Selective electroreduction of CO2 to carbon-rich products with a simple binary copper selenide electrocatalyst†
Abstract
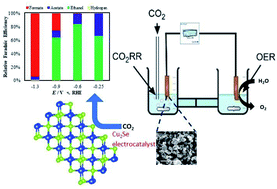
In this article solvothermally synthesized copper selenide nanostructures have been reported as highly efficient electrocatalysts for carbon dioxide reduction under ambient conditions with high selectivity for carbon-rich C2 products at a low applied potential. In addition to electrochemical measurements, density functional theory calculations were also performed to investigate the adsorption energy of the key intermediate carbon monoxide on the catalyst surface. The authors proposed that CO adsorption energy on the surface can be a critical component to determine the extent of CO2 reduction on the surface, whereby a low CO adsorption energy was expected to yield primarily C1 products while a very large adsorption energy leads to catalyst poisoning. In this article the authors have shown that by carefully designing the catalyst surface to optimize the CO adsorption energy and dwell time, selenide based electrocatalysts can indeed show more efficient CO2 reduction compared to the base metal, leading to carbon-rich products. This is one of the first reports where the Cu2Se surface has been studied in detail with experimental as well as DFT studies for CO2 reduction. Interestingly, the reduction products showed dependence on the applied potential forming exclusively formic acid at a high applied potential (1.2 V and higher vs. RHE), while ethanol and acetic acid were produced in high yield at potentials lower than 0.8 V vs. RHE. The applied potential required for CO2 with copper selenide was as low as 100 mV vs. RHE and is one of the lowest reported to date. The CO2 reduction products were analyzed through NMR and GC TCD spectroscopy which showed ethanol and acetic acid production in excess of 80% faradaic efficiency at a low applied potential.


 Please wait while we load your content...
Please wait while we load your content...
