A thermodynamic approach toward selective and reversible sub-ppm H2S sensing using ultra-small CuO nanorods impregnated with Nb2O5 nanoparticles†
Abstract
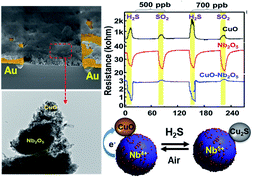
This paper provides an ideal solution to the challenges of employing CuO nanoparticles for the reversible, selective, and stable detection of sub-ppm H2S gas. This scheme presents a hidden thermodynamic advantage that makes both sulfidation and oxidation reactions reversible over a wide range of temperature (100–220 °C) by the addition of Nb2O5 nanoparticles coupled with Gibbs free energy changes. Our optimized sensor composed of CuO–Nb2O5 composites at 220 °C exhibits excellent selectivity toward H2S and SO2 gases, ultralow detection concentration of 500 ppb, fast response time (<180 s) and fast recovery, and reliable long-term stability (of over a month). Our spectroscopic investigations along with theoretical studies confirm that the CuO–Nb2O5 interface enables the formation of Cu2+/Nb4+ ↔ Cu+/Nb5+ species due to the charge transfer between the Nb and Cu species, which energetically favors CuO sulfidation and oxidation. The Gibbs free energy calculation for the sulfidation and regeneration reaction shows that the incorporation of Nb2O5 alters the reaction equilibrium over a wide range of temperature. Thus, our study provides insight into a thermodynamic strategy for designing metal oxide composite catalysts for improved catalytic reactions.


 Please wait while we load your content...
Please wait while we load your content...