled Release, 2013, 167, 92–100 CrossRef CAS.
- H. Cai, Y.-L. Huang and D. Li, Coord. Chem. Rev., 2019, 378, 207–221 CrossRef CAS.
- J. Chen, Y. Zhu and S. Kaskel, Angew. Chem., Int. Ed., 2021, 60, 5010–5035 CrossRef CAS PubMed.
- S. Huh, S.-J. Kim and Y. Kim, CrystEngComm, 2016, 18, 345–368 RSC.
- C. Zou and C.-D. Wu, Dalton Trans., 2012, 41, 3879–3888 RSC.
- J. Wang, Y. Fan, Y. Tan, X. Zhao, Y. Zhang, C. Cheng and M. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 36615–36621 CrossRef CAS PubMed.
- J. Liu, L. Zhang, J. Lei, H. Shen and H. Ju, ACS Appl. Mater. Interfaces, 2017, 9, 2150–2158 CrossRef CAS PubMed.
- S. Lee, M. Baek, H.-Y. Kim, J.-H. Ha and D.-I. Jeoung, Biotechnol. Lett., 2002, 24, 1147–1151 CrossRef CAS.
- H. Mizutani, S. Tada-Oikawa, Y. Hiraku, M. Kojima and S. Kawanishi, Life Sci., 2005, 76, 1439–1453 CrossRef CAS PubMed.
- Y. C. Barenholz, J. Controlled Release, 2012, 160, 117–134 CrossRef CAS PubMed.
- F. D. Duman, M. Erkisa, R. Khodadust, F. Ari, E. Ulukaya and H. Y. Acar, Nanomedicine, 2017, 12, 2319–2333 CrossRef CAS PubMed.
- J. Prados, C. Melguizo, R. Ortiz, C. Velez, P. J. Alvarez, J. L. Arias, M. A. Ruiz, V. Gallardo and A. Aranega, Anti-Cancer Agents Med. Chem., 2012, 12, 1058–1070 CrossRef CAS PubMed.
- J.-C. G. Bünzli and C. Piguet, Chem. Soc. Rev., 2005, 34, 1048–1077 RSC.
- M. A. Alcala, C. M. Shade, H. Uh, S. Y. Kwan, M. Bischof, Z. P. Thompson, K. A. Gogick, A. R. Meier, T. G. Strein and D. L. Bartlett, Biomaterials, 2011, 32, 9343–9352 CrossRef CAS PubMed.
- P. Nockemann, E. Beurer, K. Driesen, R. Van Deun, K. Van Hecke, L. Van Meervelt and K. Binnemans, Chem. Commun., 2005, 4354–4356 RSC.
- S. Weissman, J. Chem. Phys., 1942, 10, 214–217 CrossRef CAS.
- J. Wang, W. Sun, S. Chang, H. Liu, G. Zhang, Y. Wang and Z. Liu, RSC Adv., 2015, 5, 48574–48579 RSC.
- D. Hu, Y. Song and L. Wang, J. Nanopart. Res., 2015, 17, 310 CrossRef.
- R. Abazari, F. Ataei, A. Morsali, A. M. Slawin and C. L. Carpenter-Warren, ACS Appl. Mater. Interfaces, 2019, 11, 45442–45454 CrossRef CAS PubMed.
- A. M. Smith, M. C. Mancini and S. Nie, Nat. Nanotechnol., 2009, 4, 710–711 CrossRef CAS PubMed.
- D. Wang, C. Zhao, G. Gao, L. Xu, G. Wang and P. Zhu, Nanomaterials, 2019, 9, 1274 CrossRef CAS PubMed.
- Y. Zhuang, Y. Katayama, J. Ueda and S. Tanabe, Opt. Mater., 2014, 36, 1907–1912 CrossRef CAS.
- J. W. Chung, Z. Gerelkhuu, J. H. Oh and Y.-I. Lee, Appl. Spectrosc. Rev., 2016, 51, 678–705 CrossRef CAS.
- F. D. Duman, I. Hocaoglu, D. G. Ozturk, D. Gozuacik, A. Kiraz and H. Y. Acar, Nanoscale, 2015, 7, 11352–11362 RSC.
- I. Hocaoglu, F. Demir, O. Birer, A. Kiraz, C. Sevrin, C. Grandfils and H. Y. Acar, Nanoscale, 2014, 6, 11921–11931 RSC.
- X. Lin, G. Gao, L. Zheng, Y. Chi and G. Chen, Anal. Chem., 2014, 86, 1223–1228 CrossRef CAS PubMed.
- D. Zhao, X. Wan, H. Song, L. Hao, Y. Su and Y. Lv, Sens. Actuators, B, 2014, 197, 50–57 CrossRef CAS.
- H. Zhao, C. Serre, E. Dumas and N. Steunou, in Metal-Organic Frameworks for Biomedical Applications, ed. M. Mosafari, Elsevier, 2020, pp. 397–423 Search PubMed.
- J. Jiang, H. Gu, H. Shao, E. Devlin, G. C. Papaefthymiou and J. Y. Ying, Adv. Mater., 2008, 20, 4403–4407 CrossRef CAS.
- X. Wang, P. Li, Q. Ding, C. Wu, W. Zhang and B. Tang, J. Am. Chem. Soc., 2019, 141, 2061–2068 CrossRef CAS.
- C. Yang, X. Yin, S.-Y. Huan, L. Chen, X.-X. Hu, M.-Y. Xiong, K. Chen and X.-B. Zhang, Anal. Chem., 2018, 90, 3118–3123 CrossRef CAS PubMed.
- S. W. Botchway, M. Charnley, J. W. Haycock, A. W. Parker, D. L. Rochester, J. A. Weinstein and J. G. Williams, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 16071–16076 CrossRef CAS PubMed.
- W. A. Maza, R. Padilla and A. J. Morris, J. Am. Chem. Soc., 2015, 137, 8161–8168 CrossRef CAS PubMed.
- W. A. Maza and A. J. Morris, J. Phys. Chem. C, 2014, 118, 8803–8817 CrossRef CAS.
- R. Chen, J. Zhang, J. Chelora, Y. Xiong, S. V. Kershaw, K. F. Li, P.-K. Lo, K. W. Cheah, A. L. Rogach and J. A. Zapien, ACS Appl. Mater. Interfaces, 2017, 9, 5699–5708 CrossRef CAS PubMed.
- C. Yang, K. Chen, M. Chen, X. Hu, S.-Y. Huan, L. Chen, G. Song and X.-B. Zhang, Anal. Chem., 2019, 91, 2727–2733 CrossRef CAS PubMed.
- X. Mao, J. Xu and H. Cui, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2016, 8, 814–841 CAS.
- M. Peller, K. Böll, A. Zimpel and S. Wuttke, Inorg. Chem. Front., 2018, 5, 1760–1779 RSC.
- W. Zhang, L. Liu, H. Chen, K. Hu, I. Delahunty, S. Gao and J. Xie, Theranostics, 2018, 8, 2521–2548 CrossRef CAS PubMed.
- J. Estelrich, M. J. Sánchez-Martín and M. A. Busquets, Int. J. Nanomed., 2015, 10, 1727–1741 CAS.
- W. Lin, T. Hyeon, G. M. Lanza, M. Zhang and T. J. Meade, MRS Bull., 2009, 34, 441–448 CrossRef CAS PubMed.
- A. Pandey, N. Dhas, P. Deshmukh, C. Caro, P. Patil, M. L. García-Martín, B. Padya, A. Nikam, T. Mehta and S. Mutalik, Coord. Chem. Rev., 2020, 409, 213212 CrossRef CAS.
- M. Rogosnitzky and S. Branch, Biometals, 2016, 29, 365–376 CrossRef CAS PubMed.
- C. Olchowy, K. Cebulski, M. Łasecki, R. Chaber, A. Olchowy, K. Kałwak and U. Zaleska-Dorobisz, PLoS One, 2017, 12, e0171704 CrossRef PubMed.
- P. Caravan, J. J. Ellison, T. J. McMurry and R. B. Lauffer, Chem. Rev., 1999, 99, 2293–2352 CrossRef CAS.
- Z. Zhou and Z. R. Lu, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2013, 5, 1–18 CrossRef CAS.
- J. Ramalho and M. Ramalho, Magn. Reson. Imaging Clin. N. Am., 2017, 25, 765–778 CrossRef PubMed.
- H. S. Thomsen, P. Marckmann and V. B. Logager, Cancer Imaging, 2007, 7, 130–137 CrossRef PubMed.
- R. C. Semelka, J. P. Prybylski and M. Ramalho, Magn. Reson. Imaging, 2019, 58, 174–178 CrossRef CAS PubMed.
- L. Pasquini, A. Napolitano, E. Visconti, D. Longo, A. Romano, P. Tomà and M. C. R. Espagnet, CNS Drugs, 2018, 32, 229–240 CrossRef CAS PubMed.
- M. A. Chowdhury, J. Biomed. Mater. Res., Part A, 2017, 105, 1184–1194 CrossRef CAS PubMed.
- H.-X. Zhao, Q. Zou, S.-K. Sun, C. Yu, X. Zhang, R.-J. Li and Y.-Y. Fu, Chem. Sci., 2016, 7, 5294–5301 RSC.
- D. Wang, J. Zhou, R. Chen, R. Shi, G. Zhao, G. Xia, R. Li, Z. Liu, J. Tian and H. Wang, Biomaterials, 2016, 100, 27–40 CrossRef CAS PubMed.
- D. Wang, J. Zhou, R. Chen, R. Shi, G. Xia, S. Zhou, Z. Liu, N. Zhang, H. Wang and Z. Guo, Biomaterials, 2016, 107, 88–101 CrossRef CAS PubMed.
- D. Pan, A. H. Schmieder, S. A. Wickline and G. M. Lanza, Tetrahedron, 2011, 67, 8431–8444 CrossRef CAS PubMed.
- J. Della Rocca and W. Lin, Eur. J. Inorg. Chem., 2010, 3725–3734 CrossRef CAS.
- Y. Yang, J. Liu, C. Liang, L. Feng, T. Fu, Z. Dong, Y. Chao, Y. Li, G. Lu and M. Chen, ACS Nano, 2016, 10, 2774–2781 CrossRef CAS PubMed.
- L. Qin, Z.-Y. Sun, K. Cheng, S.-W. Liu, J.-X. Pang, L.-M. Xia, W.-H. Chen, Z. Cheng and J.-X. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 41378–41386 CrossRef CAS PubMed.
- N. Lee and T. Hyeon, Chem. Soc. Rev., 2012, 41, 2575–2589 RSC.
- F. Shu, D. Lv, X.-L. Song, B. Huang, C. Wang, Y. Yu and S.-C. Zhao, RSC Adv., 2018, 8, 6581–6589 RSC.
- A. R. Chowdhuri, D. Bhattacharya and S. K. Sahu, Dalton Trans., 2016, 45, 2963–2973 RSC.
- H.-S. Wang, Coord. Chem. Rev., 2017, 349, 139–155 CrossRef CAS.
- F. A. Mettler Jr, P. W. Wiest, J. A. Locken and C. A. Kelsey, J. Radiol. Prot., 2000, 20, 353–359 CrossRef CAS PubMed.
- A. M. Leung and L. E. Braverman, Curr. Opin. Endocrinol., Diabetes Obes., 2012, 19, 414–419 CrossRef CAS PubMed.
- M. Andreucci, R. Solomon and A. Tasanarong, BioMed Res. Int., 2014, 2014, 872574 Search PubMed.
- C. Wang, O. Volotskova, K. Lu, M. Ahmad, C. Sun, L. Xing and W. Lin, J. Am. Chem. Soc., 2014, 136, 6171–6174 CrossRef CAS PubMed.
- S. Basu and A. Alavi, PET Clinics, 2016, 11, 203–207 CrossRef PubMed.
- O. Jacobson and X. Chen, Pharmacol. Rev., 2013, 65, 1214–1256 CrossRef CAS PubMed.
- M. Hamoudeh, M. A. Kamleh, R. Diab and H. Fessi, Adv. Drug Delivery Rev., 2008, 60, 1329–1346 CrossRef CAS PubMed.
- D. Duan, H. Liu, M. Xu, M. Chen, Y. Han, Y. Shi and Z. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 42165–42174 CrossRef CAS.
- S. Zackrisson, S. Van De Ven and S. Gambhir, Cancer Res., 2014, 74, 979–1004 CrossRef CAS PubMed.
- H. F. Zhang, K. Maslov, G. Stoica and L. V. Wang, Nat. Biotechnol., 2006, 24, 848–851 CrossRef CAS PubMed.
- R. G. Kolkman, P. J. Brands, W. Steenbergen and T. G. van Leeuwen, J. Biomed. Opt., 2008, 13, 050510 CrossRef PubMed.
- J. Zhang, H. Chen, T. Zhou, L. Wang, D. Gao, X. Zhang, Y. Liu, C. Wu and Z. Yuan, Nano Res., 2017, 10, 64–76 CrossRef CAS.
- Y. Yang, Y. Chao, J. Liu, Z. Dong, W. He, R. Zhang, K. Yang, M. Chen and Z. Liu, NPG Asia Mater., 2017, 9, e344 CrossRef CAS.
- D. Zhang, H. Xu, X. Zhang, Y. Liu, M. Wu, J. Li, H. Yang, G. Liu, X. Liu and J. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 25203–25212 CrossRef CAS PubMed.
- W. Cai, J. Wang, H. Liu, W. Chen, J. Wang, L. Du, J. Hu and C. Wu, J. Alloys Compd., 2018, 748, 193–198 CrossRef CAS.
- T. Kundu, S. Mitra, D. D. Díaz and R. Banerjee, ChemPlusChem, 2016, 81, 728–732 CrossRef CAS PubMed.
- X. Gao, G. Ji, R. Cui, J. Liu and Z. Liu, Dalton Trans., 2017, 46, 13686–13689 RSC.
- Y. Li, J. Tang, L. He, Y. Liu, Y. Liu, C. Chen and Z. Tang, Adv. Mater., 2015, 27, 4075–4080 CrossRef CAS PubMed.
- C. Tian, L. Zhu, F. Lin and S. G. Boyes, ACS Appl. Mater. Interfaces, 2015, 7, 17765–17775 CrossRef CAS PubMed.
- X. Chen, M. Zhang, S. Li, L. Li, L. Zhang, T. Wang, M. Yu, Z. Mou and C. Wang, J. Mater. Chem. B, 2017, 5, 1772–1778 RSC.
- X. Song, C. Liang, H. Gong, Q. Chen, C. Wang and Z. Liu, Small, 2015, 11, 3932–3941 CrossRef CAS PubMed.
- X. Liang, Y. Li, X. Li, L. Jing, Z. Deng, X. Yue, C. Li and Z. Dai, Adv. Funct. Mater., 2015, 25, 1451–1462 CrossRef CAS.
- K. Yang, H. Xu, L. Cheng, C. Sun, J. Wang and Z. Liu, Adv. Mater., 2012, 24, 5586–5592 CrossRef CAS PubMed.
- Z. Zha, Z. Deng, Y. Li, C. Li, J. Wang, S. Wang, E. Qu and Z. Dai, Nanoscale, 2013, 5, 4462–4467 RSC.
- P. Yang, Y. Men, Y. Tian, Y. Cao, L. Zhang, X. Yao and W. Yang, ACS Appl. Mater. Interfaces, 2019, 11, 11209–11219 CrossRef CAS PubMed.
- W. Shang, C. Zeng, Y. Du, H. Hui, X. Liang, C. Chi, K. Wang, Z. Wang and J. Tian, Adv. Mater., 2017, 29, 1604381 CrossRef PubMed.
- Y. Liu, Y. Zhao and X. Chen, Theranostics, 2019, 9, 3122–3133 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |