Observing long-range non-fullerene backbone ordering in real-space to improve the charge transport properties of organic solar cells†
Abstract
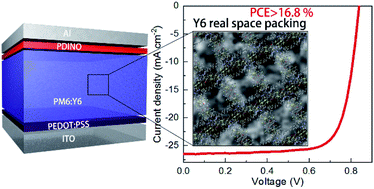
To understand the dominance of 2,2′-((2Z,2′Z)-((12,13-bis(2-ethylhexyl)-3,9-diundecyl-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2′′,3′′:4′,5′]thieno[2′,3′:4,5]pyrrolo[3,2-g]thieno[2′,3′:4,5]thieno[3,2-b]indole-2,10-diyl)bis(methanylylidene))bis(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile (Y6) and its derivatives leading to the rapid efficiency rise of organic solar cells (OSCs), solid film structures are of significant importance. Here, we employ cryo-transmission electron microscopy (Cryo-TEM) to resolve the landscape of Y6 packing in neat films (unlike single crystals) in relation to device performance, and reveal how processing with carbon disulfide and [6,6]-phenyl-C71-butyric acid methyl ester (PC71BM) influences its molecular packing, prominently the backbone ordering. We show that Y6 prefers a face-on dominant packing structure with an in-plane long-range conjugated backbone packing in films. The long-range backbone ordering is beneficial for reducing disorders on the energy distribution of the electron transport level, thereby improving the carrier lifetime in heterojunctions. We confirm that long-range energy transfer assists poly[(2,6-(4,8-bis(5-(2-ethylhexyl)-4-fluorothiophen-2-yl)-benzo[1,2-b:4,5-b′]dithiophene))-alt-(5,5-(1′,3′-di-2-thienyl-5′,7′-bis(2-ethylhexyl)benzo[1′,2′-c:4′,5′-c′]dithiophene-4,8-dione))] (PM6) excitons to reach the preferred Y6/PM6 interfaces without being quenched by PC71BM clusters, yielding no signs of bimolecular recombination and a high power conversion efficiency of 16.8%. Our results suggest an effective molecular packing structure in solid films, and the prominent role of backbone ordering in photoelectric conversion processes, which will outline the future development of OSCs.


 Please wait while we load your content...
Please wait while we load your content...