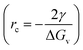Dopants modulate crystal growth in molten salts enabled by surface energy tuning†
Xiaoqiao
Li‡
ab,
Linming
Zhou‡
 c,
Han
Wang
b,
Dechao
Meng
b,
Guannan
Qian
b,
Yong
Wang
b,
Yushi
He
c,
Han
Wang
b,
Dechao
Meng
b,
Guannan
Qian
b,
Yong
Wang
b,
Yushi
He
 b,
Yongjun
Wu
b,
Yongjun
Wu
 c,
Zijian
Hong
c,
Zijian
Hong
 *c,
Zi-Feng
Ma
*c,
Zi-Feng
Ma
 b and
Linsen
Li
b and
Linsen
Li
 *bd
*bd
aCollege of Environmental & Chemical Engineering, Shanghai University of Electric Power, Shanghai 200090, China
bDepartment of Chemical Engineering, Shanghai Jiao Tong University, Shanghai 200240, China. E-mail: linsenli@sjtu.edu.cn
cSchool of Materials Science & Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: hongzijian100@zju.edu.cn
dShanghai Jiao Tong University, Sichuan Research Institute, Chengdu 610213, China
First published on 1st June 2021
Abstract
Crystalline materials are routinely produced via high-temperature synthesis and show size-dependent properties; however, a rational approach to regulating their crystal growth has not been established. Here we show that dopants traditionally used for modifying crystal lattices can also function as growth mediators in molten-salt synthesis by altering the surface energy and thus the nucleation barrier and the critical nuclei size. This was demonstrated by size-tunable synthesis of lithium cobalt oxide (LiCoO2) with a trace amount of titanium oxide or tungsten oxide as the dopant. The understanding of the dopant-mediated growth mechanism allows the rational design and control of the particle size and the doping for LiCoO2, enabling a high energy-density battery cathode with exceptional rate capability and cycle stability at high voltages intolerable for conventional LiCoO2.
Introduction
Crystal growth is a fundamental process and a key step in a wide range of applications including microelectronics, pharmaceutics, catalysis, and energy storage. Tailoring the size of individual crystals is often essential from the perspectives of performance and/or cost.1–3 For instance, increasingly larger silicon ingots are grown via the Czochralski method by controlling the temperature and pulling rate of the seed crystal so that wafers with larger diameters (now at 450 mm) can be made to enhance productivity and efficiency in semiconductor device fabrication. Nanocrystal-based or even single-atom catalysts are synthesized by colloidal chemistry or thermal pyrolysis to maximize the surface-to-volume ratio and catalytic efficiency.2,3 Electrode materials for batteries store alkali ions (e.g. Li+, Na+, and K+) at interstitial sites in the bulk and their electrochemical performance is highly dependent on the particle size.4–6 Larger particles are favorable for achieving higher packing density and volumetric energy density but there are size thresholds above which the power capability is compromised. Taking layered-oxide materials (LiMO2, M = Co, Ni–Mn–Co, or Ni–Co–Al) as examples, which are widely used in consumer electronics and electric vehicles, we may estimate the optimal grain size [L = 2(Dt)0.5] based on their typical Li diffusivity (D = 10−10–10−12 cm s−1) and the assumption that the electrochemical reaction is limited by bulk diffusion.4,7 The grain size should be in the range of 100–101 μm in order to fully utilize the capacity at practically relevant C-rates (0.2 to 2C, i.e. 5 to 0.5 h for one charge or discharge). These oxide materials are currently prepared via high-temperature solid–state reactions that have little control over the size of the individual particles and require pulverization and sieving to achieve a desirable particle size distribution. Although rational control of crystal growth has been made possible with the assistance of surfactants in low-temperature solution synthesis3 or enabled by supersaturation control,8 it remains a challenge to do the same in high-temperature synthesis.The molten-salt-assisted approach significantly enhances ion diffusion and reaction kinetics by using a molten salt as the “liquid phase” reaction medium.9–12 It offers better control over the size, elemental composition, and crystal shape of metal oxide materials but generally requires stringent control over a set of experimental variables, such as the composition of salts, salt-to-reactant ratios, reaction temperature, time, nature of metal precursors, etc. Here we present direct observation and mechanistic investigation of crystal growth regulation via a single experimental variable, using the molten-salt synthesis of LiCoO2 (LCO) as a model system. LCO is a technologically important battery material widely used in consumer electronics.13 We discovered that dopants traditionally used for modifying the lattice structure and improving the electrochemical performance of LCO can also act as growth mediators in the synthesis by altering the surface energy and thus the nucleation barrier and the critical nuclei size. Simultaneous control of the grain size and the structure of LCO leads to a high energy-density battery cathode with exceptional rate capability and cycle performance.
Results and discussion
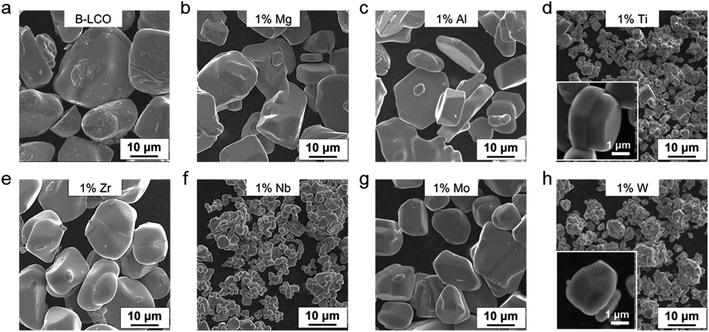
Doping is a technological process widely adopted in materials science and involves incorporating foreign atoms or ions into host lattices to achieve desirable properties and functions. LCO has been doped with numerous foreign elements to improve its cycle stability, especially when it is charged to high voltages (≥4.5 V) to increase the accessible energy density.13–15 However, little attention has been paid to the dopants' role in the crystal growth. Previous studies have revealed significantly different solubility of dopants in the LCO lattice. For example, Mg and Al show a solubility of 0.5 mol% or higher at Co sites whereas Zr is less soluble and Ti is known to segregate on the LCO particle surface or at grain boundaries (i.e. barely soluble).15–17 Since many metal oxides are known to dissolve in common molten salts,18 we reasoned that they should interact with the growing surface and thus modify the formation process of the LCO grains.To valid our hypothesis, Mg2+, Al3+, Ti4+, Zr4+, Nb5+, Mo6+, and W6+ ions were introduced into the synthesis of LCO in LiOH–Li2SO4 molten salts at 910 °C in air at the same molar fraction (1 mol% relative to Co and 0.5 mol% relative to the molten salts; see the Methods in the ESI†). Cobalt oxide (Co3O4) was used as the Co source. After the synthesis, the LCO particles were isolated from the salts by water washing. All the samples were first examined by powder X-ray diffraction (PXRD) and were indexed to the hexagonal α-NaFeO2-type structure without any detectable impurity phases (ESI Fig. 1†). Scanning electron microscopy (SEM) characterization was performed to evaluate the impact of different dopants on the crystal growth of LCO (Fig. 1). Without the dopants added, the pure LCO particles grew up to approximately 30 μm in size (Fig. 1a). The addition of Mg2+ and Al3+ had little impact on the particle size (Fig. 1b and c), whereas the size reduction was evident in all other cases. In particular, Ti4+ and W6+ doping reduced the LCO particle size by almost one order of magnitude (from ∼30 μm to ∼2–3 μm, Fig. 1d and h). More structural characterization results can be found in ESI Fig. 2.†
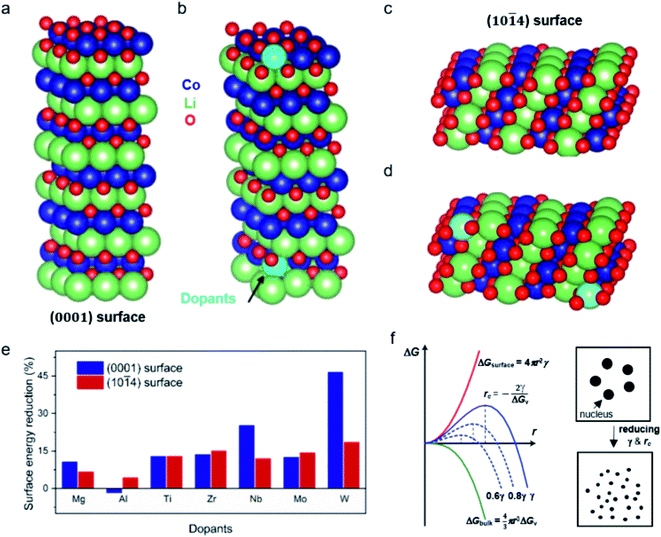
To shed more light on the effect of dopant ions in mediating the crystal growth, we performed first-principles calculations based on density functional theory (DFT, see details in the ESI†) and evaluated the surface energy change of the LCO surface due to the cation doping (Fig. 2). Two types of low-index, low-energy LCO surfaces were modeled, namely the (0001) and (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4) surfaces.19 The crystal structures of the undoped and cation doped (0001) and (10
4) surfaces.19 The crystal structures of the undoped and cation doped (0001) and (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4) surface slabs are plotted in Fig. 2a–d. Two Co ions on the top and bottom of the slab are replaced by the dopants, as shown in Fig. 2b and d. In order to minimize the charging effect, a thick (0001) surface was built with 6 Li layers. The surface energy was calculated by taking the difference of the total energy of the slab and the bulk, i.e.
4) surface slabs are plotted in Fig. 2a–d. Two Co ions on the top and bottom of the slab are replaced by the dopants, as shown in Fig. 2b and d. In order to minimize the charging effect, a thick (0001) surface was built with 6 Li layers. The surface energy was calculated by taking the difference of the total energy of the slab and the bulk, i.e.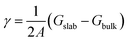 .The surface energies of undoped LCO (0001) and (10
.The surface energies of undoped LCO (0001) and (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4) surfaces were calculated to be 2.181 J m−2 and 1.111 J m−2, respectively, which agreed well with a previous report.19 We then moved on to understand the impact of the dopants on the surface energy, including Mg, Al, Ti, Zr, Nb, Mo, and W. The results are summarized in ESI Table 1.† We plot the surface energy reduction in Fig. 2e, which is calculated by extracting the energy difference for the doped and undoped surfaces. It can be observed that the substitution of Co by W can greatly change the surface energy of LCO, leading to ∼46% and ∼18% reduction of (0001) and (10
4) surfaces were calculated to be 2.181 J m−2 and 1.111 J m−2, respectively, which agreed well with a previous report.19 We then moved on to understand the impact of the dopants on the surface energy, including Mg, Al, Ti, Zr, Nb, Mo, and W. The results are summarized in ESI Table 1.† We plot the surface energy reduction in Fig. 2e, which is calculated by extracting the energy difference for the doped and undoped surfaces. It can be observed that the substitution of Co by W can greatly change the surface energy of LCO, leading to ∼46% and ∼18% reduction of (0001) and (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4) surface energies, respectively (Fig. 2e). Doping with Nb, Ti, Zr and Mo also shows a significant reduction in the surface energies, whereas Mg and Al ion substitutions have relatively weaker effects.
4) surface energies, respectively (Fig. 2e). Doping with Nb, Ti, Zr and Mo also shows a significant reduction in the surface energies, whereas Mg and Al ion substitutions have relatively weaker effects.
From classical nucleation theory (schematically shown in Fig. 2f), one can write: 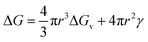 , where ΔG is the Gibbs free energy change due to the nucleation, ΔGv is the Gibbs free energy per volume due to the phase transition, r is the radius of the nuclei, and γ is the surface energy.20 The minimization of ΔG with respect to r gives:
, where ΔG is the Gibbs free energy change due to the nucleation, ΔGv is the Gibbs free energy per volume due to the phase transition, r is the radius of the nuclei, and γ is the surface energy.20 The minimization of ΔG with respect to r gives: 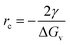 , where rc is the critical nucleus size. Since the doping level in this study is no more than 1%, the change in ΔGv is considerably small for different dopants, and rc is thus directly proportional to the surface energy γ. Reducing the surface energy could lead to smaller nuclei, which subsequently grow into smaller crystals because the total amount of the reactants is a constant in a batch synthesis. Moreover, the nucleation barrier is proportional to γ3, and reducing the surface energy could lead to a large reduction in the nucleation barrier which leads to more nuclei in the system. It can be theoretically expected that the doping with W, Ti, Nb, Mo and Zr will reduce the grain size of LCO whereas Al and Mg show minimal effect, which is consistent with our experimental observations (Fig. 1). However, we do want to point out that the dopant-induced surface energy reduction effect may not be the sole reason for the reduction of the LCO particle size. Besides the nucleation process, the dopants could also alter the particle growth process, which may explain the individual dopants' deviations from the overall trend as predicted by the DFT calculations.
, where rc is the critical nucleus size. Since the doping level in this study is no more than 1%, the change in ΔGv is considerably small for different dopants, and rc is thus directly proportional to the surface energy γ. Reducing the surface energy could lead to smaller nuclei, which subsequently grow into smaller crystals because the total amount of the reactants is a constant in a batch synthesis. Moreover, the nucleation barrier is proportional to γ3, and reducing the surface energy could lead to a large reduction in the nucleation barrier which leads to more nuclei in the system. It can be theoretically expected that the doping with W, Ti, Nb, Mo and Zr will reduce the grain size of LCO whereas Al and Mg show minimal effect, which is consistent with our experimental observations (Fig. 1). However, we do want to point out that the dopant-induced surface energy reduction effect may not be the sole reason for the reduction of the LCO particle size. Besides the nucleation process, the dopants could also alter the particle growth process, which may explain the individual dopants' deviations from the overall trend as predicted by the DFT calculations.
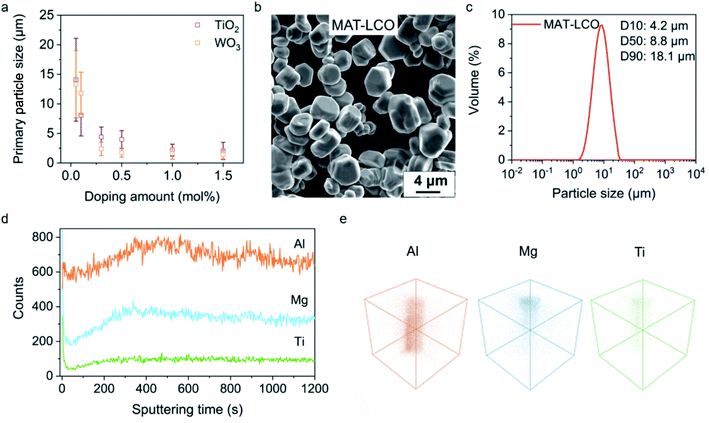
With the understanding of the dopant's role in the synthesis, we can rationalize the use of Ti4+ or W6+ not just as a dopant but also a mediator for the crystal growth of LCO at high temperature, similar to the role of surfactants or ligands in the low-temperature solution synthesis. Indeed, the size of the LCO particles could be continuously tuned by varying the Ti4+ or W6+ concentration in the molten salts. The primary particle size decreased from ∼15 μm to ∼2 μm as the Ti4+ or W6+ concentration increased from 0.01 mol% to 1.5 mol% (Fig. 3a, SEM images in ESI Fig. 3†). This dopant-mediated crystal growth approach allows us to target a certain particle size and easily achieve it by tuning a single variable (dopant concentration), which is in stark contrast to the conventional synthetic methods that often involve adjusting several variables and a large number of trial-and-error investigations. For example, we deterministically prepared a LCO sample (denoted as MAT-LCO) with a median size (D50) of ∼8.8 μm and achieved high homogeneity in the particle size and morphology (Fig. 3b and c) by using ∼0.3 mol% of Ti4+ as the mediator for crystal growth in the molten salts and having Mg2+ (0.5 mol%), Al3+ (0.5 mol%) ions as co-dopants (in addition to the Ti4+ ions). This could also be done by using W6+ as the growth mediator (ESI Fig. 4†). As we showed earlier, Mg2+ and Al3+ ions had a minimal impact on the LCO growth. They were reported to improve structural stability during deep delithiation13 and thus introduced. PXRD and Rietveld refinements confirmed that MAT-LCO had a highly ordered layered structure without detectable impurity phases (ESI Fig. 5†). Time-of-flight secondary-ion mass-spectrometry (TOF-SIMS) characterization was performed to evaluate the spatial distribution of the dopants. Depth profiling (Fig. 3d) and three-dimensional elemental mapping (Fig. 3e) show that the Al3+ ions are uniformly dispersed in the LCO particle whereas a relatively higher concentration of Mg2+ and Ti4+ ions is located near the surface.
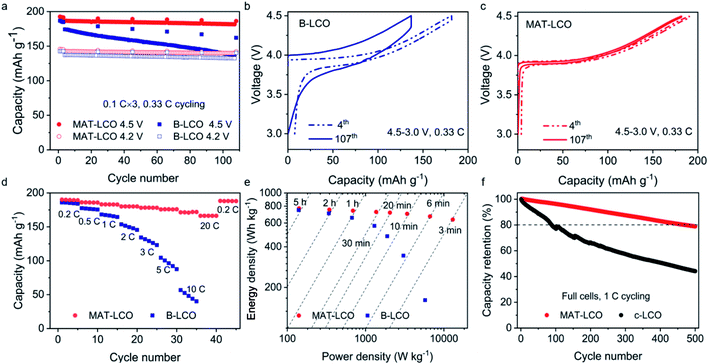
The rationally designed MAT-LCO cathode material enabled excellent electrochemical performance at a high cut-off voltage of 4.5 V (versus Li/Li+). It displayed an exceptionally high capacity of 193.6 mA h g−1 and retained more than 97% of the initial capacity over 100 cycles at 0.33C (Fig. 4a). In comparison, the undoped LCO (B-LCO) was only stable at a low cut-off voltage of 4.2 V but rapidly degraded when it was cycled to 4.5 V and lost more than 20% of the initial capacity after 100 cycles. According to the literature, the rapid performance decay of the undoped LCO during high-voltage cycling could be caused by both bulk and surface instabilities, including harmful phase transitions (O3 to H1–3 or even O1), microcrack formation, oxygen loss, and electrolyte decomposition.13 A further inspection of the voltage curves in the 4th and 107th cycles revealed a large increase in cell polarization and significant capacity loss for B-LCO (Fig. 4b) but not for MAT-LCO (Fig. 4c), proving its excellent interfacial and lattice stabilities enabled by the structural doping. Furthermore, MAT-LCO exhibited outstanding rate capability unmatched by B-LCO and delivered more than 170 mA h g−1 when it was charged and discharged at an extreme high rate of 20C (i.e. 3 min charge–discharge, Fig. 4d). The Ragone plot in Fig. 4e compares the electrochemical performance of MAT-LCO and bare LCO in terms of energy density and power density, proving that MAT-LCO is a high-energy and high-power battery cathode material. We further compared the long-term cycle performance of MAT-LCO with that of commercial LCO (c-LCO) in full cells using artificial graphite as the anode. c-LCO is the same material now widely used in high-end consumer electronics and designed for 4.35 V LCO-graphite full batteries. In our tests, the full cells were charged to 4.4 V to evaluate the high-voltage cycle stability. The full cell with MAT-LCO retained ∼80% of the initial capacity over 500 cycles, whereas c-LCO was clearly not suitable for high-voltage cycling, as evidenced by its rapid capacity decay (Fig. 4e) and much lower CEs (<99%, ESI Fig. 6†).
In conclusion, we have established a theoretical framework for regulating the crystal growth in molten salts via dopant-induced surface energy tuning and experimentally demonstrated the rational synthesis of LCO cathode materials with desirable particle size, doping, and exceptional electrochemical performance under harsh conditions. These findings enrich our understanding of crystal growth at high temperature and enable tremendous improvements in our ability to design and synthesize functional materials. We suggest that the dopant-mediated crystal growth mechanism proposed for LCO is likely general to many other materials commonly prepared via molten-salt synthesis, such as ceramics9 and complex oxides10,11 including superconductors.21
Author contributions
Conceptualization: LSL and ZH. Investigation: XL, LZ, HW, DM, GQ, YW, YH, and YW. Visualization: XL, LSL, and ZH. Funding acquisition: LSL, ZFM, and ZH. Supervision: LSL, ZFM, and ZH. Writing – original draft: LSL and HZ. Writing – review & editing: LSL, HZ, and MZF.Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
This work is supported by the Natural Science Foundation of China (22008154 to LSL), and partially by the Sichuan Science and Technology Program (2021JDRC0015 to LSL) and Sinopec (420038-1 to LSL). Z. H. would like to acknowledge a start-up grant from Zhejiang University. The DFT calculations were performed at the Shanghai Supercomputing Center, on the Mofang III cluster.Notes and references
- S. W. Boettcher, J. M. Spurgeon, M. C. Putnam, E. L. Warren, D. B. Turner-Evans, M. D. Kelzenberg, J. R. Maiolo, H. A. Atwater and N. S. Lewis, Science, 2010, 327, 185–187 Search PubMed.
- B. Qiao, A. Wang, X. Yang, L. F. Allard, Z. Jiang, Y. Cui, J. Liu, J. Li and T. Zhang, Nat. Chem., 2011, 3, 634–641 Search PubMed.
- V. F. Puntes, K. M. Krishnan and A. P. Alivisatos, Science, 2001, 291, 2115–2117 Search PubMed.
- A. Van der Ven, J. Bhattacharya and A. A. Belak, Acc. Chem. Res., 2013, 46, 1216–1225 Search PubMed.
- M. D. Radin, S. Hy, M. Sina, C. Fang, H. Liu, J. Vinckeviciute, M. Zhang, M. S. Whittingham, Y. S. Meng and A. Van der Ven, Adv. Energy Mater., 2017, 7, 1602888 Search PubMed.
- K. J. Griffith, K. M. Wiaderek, G. Cibin, L. E. Marbella and C. P. Grey, Nature, 2018, 559, 556–563 Search PubMed.
- M. S. Whittingham, Chem. Rev., 2004, 104, 4271–4302 Search PubMed.
- S. A. Morin, M. J. Bierman, J. Tong and S. Jin, Science, 2010, 328, 476–480 Search PubMed.
- T. Kimura, in Advances in Ceramics – Synthesis and Characterization, Processing and Specific Applications, ed. C. Sikalidis, IntechOpen, 2010 Search PubMed.
- D. E. Bugaris and H.-C. zur Loye, Angew. Chem., Int. Ed., 2012, 51, 3780–3811 Search PubMed.
- S. Flynn, S. Sanghvi, M. L. Nisbet, K. J. Griffith, W. Zhang, P. S. Halasyamani, S. M. Haile and K. R. Poeppelmeier, Chem. Mater., 2020, 32, 4785–4794 Search PubMed.
- A. Dash, R. Vaßen, O. Guillon and J. Gonzalez-Julian, Nat. Mater., 2019, 18, 465–470 Search PubMed.
- Y. Lyu, X. Wu, K. Wang, Z. Feng, T. Cheng, Y. Liu, M. Wang, R. Chen, L. Xu, J. Zhou, Y. Lu and B. Guo, Adv. Energy Mater., 2021, 11, 2000982 Search PubMed.
- Q. Liu, X. Su, D. Lei, Y. Qin, J. Wen, F. Guo, Y. A. Wu, Y. Rong, R. Kou, X. Xiao, F. Aguesse, J. Bareño, Y. Ren, W. Lu and Y. Li, Nat. Energy, 2018, 3, 936–943 Search PubMed.
- J.-N. Zhang, Q. Li, C. Ouyang, X. Yu, M. Ge, X. Huang, E. Hu, C. Ma, S. Li, R. Xiao, W. Yang, Y. Chu, Y. Liu, H. Yu, X.-Q. Yang, X. Huang, L. Chen and H. Li, Nat. Energy, 2019, 4, 594–603 Search PubMed.
- Y. Koyama, H. Arai, I. Tanaka, Y. Uchimoto and Z. Ogumi, J. Mater. Chem. A, 2014, 2, 11235–11245 Search PubMed.
- S. Kim, S. Choi, K. Lee, G. J. Yang, S. S. Lee and Y. Kim, Phys. Chem. Chem. Phys., 2017, 19, 4104–4113 Search PubMed.
- K. Sridharan and T. R. Allen, in Molten Salts Chemistry, ed. F. Lantelme and H. Groult, Elsevier, Oxford, 2013, pp. 241–267 Search PubMed.
- D. Kramer and G. Ceder, Chem. Mater., 2009, 21, 3799–3809 Search PubMed.
- W. K. Burton, N. Cabrera and F. C. Frank, Philos. Trans. R. Soc., A, 1951, 243, 299–358 Search PubMed.
- W. K. Ham, G. F. Holland and A. M. Stacy, J. Am. Chem. Soc., 1988, 110, 5214–5215 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ta02351a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |