Linkage engineering mediated carriers transfer and surface reaction over carbon nitride for enhanced photocatalytic activity†
Shilian
Yang‡
a,
Qian
Wang‡
a,
Qiuchen
Wang
a,
Gen
Li
a,
Tianxiang
Zhao
 a,
Peng
Chen
a,
Peng
Chen
 *a,
Fei
Liu
*a and
Shuang-Feng
Yin
*b
*a,
Fei
Liu
*a and
Shuang-Feng
Yin
*b
aProvincial Guizhou Key Laboratory of Green Chemical and Clean Energy Technology, School of Chemistry and Chemical Engineering, Guizhou University, Guiyang 550025, Guizhou, China. E-mail: pchen3@gzu.edu.cn; ce.feiliu@gzu.edu.cn
bState Key Laboratory of Chemo/Biosensing and Chemometrics, Provincial Hunan Key Laboratory for Cost-effective Utilization of Fossil Fuel Aimed at Reducing Carbon-dioxide Emissions, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, Hunan, China. E-mail: sf_yin@hnu.edu.cn
First published on 13th July 2021
Abstract
Rational tailoring of the atomic structure of photocatalysts with multiple functions to enhance the carrier transfer efficiency and surface activation of carbon nitride (C3N4) is promising and a challenge. Here, we make the first report of a facile strategy to construct amphiphilic carbon and C–O–C chain linked terminal melem units in functional carbon nitride (COCN) via copolymerizing formaldehyde with melem. By integrating the amphiphilic carrier bridge of carbon and C–O–C chains into the framework, the photogenerated carrier mobility and activated species (superoxide radicals, singlet oxygen) as well as surface interaction are significantly improved. Consequently, the optimal tailoring of C3N4 attains superior photocatalytic activity for hydrogen production (34.9 μmol h−1) and selective oxidation of sulfide to sulfoxide using air (nearly 100% conversion and selectivity after 3 h of illumination), which is about 7 times higher than that of pristine C3N4. This study provides deep insight into and strategies for the atomic tailoring of carrier transfer and surface reaction over organic-based photocatalysts.
Introduction
In recent years, photocatalysis has aroused tremendous interest due to its extensive application in the field of hydrogen evolution, CO2 reduction, environmental remediation, and organic synthesis.1 Highly efficient photocatalysts were key components propelling photocatalysis for commercial application.2,3 Given their chemically stable and nontoxic features, metal-free conjugated polymers of g-C3N4 are regarded as prospective candidates for photocatalysts.4,5 However, the high recombination rate of photogenerated carriers and low efficiency of reactant activation have severely limited the photocatalytic activity of bulk g-C3N4.6–9 Therefore, further improvement in g-C3N4 photocatalytic efficiency is a worthwhile mission.Along these lines, several pioneering approaches (e.g.: construction of heterojunctions and deficiencies, elemental doping, morphology control, and cocatalyst modification) have been carried out to overcome these obstacles.10–14 Notably, the precise and targeted atomic regulation of melem units is a fundamental and straightforward strategy to enhance the performance of pristine g-C3N4.15–17 For example, Ohno et al. reported the incorporation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups into the C3N4 matrix using melem and barbituric acid as precursors, which significantly improved the charge separation and photocatalytic H2O2 production.18 Ye et al. designed a short carbon chain linked pristine tri-s-triazine structure in the basal plane of C3N4 to improve its photocatalytic performance via facilitating charge carrier transfer in a convenient transport channel.19 Che et al. introduced a graphitic carbon ring into the in-plane of C3N4 to expedite the separation of photoexcited carriers and electron transport.20 In addition, it is reported that localized graphitized carbon nitride with an asymmetric benzene ring could form separated charge carrier centers and a localized internal field to facilitate the separation of photogenerated carriers.21 It is envisioned that the rational design of different chain linked pristine tri-s-triazine structures in C3N4 could suppress carrier recombination through forming asymmetric units. Most of the above work was entirely focused on the promotion of separation and transportation of charge carriers as well as on the construction of band structure for enhancing photocatalytic activity. However, the surface reaction associated with reactant activation is also a significant factor but is often overlooked and rebellious for improving photocatalytic efficiency.22 Precise and targeted control over different chain linked pristine tri-s-triazine structures could not only suppress carrier recombination, but could also fully consider the surface reaction property. Unfortunately, to the best of our knowledge, there has been no report about multiple chains linked to a pristine tri-s-triazine structure in C3N4.
O groups into the C3N4 matrix using melem and barbituric acid as precursors, which significantly improved the charge separation and photocatalytic H2O2 production.18 Ye et al. designed a short carbon chain linked pristine tri-s-triazine structure in the basal plane of C3N4 to improve its photocatalytic performance via facilitating charge carrier transfer in a convenient transport channel.19 Che et al. introduced a graphitic carbon ring into the in-plane of C3N4 to expedite the separation of photoexcited carriers and electron transport.20 In addition, it is reported that localized graphitized carbon nitride with an asymmetric benzene ring could form separated charge carrier centers and a localized internal field to facilitate the separation of photogenerated carriers.21 It is envisioned that the rational design of different chain linked pristine tri-s-triazine structures in C3N4 could suppress carrier recombination through forming asymmetric units. Most of the above work was entirely focused on the promotion of separation and transportation of charge carriers as well as on the construction of band structure for enhancing photocatalytic activity. However, the surface reaction associated with reactant activation is also a significant factor but is often overlooked and rebellious for improving photocatalytic efficiency.22 Precise and targeted control over different chain linked pristine tri-s-triazine structures could not only suppress carrier recombination, but could also fully consider the surface reaction property. Unfortunately, to the best of our knowledge, there has been no report about multiple chains linked to a pristine tri-s-triazine structure in C3N4.
Herein, we propose a facile strategy to embed amphiphilic carbon and C–O–C chain linked melem units in functional carbon nitride (COCN) via copolymerizing formaldehyde with melem. Notably, by integrating an amphiphilic electron bridge into the framework, the migration of photogenerated carriers, production of activated species (superoxide radicals, singlet oxygen) and surface interaction capacity of the reactant are significantly improved. Therefore, the optimized atomic tailoring of the sample attains superior photocatalytic performance by achieving a 7-fold improvement for the selective oxidation of sulfide to sulfoxide using air (nearly 100% conversion and selectivity after 3 h of illumination) and high hydrogen production (34.9 μmol h−1) as well as good stability compared with pristine C3N4.
Experimental section
Synthesis of COCN
The amphiphilic carbon and C–O–C chain linked melem units (COCN) were fabricated via a simple copolymerization method. In a typical synthesis, 5 g of melamine was added into an alumina crucible and heated at 425 °C in a muffle furnace overnight to collect melem. The obtained powder was suspended in DI water and refluxed for several hours to purify the melem. And then, 2.182 g of melem was added to 100 g of formaldehyde aqueous solutions (0.545 g, 37 wt%), and the pH value adjusted to 9 using 10 wt% triethanolamine. After stirring for 1.5 h at 80 °C, the pH value of the mixed solution was adjusted to 5 with 10 wt% HCl and it was subsequently heated for 2 h under vigorous stirring. Then the solid composite precursor could be obtained by filtering, washing, and drying. Finally, pure COCN was fabricated by annealing treatment of the precursor at a suitable temperature for 4 h under a nitrogen atmosphere. For comparison, COCN-1, COCN-2 and COCN-3 were obtained by annealing treatment of the precursor at 450, 500 and 550 °C for 4 h under nitrogen atmosphere, respectively.Synthesis of C3N4
Typically, C3N4 was generated by directly calcining melamine at 500 °C for 4 h under a nitrogen atmosphere.Catalyst characterizations
X-ray diffraction patterns (XRD, Bruker D8 Advance), Fourier transform infrared spectroscopy (FT-IR, Shimadzu IR Affinity-1) and X-ray photoelectron spectroscopy (XPS, Thermo Fisher K-Alpha Plus) were used to check the chemical structures and surface valence state of the obtained samples, respectively. The morphological structure of COCN was surveyed with a scanning electron microscope (SEM, Hitachi S-4800) and transmission electron microscopy (TEM, JEM-2100F). The Brunauer–Emmett–Teller (BET) and chemisorption data of the prepared materials were analysed by using a Micrometrics ASAP 2020 and AutoChem II 2920, respectively. The optical absorption properties of COCN and C3N4 were examined by employing a UV-vis spectrophotometer (UV-vis, Shimadzu UV-3600 plus). The photocurrent and Mott–Schottky experiments were measured on an electrochemical analyser (CHI660E, Chenhua).Photocatalytic selective oxidation of sulfide
Photocatalytic selective oxidation of sulfide to sulfoxide was performed in a glass reactor with an air dryer and reflux condenser. Typically, 0.5 mmol of substrate, 2 mL of acetonitrile and 0.02 g of photocatalyst were added into the reactor. Prior to light irradiation, the reaction system was stirred for 0.5 h to maintain a balance of absorption and desorption of air. After an appropriate period of irradiation, the reaction mixture of the catalyst and liquid was separated by centrifugation. The qualitative characteristics and quantification of the products were identified by GC-MS analysis and gas chromatography (GC 2014C), using biphenyl as internal standard.Photocatalytic H2 evolution
Photocatalytic H2 generation performance over the as-prepared catalyst was studied by employing a conventional photocatalytic water splitting system (NBET, Beijing) with a light source (125 mW cm−2). Typically, 0.02 g of catalyst, 10 mL of triethanolamine (TEOA), 250 μL of H2PtCl6 and 40 mL of water were added into the reactor. Before irradiation, the photocatalytic H2 generation system was closed to produce an airproof system and stirred for 0.5 h. After an appropriate period of irradiation, the obtained H2 was quantified via a gas chromatography system (GC9790, Fuli).Results and discussion
Preparation and characterization
The amphiphilic carbon and C–O–C chain linked melem units (COCN) have been synthesized by the simple polymerization of formaldehyde with melem (Fig. 1a). Firstly, melem was synthesized by calcination of melamine at 425 °C for 12 h and purification in boiling water. A number of gaps were discovered in the columnar structure of the melem rod (see Fig. S1 in ESI†). In general, the process of the melem and formaldehyde condensation reaction can be divided into two steps.23 In the first step, the prepared melem was treated with formaldehyde to form trimethylohmelem under alkaline conditions. A narrow gap had obviously appeared in the columnar structure. XRD (Fig. S2†), FTIR (Fig. S3†), and XPS (Fig. S4a†) analyses disclosed that CH2OH had been successfully incorporated in the melem structure. Further polymerization of the copolymer was carried out to generate a short chain-like precursor under acidic conditions. As a result of incomplete polymerization, there were abundant OH on the edges of the melem units (Fig. S4b†). Finally, an oversimplified temperature-programmed heating process was implemented to aggregate the short chain precursor, resulting in embedded multiple functionalized chains. Importantly, the melem could form C3N4 without aggregating with formaldehyde under the same conditions (Fig. S5, ESI†). | ||
| Fig. 1 (a) Schematic of COCN synthesis, (b) SEM, (c) TEM and (d–f) elemental mapping images of COCN-2. | ||
As shown in Fig. 1b, the COCN-2 sample has a rod-like appearance with a diameter of about 1500 nm. Also, the rod is made up of numerous mesopores and macropores (Fig. 1c). The specific surface area of COCN-2 is (79.4 m2 g−1), much larger than that of the C3N4 composite (11.7 m2 g−1) (Fig. S6, ESI†). It is speculated that the hierarchical pores are conducive to light absorption and product/reactant migration. In addition, the element mapping images reveal that the dispersion of C, N and O elements in COCN is uniform (Fig. 1d–f and S7†).
Successful synthesis of COCN was tested by XRD, FT-IR and 13C NMR spectral analyses. As shown in Fig. S2,† the two characterization peaks at 13.1° and 27.8° are ascribed to the in-plane repetition of (100) and the π–π reflection of (002), respectively.24 Compared with C3N4, the peak of (002) in COCN-2 moves to a higher angle, displaying a decrease in the interlayer distance, which could significantly improve interlayer exciton splitting and charge transfer.25 Some new sharp peaks at 12.4°, 19.6° and 21.8° appeared for COCN-1 and COCN-2, implying the formation of new chains. Interestingly, heat treatment at a higher temperature caused the disruption of new chains to form a conventional tri-s-triazine linked framework. The FTIR spectrum of COCN shows characteristic broad peaks at 811 and 1300–1700 ascribed to the out-of-plane bending of the triazine ring and the heptazine heterocyclic ring (C6N7) units.26 Small peaks are observed at 993 (C–N), 1088 (C–O–C), 1495 (C–H2) and 3126 cm−1 (N–H), implying the generation of N–CH2–N and C–O–C species in the structure of COCN (Fig. S3†).19,27–30 In the 13C NMR spectrum (Fig. S8†), two peaks at 161.9 and 154.4 ppm are assigned to the C–NHx and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N bonds, respectively.31 This result indicated that the heptazine units of carbon nitride were not damaged. Two smaller peaks at 67.7 and 55.5 ppm belong to the C–O–C and carbon chains, suggesting that C–O–C and carbon chains have been inserted into the framework of COCN.32 In addition, two peaks around 107.5 and 115.2 ppm are ascribed to unsaturated carbon, which derives from the dissociation of C–O–C at high temperature.33 In order to obtain the thermal behavior of the as-prepared samples, TG measurements were carried out (Fig. S9†). A small weight loss can be observed below 600 °C, which was assigned to the decomposition of the inserted chains in COCN, indicating that C–O–C and carbon species exist in the framework. According to the results acquired from XRD, IR, and 13C NMR measurements, we can confirm that the heptazine units of carbon nitride had not changed, coupled with the insertion of C–O–C and carbon chains into the framework of COCN.
C–N bonds, respectively.31 This result indicated that the heptazine units of carbon nitride were not damaged. Two smaller peaks at 67.7 and 55.5 ppm belong to the C–O–C and carbon chains, suggesting that C–O–C and carbon chains have been inserted into the framework of COCN.32 In addition, two peaks around 107.5 and 115.2 ppm are ascribed to unsaturated carbon, which derives from the dissociation of C–O–C at high temperature.33 In order to obtain the thermal behavior of the as-prepared samples, TG measurements were carried out (Fig. S9†). A small weight loss can be observed below 600 °C, which was assigned to the decomposition of the inserted chains in COCN, indicating that C–O–C and carbon species exist in the framework. According to the results acquired from XRD, IR, and 13C NMR measurements, we can confirm that the heptazine units of carbon nitride had not changed, coupled with the insertion of C–O–C and carbon chains into the framework of COCN.
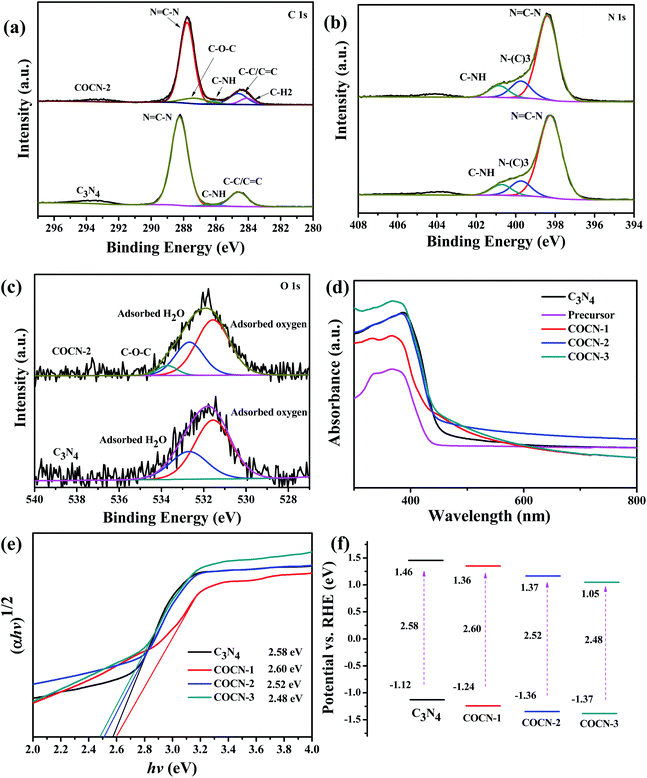
XPS survey spectra were implemented to further test the surface chemical states of COCN. As shown in Fig. 2a, the C 1s of COCN-2 possesses four peaks at 284.1 eV, 284.6 eV, 286.1 eV, 287.2 eV and 287.8 eV corresponding to C–H2, the sp2 hybridized carbon atoms, C–NH, C–O–C and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N bond, respectively.27,34–37 There was some shift toward a lower position compared with C3N4, implying the chemical environment has been changed. Moreover, it was found that the peaks of the C–O–C bonds of COCN-1 shifted to a slightly higher position for COCN-3, indicating oxidation had occurred in COCN-3 (Fig. S10, ESI†). As revealed in Table S2 (ESI†), COCN-1 possesses plenty of oxygen species and COCN-2 has the most C–H2 bonds. This can be explained by the fact that the precursor of carbon and C–O–C chains will be recombined at high temperature to form a traditional tri-s-triazine framework. Three peaks at 398.4, 399.7 and 400.9 eV were obtained by deconvoluting the spectrum of COCN-2 and related to the C–N
C–N bond, respectively.27,34–37 There was some shift toward a lower position compared with C3N4, implying the chemical environment has been changed. Moreover, it was found that the peaks of the C–O–C bonds of COCN-1 shifted to a slightly higher position for COCN-3, indicating oxidation had occurred in COCN-3 (Fig. S10, ESI†). As revealed in Table S2 (ESI†), COCN-1 possesses plenty of oxygen species and COCN-2 has the most C–H2 bonds. This can be explained by the fact that the precursor of carbon and C–O–C chains will be recombined at high temperature to form a traditional tri-s-triazine framework. Three peaks at 398.4, 399.7 and 400.9 eV were obtained by deconvoluting the spectrum of COCN-2 and related to the C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, N–(C3) and C–NH bonds, respectively.38 In addition, the peak area of C–NH decreased from 12.07% of COCN-1 to 8.73% of COCN-3, while the peak area of N–(C3) and C–N
C, N–(C3) and C–NH bonds, respectively.38 In addition, the peak area of C–NH decreased from 12.07% of COCN-1 to 8.73% of COCN-3, while the peak area of N–(C3) and C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in C3N4 increased via the rise in calcination temperature (Table S3, ESI†), suggesting C–NHx on the edges of the heptazine units. Three characteristic peaks at 531.6, 532.7 and 533.7 eV can be observed from the O 1s XPS spectra of COCN, assigned to surface adsorbed O2 and H2O, and C–O–C groups, respectively.39 According to the XPS, FTIR and 13C NMR spectrum results, high temperature will break down the carbon and C–O–C, resulting in COCN-1 and COCN-2 possessing the maximum number of C–O and carbon chains, respectively.
C in C3N4 increased via the rise in calcination temperature (Table S3, ESI†), suggesting C–NHx on the edges of the heptazine units. Three characteristic peaks at 531.6, 532.7 and 533.7 eV can be observed from the O 1s XPS spectra of COCN, assigned to surface adsorbed O2 and H2O, and C–O–C groups, respectively.39 According to the XPS, FTIR and 13C NMR spectrum results, high temperature will break down the carbon and C–O–C, resulting in COCN-1 and COCN-2 possessing the maximum number of C–O and carbon chains, respectively.
The optical absorption properties of bulk C3N4 and COCN were checked by employing UV-vis diffuse reflectance spectroscopy. As shown in Fig. 2d, COCN exhibits a slightly blue shifted absorption band but higher photoabsorption in comparison with bulk C3N4. Such a slight blue shift might be attributed to the intensive quantum confinement effect or delocalization of the π-conjugation system.40 The bandgap of the as-prepared samples is calculated from the Kubelka–Munk equation. It displayed a narrowing of the bandgap width from 2.58 eV (g-C3N4) to 2.48 eV (COCN-3). To identify the possible positions of the valence and conduction bands, Mott–Schottky measurements were investigated at different frequencies (1000 Hz, 1500 Hz, 2000 Hz) and are shown in Fig. S11.† There was a positive slope and the corresponding conduction bands were estimated as −1.12, −1.24, −1.36 and −1.37 vs. RHE for the n-type semiconductors of C3N4, COCN-1, COCN-2 and COCN-3, respectively. According to bandgap measurements of the UV-vis spectrum, the band gap of the as-prepared samples is obtained as shown in Fig. 2f. There are negative positions of the conduction band and mild oxidizing capacity by embedding carbon and C–O–C chains, which favours hydrogen evolution and photocatalytic selective oxidation.
Photocatalytic selective oxidation of sulfides and mechanisms
In this study, the selective oxidation of methyl phenyl sulfide was implemented as a model reaction using air as an oxidizing agent at room temperature and under visible light irradiation. Parallel experiments were carried out under N2 and CO2 atmospheres or without light illumination and/or photocatalyst, resulting in no products being generated, suggesting that the selective oxidation reaction is photocatalysed and O2 from the air is compulsory. As shown in Table 1, it was observed that a negligible conversion (15%) of thioanisole was acquired over C3N4, whereas carbon and C–O–C chain modified COCN exhibited markedly increased activities. Among these, near complete conversion and selectivity were achieved over COCN-2 after 3 hours of illumination, which was 7 times higher than that of pristine C3N4. In addition, it also had the highest formation rates among the reported catalysts (Table S5, ESI†). We also studied the stability of COCN-2 in ten successive runs and detected almost no loss of photoactivity or structure (Fig. S12, ESI†), indicating the high stability of COCN-2. Furthermore, COCN-2 was extended to the oxidation of various thioether compounds. Thioanisole derivatives in the presence of either electron-withdrawing or electron-donating substituent groups were successfully oxidized to the corresponding sulfoxides with excellent conversion and selectivity. The excellent conversion rate of different substituted thioanisoles is in the order ortho < para, which indicates the presence of a steric effect.41 In general, COCN-2 is a promising photocatalyst for industrial applications of the selective oxidation of sulfides.| Entry | R1 | R2 | Time (h) | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|---|
| a Reaction conditions: substrate (0.5 mmol), photocatalyst (COCN-2, 20 mg), acetonitrile (2 mL), air, room temperature, λ >420 nm. b Photocatalyst: COCN-1. c COCN-3. d Photocatalyst: C3N4. e The precursor of COCN. | |||||
| 1 | Ph | CH3 | 3 | 99 | >99 |
| 2b | Ph | CH3 | 3 | 95 | >99 |
| 3c | Ph | CH3 | 3 | 87 | >99 |
| 2d | Ph | CH3 | 3 | 15 | 92 |
| 3e | Ph | CH3 | 3 | 33 | 96 |
| 4 | Ph | Ph | 3 | 99 | >99 |
| 5 | P-MeOPh | CH3 | 3 | 99 | >99 |
| 6 | P-ClPh | CH3 | 5 | 99 | >99 |
| 7 | P-BrPh | CH3 | 8 | 99 | >99 |
| 8 | O-BrPh | CH3 | 8 | 99 | >99 |
| 9 | CH3 | CH3 | 5 | 99 | >99 |
| 10 | CH3CH2 | CH3CH2 | 5 | 99 | >99 |
Behind such an outstanding performance, it is more significant to decode the key scientific factors that affect the photocatalytic activity of COCN. Hence, we are concerned with the relationship of photogenerated charge carriers with or without modified chains in the C3N4 framework. In general, steady-state/time-resolved photoluminescence (PL) spectroscopy is an important method to monitor the recombination ability of photogenerated carriers. As shown in Fig. 3c, the photocurrent density of COCN-2 is nearly three times higher than that of C3N4. Meanwhile, EIS measurements suggest that the incorporation of carbon and C–O–C bonds into g-C3N4 can effectively enhance the electronic conductivity and charge transportation of the polymer matrix. Overall, we can reasonably conclude that the bridged carbon and C–O–C bonds act as an electron transporter to dramatically accelerate the separation and transfer of photocarriers. Furthermore, the maximum number of carbon chains is more conducive to carrier transmission. In particular, due to the different distances migrated by the charge carriers according to the appropriate ratio of carbon and C–O–C bridges, a built-in electric field could be formed to enlarge the separation of photogenerated carriers.
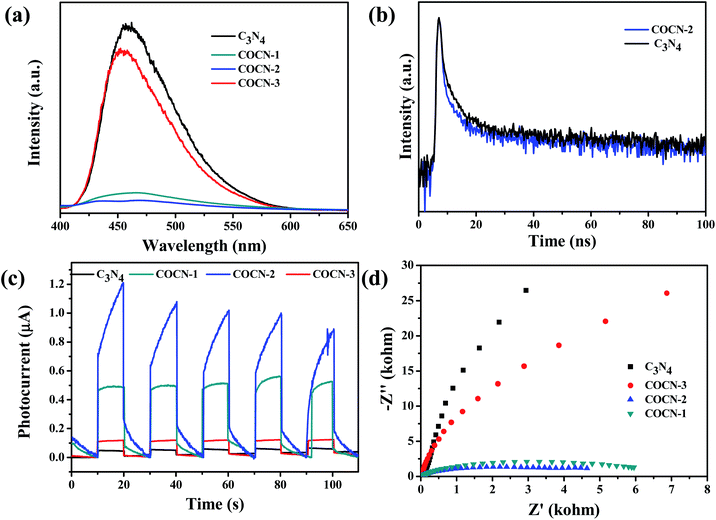 | ||
| Fig. 3 (a) PL (λEx = 380 nm), (b) transient fluorescence, (c) photocurrent and (d) electrochemical impedance spectroscopy of as-prepared samples. | ||
On the other hand, the surface properties of COCN are crucial factors affecting the photocatalytic performance. Firstly, hydrophilicity/hydrophobicity plays a very important role in the surface reaction for nanomaterials. To probe the surface properties of the as-prepared samples, the contact angle of thioanisole was measured. As shown in Fig. S13,† the contact angle of COCN is in the order C3N4 < COCN-3 < COCN-1 < COCN-2, implying a relatively strong interaction with thioanisole. This is in accordance with the content of non-polar functional groups (carbon chains) on the surface of COCN, resulting in the surface being under hydrophobic conditions. In addition, the adsorption of O2 plays an important role in the oxidation reaction. From Fig. S14,† an increase in intensity in the adsorbed oxygen molecule occurred in O2-TPD, implying the ability to activate O2 was improved by embedding chains.
To determine the crucial electron transfer pathway of the photocatalyst, we used different kinds of quenching agents to test the nature of the reaction. In this investigation, tert-butanol (TBA), L-tryptophan (LT), p-benzoquinone (BQ), potassium persulfate (KSO) and ammonium oxalate (AO) served as hydroxyl radical (˙OH), singlet oxygen (1O2), superoxide radical (O2˙−), electron (e−) and hole (h+) scavengers.42,43 According to the results (Fig. S15†), singlet oxygen, and photogenerated electrons and holes were considered to be essential active species whereas superoxide radicals participated but less significantly. Therefore, EPR analysis and radical trapping experiments were carried out for the qualitative and quantitative characterization of O2˙− and 1O2.44,45 As shown in Fig. 4a and b, all the prepared samples could generate 1O2 and O2˙−. Noticeably, the response intensity of 1O2 and O2˙− significantly increased in the order C3N4 < COCN-3 < COCN-1 < COCN-2 by incorporating carbon and C–O–C chains in C3N4. We also chose nitro blue tetrazolium (NBT) and 1,3-diphenylisobenzofuran (DPBF) as scavengers to quantify the concentration of O2˙− and 1O2 under visible light irradiation (Fig. S16†), respectively. An enhancement of about 11 and 9 times, respectively, in the amount of O2˙− and 1O2 generated from COCN-2 was achieved compared to pristine C3N4. It is deduced that high oxygen adsorption and excellent electron-transfer efficiency can be achieved to form O2˙− and 1O2 over COCN-2. To investigate whether 1O2 formation undergoes a charge transfer process, p-benzoquinone (BQ) as an efficient O2˙− trapping agent was added to the DPBF decomposition process in the presence of a COCN photocatalyst. The decomposition efficiency of DPBF was almost halved, suggesting that both energy transfer and charge transfer played important roles in 1O2 generation. In addition, in situ FT-IR measurements were conducted for COCN-2 to study the photocatalytic mechanism (Fig. S17†). Only weak peaks from 3000 to 3500 cm−1 were observed under an O2 atmosphere before light irradiation, indicating the chemical adsorption of oxygen on the surface of COCN-2. Under light illumination, the characteristic peaks of carbon and C–O–C appeared. This can be explained by the excitation and transmission of electrons and holes on the surface of COCN-2. According to the previous discussion, we can further suppose that the embedded new chains serve as a carrier transmission channel for oxygen activation.
Based on the above analysis and the principles reported in the literature,46,47 a plausible mechanism for this photocatalytic oxidation over COCN-2 is proposed in Fig. S18.† Under light illumination, COCN-2 was easily photoexcited and produced electron and hole pairs. Photogenerated electrons will react with the surface of O2 to form a radical species O2˙−. Subsequently, a quantity of O2˙− was attacked by h+ to form 1O2. Finally, the sulfoxide product could be obtained from further oxidizing sulfide using singlet oxygen. Another minor pathway is that the photogenerated holes are activated with sulfide to produce the corresponding intermediate, followed by reaction with O2˙− to generate sulfoxide.
Photocatalytic hydrogen production
The photocatalytic activities of COCN and C3N4 were also studied for H2 evolution under visible light illumination. The average H2 generation rates over C3N4, COCN-1, COCN-2 and COCN-3 were ca. 5.6, 24.6, 34.9 and 15.9 μmol h−1, respectively (Fig. 4e). The COCN-2 photocatalyst shows about six times the activity of C3N4. The hydrophilic properties on the surface of all the catalysts can also be observed in Fig. S19.† The trend of the liquid contact angle follows the order C3N4 < COCN-3 < COCN-2 < COCN-1, which is in harmony with the content of polar groups. It should be noted that COCN-1 possessed abundant C–O–C groups but had less carrier mobility. Therefore, the excellent photocatalytic activity in hydrogen production could be ascribed to ameliorated hydrophilicity and the enhancement of photoinduced charge separation. The zeta potential was measured to reflect the status of the surface charge. As shown in Table S6,† the absolute value of the zeta potential of COCN-2 is highest among the prepared samples, indicating that H+ can be significantly adsorbed over COCN by the electronegative clusters. Besides the photocatalytic performance, the cycling stability of COCN-2 was studied in eight repeated tests of photocatalytic hydrogen production and each test was maintained for 4 h (Fig. 4f). There was no significant deactivation and no structural collapse was detected (Fig. S20†), evidencing the outstanding stability of the inherent structure of COCN-2.Conclusions
In summary, we propose a facile strategy to construct novel amphiphilic carbon and C–O–C chain linked melem units in functional carbon nitride (COCN) by a facile copolymerizing method. Notably, these inserted carbon and C–O–C units served both as a carrier migration channel to enhance the transportation and separation efficiency of photo-generated carriers in the C3N4 framework. Meanwhile, there is a huge improvement in the adsorption and activation of oxygen molecules, resulting in an acceleration of the production of superoxide radicals and singlet oxygen. More importantly, the different amounts of polar and non-polar structures inserted could adjust the hydrophilic and hydrophobic properties of the material surface, which facilitates the interactions of sulfide or water. Therefore, the optimized atomic tailoring of the sample achieves superior photocatalytic performance by achieving a seven and six-fold improvement for selective oxidation of sulfide to sulfoxide using air (nearly 100% conversion and selectivity after 3 h of illumination) and hydrogen production (34.9 μmol h−1), respectively. This study provides deep insight into and strategies for the atomic tailoring of carrier transfer and surface reactions over organic-based photocatalysts.Author contributions
Shilian Yang: conceptualization, methodology, investigation, data curation. Qian Wang: conceptualization, methodology, data curation. Qiuchen Wang: methodology. Gen Li: formal analysis. Tianxiang Zhao: supervision, investigation. Peng Chen: conceptualization, supervision, writing-review & editing, funding acquisition. Fei Liu: supervision, investigation, funding acquisition. Shuang-Feng Yin: supervision, investigation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was financially supported by Scientific and Technological Innovation Talents Team Project of Guizhou Province (No. 20185607), One Hundred Person Project of Guizhou Province (No. 20165655), Natural Science Project of Department of Education of Guizhou Province (No. 2017116), Innovation Group Project of Education Department in Guizhou Province (No. 2021010) and Guizhou Provincial Science and Technology Foundation (No. 2021069). The authors would like to thank Shiyanjia Lab for materials characterizations.References
- J. Low, J. Yu, M. Jaroniec, S. Wageh and A. A. Al-Ghamdi, Adv. Mater., 2017, 29, 1601694 CrossRef PubMed
.
- Z. Wang, C. Li and K. Domen, Chem. Soc. Rev., 2019, 48, 2109 RSC
.
- T. Hisatomi and K. Domen, Nat. Catal., 2019, 2, 387 CrossRef CAS
.
- J. Fu, J. Yu, C. Jiang and B. Cheng, Adv. Energy Mater., 2018, 8, 1701503 CrossRef
.
- Y. Zheng, L. Lin, X. Ye, F. Guo and X. Wang, Angew. Chem., Int. Ed., 2014, 53, 11926 CrossRef CAS PubMed
.
- A. Indra, A. Acharjya, P. W. Menezes, C. Merschjann, D. Hollmann, M. Schwarze, M. Aktas, A. Friedrich, S. Lochbrunner, A. Thomas and M. Driess, Angew. Chem., Int. Ed., 2017, 56, 1653 CrossRef CAS PubMed
.
- Z. Teng, N. Yang, H. Lv, S. Wang, M. Hu, C. Wang, D. Wang and G. Wang, Chem, 2019, 5, 664 CAS
.
- G. Liao, Y. Gong, L. Zhang, H. Gao, G. J. Yang and B. Fang, Energy Environ. Sci., 2019, 12, 2080 RSC
.
- Y. Xiao, G. Tian, W. Li, Y. Xie, B. Jiang, C. Tian, D. Zhao and H. Fu, J. Am. Chem. Soc., 2019, 141, 2508 CrossRef CAS PubMed
.
- P. Xia, S. Cao, B. Zhu, M. Liu, M. Shi, J. Yu and Y. Zhang, Angew. Chem., Int. Ed., 2020, 59, 5218 CrossRef CAS PubMed
.
- H. Che, G. Che, P. Zhou, C. Liu, H. Dong, C. Li, N. Song and C. Li, Chem. Eng. J., 2020, 382, 122870 CrossRef CAS
.
- J. Liao, W. Cui, J. Li, J. Sheng, H. Wang, X. a. Dong, P. Chen, G. Jiang, Z. Wang and F. Dong, Chem. Eng. J., 2020, 379, 122282 CrossRef CAS
.
- Z. Tong, D. Yang, Z. Li, Y. Nan, F. Ding, Y. Shen and Z. Jiang, ACS Nano, 2017, 11, 1103 CrossRef CAS PubMed
.
- W. Wang, T. An, G. Li, D. Xia, H. Zhao, J. C. Yu and P. K. Wong, Appl. Catal., B, 2017, 217, 570 CrossRef CAS
.
- Q. Deng, G. Ba, T. Huo, H. Li and W. Hou, Appl. Catal., A, 2020, 606, 117833 CrossRef CAS
.
- H. Li, F. Li, Z. Wang, Y. Jiao, Y. Liu, P. Wang, X. Zhang, X. Qin, Y. Dai and B. Huang, Appl. Catal., B, 2018, 229, 114 CrossRef CAS
.
- V. R. Battula, S. Kumar, D. K. Chauhan, S. Samanta and K. Kailasam, Appl. Catal., B, 2019, 244, 313 CrossRef CAS
.
- Z. Teng, W. Cai, S. Liu, C. Wang, Q. Zhang, S. Chenliang and T. Ohno, Appl. Catal., B, 2020, 271, 118917 CrossRef CAS
.
- Y. Li, J. Ren, S. Ouyang, W. Hou, T. Petit, H. Song, H. Chen, D. Philo, T. Kako and J. Ye, Appl. Catal., B, 2019, 259, 118027 CrossRef CAS
.
- W. Che, W. Cheng, T. Yao, F. Tang, W. Liu, H. Su, Y. Huang, Q. Liu, J. Liu, F. Hu, Z. Pan, Z. Sun and S. Wei, J. Am. Chem. Soc., 2017, 139, 3021 CrossRef CAS PubMed
.
- G. Jia, Y. Wang, X. Cui, Z. Yang, L. Liu, H. Zhang, Q. Wu, L. Zheng and W. Zheng, Appl. Catal., B, 2019, 258, 117959 CrossRef CAS
.
- P. Chen, H. Wang, H. Liu, Z. Ni, J. Li, Y. Zhou and F. Dong, Appl. Catal., B, 2019, 242, 19 CrossRef CAS
.
- L. Kang, B. Wang, J. Zeng, Z. Cheng, J. Li, J. Xu, W. Gao and K. Chen, Green Chem., 2020, 22, 504 RSC
.
- Y. Yu, S. Wu, J. Gu, R. Liu, Z. Wang, H. Chen and F. Jiang, J. Hazard. Mater., 2020, 384, 121247 CrossRef CAS PubMed
.
- H. Tan, X. Gu, P. Kong, Z. Lian, B. Li and Z. Zheng, Appl. Catal., B, 2019, 242, 67 CrossRef CAS
.
- Q. Han, Z. Cheng, J. Gao, Y. Zhao, Z. Zhang, L. Dai and L. Qu, Adv. Funct. Mater., 2017, 27, 1606352 CrossRef
.
- Y. A. Workie, N. Sabrina, T. Imae and M. P. Krafft, ACS Biomater. Sci. Eng., 2019, 5, 2926 CrossRef CAS PubMed
.
- G. Zhang, Y. Xu, C. He, P. Zhang and H. Mi, Appl. Catal., B, 2021, 283, 119636 CrossRef CAS
.
- Y. Yu, W. Yan, X. Wang, P. Li, W. Gao, H. Zou, S. Wu and K. Ding, Adv. Mater., 2018, 30, 1705060 CrossRef PubMed
.
- Y. Ejeta and T. Imae, J. Photochem. Photobiol., A, 2021, 404, 112955 CrossRef
.
- S. Bellamkonda, R. Shanmugam and R. R. Gangavarapu, J. Mater. Chem. A, 2019, 7, 3757 RSC
.
- J. Tong, X. Han, S. Wang and X. Jiang, Energy Fuels, 2011, 25, 4006 CrossRef CAS
.
- J. Tian, L. Zhang, M. Wang, X. Jin, Y. Zhou, J. Liu and J. Shi, Appl. Catal., B, 2018, 232, 322 CrossRef CAS
.
- L. Jing, D. Wang, M. He, Y. Xu, M. Xie, Y. Song, H. Xu and H. Li, J. Hazard. Mater., 2021, 401, 123309 CrossRef CAS PubMed
.
- H. Yu, L. Shang, T. Bian, R. Shi, G. I. Waterhouse, Y. Zhao, C. Zhou, L. Z. Wu, C. H. Tung and T. Zhang, Adv. Mater., 2016, 28, 5080 CrossRef CAS PubMed
.
- L. Jing, R. Zhu, D. L. Phillips and J. C. Yu, Adv. Funct. Mater., 2017, 27, 1703484 CrossRef
.
- Y. Sui, J. Liu, Y. Zhang, X. Tian and W. Chen, Nanoscale, 2013, 5, 9150 RSC
.
- H. Che, G. Che, P. Zhou, C. Liu, H. Dong, C. Li, N. Song and C. Li, Chem. Eng. J., 2020, 382, 122870 CrossRef CAS
.
- X. Song, X. Li, X. Zhang, Y. Wu, C. Ma, P. Huo and Y. Yan, Appl. Catal., B, 2020, 268, 118736 CrossRef CAS
.
- Y. Ma, E. Liu, X. Hu, C. Tang, J. Wan, J. Li and J. Fan, Appl. Surf. Sci., 2015, 358, 246 CrossRef CAS
.
- H. Wang, S. Jiang, S. Chen, D. Li, X. Zhang, W. Shao, X. Sun, J. Xie, Z. Zhao, Q. Zhang, Y. Tian and Y. Xie, Adv. Mater., 2016, 28, 6940 CrossRef CAS PubMed
.
- M. B. Ballatore, M. B. Spesia, M. E. Milanesio and E. N. Durantini, RSC Adv., 2018, 8, 22876 RSC
.
- P. Chen, L. Chen, Y. Zeng, F. Ding, X. Jiang, N. Liu, C.-T. Au and S.-F. Yin, Appl. Catal., B, 2018, 234, 311 CrossRef CAS
.
- H. Wang, X. Yang, W. Shao, S. Chen, J. Xie, X. Zhang, J. Wang and Y. Xie, J. Am. Chem. Soc., 2015, 137, 11376 CrossRef CAS PubMed
.
- S. Zhao and X. Zhao, Appl. Catal., B, 2019, 250, 408 CrossRef CAS
.
- J. Jiang, R. Luo, X. Zhou, Y. Chen and H. Ji, Adv. Synth. Catal., 2018, 360, 4402 CrossRef CAS
.
- X. Chen, K. Deng, P. Zhou and Z. Zhang, ChemSusChem, 2018, 11, 2444 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ta03813c |
| ‡ These authors contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |