Photothermal effect enables markedly enhanced oxygen reduction and evolution activities for high-performance Zn–air batteries†
Abstract
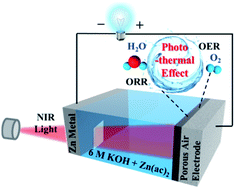
The ability to craft high-performance and cost-effective bifunctional oxygen catalysts opens up pivotal perspectives for commercialization of zinc–air batteries (ZABs). Despite recent grand advances in the development of synthetic techniques, the overall performance of electrocatalytic processes enters the bottleneck stage through focusing only on the design and modification of bifunctional catalyst materials. Herein, we report a simple yet robust strategy to markedly boost the performance of ZABs via capitalizing on the photothermal effect. Concretely, a bifunctional electrocatalyst comprising Co3O4 nanoparticles encapsulated within N-doped reduced graphene oxide (denoted as Co3O4/N-rGO) acted as both active material and photothermal component. Upon light illumination, the compelling photothermal effect of Co3O4/N-rGO rendered a localized and instant heating of the electrode with more active sites, enhanced electrical conductivity and improved release of bubbles. As such, a prominently reduced indicator ΔE of 0.635 V was realized, significantly outperforming recently reported systems (usually >0.68 V). Corresponding rechargeable ZABs based on Co3O4/N-rGO air electrodes possessed an excellent maximum power density of 299 mW cm−2 (1.8 times that of Pt/Ru-based ZABs) assisted by the photothermal effect with a superb cycling stability (over 500 cycles). This intensification strategy opens vast possibilities to ameliorate the performance of catalysts via innovatively and conveniently utilizing their photothermal feature, which may advance future application in high-performance ZABs and other energy conversion and storage systems.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...