Open Access Article
Open Access ArticleRecent advancements in solid electrolytes integrated into all-solid-state 2D and 3D lithium-ion microbatteries
Albina Jetybayevaab,
Berik Uzakbaiulyabc,
Aliya Mukanova *abc,
Seung-Taek Myung
*abc,
Seung-Taek Myung d and
Zhumabay Bakenov
d and
Zhumabay Bakenov *abc
*abc
aInstitute of Batteries, 53 Kabanbay Batyr Ave., Nur-Sultan 010000, Kazakhstan. E-mail: aliya.mukanova@nu.edu.kz
bDepartment of Chemical and Materials Engineering, Nazarbayev University, Nur-Sultan, Kazakhstan
cNational Laboratory Astana, Nazarbayev University, 53 Kabanbay Batyr Ave., Nur-Sultan 010000, Kazakhstan
dDepartment of Nano Technology and Advanced Materials Engineering, Sejong University, Gunjadong, Gwangjin-gu, Seoul 05006, South Korea
First published on 1st July 2021
Abstract
With the increasing role of microelectronics and autonomous wireless devices in everyday life, the miniaturization of power sources has attracted a lot of attention. Solid-state Li-ion microbatteries proved to be a good candidate for micro-energy storage devices due to their high energy density. As the electrolyte is one of the key components in a battery, much research has been conducted to develop high-quality materials for successful integration in the microbattery technology. Several types of solid electrolytes, including inorganic glass, crystalline and polymer materials, have been investigated in both two-dimensional (2D) and three-dimensional (3D) architecture, and these systems are reviewed in this work along with the general overview of microbatteries concepts. The latest advancements, performance and remaining issues of both 2D and 3D solid structures with different solid electrolytes are discussed. The paper also focuses on reviewing the electrochemical properties of solid electrolytes reported in various literature. So far, it was observed that LiPON electrolyte satisfying most of the electrolyte specifications appears to be one of the most studied and the most appropriate candidates for solid-state microbatteries, performing well in several 2D and innovative 3D structures. Along with that, polymer electrolytes with innovative 3D architectures deposited with effective techniques, such as electrodeposition, formed an excellent electrode–electrolyte interface and showed high power and energy densities. Therefore, these electrolytes hold great promise for further 3D microbatteries development. The important information on solid electrolytes and their application in microbatteries is systemized and provided, including the electrolyte composition, ionic conductivity, microbattery electrodes, preparation methods and conditions, architecture, electrochemical test conditions and their performance to elucidate the electrolyte candidates and their microbattery structures with high capacity and long cycle life.
1. Introduction
The rapid development of micro- and nano-electromechanical systems (MEMS/NEMS) resulted in the advancements of small-scale devices such as microsensors, micro drug delivery systems, micromachines.1–4 These devices require an autonomous power supply with a stable current or the ability to deliver high-peak currents within a confined volume (<10 mm3).1 Lithium-ion battery (LIB) technology is an excellent candidate, as it has become a mature energy storage solution with the highest energy per weight.5–7 The early microbatteries were structured in thin film, two-dimensional (2D) architectures, where the straightforward layered fabrication and easy integration in devices were advantageous for industrial production.8 However, 2D structures have power, energy, and size limitations; thus, it is challenging to satisfy the increasing demand for rapidly developing devices. As a result, a large interest in three-dimensional (3D) microbatteries arose, as 3D architectures allow power and energy density enhancement without the undesired volume increase.3,4,9–11However, to significantly reduce the size of traditional Li-ion batteries and keep the energy efficiency, it is necessary to remove inactive materials, such as separators, as well as introduce alternative electrodes and electrolytes. The commercial electrolytes, although being highly ionically conductive, cause safety issues, like leakage risks.12,13 Thus, solid-state electrolytes have been actively developed and integrated into the microbatteries. Meanwhile, a newer generation of promising electrode and electrolyte materials have been continuously investigated.
The solid electrolyte is an important component in the solid-state cell, as in most cases its unsatisfactory bulk and interfacial performance pose the main constraints in the various Li-ion microbattery (LIMBs) technologies' commercialization.12,14,15
In order to successfully implement the solid electrolyte in the microbattery, it should satisfy the following basic specifications:16,17
• High ionic conductivity 10−4 S m−1 at room temperature18 (at least >10−7 S m−1)19
• Compared to ionic conductivity, significantly lower electronic conductivity20,21
• High electrochemical, chemical and thermal stability against electrode materials19,20
• Mechanical integrity to prevent Li dendrites formation17
• Easy synthesis and manufacture on a large scale22
• Low cost and toxicity.
Solid electrolytes are typically divided into organic and inorganic types. Organic type is mainly presented by polymer-based electrolyte, which has recently been the subject of more studies due to the high achievable ionic conductivity (up to 10−4 S cm−1), relatively easy fabrication methods, flexibility, and the ability to constrain the volume changes of electrodes, such as Si.23,24
Inorganic electrolytes are usually classified into crystalline and glass-based on the material structure. Crystalline electrolytes include materials such as Li superionic conductor (LISICON), Na superionic conductor (NASICON), perovskites and garnet-type electrolytes.25,26 Glass electrolytes are mainly represented by lithium phosphate-containing compounds and amorphous phase of some crystalline electrolytes.8,27,28
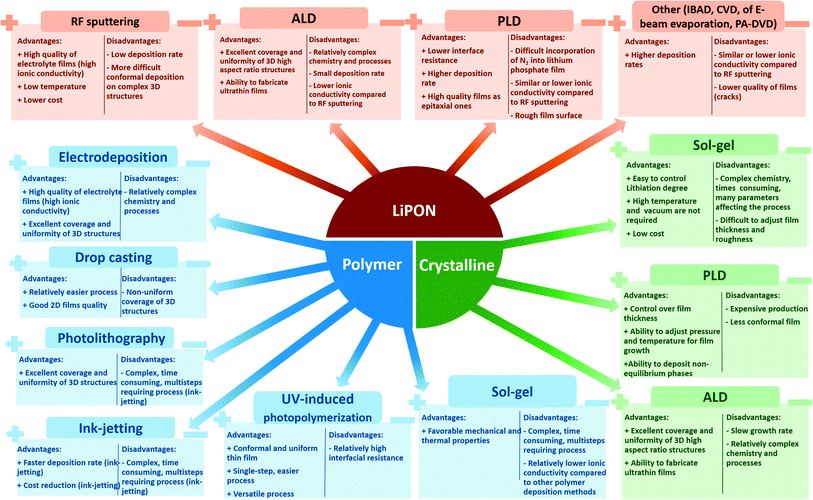
The choice of electrolyte type for microbattery depends on the application requirements. Undoubtedly, the integration of solid electrolytes into microbatteries requires specific preparation techniques. Each technique has its advantages and drawbacks that usually also have a critical impact on the decision of which type should be preferred. Among the most common techniques to obtain thin films are radio-frequency (RF) magnetron sputtering (MS), Pulsed Laser Deposition (PLD), Atomic Layer Deposition (ALD), Chemical Vapor Deposition (CVD), Electrodeposition (ED), nitrogen Ion Beam Assisted Deposition (IBAD), thermal evaporation (TE), and others.19,29–33
In fact, when ‘micro-sized’ electrolytes are developed, the same problems as for ‘bulk’ electrolytes exist along with additional specific problems. The ionic conductivity is less of a concern in microbatteries due to the shorter path for lithium ions;34 however, a lot of studies have been devoted to keeping it on a satisfying level or improving it. Equally critical is a morphology where weakness can easily result in short circuits. Another important aspect is reducing the interface resistance between the electrolyte and the interface. The structure of a 3D microbattery implies increasing the surface area allowing a decrease in the interfacial resistance.
Summarizing the above, the development of solid-state microbatteries, including attempts to create 3D types, are the focus interest of many researchers. Therefore, this work is to track the progress of the research results.
During the recent two decades, a growing number of research on Li solid electrolytes used in all-solid-state microbatteries and reviews on these studies has been done. Several review papers covered the general principles, overall information on the materials used for electrodes and electrolytes, and deposition techniques of all-solid-state thin film and 3D microbatteries.1,7,35–39 Other articles discussed all-solid-state microbatteries modelling and simulations evaluating the effect of 2D or 3D structures and mechanical stresses on the microbattery performance.23,40 The reviews with a particular focus on the microbatteries with Si anode41 and LiCoO2 (LCO) cathode42 also provided useful information on several solid electrolytes integrated into these structures. Moreover, the recent overviews for the emerging deposition techniques applied for microbatteries, such as PLD and ALD discussed the development of solid-state electrolytes.29,43,44 All these previous studies contain very important information, however, there are no specific reviews fully covering and revising the topic of solid-state electrolytes integrated into microbatteries. One review by Xia et al.19 focused on solid-state electrolytes including glass-like and crystalline materials and their properties for potential application in thin-film microbatteries. However, it was published a decade ago and did not include solid polymer electrolytes. There are many new studies published recently on glass, crystalline, polymer electrolytes, which were not covered up until now. In addition, various types of 3D-shaped microbatteries were developed and can be discussed as well.
Therefore, this review article presents an overview of the polymer, crystalline and glass-based solid electrolytes integrated and tested in LIMBs. The recent advancements, performance, and remaining challenges of thin-film and 3D solid structures with various solid electrolytes are discussed. The initial part shortly reviews the microbatteries concepts, while the following parts focus on each type of solid electrolytes in different microbattery architectures.
This review was structured as follows, first an overall brief introduction to solid-state electrolytes currently used in microbatteries and the general overview of solid-state microbattery technology with its main materials and concepts will be provided. Then the types of solid-state electrolytes will be covered in detail separately for polymer, inorganic crystalline, and inorganic glass electrolytes. Firstly, organic electrolytes, as the common electrolyte materials for batteries applications, will be reviewed. Then the materials applied mainly in their crystal structure (inorganic crystalline electrolytes) will be discussed, as they are less integrated into microbatteries. Next materials more frequently used in their amorphous state (inorganic glass electrolytes) will be covered as the most developed type for microstructures. The inorganic electrolytes are differentiated into crystalline and glass category in this review since most of the specific materials are mainly used in microbatteries in either crystalline or glass state. For example, LiPON is predominantly applied as amorphous material, while NASICON as a crystal one. Finally, the table and summarizing plots are presented to show the current situation. The provided material can serve as a good source for further research and development in solid-state microbattery technology.
2. All-solid-state microbatteries overview
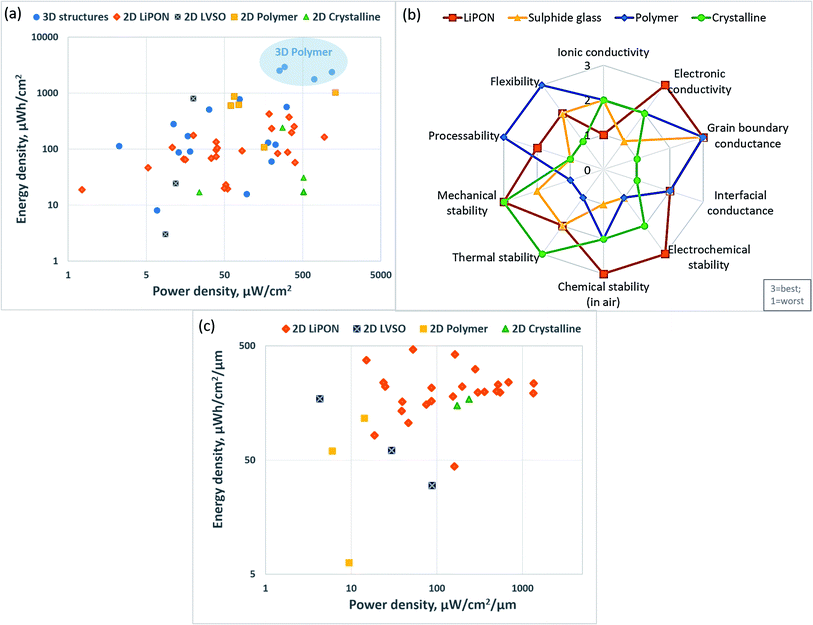
With the increasing interest in advanced micro- and nano-devices, such as radio-frequency identification tags, stand-alone sensor systems, implantable medical devices, labs-on-chip, Complementary Metal Oxide Semiconductor (CMOS) memory chips, smart cards, and others the demand for micro-energy systems is rising drastically.19,29,41,43 The energy systems can be classified based on the energy generation and storage systems, such as solar cells,45 micro-fuel cells,46 micro thermoelectric generators,47 nuclear microbatteries, and rechargeable LIMBs.48 LIMBs are considered a promising solution for powering a wide variety of small devices due to relatively higher energy density (Fig. 1a), a wider range of operating temperatures, good cycling stability, and mature manufacturing technology.41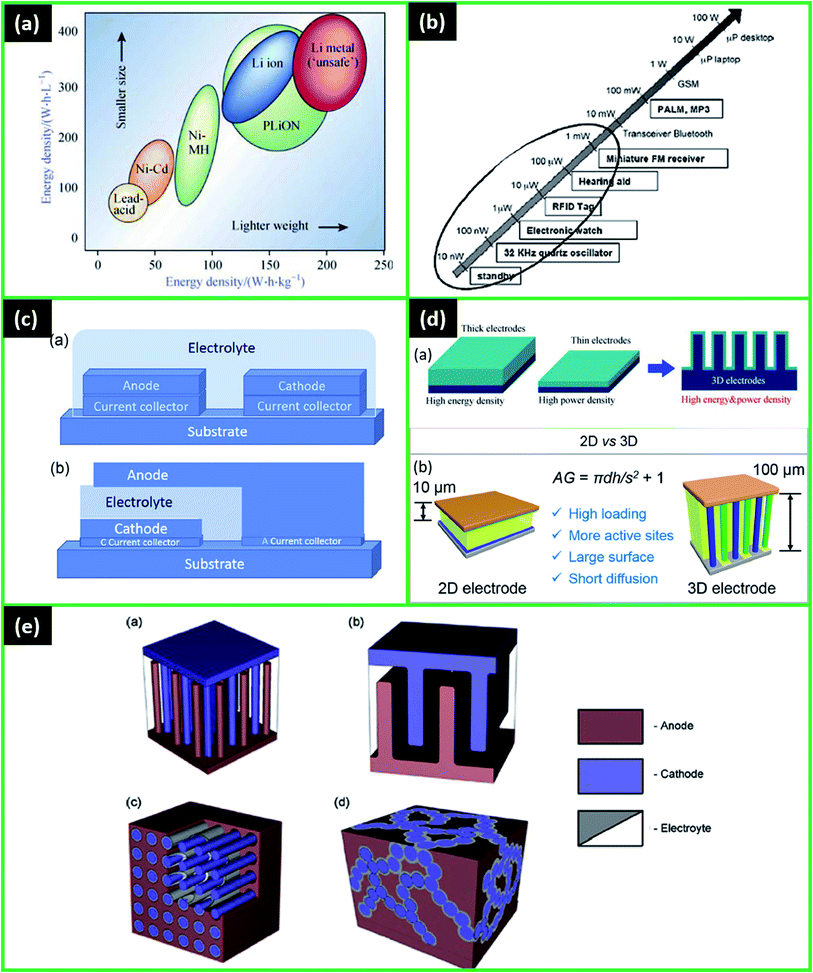 | ||
| Fig. 1 (a) Comparison of the different battery technologies in terms of volumetric and gravimetric energy density. Reproduced with permission from ref. 41; (b) typical electrical consumption for different mobile devices. Reproduced with permission from ref. 50; (c) representative configuration of (a) planar (b) stacked microbatteries. Reproduced with permission from ref. 1; (d) (a) advantages of the 3D battery structure, (b) superiority of 3D vs.2D electrodes is illustrated with schematics (where AG – area gain of 3D, d – nanorod diameter, s – nanorod spacing, and h – rod height). Reproduced with permission from ref. 41 and 49; (e) 3D micro LIB structures: (a) interdigitated rod electrodes, (b) interdigitated plates or 3D-trench, (c) concentric tube, (d) 3D aperiodic sponge. Reproduced with permission from ref. 66. | ||
The principle of work for rechargeable microbatteries is similar to standard LIBs where Li-ions transfer between anode and cathode, and charge carriers produce electrical energy. The electrodes are also separated by an electrolytic solution or solid-state electrolyte.37 The greatest difference between traditional batteries and LIMBs is the volume, where a microbattery is typically smaller than 0.01 cm3.1 As a result, geometry along with suitable materials are critical for microbatteries' power densities.1,37 The energy consumption for typical microelectronic devices is about 10 mW and may range between tens of nanowatts and tens of milliwatts (Fig. 1b).1,49,50 Most of the microbatteries can generate these energy and power densities in the span of 30–300 μW h cm−2 μm−1 and 0.0001–10 mW cm−2 μm−1, respectively, which in many cases might be insufficient to fit inside of microdevices and fulfil their energy requirements.1,49 Therefore, making the advancement in materials and cell geometry is a primary goal to increase the energy density of microbatteries.
Replacing liquid electrolytes with solid-state electrolytes in microbatteries is in more demand these days, as it is safer, it brings higher thermal and electrochemical stability, enables Li metal anode, and automatically excludes the necessity of additional electrical insulators.19,37 Separators with liquid electrolyte have an average thickness of 20 μm or more, whereas solid electrolytes typically have thicknesses of 1 μm in microbatteries.37 Furthermore, liquid electrolytes also lead to more complicated packaging to avoid safety issues.7
With the recent successful developments in solid-state ion-conducting materials, the rapid progress towards LIMBs solidification, miniaturization, and commercialization was observed. The first all-solid-state microbatteries had a 2D thin-film structure. The structure of 2D usually has several layers deposited either in a planar or a stacked way (Fig. 1c).1
The most commonly used materials for thin-film microbatteries include LCO for a cathode,51–56 Li metal for an anode,44,56–58 and liquid Li-ion compounds and solid materials, such as lithium phosphorus oxynitride (LiPON),37,56,57,59 LISICON,60 NASICON,61 poly(ethylene oxide) (PEO)-based polymer62–64 and others for electrolytes. The early studies showed that all-solid-state 2D microbattery, having a more homogeneous current distribution, proved to be more stable and more resistant to high temperatures during cycling.56,65 Moreover, good compatibility of microbatteries with the manufacturing processes of solar panels and circuits resulted in the commercialization of some thin-film batteries' designs (Table 1).7,19,29,57 Nevertheless, 2D microbatteries struggle to provide sufficient power and energy to the increasing demand of the fast-growing small power applications, especially MEMS devices.1 The possible approach to increase the energy density of 2D all-solid-state microbatteries is to increase the thickness of electrodes.43 However, this will compromise the power density, as the diffusion path of Li-ions will become longer and the expanded size will complicate integration into microdevices. Besides the major problem of large footprint area needed to deliver the required capacity, another issue is the dependency of the current density distribution on the relative distance between the electrodes.1
| Manufacturer | Electrochemical chain | Specifications |
|---|---|---|
| Cymbet Co. | EnerChip™ LiCoO2/Li | 60 μA h cm−2 μm−1/5000 cycles/4–4.15 V |
| Infinite Power Solutions | LiCoO2 or V2O5/LiPON/Li | “Thinergy” 40 μA h cm−2 μm−1/4.1 V |
| Front Edge Technology | LiCoO2/LiPON/Li | “Nanoenergy” 0.9 μA h cm−2 μm−1 |
| Ulvac Inc. | LiCoO2/Li3PO4/Li | 50 μA h cm−2 μm−1 |
| STMicroelectronics | LiCoO2/LiPON/Li | 700 μA h/discharge/5 mA/3.9 V |
| Excellatron | LiCoO2–LiMnO2–LiPON–Sn3N4 | 0.3 μm thick/0.1 mA h/2000 cycles/3.9–4.1 V |
| Enfucell | MnO2-based cell | Voltage rating > 3 V |
| GS Caltex | n/a | 300 μm thick/3.9 V/8000 cycles |
| Ilika Inc. | Stereax® M250 medical | 174 μA h cm−2, 3.5 V |
| Wyon | CP1254 | 160 μA h/3.7 V/2 mm thick |
| Varta | 60 mA h/3.7/5.4 mm thick |
For microbatteries, the key issue is to fit within the small size of microdevices and at the same time to have high energy and power densities.1 Since 3D designs result in high energy density while occupying a minimal footprint area, they have been extensively researched.9 Compared to 2D designs with the limited surface area, 3D-structured cells demonstrated higher microbattery performance compared to 2D microbatteries in several studies.7,37 In the 3D arrangement, the larger specific surface area allows usage of more active material in the same footprint area, directly increasing aerial capacity, and thus, energy and power densities as well (eqn (1) and (2)). The quantification of advantages of 3D architectures was also provided by Long et al.9 In addition, in 3D structure, the shorter Li-ion diffusion lengths result in higher power density (Fig. 1d).41,49 Thus, both the energy and power density can be increased.
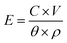 | (1) |
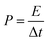 | (2) |
There are many studies on the various 3D designs for microbatteries, and the most common ones are demonstrated in Fig. 1e.1,66 Among them, interdigitated architectures are more easily manufactured and have a lower risk of short circuits between the electrodes. In other designs, pinholes in the extremely thin electrolyte/separator layer can be formed as a result of electrode materials' volume change and, thus, cause the battery to fail.1 Therefore, it is critical to find the optimal distance between the electrodes that will allow the maximum possible energy density without significantly raising the short-circuit risk. Up to now, several review papers have specifically covered the 3D architectures of batteries, their structures, modelling, and common materials.9,10,16,67–69
Undoubtedly, the choice of materials for the battery has a fundamental role to achieve desirable performance. Thus, careful selection is crucial. As a cathode material in 3D all-solid-state LIMBs compounds such as LCO,70–72 spinel structures, such as LiNi0.5Mn1.5O4 (LNMO),73–75 LiFePO4 (LFP),76,77 vanadium oxides,31,78 and others have been utilized. Currently, extensively studied LCO with relatively high capacity, stability, and mature manufacturing procedure prevails as a cathode material, while other promising materials are under development.7,37,71
For the anode, Li metal is the commonly used material due to its high specific capacity, low electrochemical potential, and weight.7,37,44,70,79 Moreover, for solid-state electrolytes, the problem with Li dendrite formation is of less concern.7 Other anode materials include elements of the IVA group (Sn, C, Si, etc.).31,80,81 For example, Li–Si alloy has demonstrated high specific capacity (3580 mA h g−1), with the only concern of Si volume expansion that can be possibly mitigated by proper architecture or other methods.37,71,72,82 Titanium-based structures (TiO2, Li4Ti5O12 (LTO)) are also among the anode candidates. LTO, for example, can show good cyclability with a small volume change and yet relatively low capacity (160 mA h g−1).7,73–75,83 As LTO suffers from a high charge–discharge potential of 1.5–1.6 V, it acts as a high-voltage anode or low-voltage cathode, and this narrows the cell potential.37 So LTO can be used in applications where stability and low-temperature applications are prioritized over energy content.37 Many studies have also suggested and tested TiO2 structures as anodes due to good lithium intercalation behaviour, reasonable capacity retention, and lower risk of Li dendrites formation compared to carbonaceous materials.68,84–86 The interest grew especially in using TiO2 for 3D designs as self-supporting oxide satisfying the criteria of 3D electrodes with high capacity and cyclability.68,84–87 In addition to the many experimental investigations, theoretical calculations of the highest achievable capacity have been done for the optimized architectures of TiO2 micropillars.88
Over the last decade, many studies have been investigating the important component for microbatteries – solid-state electrolyte.
In this review paper, the focus is to cover the recent advances in LIMBs from the point of view of various solid electrolytes and to identify new potential variants for the next-generation all-solid-state microbatteries.
3. Polymer electrolytes
Polymer electrolytes (PE) are one of the promising solid electrolytes for Li microbatteries due to several advantages including good ionic conductivity (up to 10−4 S cm−1), improved flexibility, ability to accommodate volume changes, easy processing, and the recently improved mechanical strength (106–108 Pa) which helps to prevent Li dendrites formation.23,24,89–91 The essential benefits of using a polymer electrolyte in the 2D and 3D structured microcells can be its ability to reach the very narrow spaces between the electrodes and, consequently, to provide good contacts. PEs are fabricated by dissolving Li salts (LiPF6, LiClO4, LiFSI, LiTFSI, LiDFOB, etc.) in the polymer matrix (polyethylene oxide (PEO), poly(methyl methacrylate) (PMMA), polyacrylonitrile (PAN), polyvinylidene fluoride (PVDF), and its derivatives, etc.).24,89 They can be categorized as solid polymer electrolytes, composite polymer electrolytes, and gel polymer electrolytes. Solid polymer electrolytes (SPEs) are dry polymers and are solvent-free systems in which organic liquid is not used.92 The early SPE demonstrated low ionic conductivity of approximately 10−7 S cm−1.93 Later, it was found that it is the amorphous phases rather than crystalline that act as the transmission host for Li-ions that move through polymer chains.94 Thus, several methods were suggested to increase the presence of the amorphous phase, including crosslinking, addition of large side-groups to the polymer chains, and addition of plasticizers.37,94 Composite polymer electrolytes are SPEs where polymers could be blended, cross-linked, doped, reinforced by additives and inorganic fillers,92 whereas, gel polymer electrolytes are also known as plasticized PEs.92 Polymers are plasticized and get swollen by a liquid electrolyte. Generally, PEs can be produced by several techniques, including the evaporation method, drop-casting from a polymer solution, in situ plasma polymerization, and electrodeposition.94–97 Despite being attractive alternatives to glass electrolytes, PEs have several disadvantages, such as low voltage window, unreliable electrochemical and thermal stability, and deficient mechanical integrity to suppress the Li dendrite growth.23 The minimization of these disadvantages is the aim of most studies, as well as the investigation of electrode/electrolyte compatibility and optimal cycling conditions. There are numerous in-depth reviews on SPEs based on various polymer matrices, active/inactive fillers, composite and hybrid components. The readers are asked to refer to those for further investigation.92,98–100 In this section, we are going to review PEs that are used to fabricate all solid-state LIMBs.3.1 Solid polymer/composite polymer electrolytes
Among SPEs, polyethylene-based electrolytes are one of the most commonly used polymer electrolytes due to a promising achievable ionic conductivity (10−7–10−4 S cm−1),94 good compatibility with solid-state Li microbattery materials,73 and thermal stability.101 In addition, they possess faster dynamics at room temperature and excellent capability to solvate large concentrations of lithium salts.102 Deposition of the electrolytes were mainly done by drop casting or dip coating and electrodeposition, as well as other deposition methods onto electrode materials, which were produced by traditional methods. Since dip/drop coating of PEs may not coat complex structures of electrodes uniformly for high capacity/power batteries, the research has also been focused on other electrolyte deposition methods. The electrodeposition technique was proposed to produce more conformal coating of the electrolyte. Other methods of deposition of SPEs include a range of techniques, such as infiltration of polymers into structured electrodes, spin coating, deposition onto electrodes, and patterning using photolithography or UV-polymerization, as well as CVD growth onto electrodes.3.1.1.1 Polyethylene polymers. Drop casting and dip coating are widely used deposition techniques of SPEs. Several investigations of 2D thin-film batteries with PEG-based electrolytes have been conducted, with the results being less successful compared to cells with LiPON electrolyte due to polymer electrolyte stability issues. For instance, the covalent silica–PEO–LiTFSI (SiO2–PEG) hybrid solid electrolyte was prepared via sol–gel and organic polymerization methods and tested in thin film Li/SiO2–PEG/Li4Ti5O12 cell, where the electrolyte and the cathode were dip-coated thin films.63 It was found that the produced electrolyte had good ionic conductivity of 2.6 × 10−5 S cm−1 with a Li-ion transference number of 0.37, while the microbattery demonstrated a decrease of capacity from 2.75 to 0.8 mA h g−1 after 500 cycles at the current density of 1.0 mA cm−2 and in the potential window of 1–2 V.63 Further capacity fading was observed due to the deterioration of the electrolyte/electrode interface, proved by the increased resistance of the cell.63
In another study of thin-film battery, PEO based BAB block copolymer with the improved ionic conductivity of 2 × 10−4 S cm−1 was integrated into the cell with LMO cathode and Li4/3Ti5/3O4 anode composite electrodes that were micro-injected onto Au current collectors.64 The resulting microbattery showed an initial energy density of 8.48 μW h cm−2, which was lower compared to the cell fabricated by the sputtering method. Nevertheless, the important advantage of this technique is it being a cheap and simple process.64
3D designs with SPE were more successful and received larger attention as the ability of SPE conformal deposition allowed the relatively reliable electrode–electrolyte interface formation and, as a result, improved performance. One of the first constructed 3D microbatteries with SPE contained the drop-casted polymer electrolyte polymethyl methacrylate–polyethylene glycol (PMMA–PEG) combined with titanium nanotubes (TiO2nts) anode and an LNMO cathode (Fig. 2a).73 Cells were assembled by squeezing together the TiO2nts that were grown by anodization of Ti foil and LNMO casted on Al foil. The cell demonstrated the stable capacity of 80 mA h g−1 (30 μA h cm−2 μm−1) in the potential window of 1–3.3 V and at the rate of C/10. The coulombic efficiency and capacity retention remained at 96.7% and 91.5%, respectively, for 35 cycles. Nevertheless, it was found that the performance of this microbattery can be significantly improved if the large surface area of TiO2nts would have been fully utilized.73
 | ||
| Fig. 2 (a) (a) SEM images of cross section of the all-solid-state battery composed of TiO2nts/polymer electrolyte/LNMO: (b) enlarged view of the self-organized TiO2nts. The inset shows the top view of the nanotubes. Reproduced with permission from ref. 73; (b) schematic illustrations showing the step-by-step fabrication of the all-solid-state Li-polymer 3D-microbatteries based on: (a) a LiFePO4-coated carbon foam electrode and (b) a Cu2O-coated Cu nanopillar electrode. Reproduced with permission from ref. 104. Copyright (2018) American Chemical Society. | ||
3.1.1.2 Non-polyethylene polymers. Non-polyethylene polymers have also been studied in an attempt to introduce more appealing properties of PEs, such as higher cationic transference number. Sun et al., for example, fabricated a novel polymer electrolyte of poly(ether amine) (PEA)-based monomer where one of the oligomer chains was substituted by the methacrylic group to add the polymerizable functionality.103 It was then mixed with LiTFSI salt, and the resulting SPE demonstrated ionic conductivity of 8 × 10−6 S cm−1 at 60 °C. Using UV-polymerization, SPE with a thickness of 1 μm was integrated into thin-film LFP/SPE/Li battery (with standard LFP composite electrodes), which had 140 mA h g−1 capacity that faded after 12 cycles due to the formation of Li dendrites.103
Later, the same research group investigated another SPE based on poly(trimethylene carbonate) (PTMC) in two different 3D structures: carbon foams dip-coated with LFP and electrodeposited Cu nanopillars with Li counter electrode (Fig. 2b).104 The new SPE was proposed due to the expected higher ionic conductivities (10−5 S cm−1) compared to PEO (10−5–10−8 S cm−1). It was found that, although the functionalized P(TMC-OH) electrolyte resulted in the conformal coating of LFP unlike PTMC-based electrolyte without functionalization, both showed poor electrochemical performance and the latter even led to short circuits as a result of poor contact. That unsatisfactory behaviour was also attributed to the limited interfacial contact with Li electrode, insufficient ionic conductivity and lack of electronic wiring. The Cu 3D structure with P(TMC-OH) electrolyte, on the other hand, demonstrated more promising performance, with stable cycling at a capacity of 0.2 mA h cm−2 and a current rate of 8 μA cm−2.104
Tan et al. tested a microbattery with electrodeposited nanotubes of Cu2Sb and Li metal electrodes.105 Poly(propylene glycol) diacrylate (PPDGA) and polyetheramine (glyceryl poly(oxypropylene)) (PEA) blend with LiTFSI was chosen as an electrolyte, the conductivity properties of which were found to be in the order of 10−6 S cm−1.106 The battery showed moderate electrochemical performance with the capacity of 0.05 mA h cm−2 at C/50 rate and voltage range of 0–2 V after 50 cycles. That was approximately 10 times larger than that of 2D microbattery, which proved that the bigger footprint area resulted in higher energy produced. However, the capacity for 3D structure showed a diminishing trend which was expected due to the loss of contact between the electrodes and electrolytes during cycling as a result of the electrode's volume changes.105
3.1.2.1 Polyethylene polymers. The microbattery of TiO2nts/PMMA–PEG/LNMO (Fig. 3a) demonstrated a twofold increase of capacity (to 150 mA h g−1 (70 μA h cm−2 μm−1) at C/10 after 10th cycle) due to the complete filling of electrodes by the electrodeposited electrolyte.74 Moreover, even after the 100th cycle, the capacity of electrodeposited polymer microbattery was two times larger than the one for the drop casted electrolyte.74 Similarly, as a good candidate for 3D Li-ion microbatteries, PEO–PMMA electrolyte electrodeposited on the anodized nanostructured titania electrode was tested in Li/PEO–PMMA(LiTFSI)/TiO2nts coin cell.108 In this cell, the capacity decreased from 83 to 65 μA h cm−2 μm−1 after 50 cycles at C/5. The enhanced interface between electrode and electrolyte by proper covering and filling of nts area by PEO–PMMA using the electrodeposition allowed improved capacity compared to LiPON. Moreover, the thickness of the electrolyte (<1 μm) proved the ability to withstand the volume variations of the electrodes.108 The research team under the same main investigator attempted to improve the microbattery performance by electrodepositing PMMA–PEG on porous lithium nickel manganese oxide (LNMO) spread on Al disk.75 After that, the electrodes were pressed together with a drop of electrolyte in between. The TiO2nts/PMMA–PEG/LNMO microbattery demonstrated the capacity of 89 mA h g−1 (44 μA h cm−2 μm−1) between 1–3.3 V and at C/10, corresponding to the 72% capacity retention.75 It was found that, again, the electrodeposition of polymer electrolyte on LNMO significantly improved the performance of the microbattery.75
 | ||
| Fig. 3 (a) (a) Schematic representation and (b) cross-sectional SEM image of the all-solid-state battery composed of TiO2nts(EP)/polymer/LNMO(EP). Reproduced with permission from ref. 74; (b) schematic of a three-dimensional solid-state interpenetrating cell. Reproduced with permission from ref. 1; (c) assembly of a penta-continuous interpenetrating and nanostructured hybrid from double gyroidal mesoporous carbon (GDMC) monoliths: (a) schematic illustration of the synthesis pathway, (b) photographs of the as-made BCP-organic hybrids (top left), GDMC monoliths after carbonization (top right), and GDMC monoliths electrically contacted in the edge-on geometry (bottom), (c and d) SEM images of a GDMC monolith exhibiting uniform thickness (c), surfaces with open and accessible gyroidal mesoporosity (c, inset), and uniform gyroidal cross-section (d). Reproduced with permission from ref. 81. | ||
Recently Abdelhamid et al. electrochemically grafted and polymerized (PEO)-acrylate-based electrolyte and obtained pinhole-free and homogeneous film on 3D metal cylindrical micropillars.109 The resulting electrolyte had relatively high ionic conductivity (10−4 S cm−1).109 Although the testing of microbattery has not been conducted, it is expected that homogeneous SPE film will positively impact the microbattery performance. Moreover, this approach was shown as a promising way to control electrolyte formation by varying the potential and duration of the polymerization process.
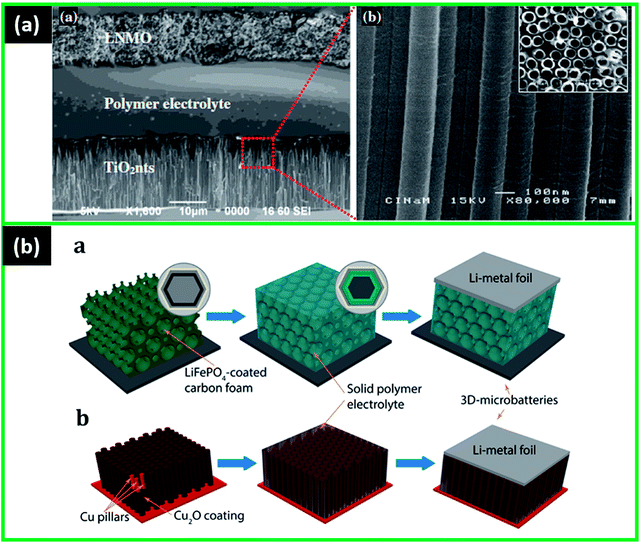
3.1.2.2 Non-polyethylene polymers. The other non-PEO based polymer electrolytes in thin film and 3D microbatteries have also been studied recently. The early investigation of the 3D structures was done on the interpenetrating cell consisting of infiltrated VO cathode ambigel assembled with a macroporous polymer electrolyte coated carbon anode.80 The poly(phenylene oxide) (PPO) was used as an electrolyte and electrodeposited on the three-dimensionally ordered (3DOM) carbon, where the macropore scale of the structure was then filled with vanadia aerogel-like material cathode (Fig. 3b).80 When cycling, the initial discharge capacity was observed to be around 9 mA h g−1, which reduced to approximately 1 mA h g−1 after 10 cycles between 1.6–3.3 V. Although the capacity was improved due to the increased area, the issue with the cathode's electronic and ionic conductivities hindered the wide implementation of this 3D battery.80
The research by Werner et al. investigated the innovative gyroidal 3D structure with poly(phenylene oxide) (PPO) solid electrolyte. The polymer was electrodeposited on gyroidal mesoporous carbon (GDMC) monoliths (anode), and then lithium sulfide–polymer composite was infiltrated as a cathode (Fig. 3c).81 The electrochemical testing of this structure demonstrated 0.225 mA h cm−2 capacity in the voltage window of 1.5–3 V and 0.125 mA cm−2 current rate with almost no fading after 10 cycles. These results, with the capacity being improved almost 45 times, were better than the previously developed solid-state 3D prototype with nanoscale dimensions.80 The polymer electrolyte (10 nm) also showed sufficient stability with a conformal and layer impermeable to the cathode, which helped to avoid short circuits.81 However, the small open-circuit voltage due to the current leakage and large polarization in electrolyte introduced some issues.81 Moreover, the volume changes of sulfur caused the loss of connection with the current collector and formed electronically disconnected parts, leading to small specific capacity (20% of theoretical value).81
Another microbattery consisted of TiO2nts and Li anode was tested with a new polymer electrolyte-p-sulfonated poly(allyl phenyl ether) (SPAPE).96 The electrolyte was electrochemically deposited on TiO2nts, which were also electrodeposited, forming a conformal layer. The cycling showed that the microbattery could deliver approximately 60 μA h cm−2 capacity after the 4th cycle (at a C/8 rate). In general, the performance of the cells had an improved areal capacity, and its retention depended on the electrochemical synthesis parameters of the polymer electrolyte.96 Later the same SPAPE electrolyte was investigated on carbon nanotubes (CNT). The CNT/SPAPE/Li microbattery delivered a capacity of 750 mA h g−1 (276 μA h cm−2) even after 110 cycles at 1C, which is 67% more than pristine CNT.110
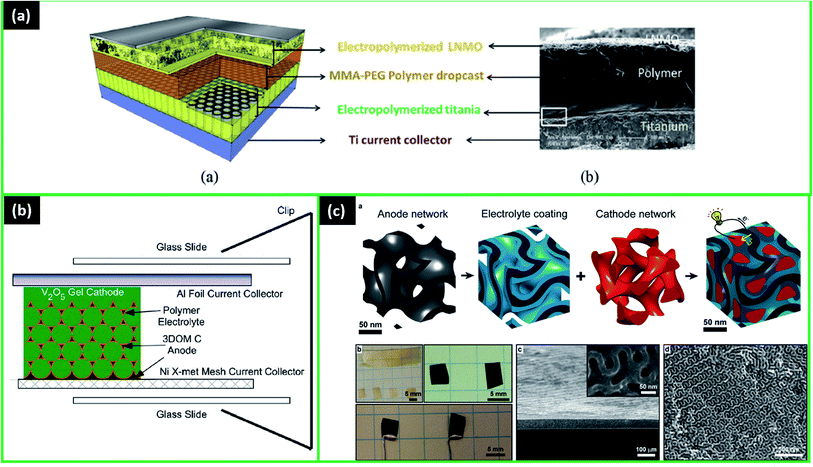
3.1.3.1 Polyethylene polymers. A few examples of particular application of the above-mentioned techniques on the microbattery will be discussed. Firstly, Hur et al. fabricated an interesting 3D microbattery Si/SU-8/LiNi0.8Co0.15Al0.05O2 (NCA), where SU-8 is a promising polymer electrolyte that has a structure similar to PEG (Fig. 4a).82 The SU-8 was photolithographically patterned on Si arrays, showing high conformity but low ionic conductivity of approximately 2.8 × 10−7 S cm−1. The cathode slurry was applied dropwise over the anode array. The microbattery's maximum delivered capacity was found to be 0.55 mA h cm−2 at the current density of 0.22 mA cm−2, and after 100 cycles, it was 0.5 mA h cm−2 (potential range 2.6–3.7 V). These results were clearly better than for 2D structured microbatteries. However, the silicon pulverization problem, causing non-uniform conductive areas, led to the electrochemical degradation of this structure.82
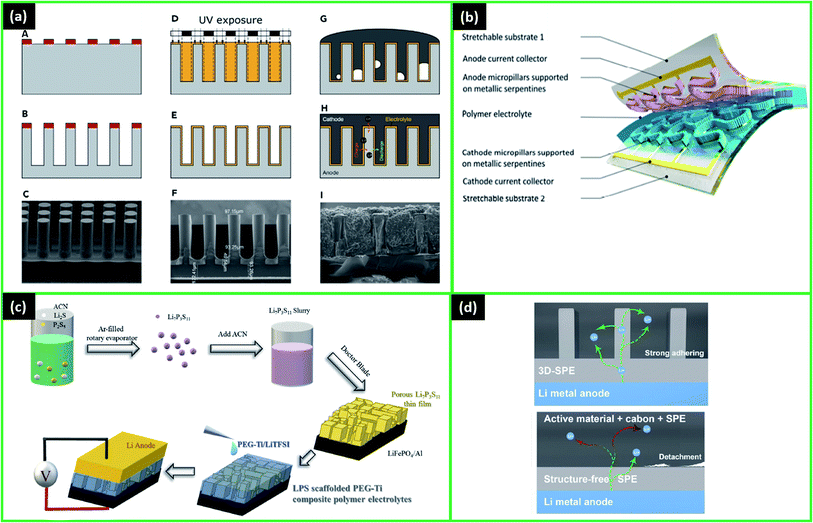 | ||
| Fig. 4 (a) Fabrication scheme for 3D battery based on SU-8-coated silicon arrays: (A) silicon wafer is coated with oxide and array pattern is etched, (B) 3D post array is etched into silicon, (C) SEM image of silicon array, (D) SU-8 photoresist is selectively cross-linked around the silicon posts by photolithography, (E) uncross-linked SU-8 is removed in a developer bath and base layer is cross-linked, (F) SEM image of SU-8-coated array, (G) vacuum infiltration of cathode slurry, (H) charging schematic of complete 3D battery, (I) SEM image of full 3D battery. Reproduced with permission from ref. 82; (b) schematic view of the stretchable LIMB. Reproduced with permission from ref. 83; (c) schematics of fabrication procedure for Li-ion batteries with LPS-scaffolded solvent-free PEG–Ti composite polymer electrolyte. Reproduced with permission from ref. 62; (d) schematic illustration of all-solid-state Li-metal battery with stereolithography 3D printing SPE and structure-free SPE. Reproduced with permission from ref. 77. Copyright (2020) American Chemical Society. | ||
There has been a research of a special stretchable design of micropillar electrodes supported on metallic serpentines. There the polymer electrolyte composed of 0.5 M of LiTFSI in MA-PEG500 was spin-coated onto the innovative structure with LNMO and LTO electrodes, which were deposited by doctor-blade technique and laser ablated to achieve micropatterns (Fig. 4b).83 Such architecture not only resulted in the increase of capacity by 2.5 times (1 mA h cm−2 at C/2) compared to the 2D structure but also showed good performance under the mechanical strain and very small capacity fading over 100 cycles.83 Thus, this microbattery proved to be a promising approach with a further target to increase the energy density by varying the electrode materials and improving the micropillars' density.
Other interesting LIBs structures with PEO-based electrolytes have also been tested in coin cells. However, due to promising results, it was concluded that the techniques could potentially be adapted for microbattery technologies. For instance, Cai et al. developed a new low-cost processing method of microbatteries, where, firstly, the Li7P3S11 (LPS) porous scaffold was prepared, and then, PEG–Ti hybrid polymer electrolyte was infiltrated into it (Fig. 4c).62 The constructed composite electrolyte showed a relatively high ionic conductivity of 1.6 × 10−4 S cm−1. The microbattery with this electrolyte, Li anode and LFP cathode, delivered 103 mA h g−1 capacity (2.5–4 V, 0.05C) stable for the first 8 cycles.62 Therefore, the functionality of this battery manufactured by the innovative method was proved. However, to evaluate the longer cycling performance, more experiments are required. In another study, He et al. proposed the adopted method of stereolithography to produce Li/SPE/LiFePO4 3D microbatteries, where SPE was PEO–succinonitrile (SCN)–LiTFSI (Fig. 4d).77 The electrolyte had high ionic conductivity (3.7 × 10−4 S cm−1) at room temperature, and cells achieved a higher capacity of 128 mA h g−1 after 250 cycles at 0.1C and stable cycling compared to 2D microbattery (32 mA h g−1),77 hence, proving that this technology is a promising candidate for microbattery fabrication.
3.1.3.2 Non-polyethylene polymers. Several composite polymer electrolytes have also been tested in 2D thin-film batteries with promising results. One of such composite polymer electrolytes included dimethacrylic oligomer bisphenol A ethoxylate dimethacrylate (BEMA), diluent poly(ethyleneglycol) methyl ether methacrylate (PEGMA), ethylene carbonate/diethyl carbonate (EC/DEC), and LiTFSI.91 It was prepared on the VO electrode using UV-induced photo-polymerization deposition and then assembled with Li anode in a thin-film cell. It delivered a capacity of 130 mA h g−1 at the first cycle and 1.5C rate and after 300 cycles at the higher 5C rate, the capacity decreased to 100 mA h g−1.91 Such good stability was attributed to the electrolyte conformal coating and intimate contact between electrode and electrolyte.91
The new method of chemical vapour deposition was applied to deposit a series of copolymer films based on hydroxyethyl methacrylate and ethylene glycol diacrylate on 3D TiO2nts.111 The results showed that conformal coating with the electrolyte can be produced, and the tuning of electrolyte properties (ionic, electronic conductivity, mechanical strength) is possible with CVD.111 However, the full cell testing for these films has not been done yet and needs further investigations.
3.2 Gel polymer electrolytes (GPEs)
GPEs are easily made by heating a mixture containing a polymer matrix, a lithium salt, and a solvent.92 The mixture is then cast in a hot state and cooled to form a thin film under the pressure of electrodes.92 GPEs have mainly been investigated for applications in traditional Li-ion batteries. Nevertheless, some studies indicated the fabrication of microbatteries using GPEs due to the possibility of increased ionic conductivity (10−3–10−4 S cm−1) compared to common polymer electrolytes as well as shape flexibility.112–114 In this review, the microbatteries with gel electrolyte having mostly solid phases are considered. Park et al. investigated the gel polymer electrolyte poly(vinylidene fluoride-hexafluoro propylene) (PVDF-HFP) fabricated by the solvent casting method and tested in a thin-film battery of Li/PVDF-HFP gel/LCO.115 The cathode was screen printed onto a platinum current collector/SiO2/Si wafer using a stainless 400-mesh screen. The cell displayed 164 μA h cm−2 capacity at the rate of 20 μA cm−2 and in the potential window of 3–4.2 V.115 This was 23 times higher than that of LiPON as a result of the improved contact area between the cathode and the electrolyte.115 However, more tests with larger numbers of cycling and stability observations need to be done for further development of this electrolyte.Similarly, another research group also studied GPE consisting of PVdF-HFP, P13FSI-pyrrolidinium bis(fluorosulfonyl)imide, and LiTFSI.116 The GPE was coated on Li and macroporous silicon (pSi) to construct the Li/GPE/LCO(sputtered) and pSi/GPE/LCO(sputtered) microbatteries, respectively.116 The Li/GPE/LCO cell demonstrated good stability in the voltage range of 3–4.2 V and showed 264 μA h cm−2 capacity at the high current rate of 333 μA cm−2 and 98.9% retention even after 30 cycles.116 At the same time, pSi/GPE/LCO had the initial capacity of 226 μA h cm−2, which decreased to approximately 180 μA h cm−2 after 30 cycles. The high ionic conductivity of the GPE (1.88 × 10−3 S cm−1 at 25 °C) and compatibility with electrode materials might have led to the successful functionality of both structures.116
Another composite polymer electrolyte (CPE) composed of LiI1P(EO)20EC, 12% (v/v) Al2O3 structure was investigated in the thin-film Li/CPE/FeS1+x cell.117 The cell demonstrated stable behaviour with the initial capacity of around 50 mA h at the current rate of 50 μA cm−2 and around 39% capacity loss after 650 cycles, which is considered as significant deterioration.117 However, a few details are available on the CPE properties and characteristics since a deeper understanding of FeS1+x cathode was targeted for further work.
One of the recent studies tested the new 3D interdigitated structure with VO cathode, Li anode, and GPE composed from PEO, LiTFSI, 1,3-dioxolane (DOL), and 1,2-dimethoxyethane (DME) (Fig. 5a).78 The innovative microbattery delivered 0.15 mA h cm−2 areal capacity, 73% of which was retained after 550 cycles at 1C, proving a good stable behaviour. The peak energy and power densities of 1.2 J cm−2 and 75.5 mW cm−2, respectively, were observed at 100C, which is considerably larger than other packaged microbatteries' performance parameters, thus making this 3D microbattery one of the promising candidates for further development and integration in the autonomous devices.78 Kil et al. have done research on 3D pillars structure with UV-curable GPE (ethoxylated trimethylolpropane triacrylate (ETPTA) monomers, liquid electrolytes, and alumina nanoparticles) with ionic conductivity of 1 mS cm−1.113 The UV curing was used here to promote cross-linking of the polymer matrix for solidification. The structure from micropillar Si anode, micropatterened GPE, and Li as a counter electrode was cycled, and it was found that the initial high-charge capacity 2680 mA h g−1 faded to approximately 700 mA h g−1 after the 10th cycle.113 Although the retention is not favorable, it was shown that optimized alumina content and fabrication technology resulted in the dimensionally stable, conformal, and bendable GPE.
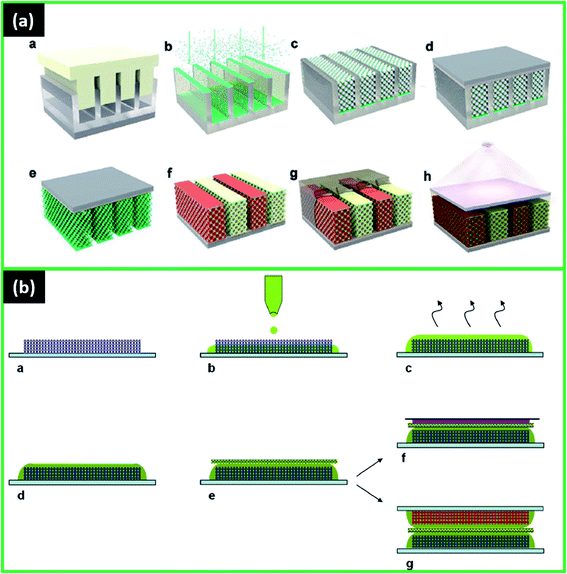 | ||
| Fig. 5 (a) Schematic of the microbattery fabrication: (a) PMMA template is fabricated by nanoimprinting, (b) vertical e-beam evaporation of Ni on the PMMA template, (c) structure after selective removal of Ni film from the top of the PMMA template, assembly of a PS opal in the PMMA template, and Ni electrodeposition through the PS opal, (d) bonding of (c) to a polyimide film using an epoxy adhesive, (e) etching of the PMMA template and polystyrene opal to expose the interdigitated 3D microelectrodes, (f) VO (cathode) and Li (anode) electrodeposited on the appropriate interdigitated Ni fingers, (g) infiltration of PEO/LiTFSI/DOL/DME gel electrolyte, (h) packaging of the cell with UV-cured NOA. Reproduced with permission from ref. 78; (b) scheme of half cell and Li-ion full cell realization: (a) tape cast porous positive (resp. negative) composite electrode on aluminum current collector, (b) ionogel precursor printing on electrode: filling of the composite electrode porosity and obtaining separator effect, (c) polycondensation of ionogel precursor, (d) all solid positive (resp. negative) electrode and ionogel assembly is obtained, (e) addition of fiberglass separator soaked in ES liquid electrolyte, (f) half-cell with lithium metal counter electrode and copper current foil, (g) Li-ion full-cell formed by face-to-face stacking of negative electrode/ionogel assembly and positive electrode/ionogel assembly separated by fiberglass soaked in ES liquid electrolyte. Reproduced with permission from ref. 14. | ||
Another type of solid polymer electrolytes – ionogel that consists of molten salt and the inorganic matrix has attracted more attention due to high Li conductivity (up to 10−3 S cm−1) and ability to create a conformal layer favourable for 3D structures.14,118,119 First, testing planar structures, Delannoy et al. applied a new method of ink-jetting the silica-based ionogel (PYR13-Li-TFSI: N-methyl-N-propylpyrrolidinium bis(trifluoromethan) suflonylimide and LiTFSI) on the electrode surfaces (Fig. 5b).14 The resulting thin-film battery of Li4Ti5O12/ionogel/LFP appeared to have a steady surface capacity of 300 μA h cm−2, approximately double of liquid electrolyte cell (145 mA h cm−2) after 100 cycles at the C/10 rate and potential window of 1.5–2.5 V.14 The satisfactory results confirmed that the ink-jet printing, being a fast and cheap technique, could be a good candidate for polymer electrolyte deposition. It might be important to highlight the recent battery is not considered a microbattery, but due to very promising results was proposed for further testing in the microbattery structure. The new 2.5D structure with LFP pillars covered with ionogel and planar Li showed excellent performance with power and energy densities of 2.8 mW cm−2 and 3.7 mW h cm−2, respectively, that were attributed to the conformal cover of pillars by the electrolyte.118 These results are currently best-reported solid-state 3D battery densities. Therefore, more studies on this and other 3D designs and promising ionogel electrolyte applications in microbatteries are required.
In general, there are many SPE preparation methods examined for various polymer materials. The deposition techniques were rather unique in each case as many polymer compositions have been tested. Among those discussed in this paper, the most common methods include electrodeposition applied for PMMA–PEG,74,75,108 SPAPE96,110 and drop-casting used for PMMA–PEG,73 PEA,105 PVDF-HFP.115 Based on the findings, electrodeposition has been proved to be an effective technique, which provided a conformal electrolyte layer and excellent interface with electrodes that directly improved the microbattery performance.74 The impact of electropolymerization cycles was observed for SPAPE with the optimum cycles determined to be 10.110 The effect of other electrodeposition conditions needs to be studied more. Parameters of other new methods such as sol–gel,63 photolithography,82 stereolithography,77 ink-jetting,14 UV-induced photopolymerization,91 and their influence on SPE characteristics have not been investigated in detail yet and require more attention in the future.
3.3 Transference number
Transference (or transport) number is defined as the proportion of electric current transported by a specific ion.34,120,121 In LIBs other ions rather than Li+ do not provide the electrical energy, as their charge cannot be transferred through the external circuit. Thus Li transference number is considered as the main parameter.120,122 A higher transference number is favourable, as it allows larger power densities by decreasing the electrolytes' concentration polarization.121 Detailed information on the ion transport mechanisms in solid electrolytes and the ways of measuring the transference number were provided in the review by Quartarone and Mustarelli.34 Generally, in inorganic ceramic electrolytes, the conduction occurs due to only one mobile ion (Li+ for LIB electrolytes) with some rare cases of charge transfer through electronic charge carriers, therefore resulting in the transference number close to 1.120,123 Thus, this parameter is mainly important for polymer electrolytes, where several other species like various anions can be mobile and contribute to the overall conduction.124 So some earlier studies observed the low cation transference number for polymer electrolytes.125,126For solid PEO-based electrolytes, it was shown that the cationic transference number varied between 0.2 and 0.3 with ionic conductivity in the range of 10−8–10−5 S cm−1.120,122,127,128 One of the best transference numbers of 0.48 with a large ionic conductivity of 10−3 S cm−1 was obtained for PEG500DME-LiTFSI electrolyte. However, its further testing in LIMB structure is required.101 To decrease the negative effect of polarization phenomena during cycling for low transport number PEO-based electrolytes, the addition of such fillers as SiO2 and Al2O3 and formation of hybrid polymer electrolytes were attempted and those changes resulted in an improved transference number up to 0.8 due to a larger number of free Li+ ions.63,123 Other researchers succeeded in demobilizing anions in PEO structure, leaving only Li ions active, which resulted in the high transport number > 0.85 (with ionic conductivity 1.3 × 10−5 S cm−1).129 Such increased value helped to improve the cell performance even though the ionic conductivity was reduced by one order of magnitude, proving the importance of this parameter.130
The relatively low transport number of PEO-based electrolytes led to the investigation of other SPEs. For instance, poly(trimethylene carbonate) (PTMC)-based electrolytes had a transference number higher than 0.6 along with ionic conductivity of 10−5 S cm−1, which was attributed to the weaker Li binding in the PTMC structure compared to PEO-based one.131,132 Another type of SPE – single ion electrolyte having Li source directly on polymer chain rather than from Li salts dissolution-demonstrated an excellent transference number (0.8–1) and high ionic conductivity (10−4 S cm−1).133,134 Such high values were obtained due to this specific attachment of anionic centres to the polymer side chains.133,134 In addition, the higher Li+ conductivity (up to 0.6) was achieved for Lewis-acidic polymers like polyboranes as a result of weaker Li+ and stronger anion coordination.135 However, more studies are undergoing to investigate other properties and to improve the complex and expensive synthesis of the above-mentioned PEs. The recent review by Zhao et al. extensively covered the transference numbers of different types of SPEs together with the various methods to improve these numbers.122
Considering the particular case of the discussions of the thin-film or 3D SPEs' transference numbers and their effect on the microbatteries performance, it is noticeable that not all studies investigate the exact values of the applied SPEs. From the available resources, it was reported that LIMBs of PEO with fillers (2D) and polycarbonate-based electrolytes (3D) with transport numbers 0.37 and 0.6, respectively, delivered relatively stable energy with further room for SPEs properties improvement and more experiments to conduct.63,104
4. Inorganic crystalline electrolytes
Ceramic materials with a crystal structure that can be designed to have high ionic conductivity and thermal stability are promising candidates for all-solid-state battery electrolytes.19,136 The bulk properties can be better compared to glass and polymer electrolytes.136 However, to obtain high total ionic conductivity, it is necessary to increase the grain boundary conductivity since the grain boundaries present a large barrier for ions' migration across the interfaces.18 As a result, diffusion with a slow ion transfer kinetics in electrolyte becomes the rate-determining step, decreasing the performance of the microbattery. Thus, it is also critical to create a good contact between the electrolyte and electrode. The lack of grain boundaries due to isotopically conductive and intrinsically soft structure in polymer and glass electrolytes helps to build stable mechanical interfaces that significantly diminish the Li-ion diffusion resistance.136 Considering these properties, it is also easier to fabricate thin films of polymer and glass electrolytes. Moreover, glass electrolytes typically have higher electrochemical decomposition potentials, adding more stability to the material.19 The above-mentioned difficulties prevented a wide application of ceramic materials with crystal structures in 2D microbatteries so far, while 3D structures presented an even bigger challenge for conformal coating with electrolytes and production of their crystal structure. Nevertheless, high thermal stability of crystalline electrolytes is one of the main advantages of these materials, which is usually gained through annealing that also helps to improve the electrode–electrolyte interface quality.19 Several types of crystalline electrolytes can be found and will be discussed below, including the main NASICON, LISICON, perovskite and garnet-type electrolytes. The other crystalline materials, such as argyrodite materials, are promising candidates for microbatteries, but they have not been integrated and studied yet.137,1384.1 Garnet
One of the candidates for SSEs are garnet-type materials with the general formula of Li3Ln3M2O12 (M = Te, W; Ln = Er, Tm, Eu, Gd, Tb, Y, Pr, Nd, Sm, Dy, Ho, Yb, Lu).18 Among them, the outstanding candidate is Li7La3Zr2O12 (LLZO)-type electrolyte. It attracted more attention due to its high ionic conductivity found to reach up to 1.02 × 10−3 S cm−1 at 30 °C.139,140 Moreover, LLZO demonstrated good thermal stability and availability of raw materials.140–143 The typical crystal structure of LLZO consists of dodecahedral LaO8 and octahedral ZrO6 (Fig. 6a).144,145 Many investigations have been done to study LLZO and improve its properties, and they are discussed, for example, in a recent extensive review by Wang et al.140 In it, the effect of phases was discovered, where the higher ionic conductivity was attributed to the prevailing presence of cubic phase over tetragonal.146 Furthermore, various substitutions and doping were done with cations Al3+, Nb5+, Ta5+, Ga3+, Te6+, Y3+, Ti4+, Ge4+, and Fe3+. In most of the cases, the doping increased the Li-ion conductivity and enhanced the stability against Li.18,145 LLZO synthesis conditions were also extensively studied, as they directly affect the important structural parameters such as grain boundaries, crystallite size, grain size, and bulk density. As LLZO is mainly synthesized by the sintering method, the optimal conditions were investigated to decrease the grain boundaries, which, when present in large amounts, significantly reduce the ionic conduction.145 Moreover, an innovative approach of Li6.25Al0.25La3Zr2O12 fabrication using a multilayer processing with Li reservoirs (Li3N) was suggested, which helped to lower the processing temperature by 400 °C while keeping the desired phase of the material and ionic conductivity relatively high (10−5 S cm−1 order).147 Similarly, another alternative method to produce LiCoO2–LLZO was proposed, where infiltration is applied to deposit cathode from metal salts directly in a porous LLZO scaffold at a low processing temperature (700 °C), forming a low resistance interface.148 Further development of these promising processes is required in the future. Another uncommon sol–gel method was applied by Chen et al. to successfully deposit thin-film LLZO, also optimizing such parameters as annealing and number of layers to obtain the highest 1.6 × 10−6 S cm−1 ionic conductivity.149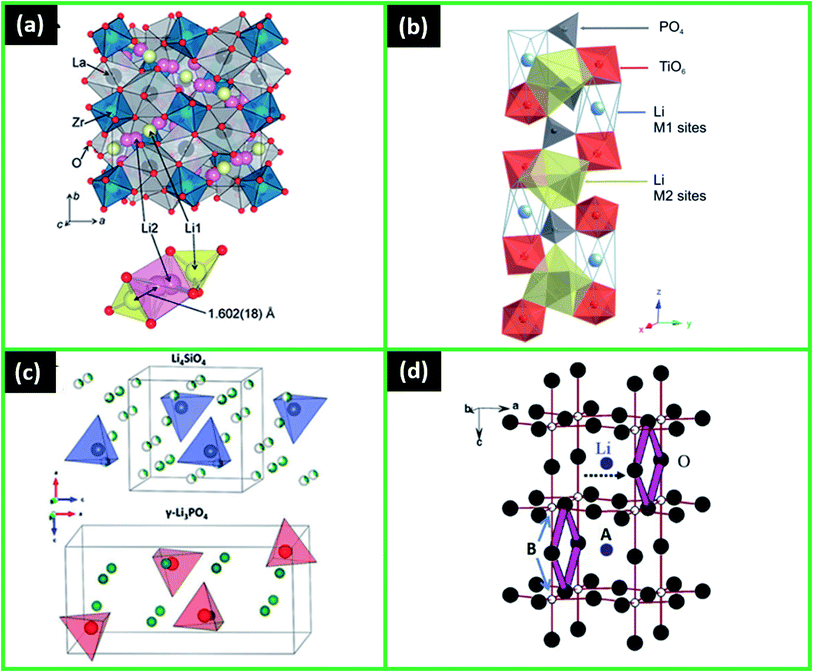 | ||
| Fig. 6 (a) Crystal structure of cubic Li7La3Zr2O12 and coordination polyhedra around the Li1 and Li2 sites. Reproduced with permission from ref. 145; (b) part of the NASICON-type crystal structure showing the M1 and M2 crystal sites. Reproduced with permission from ref. 156; (c) schematic representations of the crystal structures of the end-member phases Li4SiO4 and g-Li3PO4. Reproduced with permission from ref. 145; (d) crystal structure of perovskite-type solid electrolytes Li3xLa2/3−xTiO3. Reproduced with permission from ref. 181. Copyright (2003) American Chemical Society. | ||
Although garnet-type materials are promising electrolytes for solid-state batteries challenges like high reactivity with moisture and CO2 at an ambient atmosphere, crystalline instability at high temperatures, high production cost, and high interfacial resistance with Li need to be addressed.145,150–152 Particularly for LLZO, it was found that its relatively high electronic conductivity causes the formation of Li dendrites within the electrolyte and results in cell failure.21 Moreover, the ionic conductivity of thin-film LLZO is 1–3 orders of magnitude lower than of bulk ones due to various microstructural changes.153 Thus, a few studies are available on microbatteries with LLZO currently, and more studies are ongoing to investigate, for example, amorphous LLZO to tune its Li conductivity and avoid Li dendrites formation.153
4.2 NASICON
NASICON, one of the most studied crystal electrolytes, has attracted more attention not only due to high ionic conductivity (reaching up to 10−3 S cm−1) but also high oxidation potential, structural and thermal stability.19,26,154,155 NASICON is typically composed of LiMIV2(PO4)3 (M is a tetravalent cation: Ti, Zr, Sn, Ge, Hf).154 The structure is formed by the PO4 tetrahedra and MO6 octahedra, which are forming the 3D tunnels for Li-ions positioned at the interstitial sites (Fig. 6b).19,156The mechanical and electrical properties of NASICON mainly depend on the chemical elements present in the structure. For instance, initially developed LiTi2(PO4)3 had ionic conductivity of 10−6 S cm−1, which was enhanced to 10−3 S cm−1 by partial substitution of Ti4+ by Al.157–159 This substitution by the trivalent cations A3+ (Al, Ga, In, La, Y, Ti, Sc, Cr, Fe) forming Li1+xAxTi2−x(PO4)3 (LATP) with better electric properties happened presumably due to a higher charge carrier number the cations.157 Although the bulk ionic conductivity was found to be large, thin films were reported to have several orders of magnitude lower conductivities due to hindered glass-ceramic phase control. For example, thin-film LATP fabricated by RF sputtering had the largest Li conductivity of 2.46 × 10−5 S cm−1,160 while prepared by annealing techniques had it in the order of 10−6 S cm−1.161 Similarly, aerosol deposited thin film of Li1.5Al0.5Ge1.5(PO4)3 (LAGP) demonstrated maximum ionic conductivity of only 5 × 10−6 S cm−1.162 In addition, Ti4+ in LATP structure could be easily reduced by Li,163,164 making it unstable in batteries with lithium anodes, while structures with Al–Ge and Fe–Hf showed more compatibility with the Li electrode.18 An attempt to improve the stability of Li1.3Al0.3Ti1.7(PO4)3 LATP against Li was made recently by Liu et al. Al2O3 was coated on LATP by ALD and the electrolyte performance was checked in a symmetrical Li/LATP/Li thin-film cell.165 The results showed that a stable interface was created and the undesired Li penetration and Ti reduction were inhibited.165 Similarly, a more stable interface was formed between LATP and LCO with the artificial layer of Li3PO4 deposited by ALD, where the layer helped to suppress the elemental interdiffusion and the formation of interlayers with low Li conductivity.166 The properties of NASICON could also be improved by several techniques, such as thermal treatments, sintering process, excessive lithium introduction, and Si doping.154 For example, LiZr2(PO4)3 prepared at 1200 °C underwent the phase transition from monoclinic to rhombohedral when it was heated, resulting in increased ionic conductivity of up to 1.2 × 10−2 S cm−1 measured at 300 °C.18 In general, several reviews considered the recent advances, challenges, and perspectives of NASICON-type electrolytes, and some of their potential in microbattery applications.16,18,25,38,167,168
One of a few thin-film batteries with NASICON-type electrolyte was investigated by Hofmann et al. Li1.5Al0.5Ti1.5(PO4)3 electrolyte was deposited by PLD and analysed in the microbattery structure with a Si anode and a LiCoPO4 cathode.169 The cathode–electrolyte interface was studied particularly and significant inter-diffusion processes that are highly dependent on heat treatment were observed. The results highlighted the difficulty of producing a stable interface, which favours the Li ion diffusion, as it can be done only at low temperatures, while for crystal structures formations much higher temperatures are required.169 Thus, additional protective layers were suggested for these structures to reduce the inter-diffusion and interface resistance. As the capacity and general performance information of this thin-film battery is absent, more studies are needed in the future.
Although there are numerous NASICON-type electrolytes that have been actively investigated, only a few of them were integrated and evaluated in microbattery systems up to now. Thus, more studies are required on the electrode/electrolyte interface improvement as well as the optimal conditions for deposition and treatment of electrolytes.
4.3 LISICON
Li superionic conductor – LISICON is another good candidate for solid electrolytes due to the obtained high ionic conductivity (up to 10−2 S cm−1).145 LISICON structure is based on the Li4XO4 (X = Si, Ge, Ti) and Li3YO4 (Y = P, As, V, Cr); Li2MXO4 (M = Zn, Mg); Li2ZO4 (Z = S, W), which results in g-Li3PO4 phases19,145 (Fig. 6c).The earliest investigations were done on Li14Zn(GeO4)4 where the ionic conductivity was found to be 1.3 × 10−6 S cm−1 at 33 °C and 2 × 10−6 S cm−1 at 50 °C.170,171 In such structures, the conductivity was enhanced, as Li could diffuse not only through vacancies but also through interstitials.170,171 Several recent studies revealed other promising structures, which had several orders of magnitude higher conductivities than those of the parent phases. For instance, (1−z)Li4SiO4−(z)Li3PO4 (z = 0.25, 0.5 and 0.75) families with ionic conductivities of 10−3 S cm−1 at 300 °C,172 Li10.42Si1.5P1.5Cl0.08O11.92 and Li10.42Ge1.5P1.5Cl0.08O11.92 with 1.03 × 10−5 and 3.7 × 10−5 S cm−1, respectively.173 Various LISICONs were produced with elemental and structural changes. Although most of them had excellent thermal stability, still, the lower ionic conductivity at room temperature compared to other electrolyte candidates prevented the widespread studies of these materials up to now.145,170,171
The recent advancement of LISICON-type electrolytes was introduced with the development of thio-LISICON Li4−xGe1−xPxS4 (0 < x < 1), which had superior properties.18,174 By replacing the O2− with S2−, the new electrolytes showed higher ionic conductivities due to the weaker interaction between Li+ and S2− compared to Li+ and O2−.18 For instance, Li3.25Ge0.25P0.75S4 had improved ionic conductivity of 2.17 × 10−3 S cm−1 at room temperature along with the electrochemical stability of up to 5 V against Li,175 while Li10GeP2S12 demonstrated even higher ionic conductivity of 1.2 × 10−2 S cm−1 at 27 °C.174 The partial substitution of O2− by S2− for Li10GeP2S11.7O0.3 and Li10GeP2S11.4O0.6 led to ionic conductivities of 1.03 × 10−2 S cm−1 and 8.43 × 10−3 S cm−1 at 25 °C, respectively, bringing more stability, as sulfide adds more reactivity to the structure.176 Therefore, another research group also attempted to incorporate Cl and produce the Li9.54Si1.74P1.44S11.7Cl0.3 structure, which delivered ionic conductivity comparable to liquid electrolytes of 2.5 × 10−2 S cm−1 at 25 °C.177
Despite the fact that the recently developed thio-LISICON-based electrolytes have the desired conductivity close to liquid electrolytes, not many were tested in the microbattery structures. It was found that, generally, the sulphide in the structure brings instability at an ambient atmosphere due to its hygroscopic nature, limited operating electrochemical range, generation of H2S, and high cost of production.174,178,179
So far, Gilardi et al. deposited a thin-film LISICON-type Li4−xGe1−xPxO4 (LGPO) by PLD and characterized it.180 It was observed that although LGPO fabricated at room temperature had higher porosity, roughness and some contamination on the surface, the ionic conductivity was not much affected by the low-temperature deposition and was in the order of 10−6 S cm−1.180 Therefore, the ease of fabrication, wide electrochemical window, and relatively reasonable conductivity make LGPO a potentially interesting electrolyte for solid-state thin-film batteries, and thus, further testing of this electrolyte in microbattery required. In general, more testing of the microbattery with LISICON-based electrolytes is expected once the appropriate chemical composition of the electrolytes is found that will satisfy the conductivity and stability criteria.
4.4 Perovskite
Lithium lanthanum titanate Li3xLa2/3−xTiO3 (LLTO) is one of the perovskite-type electrolytes with a promising ionic conductivity attributed to its crystallographic structure (up to 10−3 S cm−1) (Fig. 6d).18,181 The first developed LLTO, although showing high bulk ionic conductivity of 10−3 S cm−1, had lower total ionic conductivity of 2 × 10−5 S cm−1 as a result of grain boundary effect.182 Moreover, the easy reduction of Ti4+ in the structure at low voltages (<1.8 V), brought the incompatibility of LLTO with many anodes including Li.183 Thus, several researchers attempted to substitute Ti with other elements such as Sn4+, Zr4+, Mn4+ and Ge4+ 184. It was found that ionic conductivity was increased only slightly with Mn4+ and Ge4+.184 The authors also noticed that only partial Ti4+ substitutions should be done in order to avoid the formation of the second phase.184 Another proposed method to improve LLTO characteristics is to use pulsed laser deposition (PLD) to produce amorphous LLTO films, which not only had the higher ionic conductivity (up to 10−3 S cm−1) but also better stability with Li anode.185,186Completely new perovskite structures were also tested, like Li3/8Sr7/16Ta3/4Zr1/4O3 (LSTZ), which had the total ionic conductivity of 10−4 S cm−1 at 30 °C, with improved grain boundary conductivity and stability at voltages > 1 V versus Li.20 Similarly, Li3/8Sr7/16Ta3/4Hf1/4O3 (LSTH) demonstrated good ionic conductivity (3.8 × 10−4 S cm−1 at 25 °C) and electrochemical stability > 1.4 V versus Li.187 Even though these newly developed perovskites had better performance, the problem of high-temperature preparation (>1000 °C) and interfacial issues between electrolyte and electrode require more investigations.20,187
Due to the above-mentioned challenges, a few studies are available on microbatteries with perovskite electrolytes up to now. Regardless, Lee et al., investigated amorphous LLTO potential thin film for microbatteries, which was deposited by PLD.188 By optimizing the PLD parameters, such as temperature and pressure, high Li conductivity was obtained (3 × 10−4 S cm−1).188 This electrolyte was then tested in a half-cell with LNMO that showed 98% retention of capacity after 50 cycles.188 More experiments are needed to investigate the interfacial stabilities and to examine the full thin-film cell.
Some investigations were done with the LLTO films coated with other layers, like LiPON, to enhance the stability, prevent the reactions with Li and short-circuits. To illustrate, the LLTO interlayer covered with LiPON from both sides was found to be very stable in the operating voltage window of 0–5.5 V tested by linear sweep voltammetry.189 Li et al. used e-beam evaporation to deposit LLTO thin film on LCO cathode and subsequently sputter a LiPON protection layer followed by thermal evaporation of Li to create a Li/LiPON/LLTO/LCO cell.190 This cell delivered 50 μA h cm−2 μm−1 capacity at the first cycle and 24 μA h cm−2 μm−1 after 100 cycles at electrochemical conditions of 7 μA cm−2 current and voltage window of 3–4.4 V. Though the cell provided good cyclability, a large number of grain boundaries yielded low ionic conductivity of 1.8 × 10−7 S cm−1, preventing the microbattery's better performance.190
4.5 Synthesis of crystalline SSE thin films
Although many studies have been done on the effect of synthesis and treatment of “bulk” crystalline electrolytes on their intrinsic properties,18,167,191–193 the influence of deposition methods on these electrolytes and on their microbattery performance has not been reported explicitly. For most of the crystalline materials integrated into microbatteries, the preparation method was limited to the solid-state reaction of mixtures with required composition and then deposition on the electrode. Such methods of deposition as sputtering,60,194 e-beam evaporation,190 sol–gel,149 spin-coating,195 PLD186,196 and ALD were applied to produce thin-film crystalline electrolytes, and various preparation conditions were reviewed.29,44,149,197,198 The other new deposition methods, for instance, for LLZO, are currently under development and optimization as discussed above.147,148 Up to now, it was observed that the higher e-beam evaporation power (600 W) applied for the LLTO fabrication resulted in a higher ionic conductivity.190The morphology alteration usually accompanies the crystallization process of the electrolyte during sintering at high temperatures, since the amorphous phase of the electrolyte transforms to crystalline with higher density. For the thin films, the shrinkage may also have a critical effect on their properties. For example, PLD-derived LLTO thin films were deposited and then annealed at various temperatures.196 According to the results of scanning electron microscopy (SEM), the morphology changed, since grains became more expressed in the films at the highest temperatures. However, it did not lead to the short circuit. In another work, PLD allowed deposition of uniform crystalline LLTO on the substrate heated up to 750 to 880 °C and varying gas pressure from 4 to 20 Pa during the fabrication.186
Chen et al. obtained mostly amorphous LLZO by sol–gel method despite thin film being annealed at temperatures of 600–800 °C. The final film demonstrated defects that particularly could form during the crystallization process.149 Spin-coated LLTO calcined at 550 °C during 40 hours demonstrated the clear tetragonal phase. Meanwhile, the morphology did not exhibit any cracks, needles or holes in contrast with those that were heated for 5 and 20 hours.195
Thus, the SSE thin films can be produced in a crystalline form without the critical impact of post-annealing on film morphology. Besides, the deposition of electrolyte (or its post-annealing) at high temperatures on the electrode should be made carefully, taking into account the electrode material properties, avoiding interfacial problems, and preventing Li loss when the microcell is realized.
5. Inorganic glass electrolytes
Glass electrolytes have been extensively studied as a result of having advantageous parameters, such as isotropic ionic conduction, absence of grain boundary resistance, non-flammability, easy film formation, and a variety of chemical compositions.199 Most importantly, the amorphous structure leads to higher ionic conductivity compared to the crystal one due to the presence of the so-called “open” structure (Fig. 7a).199,200 This disordered structure allows easier formation of the diffusion path as the configurational freedom is significantly higher than in the ordered structure.201 Moreover, the structure with more free space is favourable for compounds where the size of an opening, through which the ions have to diffuse, is the limiting factor, and that is the case for many crystalline materials with a low concentration of the migrating ions.202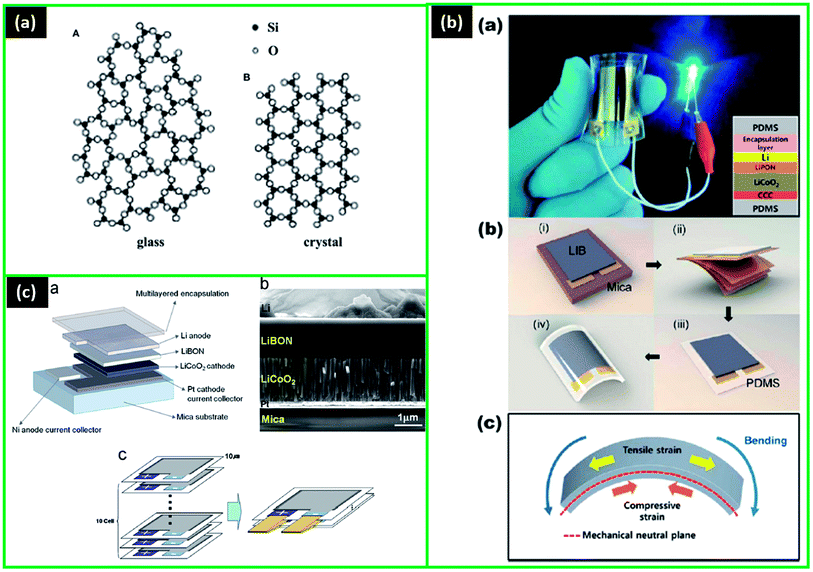 | ||
| Fig. 7 (a) SiO2 structure scheme: (A) glass, (B) crystal. Reproduced with permission from ref. 199; (b) (a) photograph of a bendable LIB turning on a blue LED in bent condition, (b) schematic illustration of the process for fabricating flexible LIBs, (c) schematic image of the mechanical neutral plane generated from the counterbalance between tensile and compressive strain. Reproduced with permission from ref. 227. Copyright (2012) American Chemical Society; (c) schematic for (a) the structure and (b) SEM image of cross-section view of the fabricated LiBON-based all-solid state microbattery, Li/LiBON/LCO, and (c) multi-stacks of the microbatteries for the required energy-density. Reproduced with permission from ref. 12. | ||
Glassy electrolytes are typically classified as oxide and sulphide glasses, and among them, lithium phosphate-based electrolytes have attracted much attention due to their stability against lithium and easy processability of thin films.37 These electrolytes' part will be covered based on the deposition techniques for easier comparison of the electrolytes' and microbatteries' performance parameters.
5.1 Lithium phosphate based electrolytes
The RF-sputtering method could produce good quality LiPON planar films without cracks. However, it has a low deposition rate (1–10 nm min−1), which was supposed to limit its widespread use and commercialization.27,205 Recently though, several industrial manufacturers, such as Front Edge Technology, succeeded to improve the deposition rate during the scale-up.206 Nevertheless, the investigation was continued to find the best parameters for film deposition. It was observed in the study by Choi et al. the decrease in power (80 W) led to an increase of N/P ratio in ∼2 μm thick LiPON film, and with slower deposition rate, the ionic conductivity could be increased even more significantly.207 Yet, ambiguous trends of ionic conductivity with N/P ratio were also shown by Hamon et al.208 and Kim et al.209 Another study by Suzuki et al. showed that when adding extra Li2O to Li3PO4 target for film sputtering, a higher number of three-coordinated N atoms were detected in the deposited LiPON film. This resulted in enhanced ionic conductivity (3.1 × 10−6 S cm−1) compared to sputtering using conventional target (2.1 × 10−6 S cm−1).210 Up to now, one of the highest ionic conductivities for LiPON with 4.9 × 10−6 S cm−1 at 22 °C was obtained by Su et al. using the RF sputtering growth rate of 14 nm min−1 (100 W).211 Generally speaking, reviewing the available works with LiPON film electrolyte showed that the used power was in the wide range of 35–350 W, while thickness was within 0.5–2 μm. The effect of sputtering target temperature was also observed, where the increase of it led to the reduction of LiPON ionic conductivity and worsened performance of the thin-film battery with this electrolyte in general.212 Such result was attributed to the formation of a closed-packed structure which hindered Li diffusion.212
The compatibility of LiPON with common electrode materials, such as Li and LCO, was actively investigated. For instance, using electroanalytical measurements, cryogenic electron microscopy, and in situ electron microscopy, it was shown that LiPON forms a stable interface (up to 80 nm) with Li, reducing the Li loss during interface formation and further cycling compared to the liquid electrolytes, therefore providing a stable (de)intercalation of Li.213–215 The composition of this layer with N and P concentration gradients and their unique spatial distribution acts as an effective passivation layer.214–216 LiPON/LCO interface, its composition, and electronic structure have been also studied by such techniques as X-ray photoemission spectroscopy, scanning transmission electron microscopy (STEM) coupled with electron energy loss spectroscopy (EELS). It was observed that the interface of a 10 Å layer is usually formed with nitrogen-containing species.217,218 Excellent cycling stability of thin-film LCO with LiPON was demonstrated, which was explained by the grain size effect with the critical particle size of the cathode being 0.3–1.1 μm.219 However, another research group found, based on the STEM/EELS analysis, that after extensive cycling Li accumulation at the interface caused an irreversible capacity losses.220 Moreover, the surface and evolution of the lithium morphologies in thin-film Li/LiPON/LCO (or LiMN2O4) have been evaluated after extensive cycling, and the cell with LCO showed more uniform Li distribution and stable behaviour while having a hold at the top of charge at 4.2 V.221 Nevertheless, the observed surface changes attributed to the Li dewetting and residual stress require more investigations on the exact mechanisms in the future to enhance safety.
5.1.1.1 2D structures. In general, the earliest developments of 2D LIMBs with LiPON electrolyte, Li and LCO electrodes had promising results. The first tests of thin-film Li/LiPON/LCO with a 2.5 μm thick LiPON demonstrated approximately 150 μA h capacity with stable cyclability of over 4000 cycles at 0.1 mA.56 Later, similar microbattery with thinner electrolyte (1 μm) also showed excellent 65 μA h cm−2 capacity with a very small 6% loss after 1800 cycles and negligible self-discharge.28 That made them an attractive candidate for industrial applications. Teledyne Electronic Technologies, for instance, proceeded to manufacture this type of microbattery on a multichip module package.56 The other commercial Li/LiPON/LCO type LIMBs with different total thicknesses of 170 and 200 μm showed the energy density of 25 and 21 mW h cm−3, respectively, along with good cyclability for at least 500 cycles with approximately 100% capacity retention.57 Testing of similar microbattery structure Li/LiPON/LCO with cathode thickness of 4.2 μm demonstrated the energy and power density of 1 mW h cm−2 and 1 mW cm−2, respectively.54 Excellent performance for Li/LiPON/LCO microbattery was observed with the capacity remaining stable (approximately 22 μA h cm−2) after 1100 cycles, indicating the importance of cathode properties and its compatibility with electrolyte.222 Another Li/LiPON/LCO thin-film battery's volumetric capacity was 63.5 μA h cm−2 μm−1, corresponding to 92% cathode utilization, which also showed almost no degradation after 500 cycles.223 Such results were attributed to the formation of crack-free and crystallographically oriented cathode films. The importance of structure–rate relationship in LCO and encapsulation that preserved film morphology was also demonstrated in the study by Song et al., where Li/LiPON/LCO cell delivered a steady volumetric capacity of 35 μA h cm−2 μm−1 with 85% retention after 800 cycles.224 Another example of successful Li/LiPON/LCOmicrocell with 45 μA h cm−2 μm−1 capacity and the retention of 88% over 800 cycles proved good integration of 1.4 μm thick LiPON (350 W) with bias sputtered and heat-treated LCO.225 Park et al. specifically tested LCO cathodes that were bias sputtered at different voltages. It was found that structure of the LCO treated by −50 V bias combined with 1.5 μm LiPON (200 W) and evaporated Li was most suitable to obtain the highest initial capacity battery of around 63 μA h cm−2 μm−1.226 There was a 16% capacity loss after 100 cycles, making it a promising treatment method of electrodes. Due to the good performance of common thin-film microbattery Li/LiPON/LCO, it was attempted to add flexibility to the battery for wearable applications by using sacrificial mica substrates and wrapping by polydimethylsiloxane (PDMS) (Fig. 7b).227 The new battery demonstrated a relatively high capacity of 106 μA h cm−2 that was stable for 100 cycles with 10% loss. Similar behaviour was also obtained for bent samples, indicating the effectiveness of the new approach and a good basis for further energy density improvements of flexible microbatteries.
Several research studies were done to evaluate other cathode materials with LiPON electrolyte. An RF sputtered LiMn2O4 (LMO) completed by 1 μm LiPON and Li metal demonstrated 48 μA h cm−2 μm−1 capacity and only 4% loss after 100 cycles in the voltage range of 3.7–4.3 V and current density of 100 μA cm−2, LMO proved to be a promising cathode candidate, though the other properties needed further studies.228 The same composed thin-film cell Li/LiPON/LMO also showed very stable behaviour with the initial capacity of 110 mA h g−1, which reduced to 105 mA h g−1 after 3500 cycles. The increase of the bulk or cathode–electrolyte interface resistances was determined to be the main issue in this battery.229 Generally, though LMO cathode was reported to have lower performance compared to LCO,28 Li et al. tested another material LNMO, which can operate at higher potential (3.2–5 V) than LMO (up to 4 V) due to partial substitution of Mn.230 The extensive electrochemical testing (up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) of Li/LiPON/LNMO thin-film battery demonstrated excellent results that showed 122 mA h g−1 capacity, retention of 90.6% at C/10, and a good rate performance of the solid-state cell due to, as the authors suggested, a reduced thickness of the electrolyte compared to the liquid one, good interfacial compatibility, as well as fast kinetics of the electrodes.230 Navone et al. has attempted to introduce a new safe crystalline cathode V2O5 (VO) and compared it with a widely used LCO. However, at the relatively similar theoretical capacity of VO and LCO, the microcell Li/LiPON/LCO delivered a higher capacity of 50 μA h cm−2 μm−1 after 140 cycles at 10 μA cm−2, while Li/LiPON/VO had 30 μA h cm−2 μm−1 capacity after 20 cycles at the same current density.231 Similarly, for another Li/LiPON/VO cell, the low performance and significant reduction of capacity were observed from 8 to 5 μA h after 500 cycles, which was attributed to the high electrode–electrolyte and charge transfer resistances.232 Better results were observed for the composite 0.5Ag:V2O5 cathode that was tested in Li/LiPON/0.5Ag:V2O5 thin-film battery with the initial capacity 72 μA h cm−2 μm−1 faded almost twice after 20 cycles.233 It was noticed that cyclability was most probably affected by the improved electronic conductivity of 0.5Ag:V2O5 film and electrode–electrolyte interfacial stability. Li et al. investigated Li/LiPON/LiCo0.8Ni0.2O2 and Li/LiPON/LiCo0.8Zr0.2O2 thin film batteries with new cathodes attempting to get better electrochemical performance than commercial LCO. The cycling of the batteries showed initial capacities of 62 μA h cm−2 μm−1 and 50 μA h cm−2 μm−1 for Ni and Zr-containing cells, respectively, which is quite close to the LCO theoretical capacity (69 μA h cm−2 μm−1), therefore these materials were proposed as the new cathode candidates.234 Zr doped cathode, however, showed better retention than Ni-doped one over 50 cycles.234 Another cathode material, amorphous LiFe(WO4)2, was integrated into Li/LiPON/LiFe(WO4)2 thin-film microbattery that had the initial high capacity of 104 μA h cm−2 μm−1, which diminished to 56 μA h cm−2 μm−1 after 150 cycles.235 The significant decrease of capacity within the first 15 cycles was a result of the unavoidable crystallization of LiFe(WO4)2 that led to lower electrochemical activity. The same research group also tested another Li/LiPON/CuWO4 thin-film battery, where the drastic reduction of the capacity from 145 μA h cm−2 μm−1 to 70 μA h cm−2 μm−1 happened at the second cycle, and only 43% of that was maintained after 100th cycle.236 The large initial irreversibility was attributed to the electrochemical reaction occurring at voltages above 2.5 V.
000 cycles) of Li/LiPON/LNMO thin-film battery demonstrated excellent results that showed 122 mA h g−1 capacity, retention of 90.6% at C/10, and a good rate performance of the solid-state cell due to, as the authors suggested, a reduced thickness of the electrolyte compared to the liquid one, good interfacial compatibility, as well as fast kinetics of the electrodes.230 Navone et al. has attempted to introduce a new safe crystalline cathode V2O5 (VO) and compared it with a widely used LCO. However, at the relatively similar theoretical capacity of VO and LCO, the microcell Li/LiPON/LCO delivered a higher capacity of 50 μA h cm−2 μm−1 after 140 cycles at 10 μA cm−2, while Li/LiPON/VO had 30 μA h cm−2 μm−1 capacity after 20 cycles at the same current density.231 Similarly, for another Li/LiPON/VO cell, the low performance and significant reduction of capacity were observed from 8 to 5 μA h after 500 cycles, which was attributed to the high electrode–electrolyte and charge transfer resistances.232 Better results were observed for the composite 0.5Ag:V2O5 cathode that was tested in Li/LiPON/0.5Ag:V2O5 thin-film battery with the initial capacity 72 μA h cm−2 μm−1 faded almost twice after 20 cycles.233 It was noticed that cyclability was most probably affected by the improved electronic conductivity of 0.5Ag:V2O5 film and electrode–electrolyte interfacial stability. Li et al. investigated Li/LiPON/LiCo0.8Ni0.2O2 and Li/LiPON/LiCo0.8Zr0.2O2 thin film batteries with new cathodes attempting to get better electrochemical performance than commercial LCO. The cycling of the batteries showed initial capacities of 62 μA h cm−2 μm−1 and 50 μA h cm−2 μm−1 for Ni and Zr-containing cells, respectively, which is quite close to the LCO theoretical capacity (69 μA h cm−2 μm−1), therefore these materials were proposed as the new cathode candidates.234 Zr doped cathode, however, showed better retention than Ni-doped one over 50 cycles.234 Another cathode material, amorphous LiFe(WO4)2, was integrated into Li/LiPON/LiFe(WO4)2 thin-film microbattery that had the initial high capacity of 104 μA h cm−2 μm−1, which diminished to 56 μA h cm−2 μm−1 after 150 cycles.235 The significant decrease of capacity within the first 15 cycles was a result of the unavoidable crystallization of LiFe(WO4)2 that led to lower electrochemical activity. The same research group also tested another Li/LiPON/CuWO4 thin-film battery, where the drastic reduction of the capacity from 145 μA h cm−2 μm−1 to 70 μA h cm−2 μm−1 happened at the second cycle, and only 43% of that was maintained after 100th cycle.236 The large initial irreversibility was attributed to the electrochemical reaction occurring at voltages above 2.5 V.
The performance and compatibility of LiPON with materials other than Li were also studied. One of the proposed materials was tin nitride (SnxNy) that was integrated into the thin-film battery SnxNy/LiPON/LCO with 7.6 μm total thickness.52 The discharge capacity was evaluated at different temperatures and was found to increase from 20 °C to 60 °C and subsequently decrease until 200 °C. Although higher temperatures caused more active ingredients to function and improve the LiPON conductivity, this also resulted in the enhancement of undesired grain diffusion. The maximum capacity of 200 μA h was observed at 60 °C, while at 100 °C, 193 μA h discharge capacity remained stable only for 15 cycles in the voltage window of 2–4 V.52
The structures with both non-commercial cathode and anode and LiPON were investigated as well. The new thin-film structure of Si/LiPON/VO–LiPO showed the capacity fading from the initial 15.7 μA h cm−2 to 7.7 μA h cm−2 after 30 cycles, which was attributed to the degradation of Si anode due to volume changes.237 Another thin-film battery ZnO/LiPON/LMO experienced low coulombic efficiency of 55% in the first cycle as a result of the reaction between ZnO and Li-ions.238 Thus, it further delivered the capacity of only 22 μA h cm−2 at the current density of 5 μA cm−2 and in the potential window 0.5–5 V. Approximately 10% of this capacity was also lost after 50 cycles. As no heat treatments were done to this battery, it was proposed to be a candidate for low-power and low-temperature substrate applications.238 Very unusual behaviour was observed for VO/LiPON/LMO thin-film battery, where the capacity significantly increased from 0.1 μA h cm−2 to 10 μA h cm−2 after 20 cycles and then remained steady.239 It was attributed to the so-called “forming process”, where the possible gradual decrease of interface resistances led to such performance. However, further studies on this phenomenon have not been done yet. Nevertheless, later, the research group Nakazawa et al. tested a similar thin-film structure battery and observed stable performance with around 80% retention of the initial capacity of 1.1 mA h after 100 cycles.240 The cell was even tested in a real digital watch, which was working for 1 month without additional charging.240 Nevertheless, as VO has some safety limitations of toxic nature, the same group also investigated Nb2O5/LiPON/Li2Mn2O4 thin-film battery with an alternative anode, which had a very thin LiPON layer (100 nm) and demonstrated stable behaviour for 500 cycles and optimal anode thickness of 100 nm.241 Nb2O5 was not actively implemented in microbatteries though due to its relatively lower capacity compared to Li. The innovative structure of a “Li-free” battery, where Li is electrochemically plated between the substrate and LiPON to form anode, was also studied in the thin-film battery of overlayer (LiPON or parylene C)/Cu/LiPON/LCO/Au.242 The cell delivered 85 μA h cm−2 of initial capacity with a 38% loss after 500 cycles. The considerable capacity fading was explained by the further development of unfavourable morphology of the plated Li and its irreversible consumption. Moreover, the importance of the overlayer, which covered the anode current collector and formed the tight gas seal, therefore reducing the formation of electrochemically inactive Li2O and LiOH, was highlighted.242 A similar “Li-free” inverted stack battery of stainless substrate (SUS)/PtLiPON/LCO/Au was also tested.243 It was observed that the cell with 7.5 μm-thick LiPON had the stable capacity of approximately 100 mA h g−1 at 5 μA cm−2 for 100 cycles due to good protection from the short circuit and the undesired reactions of plated Li. At the same time, the cell with 1.5 μm-thick LiPON was short-circuited due to insufficient mechanical strength of thin LiPON layer.243 Further investigations on the improvement of energy density in “Li-free” microbatteries are in progress nowadays.
Several ideas were proposed to improve the properties of LiPON. Xiao et al., for example, focused on the effect of fabrication conditions on LiPON properties and compared the LiPON produced by sputtering a sintered standard Li3PO4 and Li-rich Li3.3PO4 in a widely used Li/LiPON/LCO microbattery.58 It was observed that Li-rich LiPON thin-film battery, besides providing a slightly higher capacity of 64.5 μA h cm−2 μm−1 also had larger capacity retention of 98% after 26 cycles at 0.1C. In addition, at the discharge rate of 4C, Li-rich LiPON thin-film battery showed 89.5% capacity retention ratio, while standard LiPON battery had 83.1%. Such improvements were reported to be the result of the weakened space-charge layer effect induced by Li-ion defects in the Li-rich target.58 Generally, however, Lacivita et al. systematically studied the dependence of ionic conductivities on LiPON composition and actually found the optimal Li![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio to be 2.9
P ratio to be 2.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which provided the maximum ionic conductivity.244 Moreover, it was found that the small integration of boron into LiPON (LiPONB) introduced enhanced chemical and thermal stability while the electrical properties remained unchanged.245 This electrolyte was used in Li/LiPONB/TiOS thin-film battery, which had a large capacity (90 μA h cm−2 μm−1) in the voltage range of 1–3 V (versus Li) and current density of 100 μA cm−2 along with a long cycle life (>1000 cycles) and low self-discharge (<5%/year).245 Similarly, LiPONB was used in the microbattery Li/LiPONB/LCO, which had the initial capacity of 34.5 μA h cm−2 that was retained to 95.3% after 15 cycles at the current rate 10 μA cm−2 and voltage window 3.4–4.2 V.246 In another study, LiPONB with Si anode and Li cathode demonstrated excellent electrochemical performance with 571 μA h cm−2 μm−1 capacity and almost no fading for over 1500 cycles.247 Good mechanical properties and strong adhesion of LiPONB to Si prevented the initiation of cracks in the anode. Furthermore, solid electrolyte helped to avoid the formation of undesirable products at the electrode–electrolyte interface, unlike liquid electrolyte, indicating the promising use of silicon and LiPONB in solid-state microbatteries.247 Later, Song et al. have attempted to fully replace phosphorus (P) with boron and test the resulted electrolyte Li3.09BO2.53N0.52 (LiBON) in a thin-film battery Li/LiBON/LCO on a flexible substrate (Fig. 7c).12 As finding the exact electrolyte composition is very challenging with just one technique, this research team used several methods, such as inductively coupled plasma atomic emission spectroscopy (ICP-AES), elastic recoil detection-time of flight (ERD-TOF), as well as XPS to check the N-doping. The outstanding performance with the initial capacity of 55 μA h cm−2 μm−1 and 90% retention after 1000 cycles was observed for the cell. Therefore, LIBON was again noted to be a good candidate for flexible devices.12 It was also attempted to integrate Si in LiPON to improve the ionic conductivity of the electrolyte. One of the recent research papers demonstrated a higher ionic conductivity (up to 2.06 × 10−5 S cm−1) for LiSiPON compared to LiPON.248 LiSiPON produced by single-target sputtering has a potential for further development with processing optimization. However, instability of LiSiPON with Li anode remains an obstacle for a wide application.248
1, which provided the maximum ionic conductivity.244 Moreover, it was found that the small integration of boron into LiPON (LiPONB) introduced enhanced chemical and thermal stability while the electrical properties remained unchanged.245 This electrolyte was used in Li/LiPONB/TiOS thin-film battery, which had a large capacity (90 μA h cm−2 μm−1) in the voltage range of 1–3 V (versus Li) and current density of 100 μA cm−2 along with a long cycle life (>1000 cycles) and low self-discharge (<5%/year).245 Similarly, LiPONB was used in the microbattery Li/LiPONB/LCO, which had the initial capacity of 34.5 μA h cm−2 that was retained to 95.3% after 15 cycles at the current rate 10 μA cm−2 and voltage window 3.4–4.2 V.246 In another study, LiPONB with Si anode and Li cathode demonstrated excellent electrochemical performance with 571 μA h cm−2 μm−1 capacity and almost no fading for over 1500 cycles.247 Good mechanical properties and strong adhesion of LiPONB to Si prevented the initiation of cracks in the anode. Furthermore, solid electrolyte helped to avoid the formation of undesirable products at the electrode–electrolyte interface, unlike liquid electrolyte, indicating the promising use of silicon and LiPONB in solid-state microbatteries.247 Later, Song et al. have attempted to fully replace phosphorus (P) with boron and test the resulted electrolyte Li3.09BO2.53N0.52 (LiBON) in a thin-film battery Li/LiBON/LCO on a flexible substrate (Fig. 7c).12 As finding the exact electrolyte composition is very challenging with just one technique, this research team used several methods, such as inductively coupled plasma atomic emission spectroscopy (ICP-AES), elastic recoil detection-time of flight (ERD-TOF), as well as XPS to check the N-doping. The outstanding performance with the initial capacity of 55 μA h cm−2 μm−1 and 90% retention after 1000 cycles was observed for the cell. Therefore, LIBON was again noted to be a good candidate for flexible devices.12 It was also attempted to integrate Si in LiPON to improve the ionic conductivity of the electrolyte. One of the recent research papers demonstrated a higher ionic conductivity (up to 2.06 × 10−5 S cm−1) for LiSiPON compared to LiPON.248 LiSiPON produced by single-target sputtering has a potential for further development with processing optimization. However, instability of LiSiPON with Li anode remains an obstacle for a wide application.248
It is also known that LiPON interfaces mainly contributed to the internal cell resistance.54 The effect of interfacial resistance was previously described by Wang et al. for LiPON with 2 × 10−6 S cm−1 ionic conductivity.249 It was speculated that the performance of the battery was negatively affected due to increased cathode–electrolyte resistance resulting from significant strain-induced degradation of the LCO cathode at the interface region.249 Nevertheless, several researchers found that the interface resistance might not be the main limitation for some cases. For example, for the thin-film microbattery of Li/LiPON/Li4Ti5O12(LTO) cycled 5 times at 3.5 mA cm−2, the ionic charge transfer resistance between cathode and electrolyte was not a rate-limiting factor, rather the phase changes of LTO had mainly contributed to the cell impedance.250 Similarly, the study of Li/LiPON/LCO showed the stability of LiPON during ageing at 60 °C, where LCO caused a significant increase in cell resistance due to phase conversions.251 In addition, Wang et al. observed that at the highly delithiated state, the cathode tended to form a layer of rocksalt CoO and Li2O/Li2O2 structure, which accumulated Li and led to the larger amount of inactive cathode material.252
In general, the interface still plays a crucial role in battery performance and is one of the causes of the limited practical development of all-solid-state batteries. The quality of the interface and the interfacial resistance magnitude vary depending on many factors, including the deposition conditions, surface roughness, used electrode materials. Thus, the electrode–electrolyte interfaces, their behaviour, and strategies to overcome the issues have been extensively studied for various electrolytes and discussed in numerous review articles in detail.24,26,145,253–258 One proposed way to reduce the resistance was to do thermal treatment that could not only improve the ionic conductivity of LiPON but also enhance the number of electrochemically active sites at the interface of electrode and electrolyte.52,242,259 So, thermal treatment of LCO/LiPON interface at the temperature of 200 °C significantly reduced the resistance.260 In another study by Jeong et al., thermally treated Al2O3 film (400 °C) at the interface of LiPON and LCO also diminished the resistance while enlarging the capacity and stability during cycling.261
5.1.1.2 3D structures. Although 2D microbatteries are able to provide good electrochemical performance, increasing the surface area by moving to 3D architecture is the obvious way to decrease the interfacial resistance that, in a balance with a properly constructed cathode, can enhance both the power and the energy densities of microbatteries. Several 3D architectures with LiPON have been designed and studied. One of the earliest investigations was done by Xu et al., where the ionic conductivities of the planar LiPON film were found to be in the range of 1–2 × 10−6 S cm−1, whereas when trying to RF-sputter LiPON on the 3D structures (porous membranes, column arrays), inhomogeneous and rough deposition of LiPON was observed.11
Later, various micro- and nano-rod designs were tested by several research groups. For instance, successful 3D microrod patterning was done by Lethien et al., where LiPON was sputtered on the silicon nanopillars (SiNPL) negative electrodes to form SiNPL/LiPON/LFP microbattery with a conformal coverage of SiNPL (Fig. 8a and b).76 Although no electrochemical performance of the battery was discussed, LiPON ionic conductivity was measured to be 1.5 × 10−6 S cm−1.76 The functioning 3D microbatteries (Li/LiPON/LCO) with well-aligned slanted LCO nanowire structure were investigated recently (Fig. 8c).70 This 3D structure had a higher normalized discharge capacity than a 2D thin-film battery at 0.1C most probably due to the larger contact area of electrode–electrolyte. Furthermore, nanowires maintained a high specific capacity (73%, ∼70 mA h g−1) even after 400 cycles in the voltage range of 3–4.2 V due to the ability to accommodate the stress of the volume changes during cycling.70 Sun et al. have recently investigated another successful 3D structure, where vertically aligned oxygen-deficient α-MoO3−x nanoflake arrays were sputtered along with LiPON electrolyte and Li anode (Fig. 8d).79 The microbattery demonstrated good capacity (266 mA h g−1 at 500 mA g−1 and 1.5–3.5 V) and stable cycle performance (92.7% capacity retention after 1000 cycles) higher than of a 2D battery made out of the same materials.79 The following structure allowed not only a greater cathode–electrolyte interface with a short Li diffusion path but also accommodation for the volume change, which enhanced the mechanical integrity of the microbattery. Moreover, the microcolumnar 3D structures of Si/LiPON/LCO constructed with the sputtering technique were electrochemically tested (Fig. 8e).71 The results indicated that although the capacity of the 3D sample was higher (25 μA h cm−2) than that of 2D's (20 μA h cm−2), at the higher rates, the 3D sample had a significantly lower capacity (80% or less).71 It was speculated that the structural inhomogeneity with low ionic conductivity of the LiPON (2.5 × 10−7 S cm−1) led to the poor performance of this microbattery. The sputtering method was also used to deposit Ti/Pt/Ti current collector, LCO, LiPON, and Si anode on the Si nanowires (Fig. 8f) creating a microbattery with 0.5–1 μm diameter.72 This microbattery with only 110 nm LiPON film showed increased electric field in the electrolyte that resulted in the pinhole formation at the LCO/LiPON interface, rapid self-discharge, and short-circuiting. However, for the samples with larger LiPON thickness (>180 nm), the self-discharge diminished significantly, therefore confirming that the optimal thickness for electrolyte should be not smaller than 110 nm in order to avoid compromising the space-charge limited electronic conduction.72 In terms of LiPON thickness discussion, another research group Put et al. obtained the thinnest RF-sputtered plane LiPON, which was electronically insulating, retaining good ionic conductivity. Such behaviour deviating from other studies was explained by the possible difference in LiPON stoichiometry and the substrate effect, whereas in a real microbattery, LCO with sharp crystallite edges could reinforce local electric fields.262
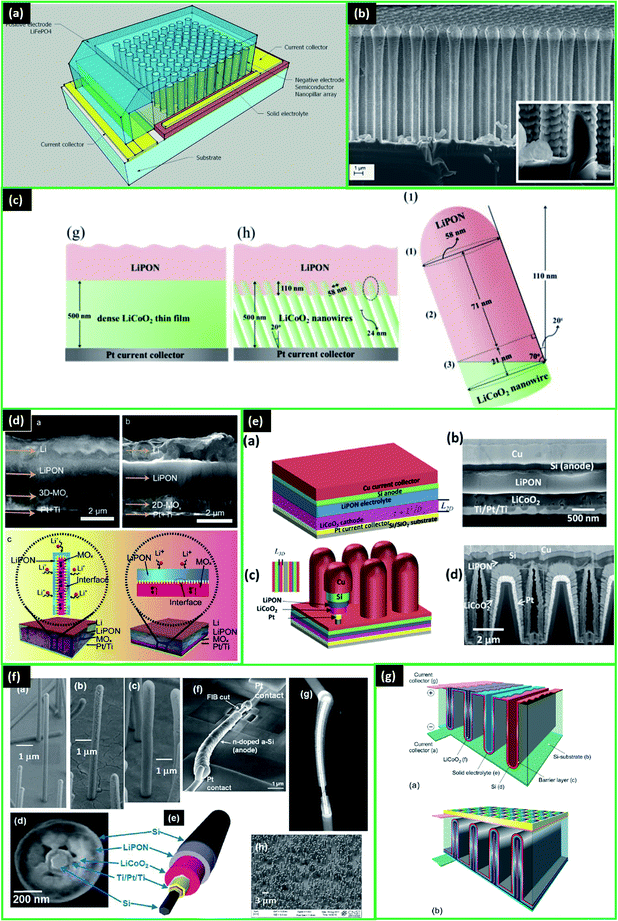 | ||
| Fig. 8 (a) Overview of the lithium ion solid state micro-battery. Reproduced with permission from ref. 76; (b) overview of the SiNPL array covered by the LiPON/LFP sputtered thin films. Reproduced with permission from ref. 76; (c) (a) dense thin film cathode, (b) slanted nanowires cathode, (i) enlarged the LiPON-deposited slanted nanowire cathode. Reproduced with permission from ref. 70; (d) cross-section field emission scanning electron microscope (FESEM) images of (a) the 3D and (b) the 2D thin film battery, (c) schematic illustration of the structural comparison between 3D and 2D thin film battery. Reproduced with permission from ref. 79; (e) (a) schematic of a planar thin film battery, (b) focused ion beam (FIB) cross section of a thin film battery, (c) schematic of a 3D battery, (d) focused ion beam cross sections of 3D battery with nominally 500 nm thick LiPON. Reproduced with permission from ref. 71. Copyright (2016) American Chemical Society; (f) FESEM images of nanowire LIBs following deposition of (a) Ti/Pt/Ti, (b) LiCoO2, and (c) LiPON/Si; (d) a FIB cut cross section FESEM image and (e) a nanowire LIB schematic; (f) a nanowire LIB contacted with Pt electrodes on a Si/SiO2 substrate, (g) HAADF STEM of a Nanowire LIB on SiNx membrane with Pt contacts showing its internal structural arrangement, and (h) a panoramic FESEM of the Nanowire LIBs on the wafer. Reproduced with permission from ref. 72. Copyright (2012) American Chemical Society; (g) (a) 3-D integrated all-solid-state Li-ion battery for which surface enlargement has been accomplished by electrochemical or reactive ion etching (RIE) of a silicon substrate, (b) autonomous energy-generating and storage device, combining a Si-solar cell with an integrated all-solid-state battery. Reproduced with permission from ref. 263. | ||
A new concept to create the 3D structure was proposed in the form of trenches using Si-substrate, TiN or TaN film to protect the Si-substrate from Li penetration and Si/LiPON/LCO components (Fig. 8g).263 It was predicted that for a surface enhancement factor of 25, the energy density can achieve 5 mW h cm−2 μm−1 for 1 μm thick LCO cathode. Moreover, LiPON film on the Si electrode had steadier cyclability due to the stable solid-electrolyte interface (SEI) compared to the liquid electrolyte, which had a significant decrease in capacity after 30 cycles.263
Baggetto et al. focused on analyzing the Ta, TiN, TaN barrier layers for that 3D structure.264 Among them, TiN has demonstrated the most promising results with Li-ions migration prevention due to the lowest reactivity with Li. In general, in the 3D Si/LiPON/LCO microbattery with TiN barrier layer, the capacity is expected to deliver energy and power density of approximately 1.5 mA h cm−2 μm−1 and 5 mW h cm−2 μm−1, respectively, at the voltage of 3.5 V.264 However, the exact electrochemical testing of this battery has not been conducted.
LiPON was also tested as a barrier layer for both sides of the lithium phosphorus tungsten oxynitride (LiPWON) a potential electrolyte. Although the protected electrolyte structure LiPON/LiPWON/LiPON was less vulnerable for short circuits, the ionic conductivity (1.2–1.5 × 10−7 S cm−1) was relatively low compared to conventional LiPON.265 Similarly, LiPON/LLTO/LiPON structure was studied, and it showed stable operation in the voltage window of 0–5.5 V. However, the same issue of low conductivity, which was in the range of 10−7 S cm−1, remained for that electrolyte.189
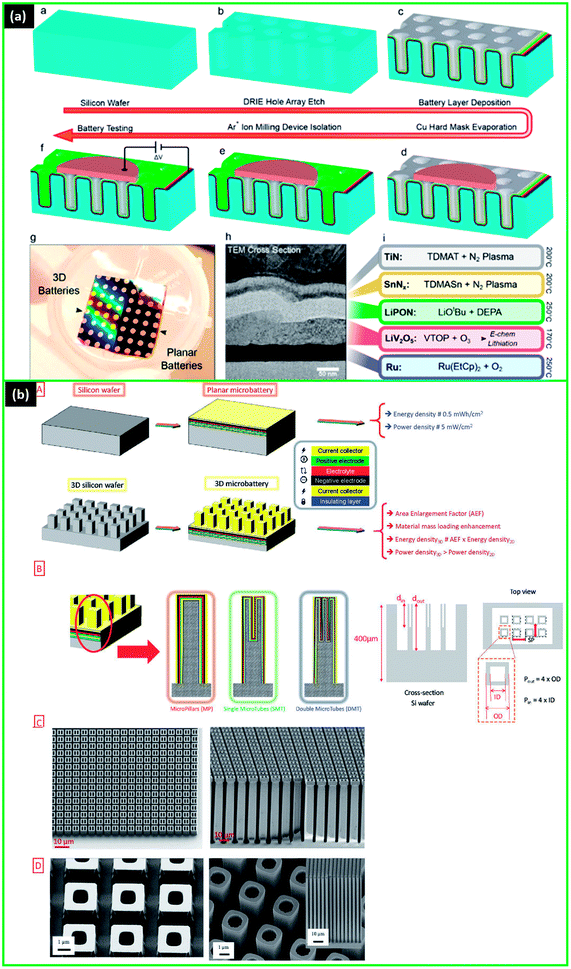 | ||
| Fig. 9 (a) Fabrication and characterization of 3D solid-state thin-film batteries. (a–d) Schematic of fabrication of devices, (e) isolation of individual batteries via Ar+ ion milling through anode current collector and anode films, (f) battery testing through contact with top electrode and cathode current collector layers, (g) optical photograph of finished battery “chip”. Each chip is dual sided, with 3D batteries on the left and planar batteries on the right. Optical iridescence from the 3D array causes the visible coloration, (h) cross-sectional TEM image of an all-ALD solid-state battery with 40 nm Ru/70 nm LiV2O5/50 nm Li2PO2N/10 nm SnNx/25 nm TiN, (i) overview of ALD chemistry and process temperature for each layer visible in (h). Reproduced with permission from ref. 31. Copyright (2018) American Chemical Society; (b) (A) schematic of 2D and 3D microbatteries fabricated on silicon wafer, (B) description of the proposed 3D scaffold: micropillars, simple microtubes and double microtubes (MP, SMT and DMT) are successfully fabricated on 3 in. silicon wafer, (C and D) SEM images of the fabricated DMT (SMT, respectively): photoresist mask (left) and 3D silicon scaffold (right) after the deep reactive ion etching of the wafer selectively to the mask. Reproduced with permission from ref. 274. | ||
In general, ALD proved to be a promising technique to develop 3D solid-state batteries due to the ability to produce the ultrathin, uniform, and conformal films for high aspect ratio structures.
As the deposition technique's parameters play a crucial role in the material properties, a lot of attention was brought to improve the methods and develop the electrolyte with the most favourable characteristics. Major techniques of solid electrolyte fabrication and their specifications are generally covered in several review reports.36–38 The RF sputtering being a dominating LiPON preparation method was discussed in numerous articles, where the effect of sputtering power, pressure, target–substrate distance, target density, N2 deposition pressure, deposition rate, and other conditions on the electrochemical properties of LiPON were investigated.58,207,208,277–280 Considering the examples of LiPON integrated into the thin-film or 3D microbattery performance, firstly, it was found that by changing the RF power, the boron content was varied in LiPONB and at the optimum 50 W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 W (Li3PO4
20 W (Li3PO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li3BO4) power, the highest ionic conductivity was observed.246 The importance of the composition of the sputtering target was also shown, where usage of Li-rich Li3.3PO4 target compared to normal Li3PO4 target led to superior LiPON conductivity and higher microbattery initial capacity.58 It was suggested that Li-rich LiPON helped to diminish the space-charge layer effect.58 Moreover, in situ (without breaking vacuum after VO electrode growth) and ex situ (with breaking vacuum) LiPON depositions demonstrated that the in situ process resulted in lower interfacial and charge transfer resistances and thus better microbattery performance.232 The full review article on the RF sputtering conditions and their impact on LiPON used in solid-state batteries has been provided recently by Ko and Yoon.281 Other techniques, like PLD and ALD, are gaining more attention in LiPON studies due to the promising results, and these techniques' features are discussed in the recent reviews by Julien et al.,29 and Liu et al.,43 Meng et al.,44 Fenech and Sharma.198 In short, for PLD, the importance of N2 gas pressure and laser fluence was highlighted so far,266 while for ALD, the precursors and the temperature window were mentioned to be important parameters.43,44 Thorough studies on PLD/ALD conditions and their impact on LiPON's direct performance in the microbatteries are still limited. Similarly, other techniques, like IBAD and CVD, also lack extensive investigations and need research on the methods' limitations in more detail.
Li3BO4) power, the highest ionic conductivity was observed.246 The importance of the composition of the sputtering target was also shown, where usage of Li-rich Li3.3PO4 target compared to normal Li3PO4 target led to superior LiPON conductivity and higher microbattery initial capacity.58 It was suggested that Li-rich LiPON helped to diminish the space-charge layer effect.58 Moreover, in situ (without breaking vacuum after VO electrode growth) and ex situ (with breaking vacuum) LiPON depositions demonstrated that the in situ process resulted in lower interfacial and charge transfer resistances and thus better microbattery performance.232 The full review article on the RF sputtering conditions and their impact on LiPON used in solid-state batteries has been provided recently by Ko and Yoon.281 Other techniques, like PLD and ALD, are gaining more attention in LiPON studies due to the promising results, and these techniques' features are discussed in the recent reviews by Julien et al.,29 and Liu et al.,43 Meng et al.,44 Fenech and Sharma.198 In short, for PLD, the importance of N2 gas pressure and laser fluence was highlighted so far,266 while for ALD, the precursors and the temperature window were mentioned to be important parameters.43,44 Thorough studies on PLD/ALD conditions and their impact on LiPON's direct performance in the microbatteries are still limited. Similarly, other techniques, like IBAD and CVD, also lack extensive investigations and need research on the methods' limitations in more detail.
5.2 Other glass electrolytes
Other glass electrolytes generally classified as oxide- and sulphide-based were attempted to be developed by various research groups using mainly RF sputtering and vacuum evaporation techniques.19 However, these materials have gained less attention compared to LiPON due to several reasons. Most of the oxide-based electrolytes produced by the conventional physical vapour deposition method showed smaller or comparable ionic conductivity and poor electrochemical stability relative to LiPON.19,65,282–284 At the same time, sulphide-based electrolytes, having a promising ionic conductivity that is much closer to liquid electrolytes, are less favourable due to hygroscopic nature and chemical instability in the air.19,285 Therefore, the number of reported studies on these materials is limited up to now.One of the glass electrolyte examples is Li2O–V2O5–SiO2 (LVSO). It showed the ionic conductivity of 10−5–10−7 S cm−1 and low electronic conductivity (10−10 S cm−1).19,286 Using this electrolyte, the microbattery Li/LVSO/MoO3 delivered 290 μA h cm−2 μm−1 capacity with 4.66 μm thickness of cathode in the voltage window of 1.5–3.5 V and at the current density of 10 μA cm−2.287 This performance was successfully maintained for 40 cycles.287 Another LVSO based microbatteries (SnO/LVSO/LCO and SnO/LVSO/LMO), where electrolyte was deposited by PLD, could deliver 9 μA h cm−2 (2–3 V) and 1.5 μA h cm−2 (1–3 V) capacities, respectively.288 In this case, the annealing of the cathodes helped to increase the roughness and thus the active interface area between the electrolyte and cathode leading to the larger capacities.288 Later, the same research group also tested SnO/LVSO/LiNi0.8Co0.2O2 microbattery with the annealed cathode and observed stable operation for 20 cycles with a small loss of the capacity from 19 to 16.1 μA h cm−2 μm−1.289 Brazier et al. studied similar PLD-fabricated-SnO/LVSO/LCO microbattery and observed that at the 4.4 μA cm−2 current, the capacity had a large irreversible loss and quickly faded due to the chemical elements' migration between LVSO and both the cathode and the anode. In addition, the delamination of SnO was detected.290 An attempt to improve LVSO's ionic conductivity was done by adding the LiBO2 in LVSO, which resulted in the maximum conductivity of 6.4 × 10−4 S cm−1 that was attributed to the porosity decrease.291 Although there were several successful tests of LVSO-based electrolytes, it still had lower or similar ionic conductivities compared to commercial LiPON in most of the cases.19,286,292 It was speculated that the possible amorphous Li2O reaction with air and the formation of LiOH or Li2CO3 decreased LVSO's conductivity.19,293 Most importantly, LVSO lacked electrochemical stability, therefore, it has not been widely utilized for microbatteries yet.
Another interesting electrolyte, Li2O–SiO2–P2O5 (LiSiPON) with the ionic conductivity of 1.24 × 10−5 S cm−1, was used in a thin-film battery of Si0.7V0.3/Li1.9Si0.28P1.0O1.1N1.0/LCO.294 The microbattery showed excellent cycling stability with the capacity of 50 μA h cm−2 μm−1 (2–3.9 V) lasting for 1500 cycles.294 However, at higher voltages (>3.9 V) the degradation of the battery happened, which was speculated to be due to the over-extraction of Li from the cathode.294,295
It was found that sulphide matrix-based oxysulfide glass electrolytes could greatly enhance Li+ conductivity (10−4–10−3 S cm−1) compared to oxide-matrix ones (10−8–10−5 S cm−1).285 This was attributed to the weaker bonding of Li with the non-bridging S anion, which resulted in easier diffusion. Nevertheless, the sulphide-based electrolytes have not been widely commercialized because of the complicated synthesis process and difficult handling of the corrosive component.285 Therefore, among the available glass electrolytes, LiPON is currently the most prominent candidate so far, since it has relatively high ionic conductivity, low electronic conductivity, and excellent electrochemical stability.
6. 2D vs. 3D LIMB
Obviously, the main difference between 2D and 3D is the geometry of the cell. However, even small shape distinctions may result in significant changes of intrinsic kinetics and thermodynamics. The main advantages of 3D architecture are an increased amount of active sites and greater surface area, free infusion and electrolyte ion access, and room for volume variations. 3D shape provides minimized ionic transport length between battery components, which simplifies the low diffusivity in solids; increased electrolyte-accessible surface, which decreases current density per unit area during cycling and lowers the overpotential required for charge transfer; mitigated mechanical degradation of the electrode. Besides, energy and areal capacity can be controlled easily by electrode height.49,296 Below are several examples where the 2D and 3D micro cells were studied and compared.For example, the larger contact area between LCO nanowire electrode and LiPON electrolyte allowed an increase of discharge capacity when compared to the corresponding planar cell.70 2D battery built with α-MoO3−x cathode, LiPON electrolyte, and Li anode was not able to deliver the performance that was achieved by its 3D counterpart.79 Along with improved electrode–electrolyte interface, accommodation for the volume change played an important role in the better mechanical properties of the cell. The microcolumnar Si/LiPON/LCO microbattery showed that the poor structural inhomogeneity and low ionic conductivity of the LiPON can bring a negative effect on cycling of 3D sample at higher rates compared to the 2D one.71
He et al. reported the cells with 3D spiral solid polymer electrolyte, the superior performance of which was caused not only by shortened Li-ion pathways between electrode and electrolyte but also due to reinforced interfacial adhesion and ability of 3D structures to maintain more mass loading of active materials.77
Edström et al. stated that the large surface area of the anode and cathode provides improved capacity per footprint area and high power capabilities if the 3D cell offers a short transport distance between the electrodes and thin layers of the electrode materials on the current collectors. Thus, the capacity can be increased by a factor of 10–30 per footprint area by using the appropriate 3D design.297 Werner et al. reported that the capacity of the gyroidal 3D nanoscaled cells was three orders of magnitude higher than a theoretical capacity of flat architecture with the same nanoscale dimensions and footprint area.81 Similarly, the 3D MoOySz/hybrid polymer electrolyte/lithiated graphite cell exhibited a capacity of about 30 times higher than that of a 2D battery keeping the same footprint and same electrode thickness. It was explained by the high surface area of the nanosize molybdenum oxysulfide. The additional reason was suggested to be the material's heightened sensitivity to the environment, since the surface of the electrodeposited cathode is usually highly oxidized.3 In another study of 3D thin film LIB with electrodes' pillars, the areal energy density was increased due to prolonged height of the pillars resulting in an increase of the loading of the active material per areal footprint.298 The simulation of three types of 3D-shaped LCO/polymer + LiTFSI/graphite cells with interdigitated, concentric, and trench electrode arrangements revealed that the former two own low polarization to higher positive electrode–electrolyte surface area with enhanced contact area in contrast with the third one. Besides, the concentric architecture provided the lowest average cell temperatures for all investigated charge rates and the highest capacity.299 The design advantage of interdigitated 3D architectures over simple thick films is that a short and uniform diffusion path maintained between the anode and cathode enables thick electrodes with high power.82
However, 3D-shaped battery structures have more challenges than 2D one for all types of electrolyte, such as manufacturing difficulties, high risks of a short circuit, compatibility of current collector–electrolyte–electrodes materials to provide a good contact between components, and an uneven current distribution that may cause nonuniform heat generation.300
7. Outlook on the 3D structures fabrication processes
It is known that for complex 3D architectures, conformal and thin uniform coating is crucial for the effective functionality of the microbatteries. Thus, the deposition techniques play an important role and can be compared based on their possibility to deposit pinhole-free, step-conformal films and applicability for battery materials. In this section, the general outlook on to the common and developing fabrication processes of 3D microbatteries' solid electrolytes is provided.For organic electrolytes, the widely applied for 2D structures drop casting and dip coating methods were attempted on 3D structures, such as nanotubes. It was observed that, for example, the deposition of SPE on TiO2nts was not conformal and the surface area of nanotubes was only partially covered by the electrolyte.73,104 Later, the electrodeposition was found to be able to deposit more homogeneous, stepwise conformal layers of electrolyte on nanotubes, which significantly improved the microbatteries' performance.74,75,96,108,110 This method also introduced easier control of film's thickness by potential and time variation and lower electrolyte–electrode interface resistances.109,301 However, this method is cumbersome and requires relatively complex material preparations. Thus, other techniques are also under development.
For instance, organic electrolyte deposition methods, such as spin coating and photolithography, also underwent testing and showed some promising results with functional microbatteries.82,83 Spin coating on micropillars, being a relatively simple process, was determined to have more adaptability for various polymer electrolytes,83 while photolithography of SU-8 photoresist electrolyte provided conformal coating of 3D arrays, allowing the use of Si as both the scaffold and anode.82
Another innovative approach is 3D printing, which is a common tool to build 3D architectures due to low-cost and various designs' flexibility.1,7 Therefore, the development of its application for electrolyte printing is expected to be a very important step for all-solid-state microbatteries. 3D printing includes such methods as stereolithography, direct ink-writing, ink-jetting, selective laser melting, and others.77,302–306 One successful investigation was done on 3D spiral SPE deposited by stereolithography, where the 3D structure tested in the coin cell had stable and better cycle performance compared to the 2D structure, proving that this technology is a promising candidate for SPE fabrication.77 This relatively simple printing method, where complex inks preparation and postprocessing of printed parts are not needed, has great potential and requires further adoption for the microbattery.77 Most probably, as a result of low viscosity requirements for the materials printing process, only a few articles are available on SPE 3D printing up to now.1 Currently, this field is in its formative stage.
Another research group demonstrated deposition of conformal SPEs on nanopillars, nanopores, and arrays by CVD.111 The main advantage of this method is the ability to tune polymer properties, like ionic conductivity, by varying the compositions and copolymer network polarity.111 Similarly, further testing of this technique is needed in a real microbatteries' environment.
UV-polymerization has also been applied to fabricate solid GPE on the Si pillars' structure. The films were proved to be not only conformable to 3D micropatterned architectures but also highly ion-conductive and bendable, thus opening more opportunities for the development of flexible devices.113 Currently, further studies on fabrication process optimization are required.
For inorganic solid electrolytes, like LiPON, the most common method applied for 3D structures' coating was RF-sputtering. Several successful structures, such as nanopillars, slanted nanowires, nanoflake arrays and microcolumns, were conformally sputtered with LiPON, and most of them have been cycled and demonstrated generally stable performance.70,71,76,79 However, in some cases, the poor performance of microbatteries was attributed to the structural inhomogeneity in deposited LiPON.71
In addition, ALD is proved to be one of the emerging and promising techniques for 3D microbatteries' and solid electrolytes' deposition. As with ALD, it is possible to tune films composition at the precision level of atoms, control the growth and thickness of the films at the atomic scale, as well as fabricate the high-quality conformal thin films on various complicated architectures.43,44 Moreover, ALD is more compatible with microbatteries' technologies and materials due to a relatively low deposition temperature.43,44 The research done using ALD to deposit LiPON on nanopore arrays and Li3PO4 on microtubes to form microbatteries showed that besides the enhanced capacity as a result of 3D structuring, the cells were electrochemically stable.31,274 Nevertheless, the relative complexity of the used chemistry and ALD process itself as well as smaller deposition rates than sputtering range prevented the wide commercialization of this technique as of today.43,44
8. Summary
Solid-state microbattery gained a lot of attention due to rapidly developing microelectronics used in various applications, such as smart cards, memory chips, biomedical devices, and others. Along with the thin-film structures, 3D structures with different materials have been developed recently to improve both the energy and power densities. One of the major components of a battery – electrolyte – plays an important role by transporting the ions between electrodes and enables the battery to function. The solid electrolyte, which is of great interest for the microbattery technology, provides additional safety by introducing a leakage-free feature and acts as a separator to prevent short circuits. Thus, in this review, the focus was on solid electrolytes of different types, including polymer, crystalline, and glass, which were successfully introduced in the 2D or 3D microbattery and electrochemically tested.The promising candidate for solid electrolytes – polymer-based one mainly demonstrated high ionic conductivity (up to 10−4 S cm−1), easy processability as well as flexibility. So, several successful 3D structures with innovative architectures and/or application of electrodeposition that helped to create a good electrode–electrolyte interface showed appealing results with both high power and energy density (Fig. 10a). Other innovative deposition techniques for SPE, such as photolithography and others, have been also tested providing some appealing results, so they are under further active investigation as well (Fig. 11). Nevertheless, cells with solid polymer electrolytes still suffer from mechanical integrity problems and thermal and electrochemical instabilities compared to LiPON, requiring further studies and development.
Similarly, most of the crystalline electrolytes, including NASICON, LISICON, garnet-based and perovskites, despite attracting high bulk ionic conductivities, still have low grain boundary conductivities and difficult processability as major issues in addition to some of the electrochemical and phase instabilities, preventing their wide usage in commercial microbatteries.
Thus, many investigations have been done on glass-based electrolytes, especially LiPON, which, among the various candidates, satisfies most of the criteria for the appropriate electrolyte, such as low electronic conductivity, good chemical, electrochemical, thermal and mechanical stabilities, relatively low interfacial resistances, and good processability (Fig. 10b). However, the critical parameter of LiPON's ionic conductivity is still significantly lower (10−7 S cm−1) than standard liquid electrolytes' (10−2 S cm−1). Although it is less critical for microbatteries with a very thin layer of electrolyte and short Li ion diffusion path length, several studies have been focusing on the improvement of this and other stability parameters by varying deposition conditions, integrating boron in the LiPON structure, and others, which were discussed in this review. Moreover, analysing the various deposition techniques of LiPON and the performance of these films in microbatteries, it was found that up until now, RF sputtering remains the prevailing method due to a higher quality of films and a less complex and energy-demanding process. However, the emerging techniques like ALD show promising results especially for 3D high-aspect-ratio structures (Fig. 11). The other glass electrolytes, such as sulphide-based, although having higher ionic conductivity (up to 10−3 S cm−1), showed unfavourable electrochemical instabilities. Generally, up until now, LiPON remained as one of the most examined and the most successful candidates for solid-state microbatteries, showing good performance in several innovative 3D structures and especially in thin-film 2D designs (Fig. 10c). However, the other electrolytes with higher ionic conductivities are under the investigation of various research groups all over the world, which put a lot of effort to tackle the existing challenges. Table 2 summarizes all critical information on Li solid electrolytes and their integration in microbatteries including the electrolyte composition, ionic conductivity, preparation methods and conditions, microbattery electrodes, structure, electrochemical test conditions, and performance in order to find favourable electrolyte candidates and their microbattery structures with high capacity and long cycle life.
| # | Electrolyte | Preparation method | Preparation conditions notes | Ionic conductivity | Microbattery | Structure (thin film, 2D/3D) | Initial discharge capacity | Voltage range | Current rate | Number of cycles | Retention | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LiPON | RF magnetron sputtering (MS) | 2.5 μm (cathode) | NA | Li/LiPON/LiCoO2 | 2D | 150 μA h | 3–4.2 V | 0.1 mA | 4000 | 99% | 56 |
| 2 | LiPON | RF MS | 170 μm | NA | Li/LiPON/LiCoO2 | 2D | 25 | 3.6–4.1 V | 1C | 500 | ∼100% | 57 |
| 200 μm (total thickness) | 21 mW h cm−3 | |||||||||||
| 3 | N–LiPON (normal) | RF MS | Li3PO4 | 2.4 μS cm−1 | Li/LiPON/LiCoO2 | 2D | 62.1 | 3.2–4.2 V | 0.1C | 26 | 98% | 58 |
| Li–LiPON (Li-rich) | Li3.3PO4 (targets); 2.7 W cm−2 | 3.2 μS cm−1 | 64.5 μA h cm−2 μm−1 | 90% | ||||||||
| 4 | LiPON | RF MS | 1 μm (LiPON) | 2 μS cm−1 | Li/LiPON/LiMn2O4 | 2D | 48 μA h cm−2 μm−1 | 3.7–4.3 V | 100 μA cm−2 | 100 | 96% | 228 |
| 5 | LiPON | RF MS | 1–1.5 μm (LiPON) | NA | Li/LiPON/LiMn2O4 | 2D | 110 mA h g−1 | 2.5–4.2 V | 20 μA cm−2 | 3500 | 95% | 229 |
| 6 | LiPON | RF MS | 1 μm (LiPON); 350 W | NA | Li/LiPON/LiCoO2 | 2D | 55 | 2.15–3.8 V | 10 μA cm−2 | 140 | 90% | 231 |
| Li/LiPON/V2O5 | 45 μA h cm−2 μm−1 | 20 | 67% | |||||||||
| 7 | LiPON | RF MS | 2.4 μm (LiPON); 35 W | NA | Li/LiPON/LiFe(WO4)2 | 2D | 104 μA h cm−2 μm−1 | 1–4 V | 28 μA cm−2 | 150 | 54% | 235 |
| 8 | LiPON | RF MS | 2.4 μm (LiPON) | NA | Li/LiPON/CuWO4 | 2D | 145 μA h cm−2 μm−1 | 1–4 V | 14 μA cm−2 | 100 | 21% | 236 |
| 9 | LiPON | RF MS | 5.5 W cm2; heat treatment 1 h at 200 °C | 3.2 μS cm−1 | SnxNy/LiPON/LiCoO2 | 2D | 193 μA h (cycling at 100 °C) | 2–4.2 V | 15 μA | 15 | ∼100% | 52 |
| 200 μA h (cycling at 60 °C) | ||||||||||||
| 10 | LiPON | RF MS | 1.5 μm (LiPON) | NA | Si/LiPON/VO–LiPO | 2D | 15.7 μA h cm−2 | 0.5–3 V | 0.3 μA | 30 | 49% | 237 |
| 11 | LiPON | RF MS | 100 W | NA | ZnO/LiPON/LiMn2O4 | 2D | 22 μA h cm−2 | 0.5–5 V | 5 μA cm−2 | 50 | 95% | 238 |
| 12 | LiPON | RF MS | 1 μm | NA | V2O5/LiPON/LiMn2O4 | 2D | 0.1 μA h cm−2 (1st cycle) | 0.5–3.5 V | 2 μA cm−2 | 35 | 98% (of 20th cycle) | 239 |
| 10 μA h cm−2 (20th cycle) | ||||||||||||
| 13 | LiPON | RF MS | 2 μm | NA | Overlayer (LiPON or parylene C)/Cu/LiPON/LiCoO2/Au | 2D | 85 μA h cm−2 | 3–4.2 V | 5 mA cm−2 | 500 | 60% | 242 |
| 14 | LiPON | RF MS | 7.5 μm; 40 W; Ar; | NA | SUS/Pt/LiPON/LiCoO2/Au | 2D | 110 mA h g−1 | 2–4.2 V | 5 μA cm−2 | 100 | ∼81% | 243 |
| 55 μA h cm−2 μm−1 | ||||||||||||
| 15 | LiPON | RF MS | 100 W | 0.7–1 μS cm−1 | Li/LiPON/Li4Ti5O12(LTO) | 2D | 31 μA h cm−2 | 1–2 V | 3.5 μA cm−2 | 5 | ∼95% | 250 |
| 16 | LiPON | RF MS | 1.5 μm | NA | Li/LiPON/LiCoO2 | 2D | 22 μA h cm−2 | 3–4.3 V | 80 μA cm−2 | 1100 | 100% | 222 |
| 100 W | ||||||||||||
| 17 | LiPON | RF MS | 1.5 μm | NA | Li/LiPON/LiCoO2 | 2D | 60 μA h cm−2 μm−1 | 3–4.2 V | 5C | 500 | 100% | 223 |
| 18 | LiPON (Li2.64P1.0O2.81N0.33) | RF MS | 1.45–1.5 μm | 1.5 μS cm−1 | Li/LiPON/LiCoO2 | 2D | 35 μA h cm−2 μm−1 | 3–4.2 V | 0.73C | 800 | 85% | 224 |
| 3.53 W cm−2 | ||||||||||||
| 19 | LiPON | RF MS | 1.4 μm | NA | Li/LiPON/LiCoO2 | 2D | 42 μA h cm−2 μm−1 | 3–4.2 V | 10 μA cm−2 | 900 | 88% | 225 |
| 350 W | ||||||||||||
| 20 | LiPON | RF MS | 1.5 μm | NA | Li/LiPON/LiCoO2 | 2D | 63 μA h cm−2 μm−1 (V = −50 V) | 3–4.2 V | 1C | 100 | 90% | 226 |
| 200 W | ||||||||||||
| 21 | LiPON | RF MS | 1.5 μm | NA | Li/LiPON/LiCoO2 | 2D | 16 μA h | 3–5 V | 10 μA cm−2 | 100 | 50% | 308 |
| 22 | LiPON | NA | NA | NA | Li/LiPON/LiCoO2 | 2D | 106 μA h cm−2 | 3–4.2 V | 46.5 μA cm−2 | 100 | 90% | 227 |
| 23 | LiPON | RF MS | In situ (no vacuum break); ex situ | 0.943 μS cm−1 | Li/LIPON/V2O5 | 2D | 8 μA h | 2.7–3.6 V | 20 μA cm−2 | 500 | 62.5% | 232 |
| 6 μA h | 58% | |||||||||||
| 24 | LiPON | RF MS | 2.4 μm | NA | Li/LiPON/LiCo0.8Ni0.2O2 | 2D | 62 μA h cm−2 μm−1 | 3–4.2 V | 14 μA cm−2 | 50 | 86% | 234 |
| 35 W | Li/LiPON/LiCo0.8Zr0.2O2 | 50 μA h cm−2 μm−1 | 98% | |||||||||
| 25 | LiPON | RF MS | 1 μm | 2 μS cm−1 | Li/LiPON/LiNi0.5Mn1.5O4 | 2D | 122 mA h g−1 | 3.2–5 V | C/10 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
90.6% | 230 |
| 26 | LiPON | RF MS | 1 μm | NA | V2O5/LiPON/Li2−xMn2O4 | 2D | 1.1 mA h | 0.3–3.5 V | 0.4 mA | 98 | 81% | 240 |
| 100 mm × 100 mm (cell size) | 8 mA h | 1 mA | 69% | |||||||||
| 200 mm × 20 0 mm | ||||||||||||
| 27 | LiPON | RF MS | 100 nm | NA | Nb2O5/LiPON/Li2Mn2O4 | 2D | 500 mA h cm−3 | 0.3–3.5 V | 0.1 mA | 500 | 76% | 241 |
| 28 | LiPON | RF MS | 1 μm | NA | Li/LiPON/LiCoO2 | 2D | 65 μA h cm−2 | 3.3–4.5 V | 100 μA cm−2 | 1800 | 94% | 28 |
| Li/LiPON/LiMn2O4 | 55 μA h cm−2 | 3–4.2 V | 500 | 77% | ||||||||
| 29 | LiPON | RF MS | 150 W; post anneal. At 550 °C | 1.5 μS cm−1 | SiNPL/LiPON/LiFePO4 | 3D; nanopillars | NA | NA | NA | NA | NA | 76 |
| 30 | LiPON | RF MS | 5 mTorr | NA | Li/LiPON/LiCoO2 | 3D, slanted nanowires | 96 mA h g−1 | 3–4.2 V | 1C | 400 | 73% | 70 |
| 31 | LiPON | RF MS | 2 μm (LiPON) | NA | Li/LiPON/α-MoO3−x | 3D, nanoflake arrays | 266 mA h g−1 | 1.5–3.5 V | 500 mA g−1 | 1000 | 92.7% | 79 |
| 32 | LiPON | RF MS | 500 nm (LiPON) | 0.25 μS cm−1 | Si/LiPON/LiCoO2 | 3D, microcolumns | 25 μA h cm−2 | 3–4 V | 0.16C | 20 | ∼87.5% | 71 |
| 4.5 μA h cm−2 | 1.2C | |||||||||||
| 33 | LiPON | Physical vapor deposition | Nanowires: 2 μm length; 0.5–1 μm diameter | NA | Si/LiPON/LiCoO2 | 3D, nanowires | NA | NA | NA | NA | NA | 72 |
| 34 | LiPON (Li2PO2N) | Atomic layer deposition (ALD) | 0.6 Å per cycle; 250 °C | NA | SnNx/LiPON/LiV2O5 | 3D, pore arrays | 30 μA h cm−2 (area enhancement factor = 10) | 0.5–3.5 V | 100 μA cm−2 | 100 | 56% | 31 |
| 35 | LiPON | ALD | 70 nm (LiPON) | 0.5 μS cm−1 | Li/LiPON/Li4Ti5O12 | 2D | 0.3 A h cm−3 | 0.8–2.6 V | 5C | 1 | NA | 271 |
| 36 | LiPON | ALD | 90 nm (LiPON) | 0.651 μS cm−1 | Si/LiPON/LiCoO2 | 2D | 20 μA h cm−3 | 2.5–4.2 V | 300 μA cm−2 | 150 | 80% | 273 |
| 37 | LiPON | Pulsed laser deposition (PLD) | N2, O2 atm | 1.6 μS cm−1 | Li/LiPON/Ag0.3V2O5 | 2D | 40 μA h cm−2 | 1–3.5 V | 7 μA cm−2 | 100 | NA | 29 |
| 38 | LiPON | E-beam reaction evaporation | 2 μm, nitrogen-plasma assisted deposition | NA | Li/LiPON/0.5Ag:V2O5 | 2D | 72 μA h cm−2 μm−1 | 1–3.5 V | 7 μA cm−2 | 20 | 58% | 233 |
| 39 | LiPON | Metalorganic-chemical vapor deposition (MOCVD) | 190 nm | 5.3 μS cm−1 | Si/LiPON/LiCoO2 | 2D | 5.2 μA h cm−2 | 2.8–3.8 V | 12.7 μA cm−2 | 1 | NA | 32 |
| 40 | LiPONB | RF MS | 2.0 W cm2; Li3PO4–LiBO2 95/5 | 2.6 μS cm−1 | Li/LiPONB/TiOySz | 2D | 90 μA h cm−2 μm−1 | 1–3 V | 100 μA cm−2 | 500 | 89% | 245 |
| 41 | LiPONB | RF MS | 50 W (Li3PO4) | 1.628 μS cm−1 | Li/LiPONB/LiCoO2 | 2D | 34.5 μA h cm−2 | 3.4–4.2 V | 10 μA cm−2 | 15 | 95.3% | 246 |
| 42 | LiPONB | RF MS | 1.4 μm; 2 W cm−2 | NA | Si/LIPONB/Li | 2D | 40 μA h cm−2 | 0.05–1 V | 100 μA cm−2 (2C) | 1500 | ∼100% | 247 |
| 571 μA h cm−2 μm−1 | ||||||||||||
| 43 | Li3.09BO2.53N0.52 (LiBON) | RF MS | 3.53 W cm−2 | NA | Li/LiBON/LiCoO2 | 2D | 55 μA h cm−2 μm−1 | 2.8–4.2 V | 1C | 1000 | 90% | 12 |
| 44 | Li2O–V2O5–SiO2 (LVSO) | RF MS | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ar/O2 atm; 100 W; 50 °C 1 Ar/O2 atm; 100 W; 50 °C |
1 μS cm−1 | Li/LVSO/MoO3 | 2D | 400 μA h cm−2 | 1.5–3.5 V | 10 μA cm−2 | 40 | 72.5% | 287 |
| 45 | LVSO | PLD | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ar/O2 atm; 100 W; 50 °C 1 Ar/O2 atm; 100 W; 50 °C |
NA | SnO/LVSO/LiCoO2 | 2D | 9 μA h cm−2 | 2–3 V | 4.4 μA cm−2 | 3 | 85% | 288 |
| SnO/LVSO/LiMn2O4 | 1.5 μA h cm−2 | 1–3 V | 93% | |||||||||
| 46 | LVSO | PLD | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ar/O2 atm; 100 W; c 1 Ar/O2 atm; 100 W; c |
0.3 μS cm−1 | SnO/LVSO/LiCoO2 | 2D | NA | 1.4–3.5 V | 4.4 μA cm−2 | 10 | NA | 290 |
| 47 | Li3.4V0.6Si0.4O4 (LVSO) | Solid state reaction | NA | NA | SnO/LVSO/LiNi0.8Co0.2O2 | 2D | 20 μA h cm−2 μm−1 | 0–3 V | NA | 20 | 84% | 289 |
| 48 | Polymethyl methacrylate–polyethylene glycol (PMMA–PEG) | Drop casting | 19 μm (PMMA–PEG) | NA | TiO2nts/PMMA–PEG/LiNi0.5Mn1.5O4 (LNMO) | 3D, nanotubes (nts) | 30 μA h cm−2 μm−1 (=80 mA h g−1) | 1–3.3 V | C/10 | 35 | 91.5% | 73 |
| 49 | PMMA–PEG | Electrodeposition of nts + drop casting | Cyclic voltammetry | NA | TiO2nts/PMMA–PEG/LiNi0.5Mn1.5O4 (LNMO) | 3D, nanotubes (nts) | 175 mA h g−1 | 1–3.3 V | C/10 | 10 | 88% | 74 |
| 50 | PMMA–PEG | Electrodeposition | 1 μm | NA | Li/PEO–PMMA(LiTFSI)/TiO2nts | 3D, nanotubes (nts) | 190 μA h cm−2 μm−1 (1st cycle) | 0.5–2.6 V | C/5 | 50 | 78% | 301 |
| 83 μA h cm−2 μm−1 (2nd cycle) | ||||||||||||
| 51 | PMMA–PEG | Electrodeposition | 100 electropolymerization cycles | NA | TiO2nts/PMMA–PEG/LNMO | 3D, nanotubes (nts) | 125 mA h g−1 | 1–3.3 V | C/10 | 10 | 72% | 75 |
| 52 | SiO2–PEG | Sol–gel; organic polymerization | 0.8–1 μm | 26 μS cm−1 | Li/SiO2–PEG/Li4Ti5O12 | 2D | 2.7 mA h g−1 | 1–2 V | 1 mA cm−2 | 500 | 30% | 63 |
| 53 | PEG based BAB block | Prepared by Nippon Soda Company Limited | NA | 0.2 mS cm−1 | Li4/3Ti5/3O4/PEG-BAB/LiMn2O4 | 2D | 8.48 μW h cm−2 | 1.6–2.7 V | NA | 1 | NA | 64 |
| 54 | LiTFSI in MA-PEG500 | Spin-coating | NA | 0.4 mS cm−1 | Li4Ti5O12/LiTFSI-MA-PEG500/LiNi0.5Mn1.5O4 | 3D, micropillars | 1 mA h cm−2 | 1–3.5 V | C/2 | 100 | 80% | 83 |
| 55 | SU-8 | Photolithography | Structure similar to PEG | 0.1–0.3 μS cm−1 | Si/SU-8/LiNi0.8Co0.15Al0.05O2 (NCA) | 3D, arrays | 0.55 mA h cm−2 | 2.6–3.7 V | 0.22 mA cm−2 | 100 | 93% | 82 |
| 56 | Poly(ether amine) (PEA)-based monomer | Chemical reaction, solution casting on electrode | 1 μm | 8 μS cm−1 | Li/SPE/LiFePO4 | 2D | 140 mA h g−1 | 2.2–4.2 V | C/50; cycled at 60 °C | 12 | 21% | 103 |
| 57 | Poly(trimethylene carbonate) (PTMC) | Chemical reaction, dip coating | Functionalized by caprolactone (CL); <5 μm | NA | Li/P(TMC-CL)–LiTFSI/Cu2O (nanopillar) | 3D, nanopillars | 0.2 mA h cm−2 | 0.2–3.2 V | 0.008 mA cm2 | 10 | 95% | 104 |
| 58 | P-Sulfonated poly(allyl phenyl ether) (SPAPE) | Electrodeposition | 300 nm | NA | Li/SPAPE/TiO2nts | 3D, nanotubes | 88 μA h cm−2 | 1–3 V | C/8 | 4 | 60% | 96 |
| 59 | SPAPE | Electrodeposition | NA | NA | Li/SPAPE/CNT | 3D, nanotubes | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mA h g−1 (1st cycle) 000 mA h g−1 (1st cycle) |
0.01–2 V | 1C | 50 | 50% (of 2nd cycle) | 110 |
| 1600 mA h g−1 (2nd cycle) | ||||||||||||
| 60 | Poly(propylene glycol) diacrylate (PPDGA) and polyetheramine (glyceryl poly(oxypropylene)) (PEA) blend with LiTFSI | Drop casting | NA | NA | Li/PPDGA–PEA–LiTFSI/Cu2Sb | 3D, nanopillar | 0.3 mA h cm−2 (1st cycle) | 0.01–1.5 V | C/50; cycled at 60 °C | 50 | 50% | 105 |
| 0.15 mA h cm−2 (2nd cycle) | ||||||||||||
| 61 | Poly(vinylidene fluoride-hexafluoro propylene) (PVDF-HFP) | Solvent casting | Addition of LiPF6 | NA | Li/PVDF-HFP(gel)/LiCoO2 | 2D | 164 μA h cm−2 | 3–4.2 V | 20 μA cm−2 | 1 | NA | 115 |
| 62 | Gel polymer electrolyte (GPE): (PVdF-HFP, P13FSI-pyrrolidinium bis(fluorosulfonyl)imide, LiTFSI) | Hand casting | Electrolyte prepared by chemical reaction | 1.88 mS cm−1 | Li/GPE/LiCoO2 | 2D | 264 μA h cm−2 | 3–4.2 V | 333 μA cm−2 | 30 | 98.9% | 116 |
| pSi/GPE/LiCoO2 | 226 μA h cm−2 | 0–1.2 V | 17 μA cm−2 | 32 | 80% | |||||||
| Li/GPE/pSi-Cu | 270 μA h cm−2 | 400 μA cm−2 | 30 | 93% | ||||||||
| 63 | GPE: PEO, LiTFSI, 1,3-dioxolane (DOL), and 1,2-dimethoxyethane (DME) | Drop casting | Stepwise infiltration of GPE components | 4 mS cm−1 | Li/GPE/V2O5 | 3D, interdigitated | 0.15 mA h cm−2 | 1.5–3.7 V | 1C | 550 | 73% | 78 |
| 64 | GPE: ethoxylated trimethylolpropane triacrylate (ETPTA) polymer matrix, liquid electrolyte (1 M LiPF6 in ethylene carbonate (EC)/propylene carbonate (PC) = 1/1 (v/v)), and Al2O3 nanoparticles | UV-curing | 18 μm | 1 mS cm−1 | Si pillars/GPE/Li | 3D, micropillars | 2680 mA h g−1 | 0.01–1.5 V | 0.5C | 10 | 26% | 113 |
| 65 | Ionogel (PYR13-Li-TFSI: N-methyl-N-propylpyrrolidinium bis(trifluoromethan) suflonylimide and LiTFSI) | Ink-jetting | NA | ∼0.05 S cm−1 | LiFePO4/ionogel/Li4Ti5O12 | 2D | 300 μA h cm−2 | 1.5–2.5 V | C/10 | 100 | 85% | 14 |
| 66 | Solid polymer electrolyte (SPE): dimethacrylic oligomer bisphenol A ethoxylate dimethacrylate (BEMA), diluent poly(ethyleneglycol) methyl ether methacrylate (PEGMA), ethylene carbonate/diethyl carbonate (EC/DEC) and LiTFSI | UV-induced photo-polymerization deposition | 25 ± 5 μm | NA | Li/SPE/V2O5 | 2D | 130 mA h g−1 | 2.5–3.8 V | 1.5C | 300 | 96% | 91 |
| 67 | Composite polymer electrolyte (CPE): LiI1P(EO)20EC, 12% (v/v) Al2O3 | NA | NA | NA | Li/CPE/FeS1+x | 2D | 50 mA h | 1–2.3 V | 50 μA cm−2 | 650 | 61% | 117 |
| 68 | Poly(phenylene oxide) (PPO) | Electrodeposition | 10–15 nm | NA | 3D carbon/PPO/V2O5 gel | 3D, microporous anode | 9 mA h g−1 | 1.6–3.3 V | 10–20 μA | 10 | 11% | 80 |
| 69 | Poly(phenylene oxide) (PPO) | Electrodeposition | 10 nm | NA | Mesoporous carbon/PPO/lithium sulfide | 3D, gyroid | 0.225 mA h cm−2 | 1.5–3 V | 0.125 mA cm−2 | 10 | ∼100% | 81 |
| 70 | Li3PO4 | PLD | 4 J cm−2 | 0.33 μS cm−1 | Li/Li3PO4/LiCoO2 | 2D | 10 μA h cm−2 | 2–4.2 V | 166 μA cm−2 | 20 | 100% | 309 |
| SnO/LVSO/LiCoO2 | 5.5 μA h cm−2 | 33% | ||||||||||
| 71 | Li3PO4 | PLD | 300 mJ | 0.8 μS cm−1 | Li/Li3PO4/LiCoO2 | 2D | 60 μA h cm−2 | 3–4.4 V | 69 μA cm−2 | 10 | 98% | 268 |
| 72 | Li3PO4 | ALD | 2 mbar | 0.62 μS cm−1 | Li/Li3PO4/TiO2 | 3D, microtubes (MT) | 100 μA h cm−2 (double DMT) | 1.1–2.3 V | C/10 | 20 | ∼100% | 274 |
| 300 μA h cm−2 (single SMT) | C/16 | |||||||||||
| 73 | Li4SiO4 | Prepared by Wako Pure Chemical Industries, Ltd | NA | 0.4 μS cm−1 | Li/Li4SiO4/LiCoO2 | 2D | 10 μA h cm−2 | 3–4.2 V | NA | 77 | 96.6% | 310 |
| 74 | Li2O–SiO2–P2O5 (LiSiPON) | RF MS | N2; 75 W | 8.8 μS cm−1 | Si0.7V0.3/Li1.9Si0.28P1.0O1.1N1.0/LiCoO2 | 2D | 60 μA h cm−2 μm−1 | 2–3.9 V | 10 μA | 1500 | 83% | 295 |
| 75 | Li3xLa2/3−xTiO3 (LLTO) | Solid-state reaction, e-beam evaporation on electrode | 1500 nm (LLTO) | 0.18 μS cm−1 | Li/LiPON/LLTO/LiCoO2 | 2D | 50 μA h cm−2 μm−1 | 3–4 V | 7 μA cm−2 | 100 | 50% | 190 |
To sum up, this review has provided information on many aspects of solid electrolytes used in various microbatteries, covering the recent advancements, performance, and current issues of such structures. To significantly improve solid-state microbatteries, it is vital to face the ongoing situation with solid electrolytes and resolve their problems. Therefore, this review objectively summarizes the information on existing studies that will help researchers to understand the actual status of solid electrolytes and find new solutions to their further development and integration in high-performance solid microbatteries.
Author contributions
A. J., A. M., B. U. conceived the idea, gathered data, analysed, processed it, and wrote the manuscript; S.-T. M. and Z. B. supervised and organized the work, revised the manuscript and assisted in the data processing and analysis. A. M. and Z. B. secured funding for this work.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a research grant AP08052231 “Development of solid-state electrolytes with high ionic conductivity for the next generation of lithium-ion batteries” from the Ministry of Education and Science of the Republic of Kazakhstan and a research project No. SOE2019001 “Development of 3D solid state thin film materials for durable and safe Li-ion microbatteries” from Nazarbayev University.References
- S. Ferrari, M. Loveridge, S. D. Beattie, M. Jahn, R. J. Dashwood and R. Bhagat, J. Power Sources, 2015, 286, 25–46 CrossRef CAS.
- W. Li, T. L. Christiansen, C. Li, Y. Zhou, H. Fei, A. Mamakhel, B. B. Iversen and J. J. Watkins, Nano Energy, 2018, 52, 431–440 CrossRef CAS.
- M. Nathan, D. Golodnitsky, V. Yufit, E. Strauss, T. Ripenbein, I. Shechtman, S. Menkin and E. Peled, J. Microelectromech. Syst., 2005, 14, 879–885 CAS.
- D. Golodnitsky, M. Nathan, V. Yufit, E. Strauss, K. Freedman, L. Burstein, A. Gladkich and E. Peled, Solid State Ionics, 2006, 177, 2811–2819 CrossRef CAS.
- N. Nitta, F. Wu, J. T. Lee and G. Yushin, Mater. Today, 2015, 18, 252–264 CrossRef CAS.
- M. Li, J. Lu, Z. Chen and K. Amine, Adv. Mater., 2018, 30, 1–24 Search PubMed.
- Y. Wang, B. Liu, Q. Li, S. Cartmell, S. Ferrara, Z. D. Deng and J. Xiao, J. Power Sources, 2015, 286, 330–345 CrossRef CAS.
- N. J. Dudney, Electrochem. Soc. Interface, 2008, 17, 44–48 CrossRef CAS.
- J. W. Long, B. Dunn, D. R. Rolison and H. S. White, Chem. Rev., 2004, 104, 4463–4492 CrossRef CAS PubMed.
- J. W. Long, B. Dunn, D. R. Rolison and H. S. White, Adv. Energy Mater., 2020, 10, 1–6 Search PubMed.
- F. Xu, N. J. Dudney, G. M. Veith, Y. Kim, C. Erdonmez, W. Lai and Y. M. Chiang, J. Mater. Res., 2010, 25, 1507–1515 CrossRef CAS.
- S. W. Song, K. C. Lee and H. Y. Park, J. Power Sources, 2016, 328, 311–317 CrossRef CAS.
- J. H. Pikul, H. Gang Zhang, J. Cho, P. V. Braun and W. P. King, Nat. Commun., 2013, 4, 1732–1735 CrossRef PubMed.
- P. E. Delannoy, B. Riou, B. Lestriez, D. Guyomard, T. Brousse and J. Le Bideau, J. Power Sources, 2015, 274, 1085–1090 CrossRef CAS.
- T. Zhang, W. He, W. Zhang, T. Wang, P. Li, Z. M. Sun and X. Yu, Chem. Sci., 2020, 11, 8686–8707 RSC.
- Y. Zhu, J. C. Gonzalez-Rosillo, M. Balaish, Z. D. Hood, K. J. Kim and J. L. M. Rupp, Nat. Rev. Mater., 2020, 1–19 Search PubMed.
- Y. Zheng, Y. Yao, J. Ou, M. Li, D. Luo, H. Dou, Z. Li, K. Amine, A. Yu and Z. Chen, Chem. Soc. Rev., 2020, 49, 8790–8839 RSC.
- F. Zheng, M. Kotobuki, S. Song, M. O. Lai and L. Lu, J. Power Sources, 2018, 389, 198–213 CrossRef CAS.
- H. Xia, H. L. Wang, W. Xiao, M. O. Lai and L. Lu, Int. J. Surf. Sci. Eng., 2009, 3, 23–43 CrossRef CAS.
- C. H. Chen, S. Xie, E. Sperling, A. S. Yang, G. Henriksen and K. Amine, Solid State Ionics, 2004, 167, 263–272 CrossRef CAS.
- F. Han, A. S. Westover, J. Yue, X. Fan, F. Wang, M. Chi, D. N. Leonard, N. J. Dudney, H. Wang and C. Wang, Nat. Energy, 2019, 4, 187–196 CrossRef CAS.
- V. Thangadurai and W. Weppner, Ionics, 2006, 12, 81–92 CrossRef CAS.
- D. Grazioli, V. Zadin, D. Brandell and A. Simone, Electrochim. Acta, 2019, 296, 1142–1162 CrossRef CAS.
- Y. Chen, K. Wen, T. Chen, X. Zhang, M. Armand and S. Chen, Energy Storage Mater., 2020, 31, 401–433 CrossRef.
- K. J. Kim, M. Balaish, M. Wadaguchi, L. Kong and J. L. M. Rupp, Adv. Energy Mater., 2021, 11, 1–63 Search PubMed.
- R. C. Xu, X. H. Xia, S. Z. Zhang, D. Xie, X. L. Wang and J. P. Tu, Electrochim. Acta, 2018, 284, 177–187 CrossRef CAS.
- X. Yu, J. B. Bates, G. E. Jellison and F. X. Hart, J. Electrochem. Soc., 1997, 144, 524–532 CrossRef CAS.
- N. J. Dudney, Mater. Sci. Eng., B, 2005, 116, 245–249 CrossRef.
- C. M. Julien and A. Mauger, Coatings, 2019, 9, 386 CrossRef CAS.
- F. Vereda, R. B. Goldner, T. E. Haas and P. Zerigian, Electrochem. Solid-State Lett., 2002, 5, 239–241 CrossRef.
- A. Pearse, T. Schmitt, E. Sahadeo, D. M. Stewart, A. Kozen, K. Gerasopoulos, A. A. Talin, S. B. Lee, G. W. Rubloff and K. E. Gregorczyk, ACS Nano, 2018, 12, 4286–4294 CrossRef CAS PubMed.
- T. Fujibayashi, Y. Kubota, K. Iwabuchi and N. Yoshii, AIP Adv., 2017, 7, 085110 CrossRef.
- C. Chen, R. A. Eichel and P. H. L. Notten, J. Electroceram., 2017, 38, 230–247 CrossRef CAS.
- E. Quartarone and P. Mustarelli, Chem. Soc. Rev., 2011, 40, 2525–2540 RSC.
- A. Levasseur, M. Menetrier, R. Dormoy and G. Meunier, Mater. Sci. Eng., B, 1989, 3, 5–12 CrossRef.
- X. Liang, F. Tan, F. Wei and J. Du, IOP Conf. Ser.: Earth Environ. Sci., 2019, 218, 012138 CrossRef.
- J. F. M. Oudenhoven, L. Baggetto and P. H. L. Notten, Adv. Energy Mater., 2011, 1, 10–33 CrossRef CAS.
- M. Balaish, J. C. Gonzalez-Rosillo, K. J. Kim, Y. Zhu, Z. D. Hood and J. L. M. Rupp, Nat. Energy, 2021, 6, 227–239 CrossRef CAS.
- L. Liu, Q. Weng, X. Lu, X. Sun, L. Zhang and O. G. Schmidt, Small, 2017, 13, 1–12 Search PubMed.
- T. M. Clancy and J. F. Rohan, J. Energy Storage, 2019, 23, 1–8 CrossRef.
- C. Yue, J. Li and L. Lin, Front. Mech. Eng., 2017, 12, 459–476 CrossRef.
- C. M. Julien, A. Mauger and O. M. Hussain, Materials, 2019, 12, 1–26 CrossRef PubMed.
- J. Liu, H. Zhu and M. H. A. Shiraz, Front. Energy Res., 2018, 6, 1–5 CrossRef.
- X. Meng, Energy Storage Mater., 2020, 30, 296–328 CrossRef.
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef.
- I. B. Sprague and P. Dutta, Appl. Phys. Lett., 2012, 101, 113903 CrossRef.
- Y. Yang, K. C. Pradel, Q. Jing, J. M. Wu, F. Zhang, Y. Zhou, Y. Zhang and Z. L. Wang, ACS Nano, 2012, 6, 6984–6989 CrossRef CAS PubMed.
- W. C. West, J. F. Whitacre, V. White and B. V. Ratnakumar, J. Micromech. Microeng., 2002, 12, 58–62 CrossRef CAS.
- J. Ni, A. Dai, Y. Yuan, L. Li and J. Lu, Matter, 2020, 2, 1366–1376 CrossRef.
- R. Salot, S. Martin, S. Oukassi, M. Bedjaoui and J. Ubrig, Appl. Surf. Sci., 2009, 256, 54–57 CrossRef.
- J. P. Carmo, R. P. Rocha, A. F. Silva, L. M. Goncalves and J. H. Correia, Procedia Chem., 2009, 1, 453–456 CrossRef CAS.
- D. Li, Z. Ma, J. Xu, Y. Li and K. Xie, Mater. Lett., 2014, 134, 237–239 CrossRef CAS.
- S. Shiraki, H. Oki, Y. Takagi, T. Suzuki, A. Kumatani, R. Shimizu, M. Haruta, T. Ohsawa, Y. Sato, Y. Ikuhara and T. Hitosugi, J. Power Sources, 2014, 267, 881–887 CrossRef CAS.
- N. J. Dudney and Y. Il Jang, J. Power Sources, 2003, 119–121, 300–304 CrossRef CAS.
- Y. N. Zhou, M. Z. Xue and Z. W. Fu, J. Power Sources, 2013, 234, 310–332 CrossRef CAS.
- J. B. Bates, N. J. Dudney, B. Neudecker, A. Ueda and C. D. Evans, Solid State Ionics, 2000, 135, 33–45 CrossRef CAS.
- B. Laïk, I. Ressejac, C. Venet and J. P. Pereira-Ramos, Thin Solid Films, 2018, 649, 69–74 CrossRef.
- D. L. Xiao, J. Tong, Y. Feng, G. H. Zhong, W. J. Li and C. L. Yang, Solid State Ionics, 2018, 324, 202–206 CrossRef CAS.
- J. B. Bates, N. J. Dudney, G. R. Gruzalski, R. A. Zuhr, A. Choudhury, C. F. Luck and J. D. Robertson, J. Power Sources, 1993, 43, 103–110 CrossRef CAS.
- J. F. Whitacre and W. C. West, Solid State Ionics, 2004, 175, 251–255 CrossRef CAS.
- H. S. Kim, Y. Oh, K. H. Kang, J. H. Kim, J. Kim and C. S. Yoon, ACS Appl. Mater. Interfaces, 2017, 9, 16063–16070 CrossRef CAS PubMed.
- T. J. Cai, Y. H. Lo and J. J. Wu, Mater. Today Energy, 2019, 13, 119–124 CrossRef.
- J. F. Vélez, M. Aparicio and J. Mosa, Electrochim. Acta, 2016, 213, 831–841 CrossRef.
- H. Nakano, K. Dokko, J. ichi Sugaya, T. Yasukawa, T. Matsue and K. Kanamura, Electrochem. Commun., 2007, 9, 2013–2017 CrossRef CAS.
- J. B. Bates, N. J. Dudney, G. R. Gruzalski, R. A. Zuhr, A. Choudhury, C. F. Luck and J. D. Robertson, Solid State Ionics, 1992, 53–56, 647–654 CrossRef CAS.
- V. Zadin, H. Kasemägi, A. Aabloo and D. Brandell, J. Power Sources, 2010, 195, 6218–6224 CrossRef CAS.
- S. Moitzheim, B. Put and P. M. Vereecken, Adv. Mater. Interfaces, 2019, 6, 1–17 Search PubMed.
- B. L. Ellis, P. Knauth and T. Djenizian, Adv. Mater., 2014, 26, 3368–3397 CrossRef CAS PubMed.
- R. W. Hart, H. S. White, B. Dunn and D. R. Rolison, Electrochem. Commun., 2003, 5, 120–123 CrossRef CAS.
- M. Yoon, S. Lee, D. Lee, J. Kim and J. Moon, Appl. Surf. Sci., 2017, 412, 537–544 CrossRef CAS.
- A. A. Talin, D. Ruzmetov, A. Kolmakov, K. McKelvey, N. Ware, F. El Gabaly, B. Dunn and H. S. White, ACS Appl. Mater. Interfaces, 2016, 8, 32385–32391 CrossRef CAS PubMed.
- D. Ruzmetov, V. P. Oleshko, P. M. Haney, H. J. Lezec, K. Karki, K. H. Baloch, A. K. Agrawal, A. V. Davydov, S. Krylyuk, Y. Liu, J. Huang, M. Tanase, J. Cumings and A. A. Talin, Nano Lett., 2012, 12, 505–511 CrossRef CAS PubMed.
- N. Plylahan, M. Letiche, M. K. S. Barr and T. Djenizian, Electrochem. Commun., 2014, 43, 121–124 CrossRef CAS.
- G. D. Salian, C. Lebouin, A. Demoulin, M. S. Lepihin, S. Maria, A. K. Galeyeva, A. P. Kurbatov and T. Djenizian, J. Power Sources, 2017, 340, 242–246 CrossRef CAS.
- G. D. Salian, C. Lebouin, A. Galeyeva, A. P. Kurbatov and T. Djenizian, Front. Chem., 2019, 7, 1–8 CrossRef PubMed.
- C. Lethien, M. Zegaoui, P. Roussel, P. Tilmant, N. Rolland and P. A. Rolland, Microelectron. Eng., 2011, 88, 3172–3177 CrossRef CAS.
- Y. He, S. Chen, L. Nie, Z. Sun, X. Wu and W. Liu, Nano Lett., 2020, 20, 7136–7143 CrossRef CAS PubMed.
- P. Sun, X. Li, J. Shao and P. V. Braun, Adv. Mater., 2020, 2006229, 1–7 Search PubMed.
- S. Sun, Q. Xia, J. Liu, J. Xu, F. Zan, J. Yue, S. V. Savilov, V. V. Lunin and H. Xia, J. Materiomics, 2019, 5, 229–236 CrossRef.
- N. S. Ergang, M. A. Fierke, Z. Wang, W. H. Smyrl and A. Stein, 2007, 1135–1139.
- J. G. Werner, G. G. Rodríguez-Calero, H. D. Abruña and U. Wiesner, Energy Environ. Sci., 2018, 11, 1261–1270 RSC.
- J. I. Hur, L. C. Smith and B. Dunn, Joule, 2018, 2, 1187–1201 CrossRef CAS.
- M. Nasreldin, R. Delattre, C. Calmes, M. Ramuz, V. A. Sugiawati, S. Maria, J. L. de B. de la Tocnaye and T. Djenizian, Energy Storage Mater., 2020, 33, 108–115 CrossRef.
- D. Fang, K. Huang, S. Liu and Z. Li, J. Alloys Compd., 2008, 464, L5 CrossRef CAS.
- W. Wei, G. Oltean, C. W. Tai, K. Edström, F. Björefors and L. Nyholm, J. Mater. Chem. A, 2013, 1, 8160–8169 RSC.
- T. Djenizian, I. Hanzu and P. Knauth, J. Mater. Chem., 2011, 21, 9925–9937 RSC.
- J. Xie, J. F. M. Oudenhoven, D. Li, C. Chen, R.-A. Eichel and P. H. L. Notten, J. Electrochem. Soc., 2016, 163, A2385–A2389 CrossRef CAS.
- S. Moitzheim, J. E. Balder, R. Ritasalo, S. Ek, P. Poodt, S. Unnikrishnan, S. De Gendt and P. M. Vereecken, ACS Appl. Energy Mater., 2019, 2, 1774–1783 CrossRef CAS.
- Q. Zhang, K. Liu, F. Ding and X. Liu, Nano Res., 2017, 10, 4139–4174 CrossRef.
- Q. Zhou, J. Zhang and G. Cui, Macromol. Mater. Eng., 2018, 303, 1–14 Search PubMed.
- C. Gerbaldi, M. Destro, J. R. Nair, S. Ferrari, I. Quinzeni and E. Quartarone, Nano Energy, 2013, 2, 1279–1286 CrossRef CAS.
- K. S. Ngai, S. Ramesh, K. Ramesh and J. C. Juan, Ionics, 2016, 22, 1259–1279 CrossRef CAS.
- K. Murata, S. Izuchi and Y. Yoshihisa, Electrochim. Acta, 2000, 45, 1501–1508 CrossRef CAS.
- R. C. Agrawal and G. P. Pandey, J. Phys. D: Appl. Phys., 2008, 41, 223001 CrossRef.
- Z. Ogumi, J. Electrochem. Soc., 1989, 136, 625 CrossRef CAS.
- I. V. Ferrari, M. Braglia, T. Djenizian, P. Knauth and M. L. Di Vona, J. Power Sources, 2017, 353, 95–103 CrossRef CAS.
- Y. Ito, K. Syakushiro, M. Hiratani, K. Miyauchi and T. Kudo, Solid State Ionics, 1986, 18–19, 277–281 CrossRef CAS.
- N. Boaretto, L. Meabe, M. Martinez-Ibañez, M. Armand and H. Zhang, J. Electrochem. Soc., 2020, 167, 070524 CrossRef CAS.
- P. Yao, H. Yu, Z. Ding, Y. Liu, J. Lu, M. Lavorgna, J. Wu and X. Liu, Front. Chem., 2019, 7, 1–17 CrossRef PubMed.
- S. B. Aziz, T. J. Woo, M. F. Z. Kadir and H. M. Ahmed, J. Sci.: Adv. Mater. Devices, 2018, 3, 1–17 Search PubMed.
- R. Bernhard, A. Latini, S. Panero, B. Scrosati and J. Hassoun, J. Power Sources, 2013, 226, 329–333 CrossRef CAS.
- L. Han, M. L. Lehmann, J. Zhu, T. Liu, Z. Zhou, X. Tang, C. Te Heish, A. P. Sokolov, P. Cao, X. C. Chen and T. Saito, Front. Energy Res., 2020, 8, 1–19 CrossRef.
- B. Sun, I. Y. Liao, S. Tan, T. Bowden and D. Brandell, J. Power Sources, 2013, 238, 435–441 CrossRef CAS.
- B. Sun, H. D. Asfaw, D. Rehnlund, J. Mindemark, L. Nyholm, K. Edström and D. Brandell, ACS Appl. Mater. Interfaces, 2018, 10, 2407–2413 CrossRef CAS PubMed.
- S. Tan, E. Perre, T. Gustafsson and D. Brandell, Solid State Ionics, 2012, 225, 510–512 CrossRef CAS.
- S. Tan, S. Walus, J. Hilborn, T. Gustafsson and D. Brandell, Electrochem. Commun., 2010, 12, 1498–1500 CrossRef CAS.
- N. Plylahan, S. Maria, T. N. T. Phan, M. Letiche, H. Martinez, C. Courrèges, P. Knauth and T. Djenizian, Nanoscale Res. Lett., 2014, 9, 1–8 CrossRef CAS PubMed.
- N. A. Kyeremateng, F. Dumur, P. Knauth, B. Pecquenard and T. Djenizian, C. R. Chim., 2013, 16, 80–88 CrossRef CAS.
- M. E. Abdelhamid, T. Rüther, J.-P. Veder, T. Rodopoulos, A. I. Bhatt, J. Lee, A. F. Hollenkamp, M. D. Horne, T. Huynh, A. Ong, K. J. Putman, G. Rowe and P. de Souza, J. Electrochem. Soc., 2019, 166, A5462–A5469 CrossRef CAS.
- V. A. Sugiawati, F. Vacandio, Y. Ein-Eli and T. Djenizian, APL Mater., 2019, 7, 031506 CrossRef.
- W. Li, L. C. Bradley and J. J. Watkins, ACS Appl. Mater. Interfaces, 2019, 11, 5668–5674 CrossRef CAS PubMed.
- R. He, M. Echeverri, D. Ward, Y. Zhu and T. Kyu, J. Membr. Sci., 2016, 498, 208–217 CrossRef CAS.
- E. H. Kil, K. H. Choi, H. J. Ha, S. Xu, J. A. Rogers, M. R. Kim, Y. G. Lee, K. M. Kim, K. Y. Cho and S. Y. Lee, Adv. Mater., 2013, 25, 1395–1400 CrossRef CAS PubMed.
- Y. Kang, H. J. Kim, E. Kim, B. Oh and J. H. Cho, J. Power Sources, 2001, 92, 255–259 CrossRef CAS.
- M. S. Park, S. H. Hyun, S. C. Nam and S. B. Cho, Electrochim. Acta, 2008, 53, 5523–5527 CrossRef CAS.
- V. Chaudoy, E. Luais, F. Ghamouss, T. Defforge, S. Desplobain, G. Gautier, J. Wolfman, J.-C. Houdbert, F. Tran-Van and J. Sakai, ECS Trans., 2015, 66, 31–39 CrossRef CAS.
- V. Yufit, K. Freedman, M. Nathan, L. Burstein, D. Golodnitsky and E. Peled, Electrochim. Acta, 2004, 50, 417–420 CrossRef CAS.
- D. S. Ashby, C. S. Choi, M. A. Edwards, A. A. Talin, H. S. White and B. S. Dunn, ACS Appl. Energy Mater., 2020, 3, 8402–8409 CrossRef CAS.
- D. S. Ashby, R. H. DeBlock, C. H. Lai, C. S. Choi and B. S. Dunn, Joule, 2017, 1, 344–358 CrossRef CAS.
- J. W. Fergus, J. Power Sources, 2010, 195, 4554–4569 CrossRef CAS.
- L. Ye and Z. Feng, Polym. Electrolytes, 2010, 550–582 CAS.
- Y. Zhao, L. Wang, Y. Zhou, Z. Liang, N. Tavajohi, B. Li and T. Li, Adv. Sci., 2021, 2003675, 2003675 CrossRef PubMed.
- P. Kurzweil and J. Garche, Overview of batteries for future automobiles, Elsevier B.V., 2017 Search PubMed.
- M. J. C. Plancha, Polym. Electrolytes, 2010, 340–377 CAS.
- K. Hayamizu, A. Yuichi, W. S. Price, N. Hiroe and N. Toshiyuki, J. Phys. Chem. B, 2004, 108, 19527–19532 CrossRef CAS.
- K. E. Thomas, S. E. Sloop, J. B. Kerr and J. Newman, J. Power Sources, 2000, 89, 132–138 CrossRef CAS.
- W. Gorecki, M. Jeannin, E. Belorizky, C. Roux and M. Armand, J. Phys.: Condens. Matter, 1995, 7, 6823–6832 CrossRef CAS.
- O. Borodin and G. D. Smith, Macromolecules, 2006, 39, 1620–1629 CrossRef CAS.
- R. Bouchet, S. Maria, R. Meziane, A. Aboulaich, L. Lienafa, J. P. Bonnet, T. N. T. Phan, D. Bertin, D. Gigmes, D. Devaux, R. Denoyel and M. Armand, Nat. Mater., 2013, 12, 452–457 CrossRef CAS PubMed.
- M. Doyle, T. F. Fuller and J. Newman, Electrochim. Acta, 1994, 39, 2073–2081 CrossRef CAS.
- J. Mindemark, B. Sun, E. Törmä and D. Brandell, J. Power Sources, 2015, 298, 166–170 CrossRef CAS.
- B. Sun, J. Mindemark, E. V. Morozov, L. T. Costa, M. Bergman, P. Johansson, Y. Fang, I. Furó and D. Brandell, Phys. Chem. Chem. Phys., 2016, 18, 9504–9513 RSC.
- M. Zhang, S. Yu, Y. Mai, S. Zhang and Y. Zhou, Chem. Commun., 2019, 55, 6715–6718 RSC.
- Q. Ma, H. Zhang, C. Zhou, L. Zheng, P. Cheng, J. Nie, W. Feng, Y. S. Hu, H. Li, X. Huang, L. Chen, M. Armand and Z. Zhou, Angew. Chem., Int. Ed., 2016, 55, 2521–2525 CrossRef CAS PubMed.
- B. M. Savoie, M. A. Webb and T. F. Miller, J. Phys. Chem. Lett., 2017, 8, 641–646 CrossRef CAS PubMed.
- L. Wang, J. Li, G. Lu, W. Li, Q. Tao, C. Shi, H. Jin, G. Chen and S. Wang, Front. Mater., 2020, 7, 1–5 CrossRef CAS.
- K. J. Kim, J. J. Hinricher and J. L. M. Rupp, Nat. Energy, 2020, 5, 278–279 CrossRef CAS.
- M. V. Reddy, C. M. Julien, A. Mauger and K. Zaghib, Nanomaterials, 2020, 10, 1–80 CrossRef PubMed.
- C. Deviannapoorani, L. Dhivya, S. Ramakumar and R. Murugan, J. Power Sources, 2013, 240, 18–25 CrossRef CAS.
- C. Wang, K. Fu, S. P. Kammampata, D. W. McOwen, A. J. Samson, L. Zhang, G. T. Hitz, A. M. Nolan, E. D. Wachsman, Y. Mo, V. Thangadurai and L. Hu, Chem. Rev., 2020, 120, 4257–4300 CrossRef CAS PubMed.
- R. Murugan, V. Thangadurai and W. Weppner, Angew. Chem., Int. Ed., 2007, 46, 7778–7781 CrossRef CAS PubMed.
- J. van den Broek, S. Afyon and J. L. M. Rupp, Adv. Energy Mater., 2016, 6, 1600736 CrossRef.
- C. Hänsel, S. Afyon and J. L. M. Rupp, Nanoscale, 2016, 8, 18412–18420 RSC.
- J. Awaka, A. Takashima, K. Kataoka, N. Kijima, Y. Idemoto and J. Akimoto, Chem. Lett., 2011, 40, 60–62 CrossRef CAS.
- L. Liang, X. Sun, J. Zhang, J. Sun, L. Hou, Y. Liu and C. Yuan, Mater. Horiz., 2019, 6, 871–910 RSC.
- C. A. Geiger, E. Alekseev, B. Lazic, M. Fisch, T. Armbruster, R. Langner, M. Fechtelkord, N. Kim, T. Pettke and W. Weppner, Inorg. Chem., 2011, 50, 1089–1097 CrossRef CAS PubMed.
- R. Pfenninger, M. Struzik, I. Garbayo, E. Stilp and J. L. M. Rupp, Nat. Energy, 2019, 4, 475–483 CrossRef CAS.
- K. J. Kim and J. L. M. Rupp, Energy Environ. Sci., 2020, 13, 4930–4945 RSC.
- R. J. Chen, M. Huang, W. Z. Huang, Y. Shen, Y. H. Lin and C. W. Nan, J. Mater. Chem. A, 2014, 2, 13277–13282 RSC.
- G. Larraz, A. Orera and M. L. Sanjuán, J. Mater. Chem. A, 2013, 1, 11419–11428 RSC.
- W. Xia, B. Xu, H. Duan, X. Tang, Y. Guo, H. Kang, H. Li and H. Liu, J. Am. Ceram. Soc., 2017, 100, 2832–2839 CrossRef CAS.
- H. Peng, Q. Wu and L. Xiao, J. Sol-Gel Sci. Technol., 2013, 66, 175–179 CrossRef CAS.
- I. Garbayo, M. Struzik, W. J. Bowman, R. Pfenninger, E. Stilp and J. L. M. Rupp, Adv. Energy Mater., 2018, 8, 1–14 Search PubMed.
- H. Rusdi, N. S. Mohamed, R. H. Y. Subban and R. Rusdi, J. Sci.: Adv. Mater. Devices, 2020, 5, 368–377 Search PubMed.
- Y. Zhu, X. He and Y. Mo, J. Mater. Chem. A, 2016, 4, 3253–3266 RSC.
- H. El-Shinawi and J. Janek, RSC Adv., 2015, 5, 14887–14891 RSC.
- P. Maldonado-Manso, E. R. Losilla, M. Martínez-Lara, M. a. G. Aranda, S. Bruque, F. E. Mouahid and M. Zahir, Chem. Mater., 2003, 15, 1879–1885 CrossRef CAS.
- J. Fu, Solid State Ionics, 1997, 96, 195–200 CrossRef CAS.
- E. C. Bucharsky, K. G. Schell, A. Hintennach and M. J. Hoffmann, Solid State Ionics, 2015, 274, 77–82 CrossRef CAS.
- H. Chen, H. Tao, X. Zhao and Q. Wu, J. Non-Cryst. Solids, 2011, 357, 3267–3271 CrossRef CAS.
- X. M. Wu, S. Chen, F. R. Mai, J. H. Zhao and Z. Q. He, Ionics, 2013, 19, 589–593 CrossRef CAS.
- R. Inada, K. Ichi Ishida, M. Tojo, T. Okada, T. Tojo and Y. Sakurai, Ceram. Int., 2015, 41, 11136–11142 CrossRef CAS.
- P. Knauth, Solid State Ionics, 2009, 180, 911–916 CrossRef CAS.
- K. Arbi, J. M. Rojo and J. Sanz, J. Eur. Ceram. Soc., 2007, 27, 4215–4218 CrossRef CAS.
- Y. Liu, Q. Sun, Y. Zhao, B. Wang, P. Kaghazchi, K. R. Adair, R. Li, C. Zhang, J. Liu, L. Y. Kuo, Y. Hu, T. K. Sham, L. Zhang, R. Yang, S. Lu, X. Song and X. Sun, ACS Appl. Mater. Interfaces, 2018, 10, 31240–31248 CrossRef CAS PubMed.
- Y. Liu, Q. Sun, J. Liu, M. Norouzi Banis, Y. Zhao, B. Wang, K. Adair, Y. Hu, Q. Xiao, C. Zhang, L. Zhang, S. Lu, H. Huang, X. Song and X. Sun, ACS Appl. Mater. Interfaces, 2020, 12, 2293–2298 CrossRef CAS PubMed.
- W. Xiao, J. Wang, L. Fan, J. Zhang and X. Li, Energy Storage Mater., 2019, 19, 379–400 CrossRef.
- M. Hou, F. Liang, K. Chen, Y. Dai and D. Xue, Nanotechnology, 2020, 31, 132003 CrossRef CAS PubMed.
- P. Hofmann, F. Walther, M. Rohnke, J. Sann, W. G. Zeier and J. Janek, Solid State Ionics, 2019, 342, 115054 CrossRef CAS.
- D. Mazumdar, D. N. Bose and M. L. Mukherjee, Solid State Ionics, 1984, 14, 143–147 CrossRef CAS.
- U. v. Alpen, M. F. Bell, W. Wichelhaus, K. Y. Cheung and G. J. Dudley, Electrochim. Acta, 1978, 23, 1395–1397 CrossRef.
- Y. Deng, C. Eames, J. N. Chotard, F. Laleìre, V. Seznec, S. Emge, O. Pecher, C. P. Grey, C. Masquelier and M. S. Islam, J. Am. Chem. Soc., 2015, 137, 9136–9145 CrossRef CAS PubMed.
- S. Song, J. Lu, F. Zheng, H. M. Duong and L. Lu, RSC Adv., 2015, 5, 6588–6594 RSC.
- N. Kamaya, K. Homma, Y. Yamakawa, M. Hirayama, R. Kanno, M. Yonemura, T. Kamiyama, Y. Kato, S. Hama, K. Kawamoto and A. Mitsui, Nat. Mater., 2011, 10, 682–686 CrossRef CAS PubMed.
- R. Kanno and M. Murayama, J. Electrochem. Soc., 2001, 148, A742 CrossRef CAS.
- Y. Sun, K. Suzuki, K. Hara, S. Hori, T. A. Yano, M. Hara, M. Hirayama and R. Kanno, J. Power Sources, 2016, 324, 798–803 CrossRef CAS.
- Y. Kato, S. Hori, T. Saito, K. Suzuki, M. Hirayama, A. Mitsui, M. Yonemura, H. Iba and R. Kanno, Nat. Energy, 2016, 1, 1–7 Search PubMed.
- H. Muramatsu, A. Hayashi, T. Ohtomo, S. Hama and M. Tatsumisago, Solid State Ionics, 2011, 182, 116–119 CrossRef CAS.
- Y. Wang, Z. Liu, X. Zhu, Y. Tang and F. Huang, J. Power Sources, 2013, 224, 225–229 CrossRef CAS.
- E. Gilardi, G. Materzanini, L. Kahle, M. Döbeli, S. Lacey, X. Cheng, N. Marzari, D. Pergolesi, A. Hintennach and T. Lippert, ACS Appl. Energy Mater., 2020, 3, 9910–9917 CrossRef CAS.
- S. Stramare, V. Thangadurai and W. Weppner, ChemInform, 2003, 34, 3974–3990 CrossRef.
- M. Itoh, Y. Inaguma, W.-H. Jung, L. Chen and T. Nakamura, Solid State Ionics, 1994, 70–71, 196–202 Search PubMed.
- C. H. Chen and K. Amine, Solid State Ionics, 2001, 144, 51–57 CrossRef CAS.
- H. T. Chung, J. G. Kim and H. G. Kim, Solid State Ionics, 1998, 107, 153–160 CrossRef CAS.
- S. I. Furusawa, H. Tabuchi, T. Sugiyama, S. Tao and J. T. S. Irvine, Solid State Ionics, 2005, 176, 553–558 CrossRef CAS.
- O. Maqueda, F. Sauvage, L. Laffont, M. L. Martínez-Sarrión, L. Mestres and E. Baudrin, Thin Solid Films, 2008, 516, 1651–1655 CrossRef CAS.
- B. Huang, B. Xu, Y. Li, W. Zhou, Y. You, S. Zhong, C. A. Wang and J. B. Goodenough, ACS Appl. Mater. Interfaces, 2016, 8, 14552–14557 CrossRef CAS PubMed.
- J. Z. Lee, Z. Wang, H. L. Xin, T. A. Wynn and Y. S. Meng, J. Electrochem. Soc., 2017, 164, A6268–A6273 CrossRef CAS.
- J. m. Lee, S. h. Kim, Y. Tak and Y. S. Yoon, J. Power Sources, 2006, 163, 173–179 CrossRef CAS.
- C. L. Li, B. Zhang and Z. W. Fu, Thin Solid Films, 2006, 515, 1886–1892 CrossRef CAS.
- R. Rajagopalan, Z. Zhang, Y. Tang, C. Jia, X. Ji and H. Wang, Energy Storage Mater., 2021, 34, 171–193 CrossRef.
- J. Huang, F. Liang, M. Hou, Y. Zhang, K. Chen and D. Xue, Appl. Mater. Today, 2020, 20, 100750 CrossRef.
- R. DeWees and H. Wang, ChemSusChem, 2019, 12, 3713–3725 CrossRef CAS PubMed.
- P. Birke, F. Salam, S. Döring and W. Weppner, Solid State Ionics, 1999, 118, 149–157 CrossRef CAS.
- K. P. Abhilash, P. Sivaraj, P. C. Selvin, B. Nalini and K. Somasundaram, Ceram. Int., 2015, 41, 13823–13829 CrossRef CAS.
- J. K. Ahn and S. G. Yoon, Electrochim. Acta, 2004, 50, 371–374 CrossRef CAS.
- S. A. Yoon, N. R. Oh, A. R. Yoo, H. G. Lee and H. C. Lee, J. Korean Ceram. Soc., 2017, 54, 278–284 CrossRef CAS.
- M. Fenech and N. Sharma, Chem.–Asian J., 2020, 15, 1829–1847 CrossRef CAS PubMed.
- C. Cao, Z. Bin Li, X. L. Wang, X. B. Zhao and W. Q. Han, Front. Energy Res., 2014, 2, 1–10 Search PubMed.
- M. Tatsumisago, Solid State Ionics, 2004, 175, 13–18 CrossRef CAS.
- T. Minami, J. Non-Cryst. Solids, 1985, 73, 273–284 CrossRef CAS.
- V. Lacivita, N. Artrith and G. Ceder, Chem. Mater., 2018, 30, 7077–7090 CrossRef CAS.
- N. J. Dudney, J. B. Bates, R. A. Zuhr, C. F. Luck and J. D. Robertson, Solid State Ionics, 1992, 53–56, 655–661 CrossRef CAS.
- B. Put, P. M. Vereecken and A. Stesmans, J. Mater. Chem. A, 2018, 6, 4848–4859 RSC.
- J. B. Bates and X. Yu, J. Vac. Sci. Technol., A, 1996, 14, 34–37 CrossRef CAS.
- V. Krasnov, K.-W. Nieh, S.-J. Ting, P. Tang, F.-H. Chang and C.-T. Lin, U.S. Pat., No. US6863699B1, 2000.
- C. H. Choi, W. I. Cho, B. W. Cho, H. S. Kim, Y. S. Yoon and Y. S. Tak, Electrochem. Solid-State Lett., 2002, 5, 14–17 CrossRef.
- Y. Hamon, A. Douard, F. Sabary, C. Marcel, P. Vinatier, B. Pecquenard and A. Levasseur, Solid State Ionics, 2006, 177, 257–261 CrossRef CAS.
- B. Kim, Y. S. Cho, J. G. Lee, K. H. Joo, K. O. Jung, J. Oh, B. Park, H. J. Sohn, T. Kang, J. Cho, Y. S. Park and J. Y. Oh, J. Power Sources, 2002, 109, 214–219 CrossRef CAS.
- N. Suzuki, S. Shirai, N. Takahashi, T. Inaba and T. Shiga, Solid State Ionics, 2011, 191, 49–54 CrossRef CAS.
- Y. Su, J. Falgenhauer, A. Polity, T. Leichtweiß, A. Kronenberger, J. Obel, S. Zhou, D. Schlettwein, J. Janek and B. K. Meyer, Solid State Ionics, 2015, 282, 63–69 CrossRef CAS.
- A. Sepúlveda, F. Criscuolo, B. Put and P. M. Vereecken, Solid State Ionics, 2019, 337, 24–32 CrossRef.
- A. S. Westover, R. L. Sacci and N. Dudney, ACS Energy Lett., 2020, 5, 3860–3867 CrossRef CAS.
- D. Cheng, T. A. Wynn, X. Wang, S. Wang, M. Zhang, R. Shimizu, S. Bai, H. Nguyen, C. Fang, M. c. Kim, W. Li, B. Lu, S. J. Kim and Y. S. Meng, Joule, 2020, 4, 2484–2500 CrossRef CAS.
- Z. D. Hood, X. Chen, R. L. Sacci, X. Liu, G. M. Veith, Y. Mo, J. Niu, N. J. Dudney and M. Chi, Nano Lett., 2021, 21, 151–157 CrossRef CAS PubMed.
- Z. D. Hood and M. Chi, J. Mater. Sci., 2019, 54, 10571–10594 CrossRef CAS.
- S. Jacke, J. Song, G. Cherkashinin, L. Dimesso and W. Jaegermann, Ionics, 2010, 16, 769–775 CrossRef CAS.
- R. Hausbrand, A. Schwöbel, W. Jaegermann, M. Motzko and D. Ensling, Z. Phys. Chem., 2015, 229, 1387–1414 CrossRef CAS.
- Y.-I. Jang, N. J. Dudney, D. A. Blom and L. F. Allard, J. Electrochem. Soc., 2002, 149, A1442 CrossRef CAS.
- D. Santhanagopalan, D. Qian, T. McGilvray, Z. Wang, F. Wang, F. Camino, J. Graetz, N. Dudney and Y. S. Meng, J. Phys. Chem. Lett., 2014, 5, 298–303 CrossRef CAS PubMed.
- N. J. Dudney, J. Electroceram., 2017, 38, 222–229 CrossRef CAS.
- M. Takahashi, M. Hayashi and T. Shodai, J. Power Sources, 2009, 189, 191–196 CrossRef CAS.
- Y. S. Yoon, S. H. Lee, S. B. Cho and S. C. Nam, J. Electrochem. Soc., 2011, 158, A1313 CrossRef CAS.
- S. W. Song, H. Choi, H. Y. Park, G. B. Park, K. C. Lee and H. J. Lee, J. Power Sources, 2010, 195, 8275–8279 CrossRef CAS.
- S. Tintignac, R. Baddour-Hadjean, J. P. Pereira-Ramos and R. Salot, Electrochim. Acta, 2014, 146, 472–476 CrossRef CAS.
- H. Y. Park, S. R. Lee, Y. J. Lee, B. W. Cho and W. I. Cho, Mater. Chem. Phys., 2005, 93, 70–78 CrossRef CAS.
- M. Koo, K. Il Park, S. H. Lee, M. Suh, D. Y. Jeon, J. W. Choi, K. Kang and K. J. Lee, Nano Lett., 2012, 12, 4810–4816 CrossRef CAS PubMed.
- Y. S. Park, S. H. Lee, B. Il Lee and S. K. Joo, Electrochem. Solid-State Lett., 1999, 2, 58–59 CrossRef CAS.
- N. J. Dudney, J. B. Bates, R. A. Zuhr, S. Young, J. D. Robertson, H. P. Jun and S. A. Hackney, J. Electrochem. Soc., 1999, 146, 2455–2464 CrossRef CAS.
- J. Li, C. Ma, M. Chi, C. Liang and N. J. Dudney, Adv. Energy Mater., 2015, 5, 1401408 CrossRef.
- C. Navone, S. Tintignac, J. P. Pereira-Ramos, R. Baddour-Hadjean and R. Salot, Solid State Ionics, 2011, 192, 343–346 CrossRef CAS.
- E. J. Jeon, Y. W. Shin, S. C. Nam, W. Il Cho and Y. S. Yoon, J. Electrochem. Soc., 2001, 148, A318 CrossRef CAS.
- F. Huang, Z. W. Fu, Y. Q. Chu, W. Y. Liu and Q. Z. Qin, Electrochem. Solid-State Lett., 2004, 7, 180–184 CrossRef.
- C. Li, W. Liu and Z. Fu, Chin. J. Chem. Phys., 2006, 19, 493–498 CrossRef CAS.
- C. L. Li and Z. W. Fu, Electrochim. Acta, 2008, 53, 6434–6443 CrossRef CAS.
- C. L. Li and Z. W. Fu, Electrochim. Acta, 2008, 53, 4293–4301 CrossRef CAS.
- S. Kanazawa, T. Baba, K. Yoneda, M. Mizuhata and I. Kanno, Thin Solid Films, 2020, 697, 137840 CrossRef CAS.
- L. Li, S. Liu, H. Zhou, Q. Lei and K. Qian, Mater. Lett., 2018, 216, 135–138 CrossRef CAS.
- M. Baba, N. Kumagai, N. Fujita, K. Ohta, K. Nishidate, S. Komaba, H. Groult, D. Devilliers and B. Kaplan, J. Power Sources, 2001, 97–98, 798–800 CrossRef CAS.
- H. Nakazawa, K. Sano and M. Baba, J. Power Sources, 2005, 146, 758–761 CrossRef CAS.
- H. Nakazawa, K. Sano, T. Abe, M. Baba and N. Kumagai, J. Power Sources, 2007, 174, 838–842 CrossRef CAS.
- B. J. Neudecker, N. J. Dudney and J. B. Bates, J. Electrochem. Soc., 2000, 147, 517 CrossRef CAS.
- T. Yamamoto, H. Iwasaki, Y. Suzuki, M. Sakakura, Y. Fujii, M. Motoyama and Y. Iriyama, Electrochem. Commun., 2019, 105, 106494 CrossRef CAS.
- V. Lacivita, A. S. Westover, A. Kercher, N. D. Phillip, G. Yang, G. Veith, G. Ceder and N. J. Dudney, J. Am. Chem. Soc., 2018, 140, 11029–11038 CrossRef CAS PubMed.
- B. Fleutot, B. Pecquenard, F. Le Cras, B. Delis, H. Martinez, L. Dupont and D. Guy-Bouyssou, J. Power Sources, 2011, 196, 10289–10296 CrossRef CAS.
- Y. Yoon, C. Park, J. Kim and D. Shin, Electrochim. Acta, 2013, 111, 144–151 CrossRef CAS.
- V. P. Phan, B. Pecquenard and F. Le Cras, Adv. Funct. Mater., 2012, 22, 2580–2584 CrossRef CAS.
- T. Famprikis, J. Galipaud, O. Clemens, B. Pecquenard and F. Le Cras, ACS Appl. Energy Mater., 2019, 2, 4782–4791 CrossRef CAS.
- B. Wang, J. B. Bates, F. X. Hart, B. C. Sales, R. A. Zuhr and J. D. Robertson, J. Electrochem. Soc., 1996, 143, 3203–3213 CrossRef CAS.
- P. Schichtel, M. Geiß, T. Leichtweiß, J. Sann, D. A. Weber and J. Janek, J. Power Sources, 2017, 360, 593–604 CrossRef CAS.
- S. Larfaillou, D. Guy-Bouyssou, F. Le Cras and S. Franger, J. Power Sources, 2016, 319, 139–146 CrossRef CAS.
- Z. Wang, D. Santhanagopalan, W. Zhang, F. Wang, H. L. Xin, K. He, J. Li, N. Dudney and Y. S. Meng, Nano Lett., 2016, 16, 3760–3767 CrossRef CAS PubMed.
- K. Nie, Y. Hong, J. Qiu, Q. Li, X. Yu and H. Li, Front. Chem., 2018, 6, 616 CrossRef CAS PubMed.
- S. Xia, X. Wu, Z. Zhang, Y. Cui and W. Liu, Chem, 2019, 5, 753–785 CAS.
- C. Sångeland, J. Mindemark, R. Younesi and D. Brandell, Solid State Ionics, 2019, 343, 115068 CrossRef.
- Z. Ding, J. Li, J. Li and C. An, J. Electrochem. Soc., 2020, 167, 070541 CrossRef.
- H. Lim, J. Park, H. Shin, J. Jeong, J. T. Kim, K. Nam, H. Jung and K. Y. Chung, Energy Storage Mater., 2020, 25, 224–250 CrossRef.
- A. Gurung, J. Pokharel, A. Baniya, R. Pathak, K. Chen, B. S. Lamsal, N. Ghimire, W. H. Zhang, Y. Zhou and Q. Qiao, Sustainable Energy Fuels, 2019, 3, 3279–3309 RSC.
- J. B. Bates, N. J. Dudney, B. J. Neudecker, F. X. Hart, H. P. Jun and S. A. Hackney, J. Electrochem. Soc., 2000, 147, 59 CrossRef CAS.
- Y. Iriyama, T. Kako, C. Yada, T. Abe and Z. Ogumi, Solid State Ionics, 2005, 176, 2371–2376 CrossRef CAS.
- E. Jeong, C. Hong, Y. Tak, S. C. Nam and S. Cho, J. Power Sources, 2006, 159, 223–226 CrossRef CAS.
- B. Put, P. M. Vereecken, J. Meersschaut, A. Sepúlveda and A. Stesmans, ACS Appl. Mater. Interfaces, 2016, 8, 7060–7069 CrossRef CAS PubMed.
- P. H. L. Notten, F. Roozeboom, R. A. H. Niessen and L. Baggetto, Adv. Mater., 2007, 19, 4564–4567 CrossRef CAS.
- L. Baggetto, R. A. H. Niessen, F. Roozehoom and P. H. L. Notten, Adv. Funct. Mater., 2008, 18, 1057–1066 CrossRef CAS.
- S. H. Jee, M. J. Lee, H. S. Ahn, D. J. Kim, J. W. Choi, S. J. Yoon, S. C. Nam, S. H. Kim and Y. S. Yoon, Solid State Ionics, 2010, 181, 902–906 CrossRef CAS.
- S. Zhao, Z. Fu and Q. Qin, Thin Solid Films, 2002, 415, 108–113 CrossRef CAS.
- W. C. West, Z. D. Hood, S. P. Adhikari, C. Liang, A. Lachgar, M. Motoyama and Y. Iriyama, J. Power Sources, 2016, 312, 116–122 CrossRef CAS.
- Y. Matsuda, N. Kuwata and J. Kawamura, Solid State Ionics, 2018, 320, 38–44 CrossRef CAS.
- M. Nisula, Y. Shindo, H. Koga and M. Karppinen, Chem. Mater., 2015, 27, 6987–6993 CrossRef CAS.
- A. C. Kozen, A. J. Pearse, C. F. Lin, M. Noked and G. W. Rubloff, Chem. Mater., 2015, 27, 5324–5331 CrossRef CAS.
- B. Put, M. J. Mees, N. Hornsveld, S. Hollevoet, A. Sepúlveda, P. M. Vereecken, W. M. M. Kessels and M. Creatore, J. Electrochem. Soc., 2019, 166, A1239–A1242 CrossRef CAS.
- S. Shibata, J. Electrochem. Soc., 2016, 163, A2555–A2562 CrossRef CAS.
- A. J. Pearse, T. E. Schmitt, E. J. Fuller, F. El-Gabaly, C. F. Lin, K. Gerasopoulos, A. C. Kozen, A. A. Talin, G. Rubloff and K. E. Gregorczyk, Chem. Mater., 2017, 29, 3740–3753 CrossRef CAS.
- M. Létiche, E. Eustache, J. Freixas, A. Demortière, V. De Andrade, L. Morgenroth, P. Tilmant, F. Vaurette, D. Troadec, P. Roussel, T. Brousse and C. Lethien, Adv. Energy Mater., 2017, 7, 1–12 Search PubMed.
- W. Y. Liu, Z. W. Fu, C. L. Li and Q. Z. Qin, Electrochem. Solid-State Lett., 2004, 7, 36–40 CrossRef.
- Y. G. Kim and H. N. G. Wadley, J. Vac. Sci. Technol., A, 2008, 26, 174–183 CrossRef CAS.
- C. S. Nimisha, K. Y. Rao, G. Venkatesh, G. M. Rao and N. Munichandraiah, Thin Solid Films, 2011, 519, 3401–3406 CrossRef CAS.
- S. Nowak, F. Berkemeier and G. Schmitz, J. Power Sources, 2015, 275, 144–150 CrossRef CAS.
- M. Haruta, S. Shiraki, T. Suzuki, A. Kumatani, T. Ohsawa, Y. Takagi, R. Shimizu and T. Hitosugi, Nano Lett., 2015, 15, 1498–1502 CrossRef CAS PubMed.
- B. Uzakbaiuly, A. Mukanova, I. Kurmanbayeva and Z. B. Bakenov, Eurasian J. Phys. Funct. Mater., 2019, 3, 174–182 CrossRef.
- J. Ko and Y. S. Yoon, Ceram. Int., 2020, 46, 20623–20632 CrossRef CAS.
- K. H. Joo, P. Vinatier, B. Pecquenard, A. Levasseur and H. J. Sohn, Solid State Ionics, 2003, 160, 51–59 CrossRef CAS.
- L. Q. Nguyen, L. Chen and V. Van Truong, Thin Solid Films, 1997, 293, 175–178 CrossRef CAS.
- B. V. R. Chowdari and K. Radhakrishnan, Solid State Ionics, 1991, 44, 325–329 CrossRef CAS.
- T. Minami, A. Hayashi and M. Tatsumisago, Solid State Ionics, 2006, 177, 2715–2720 CrossRef CAS.
- J. Kawamura, N. Kuwata, K. Toribami, N. Sata, O. Kamishima and T. Hattori, Solid State Ionics, 2004, 175, 273–276 CrossRef CAS.
- H. Ohtsuka and Y. Sakurai, Solid State Ionics, 2001, 144, 59–64 CrossRef CAS.
- N. Kuwata, R. Kumar, K. Toribami, T. Suzuki, T. Hattori and J. Kawamura, Solid State Ionics, 2006, 177, 2827–2832 CrossRef CAS.
- R. Baskaran, N. Kuwata, O. Kamishima, J. Kawamura and S. Selvasekarapandian, Solid State Ionics, 2009, 180, 636–643 CrossRef CAS.
- A. Brazier, L. Dupont, L. Dantras-Laffont, N. Kuwata, J. Kawamura and J. M. Tarascon, Chem. Mater., 2008, 20, 2352–2359 CrossRef CAS.
- S. S. Gundale and A. V. Deshpande, Electrochim. Acta, 2018, 265, 65–70 CrossRef CAS.
- S. Zhao and Q. Qin, J. Power Sources, 2003, 122, 174–180 CrossRef CAS.
- H. Ohtsuka and J. i. Yamaki, Solid State Ionics, 1989, 35, 201–206 CrossRef CAS.
- S. J. Lee, J. H. Bae, H. W. Lee, H. K. Baik and S. M. Lee, J. Power Sources, 2003, 123, 61–64 CrossRef CAS.
- S. J. Lee, H. K. Baik and S. M. Lee, Electrochem. Commun., 2003, 5, 32–35 CrossRef CAS.
- Q. Chen, R. Xu, Z. He, K. Zhao and L. Pan, J. Electrochem. Soc., 2017, 164, A1852–A1857 CrossRef CAS.
- K. Edström, D. Brandell, T. Gustafsson and L. Nyholm, Electrochem. Soc. Interface, 2011, 20, 41–46 CrossRef.
- R. Sheil and J. P. Chang, J. Vac. Sci. Technol., A, 2020, 38, 032411 CrossRef CAS.
- P. Priimägi, H. Kasemägi, A. Aabloo, D. Brandell and V. Zadin, Electrochim. Acta, 2017, 244, 129–138 CrossRef.
- B. Sun, D. Rehnlund, M. J. Lacey and D. Brandell, Electrochim. Acta, 2014, 137, 320–327 CrossRef CAS.
- N. A. Kyeremateng, F. Dumur, P. Knauth, B. Pecquenard and T. Djenizian, Electrochem. Commun., 2011, 13, 894–897 CrossRef CAS.
- K. Sun, T. S. Wei, B. Y. Ahn, J. Y. Seo, S. J. Dillon and J. A. Lewis, Adv. Mater., 2013, 25, 4539–4543 CrossRef CAS PubMed.
- D. W. McOwen, S. Xu, Y. Gong, Y. Wen, G. L. Godbey, J. E. Gritton, T. R. Hamann, J. Dai, G. T. Hitz, L. Hu and E. D. Wachsman, Adv. Mater., 2018, 30, 1–7 CrossRef PubMed.
- C. Y. Yap, C. K. Chua, Z. L. Dong, Z. H. Liu, D. Q. Zhang, L. E. Loh and S. L. Sing, Appl. Phys. Rev., 2015, 2(4), 041101 Search PubMed.
- A. Ambrosi and M. Pumera, Chem. Soc. Rev., 2016, 45, 2740–2755 RSC.
- C. Zhu, T. Liu, F. Qian, W. Chen, S. Chandrasekaran, B. Yao, Y. Song, E. B. Duoss, J. D. Kuntz, C. M. Spadaccini, M. A. Worsley and Y. Li, Nano Today, 2017, 15, 107–120 CrossRef CAS.
- N. A. Kyeremateng and R. Hahn, ACS Energy Lett., 2018, 3, 1172–1175 CrossRef CAS.
- Y. Il Jang, N. J. Dudney, D. A. Blom and L. F. Allard, J. Power Sources, 2003, 119–121, 295–299 CrossRef.
- N. Kuwata, N. Iwagami, Y. Matsuda, Y. Tanji and J. Kawamura, ECS Trans., 2009, 16, 53–60 CrossRef CAS.
- A. Nakagawa, N. Kuwata, Y. Matsuda and J. Kawamura, ECS Trans., 2010, 25, 155–161 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |