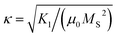Open Access Article
Open Access ArticleDesigning of magnetic MAB phases for energy applications†
Chen
Shen
 a,
Qiang
Gao
a,
Qiang
Gao
 a,
Nuno M.
Fortunato
a,
Harish K.
Singh
a,
Nuno M.
Fortunato
a,
Harish K.
Singh
 a,
Ingo
Opahle
a,
Oliver
Gutfleisch
ab and
Hongbin
Zhang
*a
a,
Ingo
Opahle
a,
Oliver
Gutfleisch
ab and
Hongbin
Zhang
*a
aInstitut für Materialwissenschaft, Technische Universität Darmstadt, 64287, Darmstadt, Germany. E-mail: hzhang@tmm.tu-darmstadt.de
bFraunhofer-Research Institution Materials Recycling and Resource Strategies IWKS, 63457, Hanau, Germany
First published on 22nd March 2021
Abstract
Based on high-throughput density functional theory calculations, we performed screening for stable magnetic MAB compounds and predicted potential strong magnets for permanent magnet and magnetocaloric applications. The thermodynamical, mechanical, and dynamical stabilities are systematically evaluated, resulting in 21 unreported compounds on the convex hull, and 434 materials being metastable considering convex hull tolerance to be 100 meV per atom. Analysis based on the Hume-Rothery rules revealed that the valence electron concentration and size factor difference are of significant importance in determining the stability, with good correspondence with the local atomic bonding. We found 71 compounds with the absolute value of magneto-crystalline anisotropy energy above 1.0 MJ m−3 and 23 compounds with a uniaxial anisotropy greater than 0.4 MJ m−3, which are potential gap magnets. Based on the magnetic deformation proxy, 99 compounds were identified as potential materials with interesting magnetocaloric performance.
1 Introduction
The modern industrial and societal demands for advanced functional magnetic materials are growing faster as we are witnessing the global expansion of hybrid-electric vehicles, robotics, wind turbines, and automation, leading to a strong incentive on the green energy revolution.1,2 Particularly, the efficient harvesting of renewable energy (such as wind energy) and endeavor to reduce the greenhouse effect (mainly through the development of e-mobility and magnetic refrigeration) have intensified the impetus to design resource-efficient magnetic materials with optimal performance, such as permanent magnets and magnetocaloric materials. For instance, one interesting question is to identify the so-called gap magnets,3i.e., permanent magnets with their energy density (BH)max4 lying between the widely applied AlNiCo and ferrites5 and the high-performance Sm–Co6 and Nd–Fe–B-based7 permanent magnets. Potential candidates can be characterized using the dimensionless figure of merit ,8 providing an effective descriptor for high-throughput screening. Moreover, following the discovery of Gd5Si2Ge2 (ref. 9) and LaFeSi13 (ref. 10) with giant magnetocaloric effect (MCE) around room temperature, magnetic refrigeration technology is assumed to be capable of competing with and hopefully surpassing conventional refrigeration in terms of energy efficiency, environmentally friendly and ecological impact in the near future.11–13 However, most permanent magnets and potential magnetocaloric materials with high performance are based on the intermetallic compounds containing rare-earths (RE), which are resource critical.14 Therefore, rare-earth-free permanent magnets and MCE materials with enhanced efficiency over a broad temperature range and useful secondary properties, such as mechanical stability, corrosion resistance, shapeability, sustainability, and recyclability, are still desirable.1,15,16
,8 providing an effective descriptor for high-throughput screening. Moreover, following the discovery of Gd5Si2Ge2 (ref. 9) and LaFeSi13 (ref. 10) with giant magnetocaloric effect (MCE) around room temperature, magnetic refrigeration technology is assumed to be capable of competing with and hopefully surpassing conventional refrigeration in terms of energy efficiency, environmentally friendly and ecological impact in the near future.11–13 However, most permanent magnets and potential magnetocaloric materials with high performance are based on the intermetallic compounds containing rare-earths (RE), which are resource critical.14 Therefore, rare-earth-free permanent magnets and MCE materials with enhanced efficiency over a broad temperature range and useful secondary properties, such as mechanical stability, corrosion resistance, shapeability, sustainability, and recyclability, are still desirable.1,15,16
The MAB phases with nanolaminated crystal structures exhibit intriguing magnetic properties and mechanical deformation behavior, which have attracted considerable attention recently.17 Such materials are ternary borides comprising stacked M–B layers (M = transition metal, B = boron) interleaved by monolayers of A atoms. In this regard, the crystal structures are quite similar to those of the well-known MAX phases Mn+1AXn (X = C and N, A denotes a main group element), which host a unique combination of metallic and ceramic properties.18 The novel magnetic nanolaminates recently discovered in the MAX phases,19 are also expected in the MAB phases. Moreover, Fe2AlB2 was found to be a promising magnetocaloric material exhibiting an interesting MCE,20 with the ordering temperature around 300 K confirmed by experimental21,22 and theoretical studies.23–25 Ke et al.26 investigated the intrinsic properties of Fe2AlB2, and found a MAE as large as −1.34 MJ cm−3, in good agreement with the experiments.22 Recently, Cr4AlB4 with a novel structure of MAB phase has been synthesized consistent with the theoretical calculations.27 Khazaei et al.28 carried out high-throughput (HTP) calculations on Al-containing non-magnetic MAB phases and predicted 9 stable compounds. More recently, Miao et al.29 reported another HTP screening for Ti–A–B, Zr–A–B, and Hf–A–B and predicted 7 thermodynamically stable compounds. Therefore, an interesting question is whether there exist more stable MAB compounds beyond the above-mentioned cases and whether are they good candidates as potential functional magnetic materials.
In this work, based on HTP density functional theory (DFT) calculations, we systematically studied the stabilities and the magnetic properties of the MAB compounds to identify possible candidates for permanent magnets and magnetocaloric materials. Six experimentally synthesized MAB phases and three non-MAB phases (as competitive phases) are considered (Fig. 1), including MAB30 (space group Cmcm), M2AB2 (ref. 31) (space group Cmcm), M3A2B2 (ref. 32) (space group Cmcm), M3AB4 (ref. 33) (space group Immm), M4AB4 (ref. 27) (space group Immm) and M4AB6 (ref. 34) (space group Cmcm); non-MAB phases are M5AB2 (ref. 35) (space group I4/mcm), M3A2B2 (ref. 36) (space group P2/m) and M4A3B2 (ref. 36) (space group P4/mmm). Three non-MAB phases are considered as competitive phases in order to make the prediction of MAB compounds more reliable. Such compounds are flexible in the chemical compositions and have tunable magnetic properties. For example, Fe5SiB2 has a TC higher than 760 K, a MS larger than 1 MA m−1, and a MAE more than 0.30 MJ m−3 at room temperature.37–41 After validating all the experimentally known phases, we identified stable and metastable ternary borides based on the systematic evaluation of the thermodynamical, mechanical, and dynamical stabilities. Taking the M2AB2-type compounds as an example, we investigated the effect of magnetic ordering on the thermodynamic stability, followed by a comprehensive analysis of the stability trend following the Hume-Rothery rules and local atomic bonding. The MAE and magnetic deformation proxy are evaluated explicitly, which help to screen for potential permanent magnets and magnetocaloric materials. Our work expands the materials library of rare-earth free permanent magnets and magnetic refrigeration, and thus provides valuable guidance to further theoretical and experimental studies to design advanced magnetic materials in transition metal-based ternary borides for energy applications.13,16
2 Computational details
The DFT calculations are performed using an in-house developed HTP environment42,43 to determine the thermodynamical stability for the above mentioned six MAB and three non-MAB phases, as demonstrated in recent studies.44–46 It is noted that the non-MAB phases are regarded as competitive phases for the MAB phase to obtain the reliable convex hull, which is also applied in designing MAX phases by considering antiperovskites as a competitive phase.45 Thermodynamical stability is evaluated by considering the formation energy (Ef) and the distance to the convex hull with respect to all the relevant competing phases available in the OQMD database.47 All the calculations are carried out using the Vienna ab initio simulation package (VASP) code.48,49 The MAE of the predicted stable phases is obtained using the full-potential local-orbital (FPLO)50 code in the force theorem regime, and the recently proposed magnetic deformation proxy51 is used to evaluate the MCE. More details of the computational processes can be found in the ESI.†3 Results and discussion
3.1 Stabilities of phases
| Phases | Space group | Lattice | ΔEf | ΔEh | Competing phases | Mag. | M | ||
|---|---|---|---|---|---|---|---|---|---|
| a | b | c | |||||||
| FeBeB | 63 | 2.648 | 12.164 | 2.925 | −0.326 | 0 | FeB, Be2Fe, B | FM | 0.422 |
| MnBeB | 63 | 2.811 | 12.252 | 2.809 | −0.378 | 0 | MnB, Be | NM | 0.002 |
| Fe2AlB*2 | 65 | 2.916 | 11.019 | 2.851 | −0.401 | 0 | FeAl6, AlB2, FeB | FM | 1.330 |
| Ref. Exp.20 | 2.928 | 11.033 | 2.868 | ||||||
| Ref. Cal.26 | 2.915 | 11.017 | 2.851 | ||||||
| Fe2BeB2 | 65 | 2.904 | 9.947 | 2.749 | −0.344 | 0 | Be2Fe, B, FeB | AFM | 0.760 |
| Cr2AlB*2 | 65 | 2.923 | 11.051 | 2.932 | −0.466 | 0 | Cr3AlB4, Cr7Al45, CrB | NM | 0.010 |
| Ref. Exp.34 | 2.937 | 11.051 | 2.968 | ||||||
| Ref. Cal.26 | 2.921 | 11.034 | 2.929 | ||||||
| Mn2AlB*2 | 65 | 2.894 | 11.080 | 2.831 | −0.471 | 0 | Mn4Al11, MnB, MnB4 | AFM | 0.765 |
| Ref. Exp.24 | 2.923 | 11.070 | 2.899 | AFM | |||||
| Ref. Cal.26 | 2.887 | 11.109 | 2.830 | AFM | |||||
| Mn2BeB2 | 65 | 2.846 | 9.969 | 2.815 | −0.435 | 0 | MnB, Be | NM | 0.011 |
| Cr3AlB*4 | 47 | 2.939 | 2.939 | 8.091 | −0.445 | 0 | Cr2AlB2, CrB4, CrB | NM | 0.049 |
| Ref. Exp.34 | 2.956 | 2.978 | 8.054 | ||||||
| Ref. Cal.56 | 2.938 | 2.943 | 8.090 | ||||||
| Cr4AlB*6 | 65 | 2.947 | 21.328 | 2.943 | −0.422 | 0.012 | CrB4, Cr3AlB4, CrB | NM | 0.003 |
| Ref. Exp.34 | 2.952 | 21.280 | 3.013 | ||||||
| Ref. Cal.57 | 2.972 | 21.389 | 2.961 | ||||||
| Fe4AlB4 | 71 | 2.927 | 18.565 | 2.870 | −0.417 | 0 | AlFe2B2, FeB | FM | 1.271 |
| Fe4BeB4 | 71 | 2.918 | 17.513 | 2.821 | −0.377 | 0 | FeB, Be2Fe, B | FM | 1.017 |
| Fe4GaB4 | 71 | 2.939 | 18.557 | 2.883 | −0.343 | 0 | FeB, Ga3Fe, B | FM | 1.288 |
| Fe4MgB4 | 71 | 2.932 | 19.626 | 2.875 | −0.354 | 0 | FeB, Mg | FM | 1.391 |
| Fe4ZnB4 | 71 | 2.931 | 18.726 | 2.872 | −0.348 | 0 | FeB, Zn | FM | 1.326 |
| Cr4AlB*4 | 71 | 2.920 | 18.856 | 2.939 | −0.510 | 0 | AlCr2B2, CrB | NM | 0 |
| Ref. Exp.27 | 2.934 | 18.891 | 2.973 | ||||||
| Ref. Cal.27 | 2.932 | 18.912 | 2.957 | ||||||
| Mn4BeB4 | 71 | 2.899 | 17.591 | 2.878 | −0.467 | 0 | MnB, Be | FM | 0.878 |
| Mn4AlB4 | 71 | 2.929 | 18.591 | 2.889 | −0.499 | 0 | MnB, Mn2AlB2 | FM | 1.014 |
| Mn4IrB4 | 71 | 2.959 | 18.716 | 2.966 | −0.450 | 0 | MnB, Ir | FM | 2.003 |
| Ni4AuB4 | 71 | 3.012 | 18.793 | 2.950 | −0.224 | 0 | Au, Ni4B3, | NM | 0 |
| Ni4CuB4 | 71 | 2.992 | 18.125 | 2.875 | −0.227 | 0 | B, Cu, Ni4B3 | NM | 0 |
| Ni4PdB4 | 71 | 2.996 | 18.453 | 2.931 | −0.265 | 0 | Ni4B3, BPd2, B | NM | 0 |
| Ni4PtB4 | 71 | 2.995 | 18.351 | 2.960 | −0.267 | 0 | BPt2, Ni4B3, B | NM | 0 |
| Ni4ZnB4 | 71 | 2.992 | 18.517 | 2.880 | −0.261 | 0 | Ni4B3, B, ZnNi3B2 | NM | 0 |
| Fe3Al2B*2 | 10 | 5.685 | 2.833 | 8.593 | −0.426 | 0 | FeAl6, AlB2, FeB | FM | 0.784 |
| Ref. Exp.36 | 5.723 | 2.857 | 2.857 | ||||||
| Fe4Al3B2 | 123 | 8.083 | 8.083 | 2.791 | −0.411 | 0 | AlFe, AlFe2B2 | NM | 0.002 |
| Co4Be3B2 | 123 | 7.586 | 7.586 | 2.586 | −0.395 | 0 | Be3Co, BeCo, CoB | NM | 0 |
| Ni4Li3B2 | 123 | 8.049 | 8.049 | 2.734 | −0.252 | 0 | Li, Ni2B | NM | 0.0002 |
| Fe5BeB2 | 140 | 5.455 | 5.455 | 9.914 | −0.292 | 0 | Be2Fe, Fe2B, Fe | FM | 1.932 |
| Fe5PB*2 | 140 | 5.570 | 5.570 | 10.436 | −0.392 | 0.033 | Fe2B, FeB, Fe2P | FM | 1.705 |
| Ref. Exp.35 | 5.548 | 5.548 | 10.332 | FM | 1.730 | ||||
| Ref. Exp.40 | 5.485 | 5.485 | 10.348 | FM | 1.720 | ||||
| Ref. Exp.58 | 5.492 | 5.492 | 10.365 | FM | 1.658 | ||||
| Ref. Cal.53 | 5.456 | 5.456 | 10.296 | FM | 1.770 | ||||
| Fe5SiB2 | 140 | 5.509 | 5.509 | 10.299 | −0.359 | 0.003 | Fe2B, FeSi | FM | 1.731 |
| Ref. Exp.38 | 5.551 | 5.551 | 10.336 | FM | 1.808 | ||||
| Ref. Exp.37 | 5.554 | 5.554 | 10.343 | FM | 1.750 | ||||
| Ref. Cal.39 | 5.546 | 5.546 | 10.341 | FM | 1.840 | ||||
| Co5PB*2 | 140 | 5.279 | 5.279 | 10.477 | −0.357 | 0.079 | Co2P, CoB, Co | FM | 0.409 |
| Ref. Exp.59 | 5.420 | 5.420 | 10.200 | ||||||
| Ref. Cal.53 | 5.284 | 5.284 | 10.541 | FM | 0.440 | ||||
| Co5SiB*2 | 140 | 5.484 | 5.484 | 9.942 | −0.337 | 0.042 | CoB, Co2Si, Co | FM | 0.394 |
| Ref. Exp.60 | |||||||||
| Ref. Cal.39 | 5.511 | 5.511 | 9.953 | FM | 0.484 | ||||
| Cr5PB*2 | 140 | 5.537 | 5.537 | 10.317 | −0.474 | 0.032 | Cr3P, CrB | NM | 0.022 |
| Ref. Exp.61 | 5.593 | 5.593 | 10.370 | ||||||
| Cr5B*3 | 140 | 5.431 | 5.431 | 9.923 | −0.418 | 0 | CrB, Cr2B | NM | 0 |
| Ref. Exp.62 | 5.460 | 5.460 | 10.460 | ||||||
| Mn5PB*2 | 140 | 5.509 | 5.509 | 10.287 | −0.480 | 0.033 | Mn2B, MnB, Mn2P | FM | 1.665 |
| Ref. Exp.59 | 5.540 | 5.540 | 10.490 | ||||||
| Ref. Exp.63 | 5.540 | 5.540 | 10.490 | ||||||
| Mn5SiB*2 | 140 | 5.559 | 5.559 | 10.293 | −0.415 | 0.003 | MnSi, Mn2B | FM | 1.583 |
| Ref. Exp.63 | 5.540 | 5.540 | 10.490 | ||||||
Furthermore, not all the compounds are magnetic, e.g., with finite magnetization larger than 0.05 μB per magnetic atom (Table 1). It is observed that the nonmagnetic compounds occur mostly for the Cr-, Mn-, and Ni-based cases, whereas Fe4Al3B2 and Co4Be3B2 are nonmagnetic as well. This can be understood based on the Stoner criteria, where Iν(EF) > 1 indicate possible itinerant magnetic ordering, with I being the Stoner parameter and ν(EF) the density of states (DOS) at the Fermi energy EF of the nonmagnetic state. For instance, the Stoner parameters of magnetic atoms range between 0.6 and 0.75 from Cr to Ni,55 thus those compounds with marginal ν(EF) smaller than 1.4 states per eV per magnetic atom end up as nonmagnetic (Fig. S3†) because Iν(EF) < 1. Moreover, the predicted results agree well with previous experimental and theoretical reports, e.g., Fe5PB2 with average magnetization 1.71 μB/Fe40 and Cr4AlB4/6 being nonmagnetic.27,34 Furthermore, it is observed that there exist nonzero induced magnetic moments on the nonmagnetic atoms which are antiparallel to the magnetic moments of the 3d atoms. For instance, Mn4BeB4 has the smallest ratio Mtot/M3d of about 0.94, where the magnetic moments of non-metal B atoms are about −0.05 μB per B atom and those of metal Be atoms are smaller than −0.01 μB per Be atom.
Interestingly, the distance to the convex hull for the experimentally synthesized compounds are finite (Table 1), e.g., Cr4AlB6, Fe5PB2 and Co5PB2 with distances to the convex hull of 12, 33 and 79 meV per atom, respectively. Co5PB5 with a distance to the convex hull being 79 meV per atom is a special case, where there is also a 3% deviation in the theoretical and experimental lattice constants. In fact, the calculated lattice constants of this compound agree well with previously reported calculations.53 Nevertheless, this suggests that a loose tolerance ΔEh < 100 meV per atom is reasonable, though it is not deliberately chosen to cover the Co5PB5 phase.
Critical tolerance with comparable values for the convex hull has also been adopted in other HTP studies.43,46,64 This leads to 434 (335 are MAB phases) stable compounds, as listed in Table S1 in the ESI.† As a consequence, our predictions become consistent with another HTP study28 focusing on Al-containing MAB phases with early transition metals on the M-sites. For instance, 8 novel MAB phases they found, i.e., CrAlB, MnAlB, Cr3Al2B2, Mn3Al2B2, Ni3Al2B2, Mn3AlB4, and Fe3AlB4, are also predicted to be stable using the loose tolerance on the convex hull, as listed in the Table S10.† It is noted that even if such compounds are metastable, they can still be synthesized using non-equilibrium methods such as MBE and ball milling. Hereafter we will consider the stability trend and magnetic properties for all those compounds. Last but not least, it is essential to consider the non-MAB phases as competing phases beyond those in the OQMD database. It is observed that the 322-MAB Fe3Al2B2 is stable with ΔEh = 0 compared with competing phases in OQMD, whereas it becomes metastable with ΔEh = 33 meV per atom after considering the non-MAB Fe3Al2B2. Certainly there are other competing phases and even novel crystal structures beyond those considered in this work, which will be saved for future investigation after experimental validations.
Another interesting question for predicting stable magnetic materials is whether the magnetic configurations would influence the thermodynamic stability, since most HTP calculations are done assuming the ferromagnetic (FM) state as in the OQMD and the Materials Project.65 This applies particularly to Mn-based compounds, as revealed by a recent work that the energy landscape of the convex hull is drastically changed after considering the magnetic ground state.66 According to the literature, the 212-type Mn2AlB2 is observed to display an AFM magnetic ground state with Néel temperature about 390 K,26,67,68 thus we performed extensive calculations on the predicted 212-type MAB compounds. As summarized in Table S2,† 15 out of 54 magnetic compounds prefer AFM magnetic configurations, including not only Mn-based but also Fe- and Co-based compounds. The magnetic ground states are consistent with those obtained from our Monte Carlo modeling based on the Heisenberg model taking exchange parameters from DFT calculations (not shown), which will be discussed in detail elsewhere. Nevertheless, the energy difference between the FM and AFM states is less than 20 meV per atom, hence the magnetic ground state has no strong impact on the thermodynamic stability for such compounds. This can be attributed to the nano-laminated crystal structure, where the magnetic interaction between the local Mn-moments is relatively weak, in comparison to the strongly frustrated fcc-lattice from the Cu3Au lattice considered in ref. 66. It is noted that systematic evaluation of the magnetic ground states is a challenge, hereafter we will focus on the physical properties of the FM states, which should be valid for most of the other compounds.
After the thermodynamic stability, mechanical and dynamical stabilities should be addressed as well in order to make systematic predictions. It is observed that mechanical stability plays a marginal role as explicitly demonstrated for 21 stable compounds on the convex hull. This is consistent with our previous studies on the antiperovskite compounds.46 For the orthorhombic MAB phases, there are nine independent elastic constants C11, C22, C33, C44, C55, C66, C12, C13, and C23. For the tetragonal non-MAB phases, there are six independent elastic constants C11, C33, C44, C66, C12, and C13. According to the mechanical stability defined in the ref. 69, none of the novel compounds predicted to be thermodynamically stable is found to be mechanically unstable. In addition, the dynamical stability is verified by examining the phonon spectra as compiled in Fig. S2† for 21 predicted and 15 known cases. Obviously, there is no imaginary modes observed for 35 compounds, indicating that those compounds are stable against local atomic displacements. The resulting phonon spectra for Cr2AlB2 and Cr3AlB4 are in good agreement with previous reported results.70 The mechanical (not shown) and dynamical (as indicated by phonon spectra in Fig. S2†) stabilities seem to be of marginal importance for the newly predicted compounds on the convex hull (cf.Table 1), which is consistent with our previous studies on the antiperovskite compounds.46 Due to the expensive computational cost, we have not performed such detailed evaluations for the other predicted compounds with the distance to the convex hull smaller than 100 meV per atom.
Nevertheless, for Ni4Li3B2 there exists an imaginary mode at the M point. This suggests that the compound may end up with other crystal structures or synthesized on certain substrates using molecular beam epitaxy.
Taking the 212-type MAB structure as an example, the stability trend with respect to the chemical composition can be understood based on the Hume-Rothery rules.71 Such rules are formulated based on the difference of size, electronegativity factors and the valence electron concentration (VEC). It is observed that the electronegativity difference between the M and A elements has no strong correlation with the stability (Fig. S5†), same as the MAX compounds.72 On the other hand, as shown in Fig. 2, both the atomic radius difference of the M and A elements 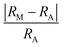 and VEC have significant influence on the stability. Clearly, most stable compounds are within the region
and VEC have significant influence on the stability. Clearly, most stable compounds are within the region  and VEC < 5.5. The newly reported novel phases in ref. 28 also prove the practicality of the current expression factors. Similar behavior is also observed for the 414-type MAB compounds with a slightly smaller tolerance for VEC < 6, as shown in Fig. S6.† The reason might be due to the fact that the M-site and boron-site contributing less valence electrons because of the extended M–B block (Fig. 1).
and VEC < 5.5. The newly reported novel phases in ref. 28 also prove the practicality of the current expression factors. Similar behavior is also observed for the 414-type MAB compounds with a slightly smaller tolerance for VEC < 6, as shown in Fig. S6.† The reason might be due to the fact that the M-site and boron-site contributing less valence electrons because of the extended M–B block (Fig. 1).
 | ||
| Fig. 2 The stability map of 212-MAB phases (circle symbols represent unstable phases in the present work; triangle symbols represent possible stable phases with convex hull distance below 100 meV per atom in the present work; square symbols represent newly reported novel phases in ref. 28). | ||
The general trend in the stability can be elucidated based on the chemical orbital Hamilton population (COHP) obtained using the LOBSTER code,73 which provides an atomic picture about the bonding. As an example which is representative for all the compounds we considered, the bond-resolved COHP is shown for M2AlB2 (where M are Cr, Mn, Fe, Co and Ni) and Fe2AB2 (where A are Be, Mg, Ca, Sr and Ba) in Fig. S8.† Focusing on varying the M elements, the number of valence electron on the M-sites increases from 6 in Cr2AlB2, to 8 in Fe2AlB2, and finally to 10 in Ni2AlB2. For Cr2AlB2, it is obvious that the values of –COHP are all positive below the Fermi energy, indicating only boning states are occupied in the corresponding bonds, which leads to a high overall stability (Fig. S8†) Increasing the number of valence electron to 10 in Ni2AlB2, the negative energies –COHP appeared below the Fermi energy in the Ni–B, Ni–Al, and Ni–Ni bonds. The occupation of such anti-bonding states weakens the bonds and therefore destabilizes the Ni2AlB2 compound. Therefore, the ICOHP of M–Al and M–B are increasing within the number of valence electron increasing, which indicates the corresponding bond strength weakens. Similar behaviour is also observed in the COPH os Fe2AB2 compounds with varying A elements being Be, Ca, and Ba (Fig. S8†). As the atomic size changes from 0.99 Å (Be), 1.74 Å (Ca) and 2.06 Å (Ba), the bond strength of those compounds becomes weaker, which are confirmed by the COHP values of Fe–Fe, Fe–B, Fe–A and A–B.
Hence, with respect to varying both M and A elements with increasing number of valence electrons and atomic size, the Fermi energy is shifted into the anti-bonding states, leading to instability. This helps to understand the trend observed in Fig. 2, which provide valuable guidance to guide the synthesis of MAB phases by substituting the M/A sites or via forming solid solutions.
3.2 Magnetic properties
K![[n with combining circumflex]](https://www.rsc.org/images/entities/i_char_006e_0302.gif) 1− 1−![[n with combining circumflex]](https://www.rsc.org/images/entities/i_char_006e_0302.gif) 2 = E 2 = E![[n with combining circumflex]](https://www.rsc.org/images/entities/i_char_006e_0302.gif) 1 − E 1 − E![[n with combining circumflex]](https://www.rsc.org/images/entities/i_char_006e_0302.gif) 2, 2, | (1) |
![[n with combining circumflex]](https://www.rsc.org/images/entities/i_char_006e_0302.gif) denotes the total energy with the magnetization direction parallel to
denotes the total energy with the magnetization direction parallel to ![[n with combining circumflex]](https://www.rsc.org/images/entities/i_char_006e_0302.gif) . In the present work, we consider
. In the present work, we consider ![[n with combining circumflex]](https://www.rsc.org/images/entities/i_char_006e_0302.gif) along three crystalline directions, namely, [100], [010] and [001], as MAB compounds have orthorhombic structures (Fig. 1). This leads to three MAEs, i.e., K001–010, K001–100 and K010–100. Fig. 3 shows the MAE with respect to the saturation magnetization (MS), in comparison with the experimentally known permanent magnets. There are in total 71 cases (cf. Table S6 in the ESI†) with the absolute value of at least one MAE greater than 1.0 MJ m−3. For instance, the MAE of Fe2AlB2 has been evaluated by different groups,21,22,26 and our result of −1.14 MJ m−3 is in good agreement with the experimental measurements of −0.9 MJ m−3 at 50 K by Barua22 and theoretical calculation −1.34 MJ m−3 by Ke.26 In the newly predicted compounds, the MAB phase Mn4PtB4 has the largest MAE as 13.498, 11.948 and −1.550 MJ m−3 for K001–010, K001–100 and K010–100. Additionally, the 111-type FePtB shows the largest MAE in non-Mn-containing compounds as −10.646, 7.225 and −3.421 MJ m−3 for K010–100, K001–010 and K001–100, suggesting the b-direction (c-direction) is easy (hard) axis. Based on the dimensionless figure of merit
along three crystalline directions, namely, [100], [010] and [001], as MAB compounds have orthorhombic structures (Fig. 1). This leads to three MAEs, i.e., K001–010, K001–100 and K010–100. Fig. 3 shows the MAE with respect to the saturation magnetization (MS), in comparison with the experimentally known permanent magnets. There are in total 71 cases (cf. Table S6 in the ESI†) with the absolute value of at least one MAE greater than 1.0 MJ m−3. For instance, the MAE of Fe2AlB2 has been evaluated by different groups,21,22,26 and our result of −1.14 MJ m−3 is in good agreement with the experimental measurements of −0.9 MJ m−3 at 50 K by Barua22 and theoretical calculation −1.34 MJ m−3 by Ke.26 In the newly predicted compounds, the MAB phase Mn4PtB4 has the largest MAE as 13.498, 11.948 and −1.550 MJ m−3 for K001–010, K001–100 and K010–100. Additionally, the 111-type FePtB shows the largest MAE in non-Mn-containing compounds as −10.646, 7.225 and −3.421 MJ m−3 for K010–100, K001–010 and K001–100, suggesting the b-direction (c-direction) is easy (hard) axis. Based on the dimensionless figure of merit  ,8 there exist quite a few compounds which can be classified as hard magnets. Particularly, the MAE of such ternary TM borides fill the gap between the widely used low performance magnets (such as AlNiCo and ferrite) and high performance magnets (such as Sm–Co and Nd–Fe–B).
,8 there exist quite a few compounds which can be classified as hard magnets. Particularly, the MAE of such ternary TM borides fill the gap between the widely used low performance magnets (such as AlNiCo and ferrite) and high performance magnets (such as Sm–Co and Nd–Fe–B).
However, not only the absolute values of the MAE but also the sign matters, e.g., the easy axis (direction with the lowest energy) is ideally aligned along a special crystalline axis. For all the MAB compounds, the [001] direction along the stacking direction of the M–B layers (Fig. 1) is chosen, corresponding to the most-probably exposed surfaces for such nano-laminated structures. For the non-MAB phases of the tetragonal space groups, the special axis is chosen to be the axis of 4-fold rotational symmetry, i.e., the [001] direction in Fig. 1(g and i). The MAE for the 322-type compounds (Fig. 1(h)) is overall small thus we do not consider them. Correspondingly, we found 16 MAB and 7 non-MAB phases with a significant out-of-plane MAE (>0.4 MJ m−3), as well as 33 (18) MAB (non-MAB) compounds with a reasonable in-plane MAE (absolute value larger than 0.4 MJ m−3), as listed in Table S7.† Among them, the 322-type MAB compound Mn3Ir2B2 has the largest out-of-plane MAE of 10.17 MJ m−3 for K010–001, and Fe2ReB2 with a large MAE of 9.00 MJ m−3 in K010–001. Interestingly, the MAE value of Fe3Zn2B2 is as large as 3.00 MJ m−3 in K100–001 while its MS is comparable to that of MnAl. It contains no expensive, toxic or critical element, which is a good candidate permanent magnet material. Moreover, Fe7B2 has a sizable MAE 0.681 MJ m−3, which is quite comparable to that of hcp Co. Such a phase is beyond the known binary Fe–B phase diagram,75 which might be synthesizable under non-equilibrium conditions. Last but not least, our results (Fig. 3 and Table S6†) provides reasonable matrix compounds whose MAE can be further enhanced by proper doping to engineer permanent magnets. For instance, our calculations reveal that Fe5PB2 has an MAE of 0.63 MJ m−3 consistent with the experimental measured value of 0.65 MJ m−3,41 whereas a recent work demonstrated that its MAE can be enhanced by substitutionally doping tungsten.76
As discussed above, most compounds with significant MAE contain 5d elements, such as Pt, Ir, and Re. This suggests that the MAE is originated from the enhanced atomic SOC strength for the 5d-shell of such elements. Following Ref. 77, the atomic resolved SOC energy changes are listed in Table S8† for the 111-type FeXB with X = Ni, Pd, and Pt. As the atomic SOC strength increases from 98 meV for Ni, 185 meV for Pd, to 533 meV for Pt,78 the contribution from the X element to the MAE is becoming more significant, as given by the change of atom-resolved SOC energy ΔESOC = ESOC(![[n with combining circumflex]](https://www.rsc.org/images/entities/i_char_006e_0302.gif) 1) − ESOC(
1) − ESOC(![[n with combining circumflex]](https://www.rsc.org/images/entities/i_char_006e_0302.gif) 2). For FeNiB, ΔESOC(Fe) (−0.492 meV per atom in [100]–[010] direction) dominants the total ΔESOC (−0.586 meV per f.u. in [100]–[010] direction) of the compound, as the SOC strength is comparable for Fe (55 meV) and Ni. Furthermore, for FeXB with X = Ni, Pd, and Pt, the ΔESOC of Ni, Pd, and Pt are −0.093, 0.702, and 2.603 meV per atom between two magnetization directions [100] and [010], corresponding to the changes in the total MAE of −0.128, 0.181, and 2.106 meV per atom, respectively. That is, ΔESOC of X has a more dominant contribution to the total ΔESOC and hence the MAE, when moving down the group from 3d to 5d elements. In the FePtB, the contribution of ΔESOC of Pt is 84% in total ΔESOC. Therefore, like FePt,79 the 5d elements have a more significant contribution to the MAE because of enhanced atomic SOC strength, though the magnetic moments on such elements are induced by those of the 3d atoms.
2). For FeNiB, ΔESOC(Fe) (−0.492 meV per atom in [100]–[010] direction) dominants the total ΔESOC (−0.586 meV per f.u. in [100]–[010] direction) of the compound, as the SOC strength is comparable for Fe (55 meV) and Ni. Furthermore, for FeXB with X = Ni, Pd, and Pt, the ΔESOC of Ni, Pd, and Pt are −0.093, 0.702, and 2.603 meV per atom between two magnetization directions [100] and [010], corresponding to the changes in the total MAE of −0.128, 0.181, and 2.106 meV per atom, respectively. That is, ΔESOC of X has a more dominant contribution to the total ΔESOC and hence the MAE, when moving down the group from 3d to 5d elements. In the FePtB, the contribution of ΔESOC of Pt is 84% in total ΔESOC. Therefore, like FePt,79 the 5d elements have a more significant contribution to the MAE because of enhanced atomic SOC strength, though the magnetic moments on such elements are induced by those of the 3d atoms.
 and
and  where P = Anonmag−1·Amag with Anonmag and Amag being the lattice constants of the nonmagnetic and magnetic unit cells. Although there is no direct scaling between ΔS and ΣM, it is suggested that ΣM > 1.5% is a reasonable cutoff to select the promising compounds.51
where P = Anonmag−1·Amag with Anonmag and Amag being the lattice constants of the nonmagnetic and magnetic unit cells. Although there is no direct scaling between ΔS and ΣM, it is suggested that ΣM > 1.5% is a reasonable cutoff to select the promising compounds.51
Fig. 4 shows the 99 potential MCMs with ΣM > 1.5% from 434 compounds with convex hull ΔE < 100 meV per atom. Among them, the reported51ΣM of Fe5SiB2 (2.14%) and Fe2AlB2 (2.05%) are confirmed in our calculations, with the resulting ΣM of 2.03% and 1.96%, respectively. Interestingly, there is positive correlation between the magnetization density and the magnitude of magnetic deformation, i.e., as the magnetic deformation increases, the magnetization of compounds also increase (Fig. 4). It is noted that 82 out of 99 potential MCMs locating at ΣM < 3.5%, and the magnetization concentrating between 500 to 1000 emu cm−3. Particularly, there are four compounds, e.g., Fe5B2 (322-MAB), Fe3Co2B2 (322-MAB), Mn3Co2B2 (322-MAB), and Fe2B (111-MAB), at the upper-right corner, which perform on both large magnetization and magnetic deformation. We suspect such compounds can exhibit significant ΔSM upon second order phase transition at the corresponding Curie temperature, which will be saved for detailed investigation in the future. Additionally, isostructural doping can be applied to improve the magnetocaloric performance80 or to fine tune the magnetostructural or metamagnetic transitions,81 which enables further design of magnetocaloric materials with optimal performance.
Several important aspects on possible MCE in such materials are noteworthy, based on the distributing map with respect to the M and A sites as shown in Fig. S9.† For instance, compounds with Fe and Mn occupying the M-site show a high possibility to posses a large MCE based on the magnetic deformation, which have been confirmed in several reported compounds.63,82,83 Based on the correlations observed in known MCMs in ref. 51, such materials are likely to show a strong magnetocaloric effect and are therefore excellent candidates for experimental study. Moreover, compounds with Mn/Fe/Co, Ru/Rh/Pd and Os/Ir/Pt occupying the A-site also show a high potential to host remarkable magnetocaloric properties. Furthermore, it is noted the fact that Fe2AlB2 is composed entirely of earth-abundant elements. This provides a major advantage at least from a cost and resource point of view, over the competing MCMs that contain expensive critical elements (e.g., Gd, Gd5Si2Ge2, FeRh). Therefore, such economic material without critical elements appears especially appealing to us, and the present system MxAyBz, when A = Al, Zn, Si and Fe should be attracted more attention, such as Fe4AlB4 (2.33%), Fe3AlB4 (2.11%), Fe4SiB4 (2.73%), Fe3ZnB4 (2.42%) and Fe5B2 (Cmmm, 6.56%) (Fig. S9†).
4 Conclusion
In summary, our high-throughput screening on 6 types of MAB phases and 3 types of competing non-MAB phases predict 434 magnetic ternary transition metal borides which are potential candidates for permanent magnets and magnetocaloric materials. After validating the 15 reported compounds, 21 novel compounds are identified to be stable based on the systematic evaluation of thermodynamic, mechanical, and dynamical stabilities, and the number of stable compounds is increased to 434 taking the tolerance of convex hull being 100 meV per atom. It is observed that the magnetic ground state for such compounds with layered structures does not have a strong influence on the thermodynamic stability. The trend of stability for the MAB phase can be understood based on the Hume-Rothery rules, where the size factor difference and the valence electron concentration play a critical role. Such a trend can be further attributed to the bond-resolved COHP, providing intuitive guidance for experimental synthesis. The detailed evaluation of the magnetocrystalline anisotropy energy and the magnetic deformations leads to 23 compounds with significant uniaxial anisotropy (>0.4 MJ m−3) and 99 systems with reasonable magnetic deformation (ΣM > 1.5%). For those compounds containing no expensive, toxic, or critical elements, it is observed that Fe3Zn2B2 is a reasonable candidate as gap permanent magnet, and Fe4AlB4, Fe3AlB4, Fe3ZnB4, and Fe5B2 as potential magnetocaloric materials. This work paves the way for designing more magnetic materials for energy applications. In particular, it also provides a good starting point to search for novel two-dimensional magnetic materials, i.e., MBene, based on detailed evaluation of the exfoliation energy and follow-up experiments.84 At last, the realistic assessment of the predicted potential MAB phases are conducting and will add to our library13,16 soon. All the raw data are freely available in the Novel Materials Discovery (NOMAD) Laboratory.85Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully thank Manuel Richter for providing the SOC strength data and helpful discussions. This work was also supported by the Deutsche Forschungsgemeinschaft – Project-ID 405553726 – TRR 270. Part of this work was supported by the European Research Council (ERC) under the European Union's Horizon 2020 Research and Innovation Program (Grant No. 743116-project Cool Innov). The Lichtenberg high performance computer of the TU Darmstadt is gratefully acknowledged for the computational resources where the calculations were conducted for this project.References
- O. Gutfleisch, M. A. Willard, E. Brück, C. H. Chen, S. Sankar and J. P. Liu, Adv. Mater., 2011, 23, 821–842 CrossRef CAS PubMed.
- K. Skokov and O. Gutfleisch, Scr. Mater., 2018, 154, 289–294 CrossRef CAS.
- J. Coey, Scr. Mater., 2012, 67, 524–529 CrossRef CAS.
- O. Gutfleisch, J. Phys. D: Appl. Phys., 2000, 33, 157 CrossRef.
- S. Sugimoto, J. Phys. D: Appl. Phys., 2011, 44, 064001 CrossRef.
- K. J. Strnat and R. M. Strnat, J. Magn. Magn. Mater., 1991, 100, 38–56 CrossRef CAS.
- J. Herbst and J. Croat, J. Magn. Magn. Mater., 1991, 100, 57–78 CrossRef CAS.
- J. Coey, J. Condens. Matter Phys., 2014, 26, 064211 CrossRef CAS PubMed.
- V. K. Pecharsky and K. A. Gschneidner Jr, Phys. Rev. Lett., 1997, 78, 4494 CrossRef CAS.
- O. Gutfleisch, A. Yan and K.-H. Müller, J. Appl. Phys., 2005, 97, 10M305 CrossRef.
- O. Gutfleisch, T. Gottschall, M. Fries, D. Benke, I. Radulov, K. P. Skokov, H. Wende, M. Gruner, M. Acet and P. Entel, et al. , Philos. Trans. R. Soc., A, 2016, 374, 20150308 CrossRef PubMed.
- F. Scheibel, T. Gottschall, A. Taubel, M. Fries, K. P. Skokov, A. Terwey, W. Keune, K. Ollefs, H. Wende and M. Farle, et al. , Energy Technol., 2018, 6, 1397–1428 CrossRef CAS.
- T. Gottschall, K. P. Skokov, M. Fries, A. Taubel, I. Radulov, F. Scheibel, D. Benke, S. Riegg and O. Gutfleisch, Adv. Energy Mater., 2019, 9, 1901322 CrossRef.
- D. Sander, S. O. Valenzuela, D. Makarov, C. Marrows, E. Fullerton, P. Fischer, J. McCord, P. Vavassori, S. Mangin and P. Pirro, et al. , J. Phys. D: Appl. Phys., 2017, 50, 363001 CrossRef.
- J. Mohapatra and J. P. Liu, Handbook of Magnetic Materials, Elsevier, 2018, vol. 27, pp. 1–57 Search PubMed.
- M. KuzâMin, K. Skokov, H. Jian, I. Radulov and O. Gutfleisch, J. Condens. Matter Phys., 2014, 26, 064205 CrossRef PubMed.
- S. Kota, M. Sokol and M. W. Barsoum, Int. Mater. Rev., 2019, 1–30 Search PubMed.
- M. W. Barsoum, Prog. Solid State Chem., 2000, 28, 201–281 CrossRef CAS.
- A. S. Ingason, M. Dahlqvist and J. Rosén, J. Condens. Matter Phys., 2016, 28, 433003 CrossRef CAS PubMed.
- X. Tan, P. Chai, C. M. Thompson and M. Shatruk, J. Am. Chem. Soc., 2013, 135, 9553–9557 CrossRef CAS PubMed.
- J. Cedervall, M. S. Andersson, T. Sarkar, E. K. Delczeg-Czirjak, L. Bergqvist, T. C. Hansen, P. Beran, P. Nordblad and M. Sahlberg, J. Alloys Compd., 2016, 664, 784–791 CrossRef CAS.
- R. Barua, B. Lejeune, L. Ke, G. Hadjipanayis, E. M. Levin, R. McCallum, M. Kramer and L. Lewis, J. Alloys Compd., 2018, 745, 505–512 CrossRef CAS.
- P. Chai, S. A. Stoian, X. Tan, P. A. Dube and M. Shatruk, J. Solid State Chem., 2015, 224, 52–61 CrossRef CAS.
- K. Kádas, D. Iuşan, J. Hellsvik, J. Cedervall, P. Berastegui, M. Sahlberg, U. Jansson and O. Eriksson, J. Condens. Matter Phys., 2017, 29, 155402 CrossRef PubMed.
- A. El Boukili, N. Tahiri, E. Salmani, H. Ez-Zahraouy, M. Hamedoun, A. Benyoussef, M. Balli and O. Mounkachi, Intermetallics, 2019, 104, 84–89 CrossRef CAS.
- L. Ke, B. N. Harmon and M. J. Kramer, Phys. Rev. B, 2017, 95, 104427 CrossRef.
- H. Zhang, F. Dai, H. Xiang, Z. Zhang and Y. Zhou, J. Mater. Sci. Technol., 2019, 35, 530–534 CrossRef.
- M. Khazaei, J. Wang, M. Estili, A. Ranjbar, S. Suehara, M. Arai, K. Esfarjani and S. Yunoki, Nanoscale, 2019, 11, 11305–11314 RSC.
- N. Miao, J. Wang, Y. Gong, J. Wu, H. Niu, S. Wang, K. Li, A. R. Oganov, T. Tada and H. Hosono, Chem. Mater., 2020, 32, 6947–6957 CrossRef CAS.
- W. Jeitschko, Monatsh. Chem., 1966, 97, 1472–1476 CrossRef CAS.
- W. Jeitschko, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1969, 25, 163–165 CrossRef CAS.
- W. Jung, K. Schweitzer and Z. Kristallogr, Cryst. Mater., 1986, 174, 109–110 Search PubMed.
- N. Chaban and I. Kuz'ma, Izvestiya Akademii Nauk SSSR, Neorganicheskie Materialy, 1973, 9, 1908–1911 Search PubMed.
- M. Ade and H. Hillebrecht, Inorg. Chem., 2015, 54, 6122–6135 CrossRef CAS PubMed.
- L. Häggström, R. Wäppling, T. Ericsson, Y. Andersson and S. Rundqvist, J. Solid State Chem., 1975, 13, 84–91 CrossRef.
- S. Hirt, F. Hilfinger and H. Hillebrecht, Z. Kristallogr. - Cryst. Mater., 2018, 233, 295–307 CrossRef CAS.
- J. Cedervall, S. Kontos, T. C. Hansen, O. Balmes, F. J. Martinez-Casado, Z. Matej, P. Beran, P. Svedlindh, K. Gunnarsson and M. Sahlberg, J. Solid State Chem., 2016, 235, 113–118 CrossRef CAS.
- M. A. McGuire and D. S. Parker, J. Appl. Phys., 2015, 118, 163903 CrossRef.
- M. Werwiński, S. Kontos, K. Gunnarsson, P. Svedlindh, J. Cedervall, V. Höglin, M. Sahlberg, A. Edström, O. Eriksson and J. Rusz, Phys. Rev. B, 2016, 93, 174412 CrossRef.
- T. N. Lamichhane, V. Taufour, S. Thimmaiah, D. S. Parker, S. L. Bud’ko and P. C. Canfield, J. Magn. Magn. Mater., 2016, 401, 525–531 CrossRef CAS.
- D. Hedlund, J. Cedervall, A. Edström, M. Werwiński, S. Kontos, O. Eriksson, J. Rusz, P. Svedlindh, M. Sahlberg and K. Gunnarsson, Phys. Rev. B, 2017, 96, 094433 CrossRef.
- I. Opahle, G. K. Madsen and R. Drautz, Phys. Chem. Chem. Phys., 2012, 14, 16197–16202 RSC.
- I. Opahle, A. Parma, E. J. McEniry, R. Drautz and G. K. H. Madsen, New J. Phys., 2013, 15, 105010 CrossRef.
- Q. Gao, I. Opahle, O. Gutfleisch and H. Zhang, Acta Mater., 2020, 186, 355–362 CrossRef CAS.
- D. Ohmer, G. Qiang, I. Opahle, H. K. Singh and H. Zhang, Phys. Rev. Mater., 2019, 3, 053803 CrossRef CAS.
- H. K. Singh, Z. Zhang, I. Opahle, D. Ohmer, Y. Yao and H. Zhang, Chem. Mater., 2018, 30, 6983–6991 CrossRef CAS.
- J. E. Saal, S. Kirklin, M. Aykol, B. Meredig and C. Wolverton, JOM, 2013, 65, 1501–1509 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 CrossRef CAS PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758 CrossRef CAS.
- K. Koepernik and H. Eschrig, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1743 CrossRef CAS.
- J. D. Bocarsly, E. E. Levin, C. A. Garcia, K. Schwennicke, S. D. Wilson and R. Seshadri, Chem. Mater., 2017, 29, 1613–1622 CrossRef CAS.
- S. Kirklin, J. E. Saal, B. Meredig, A. Thompson, J. W. Doak, M. Aykol, S. Rühl and C. Wolverton, npj Comput. Mater., 2015, 1, 15010 CrossRef CAS.
- M. Werwiński, A. Edström, J. Rusz, D. Hedlund, K. Gunnarsson, P. Svedlindh, J. Cedervall and M. Sahlberg, Phys. Rev. B, 2018, 98, 214431 CrossRef.
- I. A. Zhuravlev, V. P. Antropov and K. D. Belashchenko, Phys. Rev. Lett., 2015, 115, 217201 CrossRef CAS PubMed.
- P. Oppeneer, Handbook of Magnetic Materials, Elsevier, Amsterdam, 2001, vol. 13 Search PubMed.
- X.-H. Li, C.-H. Xing, H.-L. Cui and R.-Z. Zhang, J. Phys. Chem. Solids, 2019, 126, 65–71 CrossRef CAS.
- Y. Zhou, H. Xiang, F. Dai and Z. Feng, Mater. Res. Lett., 2017, 5, 440–448 CrossRef CAS.
- J. Cedervall, E. Nonnet, D. Hedlund, L. HaÌggstroÌm, T. Ericsson, M. WerwinÌski, A. EdstroÌm, J. Rusz, P. Svedlindh and K. Gunnarsson, et al. , Inorg. Chem., 2018, 57, 777–784 CrossRef CAS PubMed.
- S. Rundqvist, et al. , Acta Chem. Scand., 1962, 16, 1–19 CrossRef CAS.
- C. Bormio-Nunes, C. A. Nunes, A. A. Coelho, M. I. S. T. Faria, P. A. Suzuki and G. C. Coelho, J. Alloys Compd., 2010, 508, 5–8 CrossRef CAS.
- H. Baurecht, H. Boller and H. Nowotny, Monatsh. Chem., 1971, 102, 373–384 CrossRef CAS.
- C. Guy and A. Uraz, J. Less-Common Met., 1976, 48, 199–203 CrossRef CAS.
- Z. Xie, D. Geng and Z. Zhang, Appl. Phys. Lett., 2010, 97, 202504 CrossRef.
- X. Wang, R. Xiao, H. Li and L. Chen, Phys. Rev. Lett., 2017, 118, 195901 CrossRef PubMed.
- A. Jain, S. P. Ong, G. Hautier, W. Chen, W. D. Richards, S. Dacek, S. Cholia, D. Gunter, D. Skinner, G. Ceder and K. a. Persson, APL Mater., 2013, 1, 011002 CrossRef.
- I. Opahle, H. K. Singh, J. Zemen, O. Gutfleisch and H. Zhang, 2020, arXiv preprint arXiv:2001.00959.
- D. Potashnikov, E. Caspi, A. Pesach, A. Hoser, S. Kota, L. Verger, M. Barsoum, I. Felner, A. Keren and O. Rivin, J. Magn. Magn. Mater., 2019, 471, 468–474 CrossRef CAS.
- J. Cedervall, M. S. Andersson, D. Iuşan, E. K. Delczeg-Czirjak, U. Jansson, P. Nordblad and M. Sahlberg, J. Magn. Magn. Mater., 2019, 482, 54–60 CrossRef CAS.
- F. Mouhat and F.-X. Coudert, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 224104 CrossRef.
- Y. Bai, X. Qi, X. He, D. Sun, F. Kong, Y. Zheng, R. Wang and A. I. Duff, J. Am. Chem. Soc., 2019, 102, 3715–3727 CAS.
- U. Mizutani, MRS Bull., 2012, 37, 169 CrossRef.
- Y. Zhang, Z. Mao, Q. Han, Y. Li, M. Li, S. Du, Z. Chai and Q. Huang, Materialia, 2020, 12, 100810 CrossRef CAS.
- S. Maintz, V. L. Deringer, A. L. Tchougréeff and R. Dronskowski, J. Comput. Chem., 2016, 37, 1030–1035 CrossRef CAS PubMed.
- P. Bruno, Phys. Rev. B: Condens. Matter Mater. Phys., 1989, 39, 865 CrossRef.
- B. Hallemans, P. Wollants and J. Roos, Z. Metallkd., 1994, 85, 676–682 CAS.
- J. Thakur, P. Rani, M. Tomar, V. Gupta and M. K. Kashyap, AIP Conf. Proc., 2019, p. 020012 Search PubMed.
- V. Antropov, L. Ke and D. Åberg, Solid State Commun., 2014, 194, 35–38 CrossRef CAS.
- M. Richter, J. Phys. D: Appl. Phys., 1998, 31, 1017 CrossRef CAS.
- G. Daalderop, P. Kelly and M. Schuurmans, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 44, 12054 CrossRef.
- C. A. Garcia, J. D. Bocarsly and R. Seshadri, Phys. Rev. Mater., 2020, 4, 024402 CrossRef CAS.
- J. A. Arregi, M. Horkỳ, K. Fabianová, R. Tolley, E. E. Fullerton and V. Uhlíř, J. Phys. D: Appl. Phys., 2018, 51, 105001 CrossRef.
- P. Arora, M. Chattopadhyay and S. Roy, Appl. Phys. Lett., 2007, 91, 062508 CrossRef.
- M. Yu, L. Lewis and A. Moodenbaugh, J. Appl. Phys., 2003, 93, 10128–10130 CrossRef CAS.
- L. T. Alameda, P. Moradifar, Z. P. Metzger, N. Alem and R. E. Schaak, J. Am. Chem. Soc., 2018, 140, 8833–8840 CrossRef CAS.
- N. N. M. Discovery, (please search for the data using the author name “Chen Shen”), https://repository.nomad-coe.eu.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ta11026d |
| This journal is © The Royal Society of Chemistry 2021 |