Open Access Article
Open Access ArticleHow oxidation state and lattice distortion influence the oxygen evolution activity in acid of iridium double perovskites†
María
Retuerto
*a,
Laura
Pascual
b,
Oriol
Piqué
c,
Paula
Kayser
d,
Mohamed Abdel
Salam
e,
Mohamed
Mokhtar
 e,
José Antonio
Alonso
e,
José Antonio
Alonso
 d,
Miguel
Peña
a,
Federico
Calle-Vallejo
d,
Miguel
Peña
a,
Federico
Calle-Vallejo
 c and
Sergio
Rojas
c and
Sergio
Rojas
 *a
*a
aGrupo de Energía y Química Sostenibles, Instituto de Catálisis y Petroleoquímica, CSIC, C/Marie Curie 2, 28049, Madrid, Spain. E-mail: m.retuerto@csic.es; srojas@icp.csic.es
bInstituto de Catálisis y Petroleoquímica, CSIC, C/Marie Curie 2, 28049, Madrid, Spain
cDepartament de Ciència de Materials i Química Física, Institut de Química Teòrica i Computacional (IQTCUB), Universitat de Barcelona, Martí i Franquès 1, 08028 Barcelona, Spain
dInstituto de Ciencia de Materiales de Madrid, CSIC, C/ Sor Juana Inés de la Cruz 3, 28049 Madrid, Spain
eDepartment of Chemistry, Faculty of Science, King Abdulaziz University, P.O Box 80200, Jeddah 21589, Saudi Arabia
First published on 7th January 2021
Abstract
In view of iridium's scarceness and high cost, Ir-containing catalysts for water electrolyzers should have low loadings and maximal utilization. Here, we studied low-Ir-content, highly active, double perovskites (Sr2MIrO6, M = Ni, Co, Sc and Fe) for the oxygen evolution reaction (OER) in acid combining electrochemical experiments, DFT, and advanced characterization techniques. The initial OER performance depends on Ir's oxidation state and the geometric features of IrO6 frameworks, which are tuned by the choice of M. Higher oxidation states, particularly Ir6+, enhance the OER activity: Sr2NiIrO6 and Sr2CoIrO6 display potentials of ∼1.53 V at 10 mA cm−2, comparable to the best Ir-based catalysts in the literature. However, because of their less symmetric structures, perovskites with Ir6+ are less stable, prone to surface reconstruction and their cations leach under OER conditions. These results show that improved iridium-based OER electrocatalysts in acid can be designed by balancing their activity and stability.
1. Introduction
Hydrogen is a versatile energy carrier, capable of paving the way for the energy transition toward a decarbonized economy. It can enable the penetration of renewable electricity in sectors conventionally coupled to fossil fuels such as transportation, heat, (petro)chemicals, iron and steel, thereby substantially reducing carbon emissions.1,2 Polymer Membrane Water Electrolyzers (PEMWE) are among the most suitable technologies to accumulate the surplus of renewable energy into H–H chemical bonds.3 Because PEMWEs are versatile, have fast-response and are modular devices able to work at high current densities to yield high-purity hydrogen, they are suitable to cope with the intrinsic intermittency of renewable energy.4Water electrolysis comprises two half reactions: the formation of hydrogen at the cathode (in acid: 4H+ + 4e− → 2H2, hydrogen evolution reaction, HER) and the formation of oxygen at the anode (in acid: 2H2O → O2 + 4H+ + 4e−, oxygen evolution reaction, OER).5 In acidic media, the overall process is limited by the sluggish kinetics of the OER6 and the highly corrosive environment associated with water electrolysis. As a consequence, iridium- and ruthenium-based oxides are the state-of-the-art catalysts for the OER.4 Ru-based oxides typically display the lowest OER overpotentials; however, their stability in acid is insufficient.7 Therefore, benchmark catalysts are based on IrO2 and IrO2/TiO2,8,9 yielding current densities of 10 mA cm−2 at ∼1.52 V and mass activities of ∼30–50 A g−1 at 1.5 V.10–12
Since Ir is one of the rarest, ill-distributed and most expensive elements on the Earth's crust, traded in a volatile market where slight fluctuations in demand can significantly impact supply,13 it is necessary to make the best possible utilization of it. In this sense, mixed oxides are promising candidates to replace pure iridium oxides as electrocatalysts for the OER.14–17 For instance, the mass percentage of Ir in IrO2 is 86 wt%, and goes down to 59 wt% in SrIrO3 and 36–38 wt% in Sr2MIrO6, if M is a first-row transition metal. To the best of our knowledge, the iridium mixed oxides with OER activity in acid electrolyte reported so far are: (i) Ir-based perovskites such as SrIrO3/IrOx thin films,18,19 SrCo0.9Ir0.1O3,20 Ba2RIrO6 (R = Y, La, Ce, Pr, Nd, Tb),21,22 La2LiIrO6,23 6H-SrIrO3,24 Ir–SrTiO3,25 Sr2FeIrO6 and Sr2CoIrO6,26 (ii) Ir–pyrochlores such as Bi2Ir2O7, Pb2Ir2O6.5,27 Pr2Ir2O7,28 Y2Ir2O7,29 (iii) spinels such as Ni0.34Co0.46Ir0.2O4,30 and (iv) Ruddlesden–Popper phases such as Sr2IrO4 and Sr4IrO6.31
Looking at the performance of the aforementioned compounds, we hypothesize that the OER activity of Ir mixed oxides is related to the actual environment of Ir cations in such oxides. In other words, the OER activity is likely affected by the nature of the foreign metal(s) and the actual location of the cations in the oxides. However, the roles of the foreign metals, the crystal structure including conformation, the distortions, the bond strength of the IrO6 octahedra (Oh), and that of Ir's oxidation state on the OER performance of Ir mixed oxides, are not fully understood. Evidently, this knowledge void prevents the rational design of advanced Ir-based OER electrocatalysts.
A number of studies suggest a direct relationship between the environment and oxidation state of Ir in its oxides and their OER activity.25,30 The most usual Ir oxidation states in oxides are +3 and +4, as in Sr2TaIrO6, Sr2NbIrO6,32 and Sr2TiIrO6.33 However, such perovskites present cationic disorder with Ta, Nb or Ti and Ir randomly occupying the same crystallographic position, thereby preventing the study of the actual role of Ir's environment for the OER. A recent study illustrated the relevance of upper iridium oxides for the OER.22 Oxides with Ir5+ or Ir6+ are less common; however, double perovskites with Ir cations in upper oxidation states have been reported, for instance, Ba2RIrO6 (R = Ce, Tb, Y, La, Pr, Nd),21 La2LiIrO6,23 Sr2(Fe,Co)IrO6 (ref. 26) and Sr2(Ca, Mg)IrO6.34 These double perovskites exhibit cationic ordering between R, Li, Fe, Co, Ca or Mg and Ir; being Ir cations located in an independent crystallographic position than the other metals, which allows studying the impact of Ir's environment for the OER.
The suitability of OER electrocatalysts should be assessed holistically by studying their catalytic performance, including activity, durability and stability. It is well known that the activity depends on the oxygen adsorption strength of the active sites. This strength depends on several factors, such as the position of the oxygen's p-band with respect to the Fermi level, which determines the hybridization between M d orbitals and O 2p orbitals.35 In turn, the degree of hybridization is tuned by the transition metal oxidation state, the number of d electrons and the electronegativity of the metal ions.36,37 Another essential parameter to evaluate the quality of an OER catalyst is its durability. Several factors concerning the stability of mixed oxides at the harsh conditions of the OER in acid media should be considered. The first factor is the distortion of the crystal structure. For perovskites, large size differences between the cations in the A and B positions usually implies large deviations from the cubic symmetry and a marked destabilization of the mixed oxide structure under the high potentials of the OER.36 The second factor is the leaching of the non-noble components of the perovskite into the electrolyte, leading to severe reconstruction of the catalyst during the OER along with the formation of amorphous IrOx/IrOOH phases at the catalyst surface. In some cases, the reconstruction is fast and happens after only few OER cycles, or even upon immersion in the electrolyte, before the commencement of the OER experiments.20,22,23,38
In this work, we aim at establishing sound correlations between representative structural parameters of Ir perovskites, namely IrO6 Oh distortion and Ir oxidation state, and the strength of the metal–adsorbate bonds. Furthermore, we assess the effects of those structural and energetic parameters on the OER performance. We carefully tune the environment and oxidation state of Ir in double perovskites, Sr2MIrO6, by changing the nature of the M cations (M = Ni, Co, Sc, Fe). This strategy allows us to find correlations between a variety of parameters, the initial OER activity, and the stability of Sr2MIrO6, all of which provide insights for the design of improved Ir-based electrocatalysts.
2. Experimental section
2.1 Synthesis of Sr2MIrO6 perovskites
The synthesis of Sr2MIrO6 (M = Ni, Co, Sc, Fe) is detailed elsewhere.33,39 Briefly, the samples were prepared by sol–gel methods. We used a solution of citric acid and HNO3 to dissolve the starting materials. IrO2 was not dissolved but stayed in suspension under magnetic stirring. The suspension was slowly evaporated leading to organic resins containing a homogeneous distribution of the involved cations. After evaporation, the resins were dried at 140 °C and heated at 600 °C for 12 h to decompose the organic materials and nitrates. Next, Sr2NiIrO6 was annealed at 900 °C for 12 h in O2 flow and subsequently at 900 °C under 200 bar of O2 for 48 h, to obtain the Ir6+ perovskite oxide. For Sr2MIrO6 (M = Co, Sc, Fe) oxides the reactive precursors were heated in air at 1100 °C for 12 h.2.2 Physicochemical characterization
The phase identification and purity of the obtained perovskites was determined by X-ray powder diffraction (XRD) in the Bragg–Brentano reflection geometry with CuKα radiation (λ = 1.5418 Å). A detailed study of the crystal structure using Powder Neutron Diffraction (PND) and the analysis of Ir oxidation states and coordination environments using X-ray Absorption Spectroscopy (XAS) is available in the literature.33,39Transmission Electron Microscopy (TEM), high-resolution electron microscopy (HRTEM) and X-ray energy dispersive spectra (EDX) were recorded in a 200 kV (JEOL 2100F) field emission gun transmission electron microscope equipped with an EDX X-Max 80 spectrometer (Oxford Instruments). The specimens were prepared by depositing aliquots onto a Cu grid supporting a lacey carbon film.
X-ray photoelectron spectra (XPS) were acquired with a VG ESCALAB 200 R at a pass energy of 50 eV using a Mg Kα X-ray source. The kinetic energies of the photoelectrons were measured using a hemispherical electron analyser working in the constant-pass energy mode. A background pressure below 3 × 10−8 mbar was kept in the chamber during data acquisition. The binding energies were determined by setting the C 1s peak at 284.6 eV. 200 scans in increments of 0.1 eV with dwell times of 50 ms were collected in order to increase the signal-to-noise ratio.
2.3 Electrochemical characterization
An Autolab PGstat 302N potentiostat/galvanostat was used to test the electrochemical performance of the oxides. The measurements were performed using a standard three-compartment glass cell and a rotating disk electrode (RDE) (Pine Research Instruments). A graphite bar was used as counter electrode. An Ag/AgCl electrode was used as reference electrode. The perovskites were deposited as an ink on a 0.196 cm2 glassy carbon electrode. The ink was prepared by mixing 5 mg of oxide with 1 mg of carbon black (Vulcan-XC-72R) to improve the electrical conductivity. The mixture was dispersed in tetrahydrofuran (THF) and 5% Nafion and sonicated using an Ultrasonic Processor UP50H (Hielscher). The composition of the ink was 5 mgoxide, 1 mgvulcan, 0.03 mLNafion and 0.97 mLTHF. 10 μL of the ink were dropped onto the electrode to obtain a catalyst loading of 0.255 mgoxide cm−2, or ∼0.09 mgIr cm−2, depending on the oxide.The OER catalytic performance of the perovskites was evaluated by recording linear sweep voltammograms between 1.1 and 1.7 V at 10 mV s−1. The measurements were performed in an O2 saturated 0.1 M HClO4 electrolyte to assure the O2/H2O equilibrium at 1.23 V, at a rotation rate of 1600 rpm. The OER kinetic curves were capacitance-corrected by using the average of the anodic and cathodic curves and iR-corrected by using the formula E − iRcorrected = Eapplied − iR, where i is the current and R is the uncompensated resistance (R ∼ 29 Ω) as obtained from Electrical Impedance Spectroscopy (EIS) at open voltage.
Durability tests were performed by recording 50 consecutive OER cycles at 10 mV s−1 between 1.2 and 1.7 V vs. RHE, or by chronoamperometric tests by fixing the initial potential for each catalyst to the potential needed to reach 10 mA cm−2 and monitoring the evolution of the current density with time. The durability of Sr2NiIrO6 and Sr2CoIrO6 was further tested by recording 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 consecutive cycles between 1.2 and 1.7 V at 50 mV s−1.
000 consecutive cycles between 1.2 and 1.7 V at 50 mV s−1.
2.4 Computational details
The VASP code was used to perform the DFT calculations,40 RPBE was the chosen exchange-correlation functional,41 and the projector augmented-wave (PAW) method was used to describe the effect of the inner cores on the valence electron density.42 The free energies of reaction were approximated as ΔG ≈ ΔEDFT + ΔZPE − TΔS, where ΔEDFT is the DFT-calculated reaction energy and ΔZPE is the zero-point energy change between reactants and products. The ZPEs were obtained from vibrational frequency calculations using the harmonic approximation. As usual,43,44 the term TΔS includes the total entropies of the molecules involved in the OER, and neglects those of the adsorbates. The computational hydrogen electrode (CHE) was used to model proton–electron pairs.45 We assumed the following catalytic pathway for the OER:43,44| * + H2O → *OH + H+ + e− | (1) |
| *OH → *O + H+ + e− | (2) |
| *O + H2O → *OOH + H+ + e− | (3) |
| *OOH → * + O2 + H+ + e− | (4) |
Within the CHE framework, the free energies of reaction can be written as a function of the adsorption energies of the three adsorbed intermediates, namely *O, *OH, and *OOH:
| ΔG1 = ΔGOH | (5) |
| ΔG2 = ΔGO − ΔGOH | (6) |
| ΔG3 = ΔGOOH − ΔGO | (7) |
| ΔG4 = ΔGO2 − ΔGOOH | (8) |
| ηOER = max(ΔG1,ΔG2,ΔG3,ΔG4)/e− − 1.23 V | (9) |
As shown in previous publications,46,47 the concept of electrocatalytic symmetry as expressed by the electrochemical-step symmetry index (ESSI) is a good descriptor for the OER overpotential:
 | (10) |
A full description of δ–ε optimization can be found elsewhere.47 Briefly, the method is based on the introduction of two parameters into the adsorption energies: (I) δ, which is scaling-based and affects *O, *OH and *OOH, and (II) ε, which is scaling-free and affects *OOH only. Both parameters are in units of energy. Normally, reasonable values for δ are in the range [−0.3, 0.3] eV, whereas those of ε are in the range [−0.3, 0] eV. δ is either a weakening or strengthening of the adsorption energies, while ε is only a strengthening. See Section S2 of the ESI† for more details and the specific values obtained for the double perovskites containing Ni and Co.
We note that there are known inaccuracies of DFT for the OER modelling of some oxides e.g. RuO2,48,49 and some solutions have been proposed.48,50,51 In particular, the inaccuracies of DFT observed before for SrIrO3 (ref. 19) were corrected for in this study by using thick slabs. For further information on the computational details please refer to Section S1 of the ESI.†
3. Results and discussion
3.1 Double-perovskite structure and composition
The incorporation of different M metals into Sr2MIrO6 is expected to result in materials with different structural and chemical features, namely unit-cell volume, Ir oxidation state, Ir–O and M–O bond lengths, structural distortions, etc. In the following, we intend to disclose the effect of those features on the OER performance of iridium mixed oxides. Fig. S1 in the ESI† shows the XRD diffractograms for Sr2MIrO6 (M = Ni, Co, Sc and Fe) and the evolution of their unit-cell volumes. All the oxides are pure and exhibit the perovskite crystal structure. The volume of the unit cell increases with M in the order Co3+ < Fe3+ < Ni2+ < Sc3+, as expected from their ionic radii.52 Powder Neutron Diffraction (PND) data reveal that all perovskites have monoclinic symmetry with unit-cell parameters a ≠ b ≠ c and α = γ = 90° ≠ β, in the space groups P21/n (a−a−c+ tilting, Glazer notation) for Ni and Sc oxides, and I2/m (a0b−b− tilting) for Co and Fe oxides.33,39 Both P21/n and I2/m symmetries are characteristic of distorted double perovskites with alternating MO6 and IrO6 complexes along the three crystallographic directions in a long-range Oh M/Ir ordering.53,54Oxygen vacancies can play a key role in the OER activity of oxides. Therefore, the presence or absence of oxygen vacancies in the double perovskites was assessed by means of PND. This technique is one of the most reliable ones to evaluate the occupancy of light atoms such as oxygen in oxides.55 The refinement of the crystal structures reveals the absence of oxygen vacancies in Sr2MIrO6, irrespectively of the atom in the M site. PND data also reveal a stoichiometric composition of the catalysts in the bulk.33,39 The most relevant crystallographic features obtained from PND are detailed in Table S2,† for instance, oxygen vacancies, Ir oxidation state and 〈Ir–O〉 distances. The surface composition of Sr2MIrO6 was studied by XPS, providing a M/Ir ratio close to the nominal value of 1 in all samples. Except for Sr2NiIrO6, the surface is enriched in Sr in all of the studied oxides, showing Sr/Ir surface atomic ratios in the range of 1.9 to 3.1. This observation is in line with previous reports on Sr-containing perovskites, where it was reported that SrCO3 forms at the surface.56
The oxidation state of M and Ir was studied by XAS and bond-valence sum (BVS) calculations using Brown's model with PND data.33,39,57 XAS data indicate that Sr2NiIrO6 perovskites contain Ni2+ and Ir6+ cations, whereas the oxidation states in Sr2CoIrO6, Sr2ScIrO6 and Sr2FeIrO6 are close to M3+ and Ir5+. However, it should be noted that Irn+ sites are slightly more oxidized in Sr2CoIrO6 than in Sr2FeIrO6. BVS gives another estimation of the oxidation states using the bond distances calculated from PND, and the results are reliable because the following two conditions were satisfied: (i) lack of disorder between M and Ir cations in both Oh sites, and (ii) absence of oxygen vacancies. The oxidation states obtained using BVS are in good agreement with XAS results, i.e., Fe3+, Sc3+ along with the corresponding Ir5+ oxidation state; mixed valence Co3+/Co2+ (∼+2.3) with Ir5+/Ir6+ (∼+5.7); and finally, Ni2+ with Ir6+.
The oxidation state of Ir (and M) at the surface of the perovskites was obtained from XPS. As shown in Fig. S2 in the ESI,† the Ir 4f region can be fitted into two doublets, with 4f7/2 binding energies at ca. 62.5 and 64.5 eV, in good agreement with the XPS analyses of similar Ir perovskites.18,20,58 It is known that the binding energy of Ir 4f7/2 in the metallic state appears around 60.33 eV,59 and increases to values close to 61.8 eV for oxidized Ir (IrO2).60 All of the Sr2MIrO6 oxides have the main Ir 4f7/2 component at binding energies ranging from 62.3 to 62.9 eV, the actual value depending on M. These high binding energies indicate that the double perovskites contain Ir species in oxidation states higher than Ir4+. Similar binding energies were reported for Ba2PrIrO6 and La2LiIrO6, and ascribed to the presence of Ir5+.22,23 In agreement with previous works, we believe that it is not possible to obtain the precise oxidation state of the cations at the surface of perovskites based only on XPS data,22 but the results provided here suffice to conclude that the Ir species at the surface of Sr2MIrO6 perovskites have oxidation states higher than +4. In turn, the oxidation states of Ir determine the strength of Ir–O lattice bonds in each compound, and consequently the strength of Ir–adsorbed oxygen bonds, which is a key parameter for describing the OER performance.
3.2 Initial OER activity: effect of M in the catalytic performance
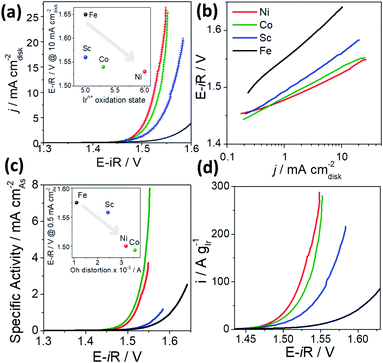
Fig. 1a shows the catalytic activity for the OER of Sr2MIrO6 (M = Ni, Co, Sc and Fe) in terms of iR-corrected current densities, normalized to the geometric area of the electrodes (0.196 cm2). The non iR-corrected curves and the R obtained from EIS at open-circuit voltage are shown in Fig. S3 and S4 in the ESI.†The current density decreases with M as Ni2+ > Co3+ > Sc3+ > Fe3+. As shown in Table 1, the current densities reached at 1.5 V are 3.3, 2.6, 1.3, and 0.3 mA cmdisk−2 for Sr2NiIrO6, Sr2CoIrO6, Sr2ScIrO6 and Sr2FeIrO6, respectively. The trends can also be observed in the Tafel plots in Fig. 1b.
| Catalyst | E − iR/V@10 mA cm−2 | j/mA cm−2@1.5 V | Tafel slope/mV dec−1 | Ir/%wt | i M AgIr−1@1.525 V | Durability tests |
|---|---|---|---|---|---|---|
| Sr2NiIrO6 | 1.525 | 3.3 | 48 | 37 | 115 | 1 h@10 mA cm−2 |
| Sr2CoIrO6 | 1.535 | 2.6 | 52 | 37 | 73 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles bt. 1.2–1.7 V@50 mV s−1 000 cycles bt. 1.2–1.7 V@50 mV s−1 |
| Sr2ScIrO6 | 1.56 | 1.3 | 67 | 38 | 35 | 1 h@10 mA cm−2 |
| Sr2FeIrO6 | 1.65 | 0.3 | 90 | 37 | 5.6 | 1 h@10 mA cm−2 |
| SrTi0.67Ir0.33O3 (ref. 25) | 1.477 | 24 | 45–80 | 27 | 820 | 20 h@10 mA cm−2 |
| 6H-SrIrO3 (ref. 24) | 1.478 | 12 | 60–120 | 58.6 | 74.7 | 30 h@10 mA cm−2 |
| Pr2Ir2O7 (ref. 28) | 1.52 | 4.5 | 50 | 49 | 351 | 1000 cycles bt. 1–1.55 V@50 mV s−1 |
| SrCo0.9Ir0.1O3−δ (ref. 20) | 1.55 | 3.0 | 40–70 | 10 | 3 h@10 mA cm−2 | |
| La2LiIrO6 (ref. 23) | 1.53 | 0.7 | 50 | 33.5 | 33.5 | 50 cycles bt. 1.1–1.7 V@10 mV s−1 |
| Ba2NdIrO6 (ref. 21) | 1.60 | 0.7 | 60–130 | 27 | 1 h@10 mA cm−2 | |
| Ni0.34Co0.46Ir0.2O4 (ref. 30) | 1.51 | ∼9 | 40 | 34 | 5.5 h@10 mA cm−2 | |
| Sr2IrO4 (ref. 31) | 1.516 | ∼5 | 45 | 44.5 | 392@1.55 V | 6 h@10 mA cm−2 |
| Sr4IrO6 (ref. 31) | 1.517 | ∼5 | 50 | 30.1 | 274@1.55 V | 6 h@10 mA cm−2 |
| IrO2 (ref. 23) | 1.56 | 0.01 | 50 | 86 | 24.5 |
Taking the potential to achieve 10 mA cm−2 as an RDE-based metric to benchmark catalysts, as suggested by Jaramillo et al.,61 we observe that Sr2NiIrO6 records the least positive potential of 1.525 V, followed by Sr2CoIrO6 and Sr2ScIrO6, with potentials of 1.535 and 1.560 V, respectively. Clearly, Sr2NiIrO6 and Sr2CoIrO6 have similar current densities; however, Sr2ScIrO6 and Sr2FeIrO6 are less active, suggesting that M plays an important role in the OER performance of Sr2MIrO6. Sr2FeIrO6 records the lowest performance in the whole polarization range, showing a potential of 1.650 V to achieve 10 mA cm−2. Similar OER activity trends have been reported for Sr2CoIrO6 and Sr2FeIrO6, showing that Co-based catalysts are more active than Fe-based catalysts and that the initial activity is related to the mixed oxide structure.26
In Table 1 we compare the electrochemical performance of the perovskites under study with state-of-the-art Ir mixed oxides. Sr2NiIrO6, Sr2CoIrO6 and Sr2ScIrO6 display comparable or higher activity than the best Ir mixed oxides in the literature for the OER in acid media, including SrCo0.9Ir0.1O3−δ,20 Pr2Ir2O7,27 Ba2LIrO6 (L = Y, La, Ce, Pr, Nd, Tb),21 and La2LiIrO6.23 Sr2NiIrO6 and Sr2CoIrO6 are only slightly less active than SrTi0.67Ir0.33O3 and 6H-SrIrO3, which have a potential of ∼1.47 V at 10 mA cm−2.24,25 In all cases, it is assumed that Ir sites are the main active sites for the OER, since the overpotentials achieved by Ir mixed oxides are comparable to Ir–O phases.8,62
The Tafel slopes obtained at low current densities are also shown in Table 1. The Tafel slopes for Sr2NiIrO6 (48 mV dec−1) and Sr2CoIrO6 (52 mV dec−1) are in line with the values reported for the best Ir mixed oxides. Indeed, most Ir- and Ru-based catalysts, including IrO2 and RuO2, display Tafel slopes between 40 and 60 mV dec−1.63
The initial OER activities were first correlated with the oxidation state of the Ir cations in each catalyst (Fig. 1a, inset). The trends in Fig. 1a reveal that the OER activity increases alongside the oxidation state of Ir in the double perovskites. Specifically, Sr2NiIrO6 with Ir6+ cations displays the highest OER activity, followed by Sr2CoIrO6 with Ir6+/5+. The perovskites with Ir5+ cations (Sr2ScIrO6 and Sr2FeIrO6) are the least active ones in the series. The importance of Ir's oxidation state for the OER performance was observed previously in the La2LiIrO6 double perovskite.23 It was claimed that Ir5+O6 Oh are not active for the OER, so that a previous activation step oxidizing Ir5+ to Ir6+ is needed to make Ir5+-mixed oxides active. This suggests that the higher OER activity of Sr2NiIrO6 and Sr2CoIrO6 phases could be related to the presence of Ir6+ cations. We note that Ir6+–O bonds are shorter and, therefore, more covalent than Ir5+–O bonds. In addition, it has been observed for metals, monoxides and several families of perovskites that, as the oxidation state of the metal centers increases, the adsorption energies of *O, *OH and *OOH become more positive, that is, weaker.37,64 Hence, we conclude that more covalent Ir–O lattice bonds are concomitant with weaker Ir–*O bonds. It is worth noting here that in a Sabatier-type analysis, the weakening of metal–adsorbate bonds is generally beneficial for the OER when the compounds are initially located on the strong-binding (left) side of a volcano activity plot and until a limit, namely, the top of the volcano. Beyond such limit, the bonds start being undesirably weak, as discussed in the next section.
The specific OER activity of the perovskites was assessed by normalizing the OER activity by the compounds' active surface area (Fig. 1c). The surface area can be calculated by several methods such as BET or electrochemical methods (ECSA). In the case of mixed oxides, in view of their usually low surface areas, it is a common practice to normalize the activity by the mass specific surface area (AS) calculated from the diameter data of TEM particles (Section S9, ESI†).14,65 The determination of AS (eqn (S5) in the ESI†) along with a comparison between AS, BET and ECSA values is provided in Section S9 in the ESI (Table S3†). The AS values obtained for the perovskites with M = Ni, Co, Sc and Fe are 2.9, 1.3, 6.8 and 1.6 m2 g−1, respectively. The different AS values obtained are in line with the different particle sizes measured, which are 276 ± 29 nm for Ni catalyst, 519 ± 152 nm for Co, 111 ± 33 nm for Sc and 376 ± 110 nm for Fe catalyst.
As observed in Fig. 1c, the specific activities follow a similar trend compared to the current densities, being Sr2NiIrO6 and Sr2CoIrO6 again the most active OER catalysts. Sr2CoIrO6 achieves a specific activity of 7.5 mA cmAs−2 at 1.55 V, which is larger than the one reported by Grimaud et al.26 of 4–5 mA cm−2 at the same potential. Another important observation is that the specific activities of Sr2CoIrO6 and Sr2FeIrO6 increase compared to the other catalysts because their surface area is lower. This means that the low current density reached by Sr2FeIrO6 stems in part from its low surface area.
We studied the effect that the IrO6 Oh distortion could have on the OER performance. The Oh distortion (Δ) in perovskites is calculated with eqn (11):57
 | (11) |
Apart from the tuning of the OER activity, one of the main purposes of designing Ir mixed oxides is to decrease the Ir loading on the electrodes. The Ir content of Sr2MIrO6 (M = Ni, Co, Sc, Fe) is ca. 38 wt%, which is similar to the content of other Ir mixed oxides and considerably lower than SrIrO3 (59 wt%) and IrO2 (see Table 1). Regarding the mass activities (in A gIr−1) of the catalysts (Fig. 1d), we note that the iridium mass fraction of all Sr2MIrO6 is similar among the analyzed double perovskites (see Table 1), so the trend is comparable to that of the current densities in Fig. 1a. As shown in Table 1, the mass activities obtained for Sr2NiIrO6, Sr2CoIrO6 and Sr2ScIrO6 at 1.525 V are comparable to state-of-the-art Ir mixed oxides and largely surpass IrO2.
3.3 Computational modelling of Sr2MIrO6 perovskites
To further rationalize the trends in OER catalytic activities of the Sr2MIrO6 double perovskites (M = Ni, Co, Sc, Fe), we resorted to DFT calculations. First, we assessed the bulk configuration of such perovskites. All calculations were performed on antiferromagnetic systems, in line with the experimental antiferromagnetic ordering on these perovskites.33,39 Among the different antiferromagnetic spin arrangements, we found that the configuration in which the spins are aligned in the (001) plane was most stable for all the analyzed systems.Furthermore, we calculated the OER overpotentials following the procedure described in the Computational Details section. Values of 0.40, 0.44, 0.75, and 0.79 V were obtained for M = Co, Ni, Fe, and Sc, respectively. Because of the inherent accuracy of DFT (given by the gray bands in Fig. 2a) and the proximity of some of the overpotentials calculated here, we safely conclude that DFT predicts that Sr2MIrO6 with Co and Ni are more active than those with Fe and Sc, in agreement with experiments. However, it is difficult to ascertain if double perovskites with Co are more active than those with Ni. We note as well that depending on the activity metrics, the ordering between M = Co and Ni changes, see Fig. 1a and c. These results are in line with the experimental observations in Fig. 1b. We rationalize the trends in two different ways. Firstly, in Fig. 2a, the overpotentials of the studied perovskites appear as a function of the descriptor ΔGO − ΔGOH, which renders a volcano-type activity plot for the OER.43,44 For the sake of comparison, we also included computational44 and experimental66 literature data for IrO2, together with Sr2CoIrO6 and Sr2NiIrO6 from our own experimental data (the addition of experimental data into the computational volcano plot is made following the method described elsewhere67,68). Fig. 2a shows that the double perovskites follow the traditional adsorption-energy scaling relations, and those with M = Co, Ni are the closest to the top of the volcano, even surpassing IrO2(110). Since most of the compounds in Fig. 2a are on the left (strong-binding) side of the volcano, weakening the adsorption energy of *O likely enhances the activity.
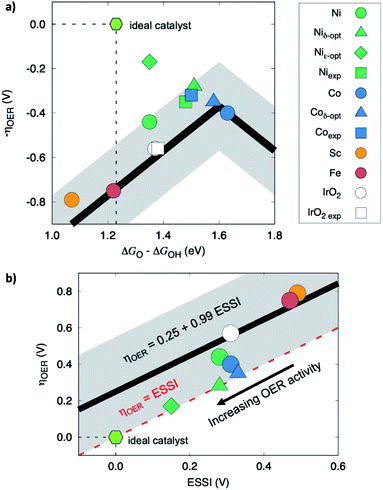 | ||
| Fig. 2 Computational modelling of the OER on Sr2MIrO6 with M = Ni, Co, Sc, Fe. (a) Volcano-type activity plot. Experimental data added following a method described previously.67,68 (b) OER overpotential as a function of the electrochemical-step symmetry index (ESSI). Optimized data calculated using the δ–ε method in ref. 47 The ideal catalyst and IrO2(110) are provided for comparison.44,66 | ||
Secondly, from a thermodynamic standpoint the ultimate goal in OER electrocatalysis is to reach thermoneutrality,46,69,70 which is achieved by a hypothetical “ideal” catalyst for a multielectron reaction. The OER reaction steps of such catalyst are fully symmetric, that is, with ΔGi = 1.23 eV (eqn (5)–(8)), which implies that ηOER = 0 V. To quantify how much a real catalyst approaches the ideal catalyst, one can use the electrochemical-step symmetry index (ESSI, see the Computational Details section).46 This metric for electrocatalytic symmetry is defined so that the OER overpotential is reduced as the symmetry of catalysts increases, namely when ESSI approaches zero. The studied Sr2MIrO6 compounds comply with this general guideline in Fig. 2b, where we observe a good correspondence between ESSI and ηOER. The OER at Ir sites seems rather asymmetric when Sc and Fe are in the M position of Sr2MIrO6, whereas Co and Ni help increase the electrocatalytic symmetry, thereby lowering the OER overpotential.
An interesting feature of ESSI is its ability to provide guidelines for the enhancement of electrocatalysts.47 This can be made following adsorption-energy scaling relations between *O, *OH and *OOH, or breaking them, respectively referred to as δ and ε optimization. The triangles in Fig. 2a and b show that the scaling-based δ-optimization of double perovskites with Ni would sizably reduce ηOER from 0.44 to 0.28 V if *OH binding energies decrease by 0.16 eV. Double perovskites with Co would only experience a small reduction from 0.40 to 0.35 V when *OH adsorption be strengthened by 0.05 eV. On the other hand, scaling-free ε-optimization would only benefit Sr2NiIrO6, for which a strengthening of 0.27 eV in *OOH adsorption with respect to *OH would lower the overpotential to 0.17 V. All this implies that Sr2NiIrO6 is more prone to further optimization, which is interesting for future studies.
3.4 Studying the initial OER activity of Ir double perovskites
Both the experimental and theoretical results presented in this work indicate that Sr2CoIrO6 and Sr2NiIrO6 are the most active catalysts among the studied Ir double perovskites. To verify that the initial OER activities reported in Fig. 1 can be ascribed to the perovskite phase and not to IrOx/IrOOH surface segregated phases, we characterized the most active perovskites (Sr2CoIrO6 and Sr2NiIrO6) after 5 cycles in OER by TEM/EDX and XPS. We specifically chose 5 cycles because no significant changes on the current densities are observed after those. The HRTEM images shown in Fig. 3a, which correspond to the catalysts recovered after 5 cycles, indicate that the materials still display the perovskite structure with only a minor fraction of Ir particles observed at the surface.We studied the surface of Sr2CoIrO6 and Sr2FeIrO6 perovskites by XPS before and after 5 cycles of reaction to compare the surface stability of a highly active catalyst and one with considerably lower activity. Both samples present Sr, Ir and M cations with Ir/M surface atomic ratios close to the initial nominal value of 1. However, the content of Sr is significantly smaller after 5 cycles than that on the initial samples (Fig. 3b), reflecting the high tendency of Sr to dissolve in the electrolyte. In the case of Sr2CoIrO6, the surface content of Ir increased compared to the content of M (Fig. 3b), indicating the incipient formation of Ir-based nanoparticles.
We also analyzed the evolution of the XPS-Ir 4f region (Fig. 3c). According to previous reports dealing with the stability of Ir perovskites for the OER in acid electrolyte,23 if the used perovskites were covered by IrOx/IrOOH nanoparticles, the oxidation state of Ir would change to +4/+3 after the reaction. However, as observed in Fig. 3c, the Ir 4f core-level region is not shifted to smaller binding energies (as expected for a reduction in Ir's oxidation state) after 5 OER cycles. Instead, it remains at the same binding energy for Sr2FeIrO6, or shifts to slightly higher binding energies for Sr2CoIrO6. In line with a previous report,23 we suggest that the oxidation from Ir5+ to Ir6+ is responsible for the high OER activity Sr2CoIrO6. This partial surface oxidation of Ir during the OER has been observed and correlated with an activity enhancement, owed to the existence of Ir in a higher oxidation state or a different molecular surface environment, caused by the oxidizing conditions of the OER.18,31 On the other hand, the oxidation of Ir5+ to Ir6+ is not observed in Sr2FeIrO6, which exhibits lower OER activity but higher stability (see below). These observations clearly indicate that the oxidation state of Ir plays a crucial role in the OER activity, which seems to be enhanced by Ir cations with upper oxidation states.
3.5 Stability and evolution of Sr2MIrO6 during the OER
The instability of mixed oxides during the OER in acidic media is an issue yet to be resolved. It has been reported that mixed oxides lose stability after several OER cycles, sometimes immediately after immersion in the electrolyte, prior to the OER cycling experiments.20,22,23,38 This lack of stability leads to catalysts that are more active than the fresh mixed oxide, likely due to the deposition of highly active IrOx/IrOOH nanosized particles at the surface of the mixed oxides. As shown in Fig. 3, we tested the catalysts after 5 cycles of reaction and, although the perovskite was still the main phase, some signs of degradation were observed. To further evaluate the stability of Sr2MIrO6, we measured 50 OER cycles at 10 mV s−1 between 1.2 and 1.7 V (Fig. 4a), in all cases showing an increment of the OER activity with cycling. To understand the origin of the observed behavior, we analyzed the structure and composition of the used catalysts after such 50 cycles.Fig. 4b shows representative HRTEM images of the perovskites after 50 OER cycles. The structure of Sr2CoIrO6 and Sr2NiIrO6 collapses, as observed in the HRTEM and in the FFT in the inset. The resulting material is formed by nanometric Ir-based particles with no signal indicative of the presence of Co, Ni or Sr in the samples after 50 cycles (EDX results, Fig. S8 in the ESI†). However, the morphology of both catalysts after 50 cycles is different (Fig. S9 in the ESI†). The Ni-containing catalyst collapses into a more open structure, less dense and apparently with more surface area (Fig. S9a†), while the Co-containing catalyst presents a more compact morphology (Fig. S9b†).
On the other hand, Sr2FeIrO6 and Sr2ScIrO6 maintain the perovskite structure as suggested by the presence of the (101) perovskite planes in the Sc perovskite and the oriented structure down the [111] zone axis in the case of the Fe-containing double perovskite. The presence of Sr, Fe(Sc) and Ir in the particles can be observed by EDX analyses, see the map in Fig. 4c. However, a careful analysis of the used perovskites also reveals the presence of IrOx nanoparticles at the surface of the latter perovskites (Fig. 4b), indicating the reconstruction of the perovskites during the OER. These results suggest that, contrary to the activity trend, the more distorted perovskites (M = Co and Ni) are less stable than the more symmetric ones (M = Sc and Fe).
Insights onto the deactivation of representative Sr2CoIrO6 and Sr2FeIrO6 samples were obtained by analysing the composition of the electrolyte by ICP-OES. We collected electrolyte before the reaction and after 5 and 50 OER cycles. The evolution of Sr, M (Co and Fe) and Ir concentrations in the electrolyte during the OER with both catalysts is shown in Fig. S10 in the ESI.† As observed, Sr and M dissolve faster in Sr2CoIrO6 than in Sr2FeIrO6 with cycling, indicating that Sr2FeIrO6 is more stable for the OER in acid electrolyte. In both cases the dissolution of Ir is low, in agreement with the formation of IrOx particles at the surface that are not dissolved during the reaction.
These observations are in good agreement with previous studies,36,71 reporting that more symmetric perovskites are more stable under the conditions of the H2O/O2 interconversion reactions, since they can be subjected to more extreme potentials without being destroyed. Conversely, distorted structures are susceptible to dissolution upon OER cycling.
We also performed chronoamperometry studies during one hour at the fixed potential where the current density is 10 mA cm−2 (Fig. 5a). The double perovskites' activity is stable during such steady-state durability test, losing only 5–10% of the initial activity in all cases. This result indicates the easier formation of Ir-nanoparticles on the surface during cycling experiments compared to experiments performed at a selected current density (i.e. 10 mA cm−2).
In view of the high OER activity of Sr2NiIrO6 and Sr2CoIrO6, we performed a more aggressive dynamic durability test consisting of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 OER cycles at 50 mV s−1 between 1.2 and 1.7 V (Fig. 5b) on both catalysts. This stability test is essentially an experiment in which the catalysts are continuously cycled between those two potentials in acid media for ∼55 h. In the case of Sr2NiIrO6, the activity loss after 10
000 OER cycles at 50 mV s−1 between 1.2 and 1.7 V (Fig. 5b) on both catalysts. This stability test is essentially an experiment in which the catalysts are continuously cycled between those two potentials in acid media for ∼55 h. In the case of Sr2NiIrO6, the activity loss after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles is small, with an increment of potential of only 10 mV to achieve the reference current density of 10 mA cm−2. For Sr2CoIrO6, the activity decreases from 13.3 to 9.8 mA cm−2 at 1.6 V, indicating severe deactivation after the durability test. The activity loss on Sr2CoIrO6 can also be followed from the evolution of the potential needed to achieve a current density of 10 mA cm−2, which increases from 1.57 to 1.60 V. These results may indicate that the surface reconstruction is different in both oxides. The effect of different surface reconstruction on the OER durability has been extensively reported.20,22–24,72 Analyzing the reconstruction of the surface after 50 cycles for both oxides (Fig. S9 in the ESI†), we conclude that the Ni-containing catalyst presents less dense Ir phases than the Co-containing catalyst. This different morphology can explain the different evolution of the catalyst after the long-term durability test. Probably, during cycling, the Co-containing catalyst forms larger Ir particles more rapidly than the Ni-containing catalyst, decreasing its current density after 10
000 cycles is small, with an increment of potential of only 10 mV to achieve the reference current density of 10 mA cm−2. For Sr2CoIrO6, the activity decreases from 13.3 to 9.8 mA cm−2 at 1.6 V, indicating severe deactivation after the durability test. The activity loss on Sr2CoIrO6 can also be followed from the evolution of the potential needed to achieve a current density of 10 mA cm−2, which increases from 1.57 to 1.60 V. These results may indicate that the surface reconstruction is different in both oxides. The effect of different surface reconstruction on the OER durability has been extensively reported.20,22–24,72 Analyzing the reconstruction of the surface after 50 cycles for both oxides (Fig. S9 in the ESI†), we conclude that the Ni-containing catalyst presents less dense Ir phases than the Co-containing catalyst. This different morphology can explain the different evolution of the catalyst after the long-term durability test. Probably, during cycling, the Co-containing catalyst forms larger Ir particles more rapidly than the Ni-containing catalyst, decreasing its current density after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.
000 cycles.
4. Conclusions
In this study we report the performance of Sr2MIrO6 (M = Ni, Co, Sc, Fe) double perovskites for the OER in acid electrolyte and rationalize the trends using a variety of experimental and computational descriptors, such as Ir oxidation state, degree of octahedral distortion, strength of Ir–adsorbate bonds, and electrocatalytic symmetry. The OER activities are comparable to those of state-of-the-art Ir-based catalysts, following the trend Sr2FeIrO6 < Sr2ScIrO6 < Sr2CoIrO6 < Sr2NiIrO6. The higher OER activity of Sr2NiIrO6 and Sr2CoIrO6 stems from their ability to stabilize Ir6+ cations during the OER. However, the stability of both oxides is lower than those of Sr2ScIrO6 and Sr2FeIrO6 in which lower Ir oxidation states are found. Thus, low Ir oxidation states, relatively strong binding energies and high structural symmetry ensure high stability.While all of the analyzed materials exhibit the perovskite structure during the first cycles of reaction, only the more symmetric phases, which are the lesser active, maintain the perovskite structure for more than 50 OER reaction cycles.
The correlations between iridium oxidation state, adsorption energies and OER activity in acid media reported in this work can serve as a guideline for designing more active and durable OER catalysts in acid electrolyte. Our results hint toward a necessary tradeoff between activity and stability, which can be provided by a careful selection of the metal M in Sr2MIrO6 double perovskites.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support from the Spanish Ministry of Economy and Competitiveness (MINECO) for the projects ENE2016-77055-C3-3-R and MAT2017-84496-R. The Deputyship for Research & Innovation, Ministry of Education of Saudi Arabia is acknowledged for funding this research work through the project number 341. To the Spanish MICIUN through PID2019-103967RJ-I00 and RTI2018-095460-B-I00 and María de Maeztu MDM-2017-0767 grants and, in part, from Generalitat de Catalunya (grants 2017SGR13 and XRQTC). F. C. V. thanks MICIUN for a Ramón y Cajal research contract (RYC-2015-18996). O. P. thanks the Spanish MICIUN for a FPI PhD grant (PRE2018-083811). The use of supercomputing facilities at SURFsara was sponsored by NWO Physical Sciences, with financial support by NWO. We thank Red Española de Supercomputación (RES) for super-computing time at SCAYLE (project QCM-2019-1-0034). We acknowledge support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI).References
- V. R. Stamenkovic, D. Strmcnik, P. P. Lopes and N. M. Markovic, Nat. Mater., 2017, 16, 57–69 CrossRef CAS.
- B. C. H. Steele and A. Heinzel, Nature, 2001, 414, 345–352 CrossRef CAS.
- O. Schmidt, A. Gambhir, I. Staffell, A. Hawkes, J. Nelson and S. Few, Int. J. Hydrogen Energy, 2017, 42, 30470–30492 CrossRef CAS.
- M. Carmo, D. L. Fritz, J. Mergel and D. Stolten, Int. J. Hydrogen Energy, 2013, 38, 4901–4934 CrossRef CAS.
- I. Katsounaros, S. Cherevko, A. R. Zeradjanin and K. J. J. Mayrhofer, Angew. Chem., Int. Ed., 2014, 53, 102–121 CrossRef CAS.
- K. Zeng and D. Zhang, Prog. Energy Combust. Sci., 2010, 36, 307–326 CrossRef CAS.
- N. Hodnik, P. Jovanovič, A. Pavlišič, B. Jozinović, M. Zorko, M. Bele, V. S. Šelih, M. Šala, S. Hočevar and M. Gaberšček, J. Phys. Chem. C, 2015, 119, 10140–10147 CrossRef CAS.
- D. F. Abbott, D. Lebedev, K. Waltar, M. Povia, M. Nachtegaal, E. Fabbri, C. Copéret and T. J. Schmidt, Chem. Mater., 2016, 28, 6591–6604 CrossRef CAS.
- C. Rozain, E. Mayousse, N. Guillet and P. Millet, Appl. Catal., B, 2016, 182, 153–160 CrossRef CAS.
- H. N. Nong, H. S. Oh, T. Reier, E. Willinger, M. G. Willinger, V. Petkov, D. Teschner and P. Strasser, Angew. Chem., Int. Ed., 2015, 54, 2975–2979 CrossRef CAS.
- P. Lettenmeier, L. Wang, U. Golla-Schindler, P. Gazdzicki, N. A. Cañas, M. Handl, R. Hiesgen, S. S. Hosseiny, A. S. Gago and K. A. Friedrich, Angew. Chem., Int. Ed., 2016, 55, 742–746 CrossRef CAS.
- H. Yu, N. Danilovic, Y. Wang, W. Willis, A. Poozhikunnath, L. Bonville, C. Capuano, K. Ayers and R. Maric, Appl. Catal., B, 2018, 239, 133–146 CrossRef CAS.
- E. Antolini, ACS Catal., 2014, 4, 1426–1440 CrossRef CAS.
- J. Suntivich, K. J. May, H. A. Gasteiger, J. B. Goodenough and Y. Shao-Horn, Science, 2011, 334, 1383–1385 CrossRef CAS.
- A. Grimaud, K. J. May, C. E. Carlton, Y.-L. Lee, M. Risch, W. T. Hong, J. Zhou and Y. Shao-Horn, Nat. Commun., 2013, 4, 2439 CrossRef.
- C. Sun, J. A. Alonso and J. Bian, Adv. Energy Mater., 2020, 2000459 CrossRef , 1–21.
- J. Bian, Z. Li, N. Li and C. Sun, Inorg. Chem., 2019, 58, 8208–8214 CrossRef.
- L. C. Seitz, C. F. Dickens, K. Nishio, Y. Hikita, J. Montoya, A. Doyle, C. Kirk, A. Vojvodic, H. Y. Hwang, J. K. Norskov and T. F. Jaramillo, Science, 2016, 353, 1011–1014 CrossRef CAS.
- R. Tang, Y. Nie, J. K. Kawasaki, D. Y. Kuo, G. Petretto, G. Hautier, G. M. Rignanese, K. M. Shen, D. G. Schlom and J. Suntivich, J. Mater. Chem. A, 2016, 4, 6831–6836 RSC.
- Y. Chen, H. Li, J. Wang, Y. Du, S. Xi, Y. Sun, M. Sherburne, J. W. Ager, A. C. Fisher and Z. J. Xu, Nat. Commun., 2019, 10, 572 CrossRef CAS.
- O. Diaz-Morales, S. Raaijman, R. Kortlever, P. J. Kooyman, T. Wezendonk, J. Gascon, W. T. Fu and M. T. M. Koper, Nat. Commun., 2016, 7, 12363 CrossRef CAS.
- S. Geiger, O. Kasian, M. Ledendecker, E. Pizzutilo, A. M. Mingers, W. T. Fu, O. Diaz-Morales, Z. Li, T. Oellers, L. Fruchter, A. Ludwig, K. J. J. Mayrhofer, M. T. M. Koper and S. Cherevko, Nat. Catal., 2018, 1, 508–515 CrossRef CAS.
- A. Grimaud, A. Demortière, M. Saubanère, W. Dachraoui, M. Duchamp, M.-L. Doublet and J.-M. Tarascon, Nat. Energy, 2017, 2, 16189 CrossRef CAS.
- L. Yang, G. Yu, X. Ai, W. Yan, H. Duan, W. Chen, X. Li, T. Wang, C. Zhang, X. Huang, J.-S. Chen and X. Zou, Nat. Commun., 2018, 9, 5236 CrossRef CAS.
- X. Liang, L. Shi, Y. Liu, H. Chen, R. Si, W. Yan, Q. Zhang, G. D. Li, L. Yang and X. Zou, Angew. Chem., Int. Ed., 2019, 58, 7631–7635 CrossRef CAS.
- R. Zhang, N. Dubouis, M. Ben Osman, W. Yin, M. T. Sougrati, D. A. D. Corte, D. Giaume and A. Grimaud, Angew. Chem., 2019, 131, 4619–4623 CrossRef.
- W. Sun, J. Y. Liu, X. Q. Gong, W. Q. Zaman, L. M. Cao and J. Yang, Sci. Rep., 2016, 6, 1–10 CrossRef.
- C. Shang, C. Cao, D. Yu, Y. Yan, Y. Lin, H. Li, T. Zheng, X. Yan, W. Yu, S. Zhou and J. Zeng, Adv. Mater., 2019, 31, 1–6 CrossRef.
- D. Lebedev, M. Povia, K. Waltar, P. M. Abdala, I. E. Castelli, E. Fabbri, M. V. Blanco, A. Fedorov, C. Copéret, N. Marzari and T. J. Schmidt, Chem. Mater., 2017, 29, 5182–5191 CrossRef CAS.
- W. Q. Zaman, W. Sun, M. Tariq, Z. Zhou, U. Farooq, Z. Abbas, L. Cao and J. Yang, Appl. Catal., B, 2019, 244, 295–302 CrossRef CAS.
- A. L. Strickler, D. Higgins and T. F. Jaramillo, ACS Appl. Energy Mater., 2019, 2, 5490–5498 CrossRef CAS.
- D. Y. Jung, G. Demazeau and J. H. Choy, J. Mater. Chem., 1995, 5, 517–519 RSC.
- P. Kayser, J. A. Alonso, F. J. Mompeán, M. Retuerto, M. Croft, A. Ignatov and M. T. Fernández-Díaz, Eur. J. Inorg. Chem., 2015, 2015, 5027–5038 CrossRef CAS.
- P. Kayser, M. J. Martínez-Lope, J. A. Alonso, M. Retuerto, M. Croft, A. Ignatov and M. T. Fernández-Díaz, Eur. J. Inorg. Chem., 2014, 2014, 178–185 CrossRef CAS.
- Y. L. Lee, J. Kleis, J. Rossmeisl, S. H. Yang and D. Morgan, Energy Environ. Sci., 2011, 4, 3966–3970 RSC.
- W. T. Hong, M. Risch, K. A. Stoerzinger, A. Grimaud, J. Suntivich and Y. Shao-Horn, Energy Environ. Sci., 2015, 8, 1404–1427 RSC.
- F. Calle-Vallejo, N. G. Inoglu, H. Y. Su, J. I. Martínez, I. C. Man, M. T. M. Koper, J. R. Kitchin and J. Rossmeisl, Chem. Sci., 2013, 4, 1245–1249 RSC.
- T. Reier, Z. Pawolek, S. Cherevko, M. Bruns, T. Jones, D. Teschner, S. Selve, A. Bergmann, H. N. Nong, R. Schlögl, K. J. J. Mayrhofer and P. Strasser, J. Am. Chem. Soc., 2015, 137, 13031–13040 CrossRef CAS.
- P. Kayser, M. J. Martínez-Lope, J. A. Alonso, M. Retuerto, M. Croft, A. Ignatov and M. T. Fernández-Díaz, Inorg. Chem., 2013, 52, 11013–11022 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS.
- B. Hammer, L. B. Hansen and J. K. Nørskov, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 7413–7421 CrossRef.
- D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef.
- J. Rossmeisl, Z. W. Qu, H. Zhu, G. J. Kroes and J. K. Nørskov, J. Electroanal. Chem., 2007, 607, 83–89 CrossRef CAS.
- I. C. Man, H. Y. Su, F. Calle-Vallejo, H. A. Hansen, J. I. Martínez, N. G. Inoglu, J. Kitchin, T. F. Jaramillo, J. K. Nørskov and J. Rossmeisl, ChemCatChem, 2011, 3, 1159–1165 CrossRef CAS.
- J. K. Nørskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J. R. Kitchin, T. Bligaard and H. Jónsson, J. Phys. Chem. B, 2004, 108, 17886–17892 CrossRef.
- N. Govindarajan, J. M. García-Lastra, E. J. Meijer and F. Calle-Vallejo, Curr. Opin. Electrochem., 2018, 8, 110–117 CrossRef CAS.
- N. Govindarajan, M. T. M. Koper, E. J. Meijer and F. Calle-Vallejo, ACS Catal., 2019, 9, 4218–4225 CrossRef CAS.
- Z. Xu and J. R. Kitchin, Phys. Chem. Chem. Phys., 2015, 17, 28943–28949 RSC.
- Z. Xu, J. Rossmeisl and J. R. Kitchin, J. Phys. Chem. C, 2015, 119, 4827–4833 CrossRef CAS.
- L. G. V. Briquet, M. Sarwar, J. Mugo, G. Jones and F. Calle-Vallejo, ChemCatChem, 2017, 9, 1261–1268 CrossRef CAS.
- C. F. Dickens and J. K. Nørskov, J. Phys. Chem. C, 2017, 121, 18516–18524 CrossRef CAS.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- M. Retuerto, J. A. Alonso, M. García-Hernández and M. J. Martínez-Lope, Solid State Commun., 2006, 139, 19–22 CrossRef CAS.
- T. Stoyanova-Lyubenova, A. J. Dos santos-García, E. Urones-Garrote, M. J. Torralvo and M. Á. Alario-Franco, Dalton Trans., 2014, 43, 1117–1124 RSC.
- M. Retuerto, A. G. Pereira, F. J. Pérez-Alonso, M. A. Peña, J. L. G. Fierro, J. A. Alonso, M. T. Fernández-Díaz, L. Pascual and S. Rojas, Appl. Catal., B, 2017, 203, 363–371 CrossRef CAS.
- M. Retuerto, F. Jiménez-Villacorta, M. J. Martínez-Lope, Y. Huttel, E. Roman, M. T. Fernández-Díaz and J. A. Alonso, Phys. Chem. Chem. Phys., 2010, 12, 13616 RSC.
- I. D. Brown and R. D. Shannon, Acta Crystallogr., 1973, 29, 266 CrossRef CAS.
- A. Grimaud, O. Diaz-Morales, B. Han, W. T. Hong, Y. L. Lee, L. Giordano, K. A. Stoerzinger, M. T. M. Koper and Y. Shao-Horn, Nat. Chem., 2017, 9, 457–465 CrossRef CAS.
- M. Peuckert, Surf. Sci., 1984, 144, 451–464 CrossRef CAS.
- V. Pfeifer, T. E. Jones, J. J. Velasco Vélez, C. Massué, M. T. Greiner, R. Arrigo, D. Teschner, F. Girgsdies, M. Scherzer, J. Allan, M. Hashagen, G. Weinberg, S. Piccinin, M. Hävecker, A. Knop-Gericke and R. Schlögl, Phys. Chem. Chem. Phys., 2016, 18, 2292–2296 RSC.
- C. C. L. McCrory, S. Jung, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 2013, 135, 16977–16987 CrossRef CAS.
- T. Reier, M. Oezaslan and P. Strasser, ACS Catal., 2012, 2, 1765–1772 CrossRef CAS.
- Y. Lee, J. Suntivich, K. J. May, E. E. Perry and Y. Shao-Horn, J. Phys. Chem. Lett., 2012, 3, 399–404 CrossRef CAS.
- F. Calle-Vallejo, O. A. Díaz-Morales, M. J. Kolb and M. T. M. Koper, ACS Catal., 2015, 5, 869–873 CrossRef CAS.
- J. Suntivich, H. A. Gasteiger, N. Yabuuchi and Y. Shao-Horn, J. Electrochem. Soc., 2010, 157, B1263 CrossRef CAS.
- K. A. Stoerzinger, L. Qiao, M. D. Biegalski and Y. Shao-Horn, J. Phys. Chem. Lett., 2014, 5, 1636–1641 CrossRef CAS.
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Nørskov and T. F. Jaramillo, Science, 2017, 355, eaad4998 CrossRef.
- M. Retuerto, L. Pascual, F. Calle-Vallejo, P. Ferrer, D. Gianolio, A. G. Pereira, Á. García, J. Torrero, M. T. Fernández-Díaz, P. Bencok, M. A. Peña, J. L. G. Fierro and S. Rojas, Nat. Commun., 2019, 10, 2041 CrossRef.
- C. H. Kjaergaard, J. Rossmeisl and J. K. Nørskov, Inorg. Chem., 2010, 49, 3567–3572 CrossRef CAS.
- M. T. M. Koper, J. Electroanal. Chem., 2011, 660, 254–260 CrossRef CAS.
- J. Suntivich, H. A. Gasteiger, N. Yabuuchi, H. Nakanishi, J. B. Goodenough and Y. Shao-Horn, Nat. Chem., 2011, 3, 546–550 CrossRef CAS.
- C. W. Song, J. Lim, H. Bin Bae and S.-Y. Chung, Energy Environ. Sci., 2020, 13, 4178–4188 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ta10316k |
| This journal is © The Royal Society of Chemistry 2021 |