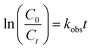Open Access Article
Open Access ArticleFacile one-step synthesis and enhanced photocatalytic activity of a WC/ferroelectric nanocomposite†
Man
Zhang
a,
Yaqiong
Wang
b,
Jianguo
Liu
 c,
Madasamy
Thangamuthu
d,
Yajun
Yue
e,
Zhongna
Yan
eg,
Jingyu
Feng
c,
Madasamy
Thangamuthu
d,
Yajun
Yue
e,
Zhongna
Yan
eg,
Jingyu
Feng
 f,
Dou
Zhang
f,
Dou
Zhang
 g,
Hongtao
Zhang
h,
Shaoliang
Guan
i,
Maria-Magdalena
Titirici
g,
Hongtao
Zhang
h,
Shaoliang
Guan
i,
Maria-Magdalena
Titirici
 f,
Isaac
Abrahams
f,
Isaac
Abrahams
 e,
Junwang
Tang
e,
Junwang
Tang
 d,
Zhen
Zhang
d,
Zhen
Zhang
 j,
Steve
Dunn
*b and
Haixue
Yan
j,
Steve
Dunn
*b and
Haixue
Yan
 *a
*a
aSchool of Engineering and Materials Science, Queen Mary University of London, Mile End Road, London E1 4NS, UK. E-mail: h.x.yan@qmul.ac.uk
bSchool of Engineering, London South Bank University, 103 Borough Road, London, SE1 0AA, UK. E-mail: dunns4@lsbu.ac.uk
cSchool of Environment, Tsinghua University, 1 Qinghuayuan, Beijing, 100084, China
dDepartment of Chemical Engineering, University College London, Torrington Place, London, WC1E 7JE, UK
eDepartment of Chemistry, Queen Mary University of London, Mile End Road, London E1 4NS, UK
fDepartment of Chemical Engineering, Imperial College London, South Kensington Campus, London SW7 2AZ, UK
gState Key Laboratory of Powder Metallurgy, Central South University, South Lushan Road, Changsha, 410083, China
hDepartment of Materials, Loughborough University, Leicestershire LE11 3TU, UK
iSchool of Chemistry, Cardiff University, Main Building, Park Place, Cardiff, CF10 3AT, UK
jDivision of Solid State Electronics, Department of Electrical Engineering, Uppsala University, Lagerhyddsvagen 1, Uppsala, Sweden
First published on 13th August 2021
Abstract
The development of noble-metal-free co-catalysts is seen as a viable strategy for improving the performance of semiconductor photocatalysts. Although the photocatalytic efficiency of ferroelectrics is typically low, it can be enhanced through the incorporation of a co-catalyst into nanocomposites. Here, we demonstrate the influence of ferroelectricity on the decolorization of rhodamine B under simulated solar light using RbBi2Ti2NbO10 and compared the performance with that of non-ferroelectric RbBi2Nb5O16. The decolorization rate for RbBi2Ti2NbO10 was 5 times greater than that of RbBi2Nb5O16. This behaviour can be explained in terms of ferroelectric polarization, which drives the separation of charge carriers. The photocatalytic activity of RbBi2Ti2NbO10 was further enhanced to over 30 times upon preparing a nanocomposite with tungsten carbide (WC) through high energy ball milling. This enhancement was attributed not only to the increased specific surface area, but also to the incorporated WC co-catalyst, which also serves as a source of plasmonic hot electrons and extends the photocatalytic activity into the visible light range. The WC/RbBi2Ti2NbO10 nanocomposite shows interesting water oxidation properties and evolves O2 with a rate of 68.5 μmol h−1 g−1 and a quantum yield of 3% at 420 nm. This work demonstrates a simple route for preparing WC containing nano-ferroelectric composites for solar energy conversion applications.
1. Introduction
The development of low cost photocatalyst materials with high photocatalytic efficiency which are also environmentally benign is viewed as a matter of global urgency.1 In this respect, the direct use of natural solar energy through photocatalysts represents an important area of technology. When refined, the approach could be used to provide useful chemicals,2 reduce CO2 in the atmosphere3 or produce fuels using narrow band-gap materials.4 Despite abundant studies on semiconductors, including a variety of inorganic metal oxides (such as TiO2, ZnO, WO3 and SnO)5–9 and sulfides (ZnS and CdS)10–14 and the development of a variety of structures,15 their photocatalytic efficiency is still far from satisfactory. A key challenge for efficiency improvement is suppressing the recombination of photo-induced charge carriers and prolonging their lifetime.10,16–18 One important strategy to address this problem is to use ferroelectric materials. Their switchable spontaneous polarization produces internal electric fields,19–22 resulting in the spatial separation of photo-induced electron–hole pairs.23,24Morris et al.25 tested the charge carrier lifetime in films of cubic (paraelectric) and tetragonal (ferroelectric) BaTiO3 using transient absorption spectroscopy and found that the charge carrier lifetime in ferroelectric BaTiO3 was four orders of magnitude longer than that in paraelectric BaTiO3. A prolonged charge carrier lifetime is also reported in the Aurivillius ferroelectric, Bi6−xSrxTi3+xFe2−xO18.26 This clearly demonstrates that the presence of internal fields can reduce the recombination of charge carriers, leading to enhanced photocatalytic efficiency. Moreover, the ferroelectric domains cause band bending of the electronic states and induce spatially selective reactivity for photochemical reactions, as demonstrated in perovskites, such as BiFeO3,27 BaTiO3,28 and Pb(Zr0.3Ti0.7)O3.29 Among various ferroelectric photocatalysts with a perovskite-like structure, bismuth-containing niobates are reported as promising candidates,30–32 due to the high energy of the Nb 4d conduction band32 and the O 2p orbitals hybridized by Bi 6s in the valence band.33,34 Moreover, depending on the Bi/Nb ratio, these compounds can adopt complex crystal structures, such as pyrochlore, layered perovskite or fluorite related structures, and thus exhibit different dielectric properties.35 Although previous studies have reported the ferroelectricity of bismuth-containing niobates with layered perovskite structures, the effect of their polarization on photocatalysis has been barely studied.36
Loading a co-catalyst onto a ferroelectric material has been proved to be an effective strategy to further boost the photocatalytic efficiency.37–39 The co-catalyst not only provides catalytic sites, but also suppresses the recombination of photo-induced charge carriers in the photocatalyst.40,41 The most popular co-catalysts are noble metals, such as Au, Pt, Rh, and Ru. However, their high cost and scarcity hamper large-scale commercial applications, and thus the development of earth-abundant and inexpensive alternatives is essential. Both tungsten (W) and carbon (C) are earth-abundant, while tungsten carbide (WC) exhibits high electronic conductivity and has Pt-like d-band electronic density states.42 Since Levy and Boudart's first report on WC in the catalysis of hydrogenolysis,43 it has been intensively studied as an electrocatalyst support for methanol oxidation, oxygen reduction, nitrophenol oxidation and hydrogen evolution.44,45 Hence, it is extremely desirable to prepare nanocomposites of ferroelectric photocatalyst particles with WC to enhance the photocatalytic activity. An intimate interfacial contact between WC and ferroelectric particles maximizes the reaction active sites and facilitates the charge transfer between the different phases. Different processing routes have been employed to produce WC particles,46,47 such as direct carburization of tungsten powder, solid-state metathesis, reduction carburization,48 mechanical grinding,49 thermal pyrolysis of tungsten-containing organic acid,47 polymeric precursor routes using metal alkoxides, hydrothermal methods,50etc. However, these processing routes are limited due to complicated apparatus and procedures, long processing times, high temperatures, and high-energy consumption.51 Depending on the processing conditions, the obtained nanocomposites can have an inhomogeneous distribution of constituent phases, uncontrollable size, and weak interactions between the semiconductor and WC phases.52 Thus, the efficient production of WC containing nanocomposites using low cost approaches remains a scientific and technological challenge.
Traditionally, the ball-milling method is used to prepare sub-micron sized particles, and high energy ball milling can be used to prepare nano-sized particles. Using the high energy ball milling approach, the ball milled material, for instance, the photocatalyst, could be coated with a secondary or foreign material which can be introduced from both the milling balls and milling jar. In this study, two Rb- and Bi-containing niobates, ferroelectric RbBi2Ti2NbO10 (ref. 53) and non-ferroelectric RbBi2Nb5O16 (ref. 54) materials, were prepared to investigate the impact of ferroelectric polarization on photocatalytic properties. To enhance the photocatalytic performance of ferroelectric RbBi2Ti2NbO10, a WC co-catalyst was loaded onto the RbBi2Ti2NbO10 nanopowder by a facile one-step high-energy ball milling processing technique.
The ferroelectric RbBi2Ti2NbO10 has a larger bandgap (3.25 eV) with a lower specific surface area (7.72 m2 g−1) compared to the values (3.02 eV and 13.85 m2 g−1) of non-ferroelectric RbBi2Nb5O16. However, the decolorization rate for RbBi2Ti2NbO10 was 5 times greater than for RbBi2Nb5O16. The prepared WC/RbBi2Ti2NbO10 nanocomposite, produced using a facile route in only 40 min, showed over 30 times higher photocatalytic activity than the non-ferroelectric RbBi2Nb5O16. Overall, this work demonstrates a novel approach to produce noble-metal-free co-catalyst loaded nanocomposites in an efficient and ultra-fast way for photocatalytic applications.
2. Results and discussion
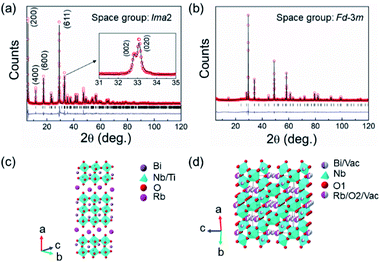
Fig. 1a and b show the fitted room temperature X-ray diffraction (XRD) patterns of the calcined RbBi2Ti2NbO10 and RbBi2Nb5O16 powders. These two materials appear to be single phases with no detectable impurities. The atomic coordinates for RbBi2Ti2NbO10 and RbBi2Nb5O16 as reported by Kim et al.53 and Ehlert et al.54 were selected as the initial models for refinement. RbBi2Ti2NbO10 is a 3-layer Dion–Jacobson phase, with orthorhombic symmetry and a polar space group of Ima2.53 The peak splitting of (002) and (020) planes is related to the orthorhombic ferroelectric lattice distortion (Fig. 1a inset). RbBi2Nb5O16 has a cubic defect pyrochlore structure with a non-polar space group of Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. Fig. 1c and d show the schematic crystal structures of the studied compounds. RbBi2Ti2NbO10 consists of 3 layers of corner sharing Ti/NbO6 octahedra with Bi3+ cations in the A-sites. These perovskite blocks are separated by a layer of Rb+ cations. On the other hand, RbBi2Nb5O16 consists of a 3-dimensional network of corner sharing NbO6 octahedra. While Bi3+ cations sit at the normal 8-coordinate pyrochlore sites (the actual coordination is lowered due to the partial occupancy of one of the oxygen sites), the Rb+ cations are located in oxygen vacancies allowing these cations to adopt an octahedral geometry with oxygen. The fitting parameters and unit cell parameters for the samples are summarized in Table 1.
m. Fig. 1c and d show the schematic crystal structures of the studied compounds. RbBi2Ti2NbO10 consists of 3 layers of corner sharing Ti/NbO6 octahedra with Bi3+ cations in the A-sites. These perovskite blocks are separated by a layer of Rb+ cations. On the other hand, RbBi2Nb5O16 consists of a 3-dimensional network of corner sharing NbO6 octahedra. While Bi3+ cations sit at the normal 8-coordinate pyrochlore sites (the actual coordination is lowered due to the partial occupancy of one of the oxygen sites), the Rb+ cations are located in oxygen vacancies allowing these cations to adopt an octahedral geometry with oxygen. The fitting parameters and unit cell parameters for the samples are summarized in Table 1.
| Composition | Crystal parameters | Refinement parameters |
|---|---|---|
| RbBi2Ti2NbO10 | Orthorhombic (Ima2) | χ 2 = 3.92 |
| a = 30.5499 (1) Å | R wp = 0.0987 | |
| b = 5.4255 (3) Å | R p = 0.072 | |
| c = 5.4656 (3) Å | R ex = 0.0502 | |
| V = 905.9 (1) Å3 | R F2 = 0.1292 | |
| RbBi2Nb5O16 | Cubic (Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) m) |
χ 2 = 4.286 |
| a = 10.5268 (1) Å | R wp = 0.1032 | |
| V = 1166.52 (1) Å3 | R p = 0.08 | |
| R ex = 0.0500 | ||
| R F2 = 0.1003 |
The relative densities of the sintered RbBi2Ti2NbO10 and RbBi2Nb5O16 ceramics were 92% and 95%. The morphological characterization of RbBi2Ti2NbO10 ceramic shows plate-like grains (Fig. S1a†), whereas RbBi2Nb5O16 ceramic was observed to have densely arranged grains in a polygonal pattern (Fig. S1b†). The temperature dependence of the dielectric permittivity and loss of RbBi2Ti2NbO10 ceramic is presented for the first time (Fig. 2a). A frequency independent peak is observed at around 506 °C in the dielectric permittivity plots, implying that RbBi2Ti2NbO10 is ferroelectric with a Curie point (Tc) at this temperature. The loss peak appears a few degrees below Tc, which has been attributed to ferroelectric domain wall movement.55 Although ferroelectric polarization–electric field loops have been reported,53 there was no evidence to show domain switching in the loops. The published loops show a dominant contribution from conductivity and the highest polarization was less than 0.3 μC cm−1. It is considered a requirement of ferroelectricity to have a measured switchable polarization loop. As such our measured loops are the first definitive evidence of ferroelectricity in the system.
The current–electric field (I–E) and displacement–electric field (D–E) loops of RbBi2Ti2NbO10 were first measured at room temperature at a frequency of 20 Hz. At this temperature, ferroelectric domain switching was not observed due to a large coercive field. The measurement was then carried out at 100 °C at the same frequency to facilitate domain switching at a reduced coercive field, as shown in Fig. 2b. The current peaks associated with ferroelectric domain switching are clearly observed in the I–E loop.56,57 Moreover, when the RbBi2Ti2NbO10 ceramics were poled at 100 °C, a piezoelectric constant (d33) value of 14.7 ± 0.2 pC N−1 was detected. All these dielectric, ferroelectric, and piezoelectric property results provide powerful evidence for the existence of ferroelectricity in RbBi2Ti2NbO10. Similarly, I–E and D–E loops were tested for RbBi2Nb5O16 at room temperature and 20 Hz (Fig. 2c). In contrast to RbBi2Ti2NbO10, no current peaks were observed in the I–E loop of RbBi2Nb5O16 ceramic, suggesting that this material has linear dielectric behaviour.56,57 This is consistent with its non-polar structure.
The as-calcined RbBi2Ti2NbO10 and RbBi2Nb5O16 powders were first milled in nylon milling jars at 180 rpm for 24 h to obtain sub-micron sized powders (Fig. S2†). The sub-micron sized RbBi2Ti2NbO10 powder is ferroelectric in nature, as confirmed by the PFM images shown in Fig. S3.† The specific surface areas of the sub-micron sized RbBi2Ti2NbO10 and RbBi2Nb5O16 powders were 7.72 and 13.85 m2 g−1 (Table 2). The light absorption properties of both powders were characterized by UV-Vis spectroscopy (Fig. 3a). It is assumed that RbBi2Ti2NbO10 and RbBi2Nb5O16 are direct band gap materials, based on an analogy with the 2-layer Dion–Jacobson phase CsBi1−xEuxNb2O7.58 The band gaps (Eg) of RbBi2Ti2NbO10 and RbBi2Nb5O16, which were calculated by using the Tauc equation,59 are 3.25 eV and 3.02 eV (Fig. 3b).
| Catalyst | RbBi2Ti2NbO10 | RbBi2Nb5O16 |
|---|---|---|
| Band gap (eV) | 3.25 | 3.02 |
| Specific surface area (m2 g−1) | 7.72 | 13.85 |
| Decolorization rate (min−1) | 3.8 × 10−3 | 7.4 × 10−4 |
The photocatalytic activity of the RbBi2Ti2NbO10 and RbBi2Nb5O16 powders was tested through the photodecomposition of RhB and the degradation profiles are shown in Fig. 3c. It can be seen that the ferroelectric RbBi2Ti2NbO10 degrades about 60% of the dye within 4 h, whereas the non-ferroelectric RbBi2Nb5O16 degrades about 12% over the same time period. The decolorization rate was calculated according to the following equation:
It was reported by Levy and Boudart43 that WC has platinum-like behaviour and facilitates redox reactions. In order to study the effect of WC on the photocatalytic activity of the RbBi2Ti2NbO10 material, the calcined RbBi2Ti2NbO10 powder was ball milled at a higher rotation speed of 600 rpm for 40 min using the same ZrO2 milling balls. For this control experiment, two different types of milling jars, made of ZrO2 and WC, were used in an attempt to introduce WC into the surface of RbBi2Ti2NbO10 powder. The ZrO2 jar was used for comparison. The XRD patterns of the ball milled powders are shown in Fig. S4.† Diffraction peaks for both RbBi2Ti2NbO10 and ZrO2 are present, which indicates the introduction of ZrO2 from the milling balls into the RbBi2Ti2NbO10 powder due to intense collision between the milling balls/jar and the ceramic powder. The photocatalytic degradation of RhB under simulated solar light irradiation using these two composite powders was studied and the results are shown in Fig. S5† and Table 3. Although the composite powder ball milled in the ZrO2 jar has a larger specific surface area (Table 3), a lower photodegradation rate was observed. The composite prepared in the WC jar shows almost 100% dye degradation within 3 h, whereas the composite prepared in the ZrO2 jar shows about 90% dye degradation in 4 h. Considering that the composite powders prepared in the WC jar and ZrO2 jar have similar specific surface areas, the enhanced photocatalytic activity of the composite powder made in the WC jar is attributed to the contribution from WC. The control experimental results indicated a route to optimize the preparation conditions for further enhancing the photocatalytic activity of RbBi2Ti2NbO10. As such, the calcined RbBi2Ti2NbO10 powder was ball milled in a WC jar under different rotation speeds and durations, details of which are listed in Table S1.† The XRD patterns of the powder are shown in Fig. S6.† With increasing rotation speed and ball milling time, the diffraction peaks of the powders became broader, indicative of the decreasing particle size of the powder. The photodegradation of RhB was carried out to characterize the photocatalytic behaviour of the obtained powders (Fig. S7†). The ball milling parameters, specific surface areas, and photodegradation rate of RhB of the obtained nanocomposites are shown in Table S1.† For an easier comparison, the powder ball milled in a WC jar at 600 rpm for 40 min (Table 3) is also shown in Table S1.† It is clear that at 600 rpm, the degradation rate of RhB firstly increases and then decreases with increasing ball-milling time. The initial increase in the photodegradation rate is attributed to the increased specific surface area; however, further reduction in the particle size reduced the photocatalytic activity. This may be due to a blocking effect associated with the ZrO2 from the milling balls and/or decreased polarization in powders with a smaller particle size, which is discussed below. The best photocatalytic activity was observed with a ball milling speed of 800 rpm and a 40 min milling time.
| Jar type | Specific surface area (m2 g−1) | Degradation rate (min−1) |
|---|---|---|
| WC | 19.47 | 0.016 |
| ZrO2 | 21.99 | 0.009 |
The role of WC in the nanocomposite prepared under these conditions was studied in detail. The obtained nanocomposite consisted of three nanocrystalline phases (RbBi2Ti2NbO10, ZrO2, and WC), but as discussed below, ZrO2 gives no contribution to the photocatalytic response under the irradiation conditions used here. Hence, we use the term “WC/RbBi2Ti2NbO10 nanocomposite” for simplicity. Compared with the sub-micron sized powder, the width of the RbBi2Ti2NbO10 diffraction peaks in the nanocomposite is broader, indicating that the average crystallite size is smaller. The introduction of WC from the WC milling jar into RbBi2Ti2NbO10 could not be confirmed by XRD due to the lack of detectable diffraction peaks (Fig. S6†). Fig. 4a shows detail of the (002) and (020) peaks in the XRD patterns of sub-micron sized RbBi2Ti2NbO10 and WC/RbBi2Ti2NbO10 nanocomposites. These peaks were modelled using a simple pseudo-Voigt peak shape. The peak splitting is clearly discernible in the sub-micron sized sample and less clear in the nanocomposite due to the peak broadening. Nevertheless, asymmetry in the distribution is obvious and the two peaks were successfully fitted indicating that the ferroelectric distortion is maintained, albeit slightly reduced in magnitude in the nanocomposite. The spontaneous polarization Ps in RbBi2Ti2NbO10 depends on the extent of orthorhombic distortion, which can be quantified from the orthorhombicity, (c − b)/b, where b and c are lattice parameters.
According to the fitting results, the orthorhombicity values for sub-micron sized RbBi2Ti2NbO10 and WC/RbBi2Ti2NbO10 nanocomposites are 0.74% and 0.46%, respectively. The smaller orthorhombicity value in the WC/RbBi2Ti2NbO10 nanocomposite suggests that the Ps is reduced compared to that in the sub-micron sized powder.24,63 This can be attributed to the size effect on the ferroelectric domain structure.64,65 When the sizes of ferroelectric particles/grains are in the micrometre range, domain walls are generated to minimize the internal stresses induced by phase transition from the paraelectric to the ferroelectric phase at the Curie point. In this case, Ps and orthorhombicity are insensitive to changes in the particle/grain size. When the particle/grain size of ferroelectric powders is reduced to the nanometre range, the orthorhombic distortion reduces. Therefore, there is no necessity for domain wall formation to minimize the internal stresses, which are already reduced as the Ps is decreased.66,67
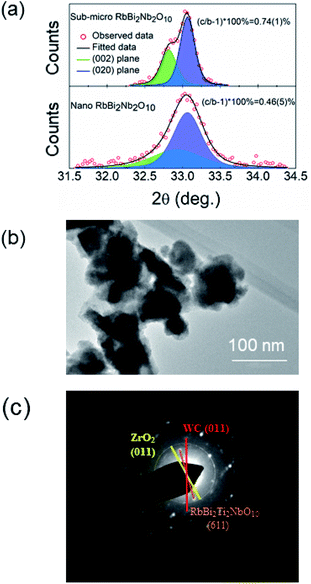
Fig. 4b shows a TEM image of the WC/RbBi2Ti2NbO10 nanocomposite. Granular powders with an average particle size of ca. 80 nm can be observed. The specific surface area determined by the BET method was 19.77 m2 g−1 (Table S1†). The selected area electron diffraction (SAED) image (Fig. 4c) shows the diffraction spots from the (611) plane of RbBi2Ti2NbO10. Although ZrO2 and WC particles were not noticeable from the TEM images, the diffraction spots from the (011) plane of WC68 and the (011) plane of ZrO2 (ref. 69) are visible. This confirms the existence of WC and ZrO2 nanoparticles within the nanocomposite.
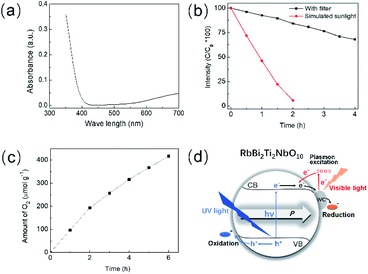
UV absorption (Fig. 5a) and valence band X-ray photoelectron spectroscopy (XPS) (Fig. S9†) were used to determine the band structure of the WC/RbBi2Ti2NbO10 nanocomposite. ZrO2 was expected to have no contribution to the photocatalytic response under simulated solar irradiation with a wavelength longer than 280 nm due to its large band gap (∼5.7 eV)70 and therefore the discussion below is focused on the photocatalytically active components. The WC/RbBi2Ti2NbO10 nanocomposite not only showed strong absorption at wavelengths shorter than 400 nm, but also showed weak but broad absorption in the longer wavelength range (Fig. 5a), which indicates the absorption of visible light. The band gap for the WC/RbBi2Ti2NbO10 nanocomposite was calculated to be 3.25 eV (Fig. S8†), confirming that the powder particle size reduction in RbBi2Ti2NbO10 does not alter the band gap value. The valence band XPS spectrum in Fig. S9a† confirms the metal like behaviour of WC.71 By comparing Fig. S9a and b,† it is concluded that the Fermi level of WC is lower than the conduction band level of RbBi2Ti2NbO10 (Fig. S9c†). Morphological characterization via TEM reveals that the WC nanoparticles were homogeneously distributed within the WC/RbBi2Ti2NbO10 nanocomposite (Fig. S10†).
The photodegradation of RhB using the WC/RbBi2Ti2NbO10 nanocomposite under simulated sun light and visible light irradiations was investigated, and the results are shown in Fig. 5b. Almost 100% of the RhB dye was degraded at a rate of 2.3 × 10−2 min−1 (Table 4) within 2 hours while irradiated using simulated sunlight, which is 6 times higher than that with sub-micron sized RbBi2Ti2NbO10 and over 30 times higher than that with non-ferroelectric RbBi2Nb5O16. As discussed above, the driving force for charge carrier separation in RbBi2Ti2NbO10 powders would reduce with decreasing particle size down to the nanoscale owing to the reduced orthorhombicity and Ps. The size reduction of RbBi2Ti2NbO10 is expected to compromise the photocatalytic activity to some extent. However, the positive contribution from the greater specific surface area of the ferroelectric nanoparticles, the efficient charge separation and transport by the WC incorporation and possibly severe plastic deformation caused by high-speed ball milling72 overwhelm the decreased ferroelectric contribution in the WC/RbBi2Ti2NbO10 nanocomposite and contribute to its enhanced photocatalytic activity.
| Photocatalyst | Band gap/eV | Catalytic conditions | Catalytic application | Catalytic activity | Ref. |
|---|---|---|---|---|---|
| KNbO3 nanowire | 3.25 | UV light | RhB degradation | K obs = 4.2 × 10−3 min−1 | 77 |
| Au/KNbO3 nanowire | 3.81 | UV light | RhB degradation | K obs = 1.6 × 10−2 min−1 | 78 |
| Bi2WO6 nanoparticle | 2.9 | Visible light | RhB degradation | K obs = 1.2 × 10−2 min−1 | 79 |
| Ag/Bi2WO6 nanoparticle | 2.9 | Visible light | RhB degradation | K obs = 3.3 × 10−2 min−1 | 79 |
| Ag/Bi4Ti3O12 | 2.9 | UV light | RhB degradation | K obs = 1.3 × 10−2 min−1 | 80 |
| Pt/Bi4Ti3O12 | 2.9 | UV light | RhB degradation | K obs = 1.5 × 10−2 min−1 | 80 |
| Ag/BaBi4Ti4O15 | 3.2 | Simulated solar light | RhB degradation | Complete within 3.5 h | 81 |
| Ag/BaBi2Nb2O9 | 3.2 | Simulated solar light | RhB degradation | Complete within 3 h | 82 |
| Ag/Ca2Bi4Ti5O18 | 3.2 | Simulated solar light | RhB degradation | 50% degradation within 4 h | 83 |
| Ag/RbBiNb2O7 nanosheet | 3.45 | UV-light | RhB degradation | K obs = 8.7 × 10−3 min−1 | 84 |
| Ag/Ba0.8Sr0.2TiO3 | 3.24 | Simulated solar light | RhB degradation | K obs = 0.18 min−1 | 67 |
| WC/RbBi2Ti2NbO10 | 3.25 | Simulated solar light | RhB degradation | K obs = 2.3 × 10−2 min−1 | This work |
| Pt/LiNbO3 nanowire | — | UV-light | O2 evolution | 19 μmol g−1 h−1 | 85 |
| WC/RbBi2Ti2NbO10 | 3.25 | Simulated solar light | O2 evolution | 68.5 μmol g−1 h−1 | This work |
To prove the above claim and determine the role of WC, we further characterized the nanocomposite of WC/RbBi2Ti2NbO10. Under visible light irradiation, the WC/RbBi2Ti2NbO10 nanocomposite photo-catalytically degrades 32% of RhB within 4 h (Fig. 5b), whereas sub-micron sized RbBi2Ti2NbO10 shows no photocatalytic activity. These results suggest that the observed photocatalytic activity under visible light is originated from the WC. It is worth noting that the nanoparticles of WC show surface plasmon properties.73 Various plasmonic properties such as plasmonic scattering, near-field coupling, and hot electrons have been reported for photocatalysis.74,75 However, in the present case, we expect that the plasmonic hot electrons generated from the WC nanoparticles may transfer to the RhB molecule directly to degrade or enter into the conduction band (CB) of RbBi2Ti2NbO10, which later reduces the RhB molecule. The broad visible absorption peak observed for WC/RbBi2Ti2NbO10 (Fig. 5a), in the range of 500 nm to 700 nm, is attributed to the plasmonic WC particles with various sizes.73,76
To verify the stability of the material, 3 cycles of RhB degradation were performed under simulated sunlight (Fig. S11†) and no significant changes were observed, suggesting that the WC/RbBi2Ti2NbO10 nanocomposite is stable enough under the present experimental conditions.
The photocatalytic water oxidation activity of the WC/RbBi2Ti2NbO10 nanocomposite was studied in the presence of Ag as an electron scavenger under full arc (similar to solar light) conditions. A significant O2 evolution was observed at pH 7.0 with an average gas evolution rate of 68.5 μmol h−1 g−1 (Fig. 5c). In the absence of a photocatalyst as well as in the absence of an electron scavenger, no O2 evolution was observed, in addition to no activity under dark conditions. It can be seen from Fig. 5c that the rate of O2 evolution was higher during the early hours of the reaction, which may be due to the prearrangements of the photocatalytic system to produce O2 with a steady rate after 3 h of irradiation. It exhibits an apparent quantum yield (AQY) of 3% at 420 nm. This value is significant because the photocatalytic water oxidation using the present ferroelectric material is relatively new and competitive to conventional water oxidation photocatalysis. These results are encouraging for the use of such ferroelectric materials in solar-driven photocatalysis.
Fig. 5d schematically describes the redox reaction on the surface of the WC/RbBi2Ti2NbO10 nanocomposite. Under UV light, electrons produced from RbBi2Ti2NbO10 participate in the degradation reactions with WC acting as a cocatalyst to enhance charge carrier separation and transfer. In addition, it is expected that the Fermi energy of WC with Pt-like electronic features would be lower than the conduction band of RbBi2Ti2NbO10.76 The plasmonic hot electrons generated in the WC nanoparticles contribute to photodegradation in the visible light range. The approach used here for enhanced semiconductor visible light photocatalysis using nano-sized WC could be applied to a broad range of semiconductor/co-catalyst materials for high performance photocatalysis.
Although the ferroelectric RbBi2Ti2NbO10 material has a larger band gap, it can be used for photocatalytic applications using the present facile one step high speed ball milling method. Table 4 lists the photocatalytic properties of ferroelectric photocatalysts. The photodegradation rate of RhB and the O2 production rate are much higher than those of reported ferroelectric photocatalysts.
3. Experimental
RbBi2Ti2NbO10 and RbBi2Nb5O16 powders were synthesized by a conventional solid-state reaction. Rb2CO3 (99.8%), Bi2O3 (99.9%), Nb2O5 (99.5%), and TiO2 (99%) powders were mixed in a stoichiometric ratio (with 4 wt% excess of Rb2CO3) by planetary ball milling (Fritsch Pulverisette 6) in ethanol using nylon milling jars and ZrO2 balls with a diameter of 10 mm for 4 h at a speed of 180 rpm. The resulting slurry was dried overnight at 80 °C. The dried powders were calcined at 920 °C for 4 h for RbBi2Ti2NbO10 and 980 °C for 4 h for RbBi2Nb5O16 followed by dry grinding using an agate mortar and pestle.The calcination and grinding processes were carried out three times. Calcination at high temperature resulted in an agglomeration of powders. In order to reduce the agglomerate size and to facilitate subsequent sintering, the calcined powders were re-milled by planetary ball milling in ethanol for 4 h. The resulting powders were pressed into cylindrical discs of 10 mm diameter and ca. 2 mm thickness, with poly (ethylene glycol) (PEG) as a binder, at 200 MPa. The pressed pellets were sintered at 950 °C for RbBi2Ti2NbO10 and 1050 °C for RbBi2Nb5O16 for 2 h in air. The surfaces of the sintered ceramics were polished and coated with Pt paste for dielectric measurements and Ag paste for ferroelectric and piezoelectric measurements.
For photocatalytic studies, the calcined RbBi2Ti2NbO10 and RbBi2Nb5O16 powders were milled in ethanol using ZrO2 milling balls with a diameter of 10 mm in nylon milling jars at a rotation speed of 180 rpm for 24 h, producing sub-micron sized powders. In order to investigate the effect of WC on the photocatalytic performance of RbBi2Ti2NbO10, control experiments, which were designed to load the RbBi2Ti2NbO10 powder with WC using high energy ball milling, were carried out. The high energy ball milling machine used is a Fritsch Premium line P7 planetary ball milling machine. The difference in the two parallel control experiments is the milling jars, with one experiment using a WC jar and the other using a ZrO2 jar for comparison. The same amount of calcined RbBi2Ti2NbO10 powder was loaded into the milling jar and ball milled at a speed of 600 rpm for 40 min. The milling medium is DI water and the milling balls were 1 mm ZrO2 balls. Although attempts were made to use WC milling balls in the WC jar to get a better composition, they were not successful due to the limited commercial availability of small diameter WC milling balls (high rotation speed and hard milling balls with a small diameter are required for the production of nanopowders via planetary ball milling86). Moreover, to optimize the properties of WC/RbBi2Ti2NbO10 composite powder, the RbBi2Ti2NbO10 powder was high energy ball milled in a WC jar for different durations (20 min and 120 min) and different rotation speeds (800 rpm).
Room temperature X-ray powder diffraction measurements were performed on a PANalytical X'Pert Pro diffractometer using Ni filtered Cu-Kα radiation (λ = 1.5418 Å). Data were collected in flat plate Bragg–Brentano geometry over the 2θ range of 5 to 120° in steps of 0.033°, with a count time of 200 s per step. Structure refinement was carried out by Rietveld analysis using the GSAS suite of programs.87 The morphologies and microstructures of the samples were observed using scanning electron microscopy (FEI Inspect-F) and transmission electron microscopy [TEM, FEI Titan Themis (200 kV)], coupled with energy dispersive X-ray spectroscopy (EDX). Piezoresponse Force Microscopy (PFM) (NT-MDT, Ntegra systems, Russia) was used to characterize the domain structure. The specific surface area of the powders was determined using the Brunauer–Emmett–Teller (BET) (Gemini VII) method. The temperature dependencies of dielectric permittivity and loss were measured using an LCR meter (Agilent Technologies Ltd, 4284A, Kobe, Hyogo, Japan) connected to a furnace. The current–electric field (I–E) and displacement–electric field (D–E) loops were measured at selected temperatures using a ferroelectric hysteresis measurement tester (NPL, UK) at 20 Hz. A triangular voltage waveform was used. For piezoelectric measurements, ceramic samples were immersed in a silicone oil bath and poled under a DC field of 130 kV cm−1 at 100 °C. The piezoelectric coefficient, d33, was measured on a quasi-static d33 meter (CAS, ZJ-3B) at room temperature.
The band structures of WC and RbBi2Ti2NbO10 were measured using valence band X-ray photoelectron spectroscopy (Nexsa, XPS system). The photocatalytic activity of the samples was evaluated by photodegradation of Rhodamine B (RhB) under simulated sunlight with wavelengths longer than 280 nm and visible light using an AM 1.5 filter. 150 mg of catalyst was added to 50 ml of aqueous RhB solution (10 ppm). The suspension was stirred continuously in the dark for 0.5 h before it was exposed to a solar simulator (Newport, class ABB). Under irradiation, 2 ml of solution was collected at intervals of 30 min. The ceramic particles were removed from the solution using a centrifuge before testing the change in the concentration of RhB by recording the absorbance at 554 nm using a UV-vis spectrophotometer. Recyclability experiments were carried out to test the stability of the WC/RbBi2Ti2NbO10 nanocomposite. The quantity of the photocatalyst and RhB solution used was the same as that in previous photodegradation experiments. After 1.5 hours of the first photocatalytic test, the WC/RbBi2Ti2NbO10 nanocomposite was washed and centrifuged three times for a second 1.5 hour cycle. This procedure was repeated a third time before being centrifuged and measuring the light absorbance.
Photocatalytic water splitting was carried out in a custom-made glass batch reactor with a quartz top window. 30 mg of the photocatalyst was dispersed in 70 ml of DI water containing 10 mM AgNO3 using ultrasonication for 15 min. The reactor was sealed and purged with high purity argon gas for 1 h to remove the air/dissolved oxygen in the solution and headspace. The reactor was irradiated using a 300 W Xe lamp (Newport, USA). The reactor was placed in a water bath during irradiation to eliminate the influence of heat. The production of O2 gas was quantified at regular intervals using a gas chromatograph (Varian 430-GC, TCD, and argon carrier gas) equipped with a molecular 5A column using Ar as the carrier gas. The apparent quantum yield (AQY) was determined by performing photocatalytic experiments using a 420 nm bandpass filter (λ ± 10 nm at 10% of peak height, Comar Optics). The AQY was calculated using the following equation:
4. Conclusions
In summary, we have successfully demonstrated a ferroelectric material, RbBi2Ti2NbO10, for enhancing photocatalytic RhB degradation compared to non-ferroelectric RbBi2Nb5O16. The observed higher activity is due to the effective charge carrier separation by the ferroelectric polarization induced internal field. Furthermore, the photocatalytic activity of RbBi2Ti2NbO10 is increased significantly by incorporating WC, which also extended the activity to the visible range. The WC/RbBi2Ti2NbO10 nanocomposite shows a 6 times higher RhB degradation rate under simulated solar light than the sub-micron sized RbBi2Ti2NbO10. This is attributed to the greater specific surface area, and the better charge carrier separation by the WC co-catalyst. The visible light photocatalytic activity of the WC/RbBi2Ti2NbO10 nanocomposite is due to plasmonic hot electron generation from WC. More importantly, the present nanocomposite shows water oxidation activity suggesting that it can be used for solar fuel synthesis applications. Our promising results indicate the feasibility to use the earth abundant WC as a competitive co-catalyst to replace expensive noble metals. The novel approach for the incorporation of WC into semiconductor materials is a facile and low-cost way of producing noble-metal-free photocatalyst composites, and the strategy could be readily adapted to other classes of materials for a variety of applications.Author contributions
M. Zhang: conceptualization, data curation, formal Analysis, investigation, methodology, validation, visualization, and writing – original draft; Y. Wang: data curation, formal analysis, investigation, methodology, and writing – review & editing; J. Liu: data curation, formal analysis, and writing – review & editing; M. Thangamuthu: data curation, formal analysis, and writing – review & editing; Y. Yue: data curation, formal analysis, and writing – review & editing; Z. Yan: data curation, formal analysis, and writing – review & editing; J. Feng: data curation, formal analysis, and writing – review & editing; D. Zhang: supervision, funding acquisition, and writing – review & editing; H. Zhang: data curation, formal analysis, supervision, and writing – review & editing; S. Guan: data curation, formal analysis, and writing – review & editing; M. Titirici: data curation, formal analysis, and writing – review & editing; I. Abrahams: formal analysis, supervision, and writing – review & editing; J. Tang: data curation, formal analysis, and writing – review & editing; Z. Zhang: data curation, formal analysis, and writing – review & editing; S. Dunn: conceptualization, formal analysis, investigation, methodology, supervision, and writing – review & editing; H. Yan: conceptualization, formal analysis, investigation, methodology, funding acquisition, supervision, and writing – review & editing.Conflicts of interest
The authors declare no competing interests.Acknowledgements
The authors are grateful for financial support from the China Scholarship Council (CSC, 201706370172, and 201608060162) and Guangzhou Guangding Technology Group Co. Ltd. Dr Yaqiong Wang was partly funded by London South Bank University. The X-ray photoelectron (XPS) data collection was performed at the EPSRC National Facility for XPS (“HarwellXPS”), operated by Cardiff University and UCL, under Contract No. PR16195.Notes and references
- H. Tong, S. Ouyang, Y. Bi, N. Umezawa, M. Oshikiri and J. Ye, Adv. Mater., 2012, 24, 229–251 CrossRef CAS.
- C. Y. Toe, C. Tsounis, J. Zhang, H. Masood, D. Gunawan, J. Scott and R. Amal, Energy Environ. Sci., 2021, 14, 1140–1175 RSC.
- S. C. Shit, I. Shown, R. Paul, K. H. Chen, J. Mondal and L. C. Chen, Nanoscale, 2020, 12, 23301–23332 RSC.
- J. Zheng, H. Zhou, Y. Zou, R. Wang, Y. Lyu, S. P. Jiang and S. Wang, Energy Environ. Sci., 2019, 12, 2345–2374 RSC.
- C. Shifu, C. Lei, G. Shen and C. Gengyu, Mater. Chem. Phys., 2006, 98, 116–120 CrossRef.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269–271 CrossRef CAS PubMed.
- P. M. Rao, L. Cai, C. Liu, I. S. Cho, C. H. Lee, J. M. Weisse, P. Yang and X. Zheng, Nano Lett., 2014, 14, 1099–1105 CrossRef CAS PubMed.
- Q. Wu, F. Huang, M. Zhao, J. Xu, J. Zhou and Y. Wang, Nano Energy, 2016, 24, 63–71 CrossRef CAS.
- S. Sun, X. Yu, Q. Yang, Z. Yang and S. Liang, Nanoscale Adv., 2019, 1, 34–63 RSC.
- R. Macquart, B. J. Kennedy, T. Kamiyama and F. Izumi, J. Phys. Condens. Matter., 2004, 16, 5443 CrossRef CAS.
- Z. Xie, X. Liu, W. Wang, X. Wang, C. Liu, Q. Xie, Z. Li and Z. Zhang, Nano Energy, 2015, 11, 400–408 CrossRef CAS.
- Q. Sun, N. Wang, J. Yu and J. C. Yu, Adv. Mater., 2018, 30, 1–7 Search PubMed.
- Z. Zhang, Q. Qian, B. Li and K. J. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 17419–17426 CrossRef CAS PubMed.
- L. Cheng, Q. Xiang, Y. Liao and H. Zhang, Energy Environ. Sci., 2018, 11, 1362–1391 RSC.
- X. Li, J. Yu and M. Jaroniec, Chem. Soc. Rev., 2016, 45, 2603–2636 RSC.
- J. Li, L. Cai, J. Shang, Y. Yu and L. Zhang, Adv. Mater., 2016, 28, 4059–4064 CrossRef CAS PubMed.
- H. Lei, M. Wu, Y. Liu, F. Mo, J. Chen, S. Ji, Y. Zou and X. Dong, Chinese Chem. Lett., 2021, 32, 2317–2321 CrossRef CAS.
- M. Wang, B. Wang, F. Huang and Z. Lin, Angew. Chem., Int. Ed., 2019, 58, 7526–7536 CrossRef CAS PubMed.
- X. Huang, K. Wang, Y. Wang, B. Wang, L. Zhang, F. Gao, Y. Zhao, W. Feng, S. Zhang and P. Liu, Appl. Catal., B, 2018, 227, 322–329 CrossRef CAS.
- B. Dai, Y. Yu, Y. Chen, H. Huang, C. Lu, J. Kou, Y. Zhao and Z. Xu, Adv. Funct. Mater., 2019, 29, 1–9 Search PubMed.
- B. Dai, Y. Chen, S. M. Hao, H. Huang, J. Kou, C. Lu, Z. Lin and Z. Xu, J. Phys. Chem. Lett., 2020, 11, 7407–7416 CrossRef CAS.
- B. Dai, J. Fang, Y. Yu, M. Sun, H. Huang, C. Lu, J. Kou, Y. Zhao and Z. Xu, Adv. Mater., 2020, 32, 1–9 Search PubMed.
- R. Su, Y. Shen, L. Li, D. Zhang, G. Yang, C. Gao and Y. Yang, Small, 2015, 11, 202–207 CrossRef CAS PubMed.
- L. Li, P. A. Salvador and G. S. Rohrer, Nanoscale, 2014, 6, 24–42 RSC.
- M. R. Morris, S. R. Pendlebury, J. Hong, S. Dunn and J. R. Durrant, Adv. Mater., 2016, 28, 7123–7128 CrossRef CAS PubMed.
- J. Malik, S. Kumar, P. Srivastava, M. Bag and T. K. Mandal, Mater. Adv., 2021, 2, 4832–4842 RSC.
- H. T. Yi, T. Choi, S. G. Choi, Y. S. Oh and S. W. Cheong, Adv. Mater., 2011, 23, 3403–3407 CrossRef CAS.
- J. L. Giocondi and G. S. Rohrer, J. Phys. Chem. B, 2001, 105, 8275–8277 CrossRef CAS.
- Y. Inoue, K. Sato and K. Sato, J. Chem. Soc., Faraday Trans. 1, 1989, 85, 1765–1774 RSC.
- J. Chen, H. Lu, H. J. Liu, Y. H. Chu, S. Dunn, K. Ostrikov, A. Gruverman and N. Valanoor, Appl. Phys. Lett., 2013, 102, 1–5 Search PubMed.
- H. G. Kim, D. W. Hwang and J. S. Lee, J. Am. Chem. Soc., 2004, 126, 8912–8913 CrossRef CAS PubMed.
- L. Wang, W. Wang, M. Shang, S. Sun, W. Yin, J. Ren and J. Zhou, J. Mater. Chem., 2010, 20, 8405–8410 RSC.
- A. Kudo, K. Ueda, H. Kato and I. Mikami, Catal. Lett., 1998, 53, 229–230 CrossRef CAS.
- A. Kudo, K. Omori and H. Kato, J. Am. Chem. Soc., 1999, 121, 11459–11467 CrossRef CAS.
- W. Zhou, D. A. Jefferson and J. M. Thomas, J. Solid State Chem., 1987, 70, 129–136 CrossRef CAS.
- C. Chen, H. Ning, S. Lepadatu, M. Cain, H. Yan and M. J. Reece, J. Mater. Chem. C, 2015, 3, 19–22 RSC.
- I. M. Arabatzis, T. Stergiopoulos, M. C. Bernard, D. Labou, S. G. Neophytides and P. Falaras, Appl. Catal., B, 2003, 42, 187–201 CrossRef CAS.
- V. Subramanian, E. Wolf and P. V. Kamat, J. Phys. Chem. B, 2001, 105, 11439–11446 CrossRef CAS.
- C. G. Silva, R. Juá rez, T. Marino, R. Molinari and H. García, J. Am. Chem. Soc., 2011, 133, 595–602 CrossRef PubMed.
- J. Yang, D. Wang, H. Han and C. Li, Acc. Chem. Res., 2013, 46, 1900–1909 CrossRef CAS.
- J. S. Jang, D. J. Ham, N. Lakshminarasimhan, W. yong Choi and J. S. Lee, Appl. Catal., A, 2008, 346, 149–154 CrossRef CAS.
- A. Y. Liu, R. M. Wentzcovitch and M. L. Cohen, Phys. Rev. B, 1988, 38, 9483–9489 CrossRef CAS PubMed.
- R. B. Levy and M. Boudart, Science, 1973, 181, 547–549 CrossRef CAS PubMed.
- Y. Wang, S. Song, V. Maragou, P. K. Shen and P. Tsiakaras, Appl. Catal., B, 2009, 89, 223–228 CrossRef CAS.
- K. He, J. Xie, Z. Yang, R. Shen, Y. Fang, S. Ma, X. Chen and X. Li, Catal. Sci. Technol., 2017, 7, 1193–1202 RSC.
- V. G. Pol, S. V. Pol and A. Gedanken, Eur. J. Inorg. Chem., 2009, 2009, 709–715 CrossRef.
- S. Shanmugam, D. S. Jacob and A. Gedanken, J. Phys. Chem. B, 2005, 109, 19056–19059 CrossRef CAS PubMed.
- F. H. Ribeiro, R. A. D. Betta, G. J. Guskey and M. Boudart, Chem. Mater., 1991, 3, 805–812 CrossRef CAS.
- Y. Oosawa, J. Chem. Soc. Chem. Commun., 1982, 4, 221–222 RSC.
- M. J. Jacinto, P. K. Kiyohara, S. H. Masunaga, R. F. Jardim and L. M. Rossi, Appl. Catal., A, 2008, 338, 52–57 CrossRef CAS.
- Z. Zhang, Y. Ma, X. Bu, Q. Wu, Z. Hang, Z. Dong and X. Wu, Sci. Rep., 2018, 8, 1–11 CrossRef.
- A. T. Garcia-Esparza, D. Cha, Y. Ou, J. Kubota, K. Domen and K. Takanabe, ChemSusChem, 2013, 6, 168–181 CrossRef CAS PubMed.
- H. G. Kim, T. T. Tran, W. Choi, T. S. You, P. S. Halasyamani and K. M. Ok, Chem. Mater., 2016, 28, 2424–2432 CrossRef CAS.
- M. K. Ehlert, J. E. Greedan and M. A. Subramanian, J. Solid State Chem., 2016, 28, 2424 Search PubMed.
- H. Yan, H. Zhang, M. J. Reece and X. Dong, Appl. Phys. Lett., 2005, 87, 1–4 Search PubMed.
- J. Wu, A. Mahajan, L. Riekehr, H. Zhang, B. Yang, N. Meng, Z. Zhang and H. Yan, Nano Energy, 2018, 50, 723–732 CrossRef CAS.
- L. Jin, F. Li and S. Zhang, J. Am. Ceram. Soc., 2014, 97, 1–27 CrossRef CAS.
- H. G. Kim, J. S. Yoo and K. M. Ok, J. Mater. Chem. C, 2015, 3, 5625–5630 RSC.
- J. Tauc, R. Grigorovici and A. Vancu, Phys. Status Solidi, 1966, 15, 627 CrossRef CAS.
- Y. Cui, J. Briscoe and S. Dunn, Chem. Mater., 2013, 25, 4215–4223 CrossRef CAS.
- Q. Fu, X. Wang, C. Li, Y. Sui, Y. Han, Z. Lv, B. Song and P. Xu, RSC Adv., 2016, 6, 108883–108887 RSC.
- R. Li, F. Zhang, D. Wang, J. Yang, M. Li, J. Zhu, X. Zhou, H. Han and C. Li, Nat. Commun., 2013, 4, 1432–1437 CrossRef PubMed.
- S. C. Abrahams, S. K. Kurtz and P. B. Jamieson, Phys. Rev., 1968, 172, 551–553 CrossRef CAS.
- G. Viola, K. Boon Chong, M. Eriksson, Z. Shen, J. Zeng, Q. Yin, Y. Kan, P. Wang, H. Ning, H. Zhang, M. E. Fitzpatrick, M. J. Reece and H. Yan, Appl. Phys. Lett., 2013, 103, 4 CrossRef.
- Q. Jiang, H. Ning, Q. Zhang, M. Cain, M. J. Reece and H. Yan, J. Mater. Chem. C, 2013, 1, 5628 RSC.
- Z. Zhao, V. Buscaglia, M. Viviani, M. T. Buscaglia, L. Mitoseriu, A. Testino, M. Nygren, M. Johnsson and P. Nanni, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 1–8 Search PubMed.
- Y. Wang, M. Zhang, J. Liu, H. Zhang, F. Li, C. W. Tseng, B. Yang, G. Smith, J. Zhai, Z. Zhang, S. Dunn and H. Yan, Adv. Energy Mater., 2020, 10, 6–11 CAS.
- K. Page, J. Li, R. Savinelli, H. N. Szumila, J. Zhang, J. K. Stalick, T. Proffen, S. L. Scott and R. Seshadri, Solid State Sci., 2008, 10, 1499–1510 CrossRef CAS.
- B. Bondars, G. Heidemane, J. Grabis, K. Laschke, H. Boysen, J. Schneider and F. Frey, J. Mater. Sci., 1995, 30, 1621–1625 CrossRef CAS.
- A. Liibert and B. Leibold, Sens. Actuators, B, 1992, 8, 253–256 CrossRef.
- L. H. Bennett, J. R. Cuthill, A. J. Mcalister, N. E. Erickson and R. E. Watson, Science, 1974, 184, 563–565 CrossRef CAS PubMed.
- I. Fujita, P. Edalati, Q. Wang, M. Watanabe, M. Arita, S. Munetoh, T. Ishihara and K. Edalati, Scr. Mater., 2020, 187, 366–370 CrossRef CAS.
- W. Huang, H. Meng, Y. Gao, J. Wang, C. Yang, D. Liu, J. Liu, C. Guo, B. Yang and W. Cao, J. Mater. Chem. A, 2019, 7, 18538–18546 RSC.
- M. Thangamuthu, C. Santschi and O. J. F. Martin, Faraday Discuss., 2019, 214, 339 RSC.
- M. Wang, M. Ye, J. Iocozzia, C. Lin and Z. Lin, Adv. Sci., 2016, 3, 1600024 CrossRef PubMed.
- S. Linic, P. Christopher and D. B. Ingram, Nat. Mater., 2011, 10, 911–921 CrossRef CAS PubMed.
- T. Zhang, W. Lei, P. Liu, J. A. Rodriguez, J. Yu, Y. Qi, G. Liu and M. Liu, Chem. Sci., 2015, 6, 4118 RSC.
- J. Lan, X. Zhou, G. Liu, J. Yu, J. Zhang, L. Zhi and G. Nie, Nanoscale, 2011, 3, 5161 RSC.
- A. Phuruangrat, A. Maneechote, P. Dumrongrojthanath, N. Ekthammathat, S. Thongtem and T. Thongtem, Mater. Lett., 2015, 159, 289–292 CrossRef CAS.
- G. Yuan, G. Zhang, K. Li, F. Li, Y. Cao, J. He, Z. Huang, Q. Jia, S. Zhang and H. Zhang, Nanomaterials, 2020, 10, 1–15 Search PubMed.
- J. D. Bobić, M. M. Vijatović, S. Greičius, J. Banys and B. D. Stojanović, J. Alloys Compd., 2010, 499, 221 CrossRef.
- W. Qi, Y. Wang, J. Wu, Z. Hu, C. Jia, G. Viola, H. Zhang and H. Yan, J. Am. Ceram. Soc., 2020, 103, 28 CrossRef CAS.
- Y. Wang, M. Zhang, J. Wu, Z. Hu, H. Zhang and H. Yan, J. Am. Ceram. Soc., 2021, 104, 322–328 CrossRef CAS.
- W. Xiong, H. Porwal, H. Luo, V. Araullo-Peters, J. Feng, M. M. Titirici, M. J. Reece and J. Briscoe, J. Mater. Chem. A, 2020, 8, 6564–6568 RSC.
- K. Saito, K. Koga and A. Kudo, Dalt. Trans., 2011, 40, 3909 RSC.
- C. Suryanarayana, Prog. Mater. Sci., 2001, 46, 1–184 CrossRef CAS.
- A. C. Larson and R. B. Von Dreele, GSAS Genralized Structure ANalysis System, Los Alamos National Laboratory Report LAUR-86-748, 1987 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ta04131b |
| This journal is © The Royal Society of Chemistry 2021 |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)