Open Access Article
Open Access ArticleAlkylamino-terephthalate ligands stabilize 8-connected Zr4+ MOFs with highly efficient sorption for toxic Se species†
Anastasia D.
Pournara‡
a,
Sofia
Rapti‡
a,
Alexandros
Valmas
 b,
Irene
Margiolaki
b,
Euaggelos
Andreou
c,
Gerasimos S.
Armatas
b,
Irene
Margiolaki
b,
Euaggelos
Andreou
c,
Gerasimos S.
Armatas
 c,
Athanassios C.
Tsipis
c,
Athanassios C.
Tsipis
 a,
John C.
Plakatouras
ad,
Dimosthenis L.
Giokas
a,
John C.
Plakatouras
ad,
Dimosthenis L.
Giokas
 ad and
Manolis J.
Manos
ad and
Manolis J.
Manos
 *ad
*ad
aDepartment of Chemistry, University of Ioannina, GR-45110 Ioannina, Greece. E-mail: emanos@uoi.gr
bDepartment of Biology, Section of Genetics, Cell Biology and Development, University of Patras, GR-26500 Patras, Greece
cDepartment of Materials Science and Technology, University of Crete, GR-71003 Heraklion, Greece
dInstitute of Materials Science and Computing, University Research Center of Ioannina, Ioannina, GR-45110, Greece
First published on 12th January 2021
Abstract
Zirconium(IV) metal organic frameworks (MOFs) with low net connectivity and available intra-framework sorption sites constitute excellent sorbents for toxic anions. To expand this family of highly promising sorbents, it would be desired to develop new synthetic strategies aiming towards such materials. Here we show that the utilization of terephthalate ligands with small to medium size alkyl-amino functional groups comprises an effective approach towards microporous Zr4+ MOFs with 8-connected frameworks. The new MOFs were proved the most effective Se(IV) and Se(VI) sorbents ever reported, with exceptional sorption capacities (up to 272 mgSe(IV) g−1 and 290 mgSe(VI) g−1), reusability, rapid sorption kinetics (≤3 min) and capability to sorb efficiently these anions in a very wide pH range (1–10), even in the presence of various competitive anions. The MOFs also display highly efficient sorption capability for the particularly toxic SeCN−, with such property demonstrated for the first time for MOF materials.
Introduction
In the last two decades, metal–organic frameworks (MOFs), based on metal ions or clusters connected via extended polytopic ligands, have attracted considerable attention due to their structural diversity, high porosity and potential applications in various fields.1–6 MOFs with ion-sorption properties seem very promising for use in wastewater treatment.7–12 Such MOFs exhibit a highly ordered porous structure (in contrast to amorphous organic resins) in combination with a large variety of organic binding groups (not present in purely inorganic materials) and thus, they may be considered as a next generation of ion-sorbents. Among metal organic ion sorbents, Zr4+ MOFs are probably the most promising ones combining a number of important features, such as robustness (stability from extremely acidic to alkaline conditions), high porosity, capability to incorporate various functional groups and ease of synthesis.13,14 In a recent work, we described H16[Zr6O16(H2PATP)4]Cl8·xH2O (MOR-2) (H2PATP = 2-((pyridin-1-ium-2-ylmethyl)ammonio)terephthalate), a new microporous Zr4+ MOF featuring an 8-connected net.15 The isolation of MOR-2 with a lower net connectivity than the usual (12-connected) for Zr4+ MOFs with terephthalate ligands was attributed to the presence of the bulky substituent in the H2PATP ligand, which likely prevents incorporation of more than 8 ligands per Zr6 cluster due to steric interactions. MOR-2 showed an extraordinary sorption capacity for Cr(VI), which is attributed not only to the favourable ion exchange of guest Cl− anions by Cr(VI) species and Cr(VI)-functional group interactions but also to the ligation of Cr(VI) anions into the Zr6 cluster replacing the labile terminal ligands of the pristine material. Thus, the decreased net connectivity of MOR-2 led to enhanced porosity (compared to an hypothetical Zr4+–H2PATP 12-connected MOF which would be non-porous) and in a substantial improvement of the Cr(VI) sorption properties related to those of usual 12-connected Zr4+ MOFs. Furthermore, MOR-2 has shown remarkably high sorption capacities for ReO4−/TcO4−, which were due to the capability of the MOF to bind Re(VII)/Tc(VII) into the Zr6 clusters.16 These results reported from our group and also the pioneering work on the anion sorption properties of NU-1000 (ref. 17–19) (which also exhibit an 8-connected framework) indicate Zr4+ MOFs with decreased net-connectivity as a source of highly promising sorbent materials for anionic pollutants. Thus, it appears very attractive to develop synthetic strategies towards new Zr4+ MOFs with low-net connectivity in order to discover new materials with enhanced properties and potential for practical applications in environmental remediation.Herein, we report four new microporous Zr4+ MOFs with the general formula H16[Zr6O16(RNH-BDC)4]·solvent (RNH-BDC2− = 2-alkyl-amine-terephthalate; R = ethyl-, ET-MOF; R = propyl-, PROP-MOF; R = isobutyl-, SBUT-MOF; R = n-butyl, BUT-MOF). The new MOFs exhibit an 8-connected framework and display enhanced porosity (BET surface areas up to 832 m2 g−1) compared to MOR-2 (BET surface area = 354 m2 g−1), the only previously reported Zr4+-terephthalate MOF with 8-connected net. These materials have shown excellent Se(IV/VI) sorption properties, with fast/selective capture of these anionic species under a variety of conditions. The Se(IV)/(VI) maximum sorption capacities of alkylamino-functionalized MOFs are the highest reported for MOF materials and most of other sorbents. Moreover, these MOFs were proved highly efficient for sorption of SeCN− species, which is one of the most dangerous pollutants found in oil refinery and mining wastewater.20 In fact, this is the first time that MOF materials have been tested for SeCN− capture. Overall, the present work demonstrates the use of terephthalic ligands with relatively small-sized functional groups as an effective method towards the isolation of porous Zr4+ MOFs with low net-connectivity and highly promising anion sorption properties.
Results and discussion
(a) Synthesis and structural characterization
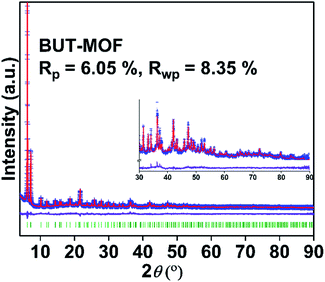
As we mentioned in the introduction, the stabilization of an 8-connected framework in MOR-2 was explained on the basis of the steric interactions between the bulky 2-picolylamino functional groups of the MOF that do not favour the formation of the usual 12-connected framework of Zr4+-terephthalate materials.15 As a next step in this research, it would be interesting to check if the presence of relatively small-sized functional groups (instead of bulky ones) in the terephthalate ligands will also lead to the stabilization of a framework with net connectivity <12. In that case, the resulting materials would combine low net connectivity-intra-framework anion sorption sites and enhanced surface areas compared to that of our previously reported MOR-2 material. In this context, we decided to synthesize Zr4+ MOFs with alkyl (ethyl, propyl, isobutyl, n-butyl)-amino-BDC2−, i.e. ligands with functional groups which are only slightly to moderately elongated compared to the functional groups (i.e. –NH2) of the well-known UiO-66-NH2 displaying 12-connected framework. A variety of synthetic approaches have been applied in order to isolate crystalline Zr4+ MOFs with the alkyl-amine-BDC2− ligands and finally, highly crystalline Zr4+ MOFs with ethyl, propyl, isobutyl or n-butyl-amino-terephthalate ligands were isolated in good yield and purity. The results from thermal analyses indicated that these Zr4+ MOFs contain 4 terephthalate ligands per formula unit, which is consistent with 8-connected framework structures (Fig. S1–S4, Tables S1 and S2†). Unfortunately, these MOFs could not be isolated as single crystals and thus, powder X-ray diffraction (PXRD) methods were applied to determine and refine their structures. The PXRD patterns of the MOFs were indexed successfully in the tetragonal crystal system and structureless refinement was performed choosing the space group I4/m. Then, we built 8-connected models of the MOFs' structures, which were further optimized via simulated annealing methods. These models were used as starting points for Rietveld refinements. The results of these refinements confirmed the accuracy of the selected structural models (Fig. 1, S5–S8 and Table S3†).In the structures of the MOFs (Fig. 2 and S9–S12†), each Zr6 cluster contains eight terminal (OH−/H2O) ligands on the equatorial plane, besides the bridging O2−/OH− and alkylamino-BDC2− groups. The connectivity of the clusters is consistent with a bcu network (Fig. 2 and S9–S11†). The 2-alkylamino substituents of the ligands are directed to the center of the network pores and solvent molecules (DMF and H2O) fill the space left available in the structures of the MOFs (Fig. S12†).
(b) Other characterizations
Field emission-scanning electron microscopy (FE-SEM) revealed that the new MOFs are composed of nanoparticles with a polyhedral shape and average size of ∼66 nm (Fig. 3 and S13†). FT-IR spectra (Fig. S14†) showed characteristic absorptions of the alkyl-amino ligands (e.g. intense peaks at ∼2900 cm−1 due to alkyl-chain of the ligands). 1H NMR data (ESI†) obtained after digesting the MOFs in highly alkaline D2O solution confirm the molecular integrity of the linkers in the MOFs, i.e. no loss of alkyl chains of the ligands was identified. Nitrogen physisorption measurements carried out at 77 K for the activated MOF samples showed typical type-I adsorption–desorption isotherms, characteristic of microporous solids (Fig. 4). The Brunauer–Emmett–Teller (BET) surface area of ET-MOF, PROP-MOF, SBUT-MOF and BUT-MOF were found equal to 832, 580, 556 and 609 m2 g−1 respectively. The BET surface areas are in very good agreement with those predicted based on the crystal structure of the MOFs, thus providing a strong evidence for the accuracy of the determined structural models. Specifically, the theoretical surface areas calculated using the poreblazer program21,22 were found 908, 573, 563 and 547 m2 g−1 for ET-MOF, PROP-MOF, SBUT-MOF and BUT-MOF respectively. CO2 adsorption isotherms recorded up to 1 bar at 273 K indicated sorption capacities of 2.78, 1.08, 1.26 and 1.34 mmol g−1 for the ET-MOF, PROP-MOF, SBUT-MOF and BUT-MOF materials respectively (Fig. S15†). Analysis of CO2 adsorption data with the density functional theory (DFT) suggests that ET-MOF, PROP-MOF, SBUT-MOF and BUT-MOF display microporous structure with pore sizes of 5.5–8.5 Å (Fig. S16†). The pores sizes for ET-MOF, PROP-MOF, SBUT-MOF and BUT-MOF found from poreblazer were 4–7 Å, which are relatively close to those determined from the gas sorption data. We should also note that the MOFs show excellent stability from extremely acidic (4 M HCl) to alkaline conditions (pH ∼ 11) (Fig. S17 and S18†). In fact, the acid and base-treated materials are highly crystalline, so we were able to provide Rietveld refinement data (Fig. S17 and Table S3†).(c) Anion sorption properties
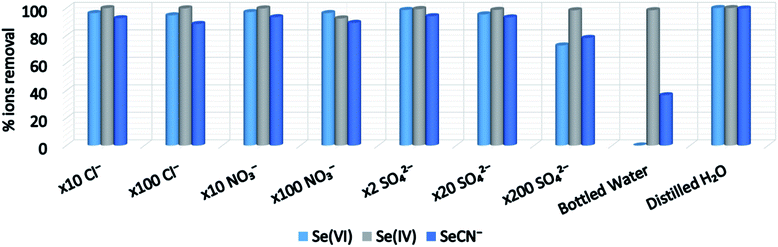
The new MOFs with labile terminal ligands in the Zr6 clusters and relatively high surface areas seem ideal sorbents for capture of anions. In the present work, we targeted on the sorption of Se anionic species, specifically selenite or Se(IV) (being in the form of HSeO3− and SeO32− at neutral and alkaline solutions (pH > 9) respectively), selenate or Se(VI) (being in the form of SeO42− for pH > 2) and SeCN− anions.23,24 Se species are considered particularly toxic and as a result, World Health Organization (WHO) and the European Union (EE) recommended a maximum selenium concentration in drinking water of only 10 ppb (the corresponding value suggested by US-EPA is 50 ppb). Though the attention has been mainly paid on the selenite and selenate, the selenocyanate anions (SeCN−) are also present in wastewaters from oil refining and mining industries.20The activation of the MOFs was performed by treatment with HCl, in order to protonate the amine groups and induce cationic charge to the frameworks.15,16 Although sorption investigations have been conducted for all reported MOFs, detailed studies were performed only for BUT-MOF.
 | ||
| Fig. 5 Sorption of Se(IV), Se(VI) and SeCN− by BUT-MOFvs. time (initial total Se concentration was 1000 ppb, pH ∼ 7). | ||
Motivated by the fact that no SeCN− sorption data were reported for MOFs, we have also studied the sorption of SeCN− by BUT-MOF. The kinetics study revealed that the sorption of SeCN− by BUT-MOF was very fast with an equilibrium time of 2 min and removal capacity ≥95.6%. Due to the particularly rapid capture of SeCN− by BUT-MOF, fitting of the SeCN− sorption kinetics was not feasible.
(d) Mechanism of ion sorption
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl atomic ratio was ∼6
Cl atomic ratio was ∼6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). This Cl content was similar in the anion-loaded materials, thus indicating that Cl is not exchangeable and may be in the form of HCl strongly bound to the surface of particles. Therefore, it is apparent that the capability of BUT-MOF and its analogues to absorb anionic Se species is not due to exchange process involving extra-framework anions. Nevertheless, the protonation of the MOF enhances the sorption kinetics as the deprotonated MOF (MOF pre-treated with a base) requires ∼4 h to achieve the sorption equilibrium (whereas the equilibrium is achieved within 1–3 min by the protonated MOF). This finding reveals that the sorption process involves in some degree binding of anions into the charged surface of the MOF particles. Indeed, zeta potential measurements indicated a value of +24.2 ± 5.0 mV revealing the positive surface charge of MOF particles. However, the exceptional capture of Se-anions by the MOFs could not be explained only on the basis of surface sorption. According to previous works describing anion sorption by 8-c Zr4+ MOFs,15–19 the terminal OH/H2O terminal ligands of the Zr6 clusters constitute the sorption sites in which the anions are grafted. Specifically, the insertion of various anions is accompanied by release of terminal OH− ligands and thus, the sorption process proceeds via an anion exchange mechanism. Therefore, such intra-framework anion sorption can also contribute to the highly efficient capture of Se anionic species by the MOFs, besides the contribution of surface binding.
1). This Cl content was similar in the anion-loaded materials, thus indicating that Cl is not exchangeable and may be in the form of HCl strongly bound to the surface of particles. Therefore, it is apparent that the capability of BUT-MOF and its analogues to absorb anionic Se species is not due to exchange process involving extra-framework anions. Nevertheless, the protonation of the MOF enhances the sorption kinetics as the deprotonated MOF (MOF pre-treated with a base) requires ∼4 h to achieve the sorption equilibrium (whereas the equilibrium is achieved within 1–3 min by the protonated MOF). This finding reveals that the sorption process involves in some degree binding of anions into the charged surface of the MOF particles. Indeed, zeta potential measurements indicated a value of +24.2 ± 5.0 mV revealing the positive surface charge of MOF particles. However, the exceptional capture of Se-anions by the MOFs could not be explained only on the basis of surface sorption. According to previous works describing anion sorption by 8-c Zr4+ MOFs,15–19 the terminal OH/H2O terminal ligands of the Zr6 clusters constitute the sorption sites in which the anions are grafted. Specifically, the insertion of various anions is accompanied by release of terminal OH− ligands and thus, the sorption process proceeds via an anion exchange mechanism. Therefore, such intra-framework anion sorption can also contribute to the highly efficient capture of Se anionic species by the MOFs, besides the contribution of surface binding.
| H16[Zr6O16(BUTNH-BDC)4] + 2SeO42− → H12[Zr6O12(SeO4)2(BUTNH-BDC)4] + 4OH− | (1) |
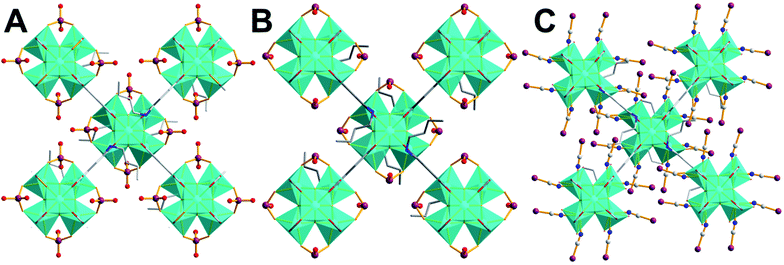
Thus, we built a model in which 2 SeO42− anions replace four terminal ligands on the equatorial plane of the Zr6 core and connect to the Zr4+ metal ions in a n1, n1:μ2-fashion (such coordination mode is commonly observed in Se(IV/VI)-loaded Zr4+ MOFs).17,18,32 We have also included water molecules in the pores of BUT-MOF/SeO42− material. After the successful optimization of this model, Rietveld refinement was carried out to obtain the final structure (Fig. 8A). The results of the refinement were very satisfying (Fig. S28†) indicating the correctness of the proposed structural model.
As mentioned above, Se(IV) exists as HSeO3− and SeO32− at neutral and alkaline (pH > 9) solutions respectively. Thus, the predicted sorption capacities of BUT-MOF would be 4 and 2 moles of Se(VI) per formula unit of BUT-MOF at pH ∼ 7 and pH > 9 respectively:
| H16[Zr6O16(BUTNH-BDC)4] + 4HSeO3− → H4[Zr6O8(HSeO3)4(BUTNH-BDC)4] + 4OH− + 4H2O (pH ∼ 7) | (2) |
| H16[Zr6O16(BUTNH-BDC)4] + 2SeO32− → H12[Zr6O12(SeO3)2(BUTNH-BDC)4] + 4OH− (pH > 9) | (3) |
We have found somewhat higher HSeO3− sorption capacity (5.0 mol mol−1 or 272 mg g−1 of BUT-MOF) than the predicted one (4.0 mol mol−1 or 217 mg g−1 of BUT-MOF). It is likely that 4 HSeO3− replaced the terminal OH/H2O ligands from the Zr6 clusters and the remaining HSeO3− (1 mol mol−1 or 55 mg g−1 of BUT-MOF) was captured at the external surface of particles through –NH2+(R)⋯HSeO3− electrostatic interactions (and hydrogen bonds between HSeO3− and carbonyl oxygen atoms, see below). The external surface sorption of HSeO3− is relatively limited (being only 20% of the total HSeO3− sorption capacity of BUT-MOF) and as a result, it is not appeared as a separate step in the HSeO3− sorption isotherm (Fig. 6). For comparison, the external surface sorption of Se(VI) or SeCN− species by BUT-MOF reaches up to 50% of the total sorption capacities, thus resulting in sorption isotherms with two components (Fig. 6).
In contrast, the experimental SeO32− sorption capacity (2.2 mol mol−1BUT-MOF, pH ∼ 9.5) was nearly equal to the calculated. At alkaline conditions, amine groups are deprotonated and thus, surface sorption is less favoured. As a result, the sorption of Se(IV) anions occurs mainly into the Zr6 clusters of the framework.
Based on the above, the structural model we built for the BUT-MOF/HSeO3− included 4 Se(IV) species coordinated in n1, n1:μ2-fashion to the Zr4+ metal centers as well as water molecules in the pores (Fig. 8B). The model was optimized and used as starting point for Rietveld refinement, which gave satisfactory results (Fig. S29†).
Based on isotherm sorption studies, BUT-MOF captures SeCN−via a two-step process: (i) surface sorption (lower concentration range) and (ii) intra-framework sorption (higher concentration range). The intra-framework SeCN− sorption can be described by the following equation:
| H16[Zr6O16(BUTNH-BDC)4] + 4SeCN− → H12[Zr6O12(SeCN)4(BUTNH-BDC)4] + 4OH− | (4) |
We thus built and optimize a model in which four SeCN− coordinate to the Zr4+ metal ions in a monodentate fashion (Se–CN⋯Zr) replacing half of the terminal water/OH− ligands (Fig. 8C). Such coordination mode is also supported by IR data (Fig. S26†). This structural model was used as starting point for Rietveld refinement, which provided fairly good results (Fig. S30†).
According to the sorption data described above, the anions can bind to the positively charged surface of the particles, i.e. to the protonated amine groups of the surface ligands, as well as ligate with the Zr4+ metal ions. In order to explain the higher selectivity of the MOFs towards HSeO3−, we should consider both ligation capability of the various anionic species and their interactions with the functional groups of the ligands. To this end, we have performed theoretical calculations.
Fig. S31† shows the natural atomic charges of SeO42−, HSeO3−, SeCN−, SO42− and HCO3− calculated by the natural bond orbital (NBO) population analysis using Weinhold's methodology.36,37 Based on the charge values of donor atoms (O or N) of the anionic species, we may have an estimation of the ligation efficiency of the anions towards the hard Zr4+ metal ions. Thus, SeO42−, HSeO3− and SO42− including O atoms with quite close charge values (−1.044 to −1.161) are expected to display similar ligation capability for Zr4+ and higher than that of HCO3− containing O atoms with lower charge values (−0.822 to −0.844). In addition, the negative charge of N atom (−0.581) of SeCN− is significant smaller than that of O atoms of the other anionic species and thus, SeCN− is anticipated to be less effective ligand for Zr4+ compared to other anions. Therefore, the higher affinity of the MOFs for HSeO3− cannot be explained only on the basis of its capability for ligation with Zr4+.
To get insight into the interactions of the various anions with the ligands of the MOF materials, we calculated the interaction energies of HSeO3−, SeO42−, SeCN−, SO42− and HCO3− anions with N-alkyl-2,5-bis(methoxycarbonyl)benzenaminium, as a model for the MOF's ligand, at the B3LYP/6-31+G(d)/PCM level of theory in aqueous solution using the Gaussian 09, version D.01 program suite.38 The optimized geometries of the possible MOF@SeCN−, MOF@HSeO3−, MOF@SeO42−, MOF@SO42− and MOF@HCO3− weak associations supported by hydrogen bonds and Coulomb (electrostatic) forces, along with selected structural parameters and the interaction energies (ΔE in kcal mol−1) and enthalpy changes (ΔH in kcal mol−1) calculated at the B3LYP/6-31+G(d)/PCM level of theory in aqueous solution, are shown in Fig. S32.†
It can be seen that the SeO42− dianions are the most strongly associated species with the MOF's ligand, followed by the SO42− dianions and the SeCN− anionic species. Notice that the interactions are primarily electrostatic in nature in synergy with hydrogen bond formation. The HSeO3− and HCO3− anions can be associated with the ligand by three different interaction modes (Fig. S32†), since the OH group of these species can participate in hydrogen bond formation with the carbonyl oxygen atoms of the ligand. The estimated interactions energies for the three interaction modes of the HSeO3− and HCO3− species with the MOF's ligand are smaller than the corresponding interactions energies of the selenate, sulfate and selenocyanate anions. However, the possibility of the HSeO3− and HCO3− anions to interact with the ligand in three different interaction modes allows more (HSeO3− or HCO3−) species to be associated to the MOF's moiety than the SeCN−, SeO42− and SO42− species, which likely explains (a) the higher selectivity of the MOFs for HSeO3−vs. SeCN−, SeO42− and SO42− and (b) the negligible SeCN−, SeO42− sorption capacity of the MOFs in the presence of excess of HCO3−. Finally, the fact that HSeO3− may be more efficient ligand for Zr4+ than HCO3− (see above) accounts for the observed selectivity of the MOFs for HSeO3− in the presence of excess of HCO3−.
Conclusions
In the present work, we demonstrated that the utilization of terephthalic linkers with slightly to moderately elongated functional groups, such as RNH- (R = ethyl, propyl, isobutyl, n-butyl), constitutes an effective synthetic strategy towards the isolation of Zr4+ MOF with decreased net (8-c) connectivity. The new MOFs with relatively small-sized functional groups showed substantially higher surface areas (up to 832 m2 g−1) than that (354 m2 g−1) of MOR-2 (the only other known Zr4+-MOF with a terephthalate linker and 8-c framework) incorporating bulky (2-picolylamino) functionalities. Presumably, even small-sized groups, such as ethyl-, propyl-amino etc., cause significant steric interactions between the linkers resulting to the stabilization of the observed 8-connected rather than the usual 12-connected frameworks. The targeted synthesis of Zr4+ MOFs with low-net connectivity is highly desirable, not only as a means for the isolation of materials with enhanced porosity, but also as an approach to introduce intra-framework anion sorption sites (i.e. labile terminal water/hydroxy ligands). Actually, the sorption capacities of the reported MOFs for the toxic Se(IV/VI)-based anions surpass that of known MOFs and most of other materials. Remarkably, the Se(VI) sorption capacities of the MOFs are more than double compared to other MOF sorbents and the Se(IV) sorption by the reported alkylamino-functionalized MOFs is extraordinary (removal capacities >98% in ≤3 min) even in the presence of a tremendous (>200-fold) excess of various competitive anions. The highly efficient Se(IV) and Se(VI) capture by the MOFs is due to a dual sorption process involving both surface binding (via electrostatic-hydrogen bonding interactions) and intra-framework sorption via replacement of the terminal H2O/OH− ligands by the inserted Se anions. In addition, the new MOFs showed an exceptional capability to sorb SeCN− species, a particularly toxic Se form, with such sorption property presented for the first time for MOF materials. Again, the SeCN− capture involves both surface and intra-framework sorption. Importantly, the SeCN− sorption capacities of the new MOFs are superior to those of other sorbents tested so far. Additional toxic anionic species may be efficiently sorbed by the alkylamino-functionalized MOFs. Such investigations are underway.Author contributions
A. D. Pournara: investigation, formal analysis, writing-original draft, S. Rapti: investigation, formal analysis, A. Valmas: investigation, writing-original draft, I. Margiolaki: investigation, resources, E. Andreou: investigation, formal analysis, G. S. Armatas: investigation, formal analysis, writing-review & editing, resources, A. C. Tsipis: investigation, formal analysis, resources, writing-original draft, J. C. Plakatouras: investigation, formal analysis, writing-review & editing, D. L. Giokas: investigation, formal analysis, resources, writing-review & editing, M. J. Manos: conceptualization, supervision, resources, writing-original draft.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research work was supported by the Hellenic Foundation for Research and Innovation (H.F.R.I.) under the “First Call for H.F.R.I. Research Projects to support Faculty members and Researchers and the procurement of high-cost research equipment grant” (Project Number: 348).Notes and references
- M. Eddaoudi, D. B. Moler, H. Li, B. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319–330 CrossRef CAS.
- G. Férey, Chem. Soc. Rev., 2008, 37, 191–214 RSC.
- S. Horike, S. Shimomura and S. Kitagawa, Nat. Chem., 2009, 1, 695–704 CrossRef CAS.
- D. Bradshaw, J. B. Claridge, E. J. Cussen, T. J. Prior and M. J. Rosseinsky, Acc. Chem. Res., 2005, 38, 273–282 CrossRef CAS.
- O. K. Farha and J. T. Hupp, Acc. Chem. Res., 2010, 43, 1166–1175 CrossRef CAS.
- S. Zheng, T. Wu, J. Zhang, M. Chow, R. A. Nieto, P. Feng and X. Bu, Angew. Chem., Int. Ed., 2010, 49, 5362–5366 CrossRef CAS.
- D. Sheng, L. Zhu, X. Dai, C. Xu, P. Li, C. I. Pearce, C. Xiao, J. Chen, R. Zhou, T. Duan, O. K. Farha, Z. Chai and S. Wang, Angew. Chem., Int. Ed., 2019, 58, 4968–4972 CrossRef CAS.
- C. Xiao, A. Khayambashi and S. Wang, Chem. Mater., 2019, 31, 3863–3877 CrossRef CAS.
- P. Kumar, A. Pournara, K. H. Kim, V. Bansal, S. Rapti and M. J. Manos, Prog. Mater. Sci., 2017, 86, 25–74 CrossRef CAS.
- J. Li, X. Wang, G. Zhao, C. Chen, Z. Chai, A. Alsaedi, T. Hayat and X. Wang, Chem. Soc. Rev., 2018, 47, 2322–2356 RSC.
- A. V. Desai, S. Sharma and S. K. Ghosh, in Metal-Organic Frameworks (MOFs) for Environmental Applications, Elsevier, Amsterdam, 2019, pp. 95–140 Search PubMed.
- Z. L. Magnuson and S. Ma, in Metal-Organic Frameworks (MOFs) for Environmental Applications, Elsevier, Amsterdam, 2019,pp. 63–93 Search PubMed.
- Y. Bai, Y. Dou, L. H. Xie, W. Rutledge, J. R. Li and H. C. Zhou, Chem. Soc. Rev., 2016, 45, 2327–2367 RSC.
- Z. Hu and D. Zhao, Dalton Trans., 2015, 44, 19018–19040 RSC.
- S. Rapti, D. Sarma, S. A. Diamantis, E. Skliri, G. S. Armatas, A. C. Tsipis, Y. S. Hassan, M. Alkordi, C. D. Malliakas, M. G. Kanatzidis, T. Lazarides, J. C. Plakatouras and M. J. Manos, J. Mater. Chem. A, 2017, 5, 14707–14719 RSC.
- S. Rapti, S. A. Diamantis, A. Dafnomili, A. Pournara, E. Skliri, G. S. Armatas, A. C. Tsipis, I. Spanopoulos, C. D. Malliakas, M. G. Kanatzidis, J. C. Plakatouras, F. Noli, T. Lazarides and M. J. Manos, J. Mater. Chem. A, 2018, 6, 20813–20821 RSC.
- A. J. Howarth, M. J. Katz, T. C. Wang, A. E. Platero-Prats, K. W. Chapman, J. T. Hupp and O. K. Farha, J. Am. Chem. Soc., 2015, 137, 7488–7494 CrossRef CAS.
- A. J. Howarth, Y. Liu, J. T. Hupp and O. K. Farha, CrystEngComm, 2015, 17, 7245–7253 RSC.
- R. J. Drout, K. Otake, A. J. Howarth, T. Islamoglu, L. Zhu, C. Xiao, S. Wang and O. K. Farha, Chem. Mater., 2018, 30, 1277–1284 CrossRef CAS.
- E. D. Van Hullebusch, Bioremediation of Selenium Contaminated Wastewater, Springer, Switzerland, 2017, pp. 1–130 Search PubMed.
- L. Sarkisov and A. Harrison, Mol. Simul., 2011, 37, 1248–1257 CrossRef CAS.
- The calculations of the surface areas and pore sizes using poreblazer were done using a lower symmetry model (I
![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) ) of the structures, without guests in the pores. In these models, there are no positional disordered groups. In the structural models with the I4/m space group, there are duplicates of atoms due to their positional disorder. Thus, the structures appear erroneously denser and the calculations lead to incorrect results.
) of the structures, without guests in the pores. In these models, there are no positional disordered groups. In the structural models with the I4/m space group, there are duplicates of atoms due to their positional disorder. Thus, the structures appear erroneously denser and the calculations lead to incorrect results. - J. Torres, V. Pintos, S. Domínguez, C. Kremer and E. Kremer, J. Solution Chem., 2010, 39, 1–10 CrossRef CAS.
- J. Kretzschmar, N. Jordan, E. Brendler, S. Tsushima, C. Franzen, H. Foerstendorf, M. Stockmann, K. Heim and V. Brendler, Dalton Trans., 2015, 44, 10508–10515 RSC.
- Y. Georgiou, S. Rapti, A. Mavrogiorgou, G. Armatas, M. J. Manos, M. Louloudi and Y. Deligiannakis, Sci. Rep., 2020, 10, 1–12 CrossRef.
- S. Soda, M. Kashiwa, T. Kagami, M. Kuroda, M. Yamashita and M. Ike, Desalination, 2011, 279, 433–438 CrossRef CAS.
- C. Mac Namara, J. Torroba and A. Deacon, Johnson Matthey Technol. Rev., 2015, 59, 334–352 CrossRef.
- J. Wei, W. Zhang, W. Pan, C. Li and W. Sun, Environ. Sci.: Nano, 2018, 5, 1441–1453 RSC.
- H. Ouyang, N. Chen, G. Chang, X. Zhao, Y. Sun, S. Chen, H. Zhang and D. Yang, Angew. Chem., Int. Ed., 2018, 57, 13197–13201 CrossRef CAS.
- J. Li, Y. Liu, X. Wang, G. Zhao, Y. Ai, B. Han, T. Wen, T. Hayat, A. Alsaedi and X. Wang, Chem. Eng. J., 2017, 330, 1012–1021 CrossRef CAS.
- S. Sharma, A. V. Desai, B. Joarder and S. K. Ghosh, Angew. Chem., Int. Ed., 2020, 59, 7788–7792 CrossRef CAS.
- R. J. Drout, A. J. Howarth, K. I. Otake, T. Islamoglu and O. K. Farha, CrystEngComm, 2018, 20, 6140–6145 RSC.
- L. Ma, S. M. Islam, C. Xiao, J. Zhao, H. Liu, M. Yuan, G. Sun, H. Li, S. Ma and M. G. Kanatzidis, J. Am. Chem. Soc., 2017, 139, 12745–12757 CrossRef CAS.
- N. Gezer, M. Gülfen and A. O. Aydín, J. Appl. Polym. Sci., 2011, 122, 1134–1141 CrossRef CAS.
- L. Zhu, L. Zhang, J. Li, D. Zhang, L. Chen, D. Sheng, S. Yang, C. Xiao, J. Wang, Z. Chai, T. E. Albrecht-Schmitt and S. Wang, Environ. Sci. Technol., 2017, 51, 8606–8615 CrossRef CAS.
- A. E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899–926 CrossRef CAS.
- F. Weinhold, in Encyclopedia of Computational Chemistry, Wiley, Chichester, UK, 1998, pp. 1792–1811 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, M. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas; J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2010 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures; thermal analyses data; X-ray powder diffraction studies and other characterization data for the reported MOFs; ion sorption data; characterization of ion-loaded materials; theoretical calculations. CCDC 2017777–2017786. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ta11653j |
| ‡ These two authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2021 |