Tailoring oxygen evolution reaction activity of metal-oxide spinel nanoparticles via judiciously regulating surface-capping polymers†
Abstract
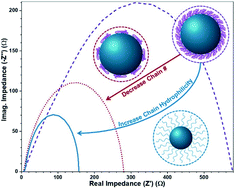
High cost and scarcity of the state-of-the-art noble metal-based catalysts represents one of the critical hurdles to be overcome in electrocatalysis. A promising direction is to utilize transition metal-based nanoparticles (NPs), which offer superior electrocatalytic performance over their bulk counterparts. Capping the surface of NPs with polymers is widely recognized as an effective means towards their dispersion and stabilization. However, it is often circumvented due to its tendency to lower the electrocatalytic activity of the ligated NPs. Here, we report the first systematic investigation into the impact of the chain density and hydrophilicity of the surface-capping polymers, which can be judiciously regulated, on the oxygen evolution reaction (OER) activity. By capitalizing on star-like diblock copolymers as nanoreactors, spinel CoFe2O4 (CFO) NPs permanently ligated with polymers of interest (i.e., varied chain density and characteristic) are crafted. The correlation between the chain density and hydrophilicity of surface-capping polymers and the OER activity of CFO NPs are scrutinized. Intriguingly, decreasing the number of surface-capping chains and increasing the chain hydrophilicity result in significantly decreased overpotential, caused by an increased exposure of the active material (CFO) to the electrolyte and reduced diffusion resistance. This study provides insight into the strategies for mitigating the activity-limiting properties of surface polymers and tailoring the electrocatalytic properties of polymer-ligated NPs.
- This article is part of the themed collection: Special issue in honour of Seth Marder


 Please wait while we load your content...
Please wait while we load your content...
