 Open Access Article
Open Access ArticleUnravelling moisture-induced CO2 chemisorption mechanisms in amine-modified sorbents at the molecular scale†
Mariana
Sardo
 *a,
Rui
Afonso
a,
Joanna
Juźków
a,
Marlene
Pacheco
b,
Marta
Bordonhos
*a,
Rui
Afonso
a,
Joanna
Juźków
a,
Marlene
Pacheco
b,
Marta
Bordonhos
 b,
Moisés L.
Pinto
b,
José R. B.
Gomes
b,
Moisés L.
Pinto
b,
José R. B.
Gomes
 a and
Luís
Mafra
a and
Luís
Mafra
 *a
*a
aCICECO – Aveiro Institute of Materials, Department of Chemistry, University of Aveiro, Campus Universitário de Santiago, 3810-193 Aveiro, Portugal. E-mail: msardo@ua.pt; lmafra@ua.pt
bCERENA, Instituto Superior Técnico, University of Lisbon, Av. Rovisco Pais, 1049-001 Lisboa, Portugal
First published on 8th January 2021
Abstract
This work entails a comprehensive solid-state NMR and computational study of the influence of water and CO2 partial pressures on the CO2-adducts formed in amine-grafted silica sorbents. Our approach provides atomic level insights on hypothesised mechanisms for CO2 capture under dry and wet conditions in a tightly controlled atmosphere. The method used for sample preparation avoids the use of liquid water slurries, as performed in previous studies, enabling a molecular level understanding, by NMR, of the influence of controlled amounts of water vapor (down to ca. 0.7 kPa) in CO2 chemisorption processes. Details on the formation mechanism of moisture-induced CO2 species are provided aiming to study CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O binary mixtures in amine-grafted silica sorbents. The interconversion between distinct chemisorbed CO2 species was quantitatively monitored by NMR under wet and dry conditions in silica sorbents grafted with amines possessing distinct bulkiness (primary and tertiary). Particular attention was given to two distinct carbonyl environments resonating at δC ∼161 and 155 ppm, as their presence and relative intensities are greatly affected by moisture depending on the experimental conditions. 1D and 2D NMR spectral assignments of both these 13C resonances were assisted by density functional theory calculations of 1H and 13C chemical shifts on model structures of alkylamines grafted onto the silica surface that validated various hydrogen-bonded CO2 species that may occur upon formation of bicarbonate, carbamic acid and alkylammonium carbamate ion pairs. Water is a key component in flue gas streams, playing a major role in CO2 speciation, and this work extends the current knowledge on chemisorbed CO2 structures and their stabilities under dry/wet conditions, on amine-modified solid surfaces.
H2O binary mixtures in amine-grafted silica sorbents. The interconversion between distinct chemisorbed CO2 species was quantitatively monitored by NMR under wet and dry conditions in silica sorbents grafted with amines possessing distinct bulkiness (primary and tertiary). Particular attention was given to two distinct carbonyl environments resonating at δC ∼161 and 155 ppm, as their presence and relative intensities are greatly affected by moisture depending on the experimental conditions. 1D and 2D NMR spectral assignments of both these 13C resonances were assisted by density functional theory calculations of 1H and 13C chemical shifts on model structures of alkylamines grafted onto the silica surface that validated various hydrogen-bonded CO2 species that may occur upon formation of bicarbonate, carbamic acid and alkylammonium carbamate ion pairs. Water is a key component in flue gas streams, playing a major role in CO2 speciation, and this work extends the current knowledge on chemisorbed CO2 structures and their stabilities under dry/wet conditions, on amine-modified solid surfaces.
1. Introduction
General recognition of the environmental impact of large concentrations of CO2 present in the atmosphere has continuously risen, leading to increased efforts to mitigate greenhouse effects.1 In order to reduce CO2 emissions, new solid adsorbent materials are actively being sought for capturing CO2 from stationary sources with dilute CO2 concentrations, in replacement of liquid-phase amine adsorbents, to overcome the inherent chemical degradation, low loading capacities, corrosion and low energy efficiency issues related to the use of aqueous amine solutions.2–7 Amine-based solid adsorbents have been extensively studied for carbon capture and storage (CCS) and also shown to be good candidates for direct air capture (DAC) applications, involving extremely low CO2 concentrations (ca. 400 ppm),1 based on negative carbon emission technologies.Combustion products from burning fossil fuels in air, and the resulting flue gas, typically contain low concentrations of CO2 (<20%) and about 80% N2. In both exhaust gases and air, moisture is a ubiquitous component and depending on the type of fuels and the combustion conditions, flue gas contains about 8–20% moisture (water vapor).8 For any large-scale real-world CO2 capture or separation application, the effect of moisture on adsorbents must therefore be taken under consideration.
Assessing the type of chemisorbed CO2 structure, under moist conditions, formed in such porous adsorbents with amines is therefore of paramount importance to design and optimise effective sorbents for CO2/N2 separation from industrial flue gas streams as well as CO2/H2 and CO2/CH4 separation processes. Despite numerous contributions, understanding the stability and chemical nature of these CO2 species is not straightforward,9 due to the many variables (temperature, gas pressure, amine coverage, silanol density, pore textural properties) that may influence CO2 speciation (not to mention the number of process variables that should also guide the adsorbent choice). Although CO2-chemisorbent materials such as MOFs10–14/postsynthetically modified MOFs15–17 or alkaline ceramics18,19 are emerging as potential candidates for CO2 captors from wet gas mixtures, mesoporous silicas, such as SBA-15 functionalized with amines, reported in this work, are among the most relevant materials being developed2,4 due to their high selectivity, capacity towards dilute CO2 sources and moisture tolerance.3,4,20
The reaction mechanism and products, resulting from co-adsorption of CO2 and moisture by silica supported amine adsorbents, may vary based on the type of amino groups, amine loading, moisture content and sometimes whether the amine is chemically grafted, physically impregnated on the support or a mixture of both.21–23 The role of water in the CO2 capture efficiency is somewhat controversial because some authors claim that the presence of moisture enhances CO2 adsorption capacity21,24 while others report no synergy effects between moisture and an increase of CO2 adsorption capacity by amine-grafted solid sorbents.25–30 The enhancement of CO2 capacity under moist conditions has been many times associated with the increase of amine efficiency (CO2/N) from ∼0.5 (dry) to 1 (wet) induced by the formation of bicarbonate species in the presence of water molecules.31 It is known that basic surface amines and acidic CO2 molecules interact to form surface carbamate and carbamic acid species under anhydrous conditions and bicarbonate/carbonate species when water is present.3,4,32,33 Nevertheless, which CO2 species is really contributing to increase CO2 adsorption efficiency, under wet conditions, has been a subject of high interest.1,28,34,35 In fact, the increase of CO2 capacity under humid conditions has been attributed, by some authors, to the formation of more carbamate species in the presence of humidity, rather than formation of bicarbonate species.1,28,34,35 Bacsik and co-workers28 explained that water could induce disruption of amine H-bonds with neighbouring surface species thus increasing their accessibility to CO2 molecules, leading to the formation of more ammonium carbamate ion pairs.
Differentiating moisture-induced CO2 species (e.g., bicarbonate) from carbamic acid or carbamates is a difficult task, mainly because the signals arising from carbamic acid and carbamate species often dominate the FTIR and NMR spectra. Several experimental studies detected bicarbonate species, mainly through FTIR spectroscopy, in aqueous amine solutions36,37 as well as on porous solids functionalized with amines.38–4113C solid-state NMR has been a valuable complementary method to identify surface bicarbonates in studies involving amine functionalized sorbents, but the assignment of CO2 species is complicated due to ill-resolved spectra in the CO2 chemisorption region. This is because of the 13C isotropic chemical shift (CS) overlap between resonances associated with different CO2 species.34,42,43
Recently, Foo and co-workers38 hinted on the presence of bicarbonate ions on primary amine grafted-SBA-15 through a combined FTIR/NMR study. The existence of bicarbonate ions was attributed to the presence of residual physisorbed water on the amine-grafted sample, exhibiting a resonance near 161 ppm in the 13C NMR spectrum. Overall, ammonium bicarbonate species have been previously assigned in the literature based on 13C NMR resonances appearing between 161 and 165 ppm,40,43–45 in tertiary amine-grafted mesoporous SBA-15, as a solid product of the reaction between NH3 and CO2 under humid conditions and in amine-modified COFs.41 However, the assignment of such species in tertiary amine-grafted SBA-15 was performed on samples without rigorous control of the CO2 and H2O partial pressures. This may lead to significant changes in the NMR spectra as shown in this work.
In this work, we present a comprehensive study involving the species formed upon CO2 and H2O adsorption on primary and tertiary amine-grafted SBA-15 (3-aminopropyltriethoxysilane (APTES) and [3-(diethylamino)propyl]trimethoxysilane (DEAPTES)). For that purpose, binary mixtures of 13C-labeled CO2 (13CO2) and water were used. A combined high-resolution 13C/1H solid-state NMR and computational study is presented to access all the variables involving the formation of moisture-induced species. Although tertiary amines are not usually considered of practical relevance in carbon capture applications due to their low CO2 uptake under dry conditions, they were used in this work since they are not expected to form the “dominant” alkylammonium carbamate species, thus facilitating the detection and assignment of signals arising from dilute CO2 species. The method we explore is significantly different from previously reported methods,40,44 as all wet samples were prepared without the use of slurries, which may give rise to additional species formed by dissolution in water. Instead, our method consists in dosing, in a controlled environment, specific amounts of water and CO2 partial pressures, as explained in detail in Section 3.3. Molecular models of the silica surface functionalized with amines engaged in different intermolecular interactions were assessed using DFT methods, whose results are compared with solid-state NMR evidence. This combination facilitates the assessment of computer models accuracy thus helping in the identification of the observed moisture-induced CO2 species.
2. Methods
2.1. Materials preparation and characterization
SBA-15 (8 nm pore size) was purchased from Sigma-Aldrich and used without further purification. SBA-15 functionalization was carried out with amino-organosilanes; APTES (purity > 98%) and 3-DEAPTES (96%) were acquired from Sigma-Aldrich. It is worth mentioning that the dryness conditions of the reaction media and material are of paramount importance to prevent extension of lateral silane polymerization within the materials and to allow efficient silane functionalization. To achieve this, typically 2 g of SBA-15 was introduced in a closed reflux apparatus connected to a vacuum line and heated to 150 °C for 2 h. After cooling, nitrogen was introduced into the system prior to the opening of the reflux apparatus, and SBA-15 was refluxed with 100 cm3 of dry toluene (Aldrich, 99.8%) containing 9 mmol of the amino-organosilane for 24 h in a nitrogen atmosphere. The resulting material was purified by Soxhlet extraction with dry toluene, to remove the unreacted amino-organosilanes, and finally dried under vacuum at 120 °C for 24 h. Functionalized materials were named APTES@SBA-15 and DEAPTES@SBA-15.The thermogravimetric, differential scanning calorimetry experiments, elemental analysis as well as the porous texture characterization of the materials (via N2 adsorption at −196 °C) were performed using the same protocol as described in our previous work46 (details in the ESI†).
2.2. Sorption apparatus for loading samples with 13CO2
The sorption apparatus (Scheme S1†) comprises a laboratory-made high-vacuum line, connected to a turbomolecular pumping station (HiCube 80, Pfeiffer Vacuum), capable of vacuum greater than 10−2 Pa. A borosilicate glass cell, adapted from the description in the literature, was connected to the vacuum line and served as an enclosure for an NMR rotor allowing the degassing and heating of zirconia NMR rotors up to 300 °C under high vacuum. The heating was performed with a laboratory-made oven connected to a power controller (Eurotherm 3116), and the temperature measured with a thermocouple. The desired gas was introduced into the system from the canister connected to the vacuum line and the cell. The pressure inside the cell was measured with a capacitance transducer (MKS Instruments, Baratron 722B).All samples of APTES@SBA-15 and DEAPTES@SBA-15 were packed in zirconia NMR rotors, enclosed into the sorption apparatus and dried by degassing and heating (150 °C, 3 h, ramp of 2.5 °C min−1) under vacuum (Scheme S1†). After cooling down under vacuum, 13CO2 (Cortecnet, 99 atom% 13C; <3 atom% 18O) and water vapor (see Section 3.3 for further details) were introduced into the system up to the desired partial pressures and allowed to equilibrate for 4.5 h. Water vapor was introduced into the cell from a glass container with liquid water connected to the vacuum line by opening a valve on the glass container. Prior to use, deionized water (Millipore Milli-Q) was further purified by freeze–vacuum–thaw cycles. After adsorption of water vapor and CO2 with the desired partial pressures, the cell was then filled with helium (Air Liquide, 99.999%), if needed, up to the atmospheric pressure. Finally, the NMR rotor was closed inside the cell and only then the cell was opened to remove the rotor for NMR measurements.
2.3. Computational details
The clusters used to model the silica surface are based on the experimental crystallographic structure of alpha-quartz47 using the atomic positions of the silicon and oxygen atoms. Dangling bonds at the edges of the cluster models due to elimination of Si atoms were saturated with H atoms along the O–Si directions of the perfect crystal and imposing an O–H distance equal to 0.96 Å.48 Note that although the real surface of mesoporous silicas is amorphous, using a surface built from a crystalline structure should not dramatically influence the results from the calculations, as we are dealing with relatively small clusters.The silylpropylamines were grafted (through optimisation) on the clusters where OH groups existed, each binding three surface OH groups. Subsequent optimisations of different species involved the relaxation of the alkyl chain (and the respective functional group at its end), water or CO2 molecules (when present), the SiO3 moieties binding the alkylamines, and the surface OH groups, while the remaining Si and O atoms were kept frozen at their crystallographic positions. The fixation of some atomic positions provides a simple but effective representation of the mechanical embedding of the solid covalent oxide surface.49,50 As common practice, several different initial conformations were studied (please refer to ref. 9 for additional details), with all discussions below considering only the most stable conformations of each kind. The absence of imaginary values in the vibrational modes involving the atoms optimised in the different structural models ensured that the structures are true minima on their potential energy surfaces.
The M06-2X hybrid functional of Truhlar and Zhao,51,52 based on the meta-generalized gradient approximation, and the standard 6-31G(d) basis set53,54 with a single polarisation function in all the atoms except hydrogen, as included in the Gaussian 09 software,55 were used in all the structural optimisations, in the calculation of electronic energies or Gibbs energies at T = 298.15 K, and vibrational frequencies. In all calculations, the default integration grids and convergence thresholds in the Gaussian 09 software were employed.
NMR shielding tensors of the optimized geometries have been computed with the GIAO method,56,57 also using the M06-2X functional and the 6-31G(d) basis set. These conditions typically create relatively small root-mean-square errors of the calculated 13C chemical shifts (cf. 3.2 ppm).58 The isotropic magnetic shielding tensors calculated for the clusters were subtracted from those calculated for gas-phase tetramethylsilane (at 0 ppm, as in our previous work4,9,20).
The M06-2X functional has been the default choice in our work with amine-functionalised silicas,9 with calculated NMR and infrared values in excellent agreement with the experimental results. In fact, tests with larger basis sets, e.g. 6-31G(d,p), 6-31+G(d,p) or 6-311++G(d,p) were found to lead to systematic overestimations of the experimental 1H and 13C shifts of the three carbon species reported in our previous work,4 when considering tetramethylsilane calculated at the same level of theory as the reference (Tables S4–S7 in the ESI†), with the overestimations increasing with the increase of the basis set size. These observations are aligned with those in other studies reported in the literature, where it was found that the increase of the basis set size from 6-31G(d) to 6-311G(2d,2p) or def2-TZVPP54,59 did not lead to any visible increment in the accuracy of the calculated NMR shifts.4,9,20,56–58
2.4. Solid-state NMR measurements
1H, 29Si, and 13C NMR spectra were acquired on a Bruker Avance III 400 and 700 spectrometers operating at B0 fields of 9.4 and 16.4 T, respectively, with 1H/29Si/13C Larmor frequencies of 400.1/79.5/100.6 MHz and 700.1/139.1/176.1 MHz, respectively. All experiments were performed on a double-resonance 4 mm Bruker MAS probe. Samples were packed into ZrO2 rotors with Kel-F caps. Spinning rates between 10 and 15 kHz were employed to record all the spectra (see figure captions for details). 1H and 13C CSs are quoted in ppm from TMS (0 ppm) and α-glycine (secondary reference, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 176.03 ppm), respectively. 29Si CSs were referenced with respect to the external secondary reference Q8M8 (octa(dimethylsiloxy)silsesquioxane) setting the high frequency signal (left side resonance) to 11.5 ppm. 1H single-pulse excitation magic angle spinning (MAS) NMR spectra were recorded at a spinning rate of 15 kHz using a pulse width of 2.66 μs (90° flip angle) corresponding to a radio frequency (rf) field strength of ca. 94 kHz. A recycle delay (RD) of 8 s was found to be sufficient and used for all samples. The 13C CPMAS spectra were acquired under the following experimental conditions: 1H 90° pulse set to 3.0 μs corresponding to an rf of ∼83 kHz; the CP step was performed with a contact time (CT) of 2000 μs using a 70–100% RAMP shape at the 1H channel and using a 50 kHz square shape pulse on the 13C channel; RD was 5 s. During the acquisition, a SPINAL-64 decoupling scheme was employed using a pulse length for the basic decoupling units of 7 μs at an rf field strength of 83 kHz. The 1H–13C LG-CP HETCOR spectra were acquired using frequency-switched Lee–Goldburg (FSLG) 1H homonuclear decoupling during the indirect dimension (t1). For LG-CP the conditions used were CT of 2000 μs, 13C rf amplitude ramped at 50–100%, and an rf amplitude for 1H spin-lock of 50 kHz at an LG offset irradiation of −50
O at 176.03 ppm), respectively. 29Si CSs were referenced with respect to the external secondary reference Q8M8 (octa(dimethylsiloxy)silsesquioxane) setting the high frequency signal (left side resonance) to 11.5 ppm. 1H single-pulse excitation magic angle spinning (MAS) NMR spectra were recorded at a spinning rate of 15 kHz using a pulse width of 2.66 μs (90° flip angle) corresponding to a radio frequency (rf) field strength of ca. 94 kHz. A recycle delay (RD) of 8 s was found to be sufficient and used for all samples. The 13C CPMAS spectra were acquired under the following experimental conditions: 1H 90° pulse set to 3.0 μs corresponding to an rf of ∼83 kHz; the CP step was performed with a contact time (CT) of 2000 μs using a 70–100% RAMP shape at the 1H channel and using a 50 kHz square shape pulse on the 13C channel; RD was 5 s. During the acquisition, a SPINAL-64 decoupling scheme was employed using a pulse length for the basic decoupling units of 7 μs at an rf field strength of 83 kHz. The 1H–13C LG-CP HETCOR spectra were acquired using frequency-switched Lee–Goldburg (FSLG) 1H homonuclear decoupling during the indirect dimension (t1). For LG-CP the conditions used were CT of 2000 μs, 13C rf amplitude ramped at 50–100%, and an rf amplitude for 1H spin-lock of 50 kHz at an LG offset irradiation of −50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000/√2 = −35
000/√2 = −35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 354 Hz. 70 t1 points with 1024 scans each were recorded along the indirect dimension. An 1H rf field strength of 83 kHz and asymmetric LG offset of 54
354 Hz. 70 t1 points with 1024 scans each were recorded along the indirect dimension. An 1H rf field strength of 83 kHz and asymmetric LG offset of 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 925 Hz and −62
925 Hz and −62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 925 Hz were used for FSLG decoupling employing an LG pulse of 9.8 μs. The indirect dimension dwell time was set equal to four FSLG blocks (78.4 μs). Quadrature detection in t1 was achieved by the States-TPPI method. The 1H CSs were corrected assuming a scaling factor of 1/√3 for FSLG decoupling. The 1H–29Si CP HETCOR spectra were acquired with CT of 8000 μs; 29Si rf power amplitude ramped at 50−100% and an 1H spin-lock rf amplitude of 50 kHz. 20 t1 points with 3072 scans each were recorded along the indirect dimension. All reported 1H NMR CSs extracted from the HETCOR spectra have an average estimated error of ±0.5 ppm due to the scaling factors used for FSLG decoupling to calibrate the 1H CSs at the indirect dimension. Further relevant experimental details can be found in the corresponding figure captions. Fitting of the APTES@SBA-15 NMR spectra recorded at 12 kHz MAS was performed with DMFIT60 software. Fitting errors were below 5%.
925 Hz were used for FSLG decoupling employing an LG pulse of 9.8 μs. The indirect dimension dwell time was set equal to four FSLG blocks (78.4 μs). Quadrature detection in t1 was achieved by the States-TPPI method. The 1H CSs were corrected assuming a scaling factor of 1/√3 for FSLG decoupling. The 1H–29Si CP HETCOR spectra were acquired with CT of 8000 μs; 29Si rf power amplitude ramped at 50−100% and an 1H spin-lock rf amplitude of 50 kHz. 20 t1 points with 3072 scans each were recorded along the indirect dimension. All reported 1H NMR CSs extracted from the HETCOR spectra have an average estimated error of ±0.5 ppm due to the scaling factors used for FSLG decoupling to calibrate the 1H CSs at the indirect dimension. Further relevant experimental details can be found in the corresponding figure captions. Fitting of the APTES@SBA-15 NMR spectra recorded at 12 kHz MAS was performed with DMFIT60 software. Fitting errors were below 5%.
2.5. Adsorption of CO2
The CO2 (99.998%, Air Liquide) adsorption isotherms were collected at 25 °C in increasing pressures up to ca. 1000 kPa (10 bar), using the manometric method. These experiments were performed in a lab-made stainless-steel volumetric apparatus with a pressure transducer (MKS, Baratron 627D14TBC1B), and equipped with a vacuum system (Pfeiffer Vacuum, HiCube 80) that achieves vacuum pressures better than 10−4 Pa. Prior to collecting experimental data, all samples were degassed at 150 °C for 2 h, at a vacuum pressure below 10−4 Pa. During the experiments, the temperature of the adsorption system and of each sample was controlled with a thermostatic water bath with an accuracy of 0.01 °C (Julabo, MB-5). Experimental adsorption isotherms were calculated considering the non-ideality of the gas phase using the second and third virial coefficients. Additionally, the experimental excess adsorbed amounts were converted to the absolute adsorbed amounts by accounting the porous volume of the material and the density of the gas phase.For the adsorption of CO2 in samples with a known amount of preadsorbed water, a given pressure of water vapor was introduced in the calibrated volume (about 2.08 kPa) and allowed to equilibrate at room temperature. The value of the pressure was recorded and the vapor dosed to the freshly activated sample by the opening of the sample valve. After equilibration, the final pressure was below the sensitivity of the pressure transducer (<6 Pa) and it was assumed that all water was adsorbed in the sample. For the calculations of the adsorbed amounts , the non-ideality of the water vapor was accounted with the second viral coefficient. Prior to use, the deionized water (Millipore Milli-Q) was further purified by freeze–vacuum–thaw cycles.
3. Results and discussion
3.1. Characterization of the adsorbent materials
The materials were first characterized to assess the porosity of the samples, thermal stability and amine content after functionalization with the amines. Textural properties of the studied porous samples were obtained by nitrogen adsorption isotherms (Fig. S1 and Table S1†). These data indicate an effective functionalization on the SBA-15 pores and are in agreement with those obtained in our previous work.4The BET surface area of the parent sample (SBA-15) decreased from ca. 743 to 340 and 329 m2 g−1 upon functionalization with APTES and DEAPTES, respectively. The pore volume also drops upon functionalization with both amines with a slightly higher decrease for the material functionalized with DEAPTES (bulkier amine). Further indications of the successful functionalization were given by the nitrogen content of the samples obtained by chemical analysis (Table S1†). The infrared spectra (Fig. S2†) show the typical Si–O stretching band at ∼1100 cm−1 from silica, and additional vibrations in the regions of 3500–3300 cm−1 (stretching of O–H/N–H bond) and 1650–1580 cm−1 (O–H/N–H amine bending).
Thermal analysis of the samples by thermogravimetry (TGA) and differential scanning calorimetry (DSC) provides information on the stability of the samples with temperature, which is relevant to define the activation conditions for the samples. A first mass loss corresponding to an endothermic process is observed until about 120 °C (Fig. S3†), attributed to the loss of water and other gases or solvents adsorbed on the sample. From about 230 °C onwards, a second mass loss is observed on the samples functionalized with amines (absent in the parent SBA-15), which corresponds to the exothermic decomposition of the grafted amines. In fact, the mass loss between 150 and 500 °C agrees with the amine content obtained from the chemical analysis; the amine content from TG data gives an estimation of 1.2 mmol g−1 and 1.0 mmol g−1 for APTES@SBA-15 and DEAPTES@SBA-15, respectively. Considering these results, the activation of the samples before adsorption and NMR studies was performed at 150 °C to ensure the proper cleaning of the surface without degrading the samples. These findings are in line with our previous studies4,20 and further confirm the presence of the amines on the surface of SBA-15.
The presence of amines in the samples was further confirmed by 13C ssNMR (Fig. S4†). Peaks with chemical shifts in the 60 to 0 ppm range were observed; APTES@SBA-15 presented three peaks at 8.7, 21.5 and 42.5 ppm corresponding to the propyl chain and DEAPTES@SBA-15 presented peaks at 9.6, 19.4, 46.2 and 56.7 ppm corresponding to the propyl chain and ethyl groups. These values agree with our previous results.4
3.2. Adsorption of CO2 in dry and wet amine-functionalized silicas
The adsorption of CO2 on the sample functionalized with primary amine (APTES@SBA-15) was carried out in the dry sample and in the sample after pre-adsorption of 0.157 mmol g−1 of water (Fig. 1, top). We opted to only use a small amount of water (low coverings) because we are interested in looking at the effect on the pore surface and not on the filling of the pores, when CO2 is adsorbed. The results clearly show that the small amount of water at the surface does not have a noticeable effect on the CO2 adsorption in all pressure ranges (up to 1000 kPa), since both isotherms overlap. Thus, for the present material and conditions, in contrast to other reports in the literature,21,24,28 an enhancement of the CO2 adsorption capacity was not observed due to the presence of moisture. Such differences may be attributed to a higher moisture content and to the dynamic adsorption method used in other studies which cannot guarantee that the material is in equilibrium with the CO2 content in the gas phase.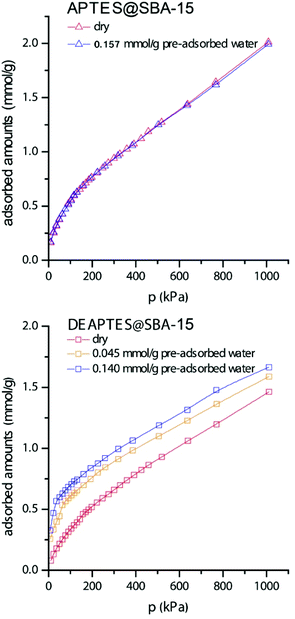 | ||
| Fig. 1 Adsorption isotherms of CO2 at 25 °C in dry and wet materials. Results for SBA-15 grafted with primary and tertiary amines at the top and bottom, respectively. | ||
A different situation was observed for the sample functionalised with tertiary amine (DEAPTES@SBA-15). The adsorption of CO2 on the sample with 0.140 mmol g−1 of pre-adsorbed water (this amount is close to the one pre-adsorbed on the sample with primary amine) was significantly enhanced compared with the adsorption on the dry sample (Fig. 1, bottom). The amounts of adsorbed CO2 were increased by about 0.24 mmol g−1 at low pressures and this difference was maintained in all pressure ranges between the dry sample and the sample with 0.140 mmol g−1 of water. It must be noticed that the amount of CO2 adsorbed at the surface approaches a ratio of one CO2 molecule for two amines, in the low-pressure region (below 21 kPa), when water is present in the system (0.140 mmol g−1). An experiment with significantly less amount of pre-adsorbed water (0.045 mmol g−1) was also performed and the effect on the CO2 adsorption was still quite significant, since an increase of about 0.17 mmol g−1 of CO2 was observed in all pressure values compared with the dry sample (Fig. 1, bottom).
The results in Fig. 1 for the adsorption of CO2 on both samples with primary and tertiary amines clearly show that the water causes a significant change in the mechanism of adsorption of CO2 on surfaces with tertiary amine which leads to increased amounts of adsorbed CO2. This effect is observed at very low pressures for the tertiary amine (i.e., water increases the affinity of the CO2 for the surface) but is absent in the case of the primary amine. In fact, no noticeable CO2 adsorption change was observed for the primary amine in the presence of small amounts of water.
3.3. Experimental strategy to study the effect of moisture on CO2 speciation
To perform NMR characterization study, all the samples were packed into the NMR sample holder in a controlled atmosphere (see the experimental section for further details). It was found that such rigorous sample handling is of utmost importance as it enables the observation of 13C resonances associated with certain CO2 species that are otherwise not detected.4,20Tertiary amines are not expected to react directly with CO2 as they cannot be deprotonated and are bulkier than primary or secondary amines.61–63 Instead, tertiary amines capture CO2 in the aqueous phase through a different mechanism (via bicarbonate formation), first reported by Donaldson31 and later reviewed by Kenig.62 First, in water, the tertiary amine dissociates H2O to form a quaternary cationic species and OH−. Hydroxide ion then attacks CO2 to form the bicarbonate anion, which may also form with primary and secondary amines although the rate constants for this base catalysed bicarbonate formation in water are typically smaller than those of the zwitterion mechanism described above.31,62 While this mechanism has been mostly studied in the solution state, not much is known about it at the surface of porous materials. One of the goals of this work is to obtain a better molecular-level understanding of the formation of such species on solid surfaces functionalized with amines. For that reason, an experimental protocol was optimised in which primary and tertiary amines are grafted onto SBA-15 and exposed to pure CO2 (for comparison) and binary 13CO2/water mixtures, with different compositions. One sample of APTES@SBA-15 was prepared by loading the material with 2.7 kPa of water vapor followed by adsorption of 100 kPa of 13CO2. For the case of tertiary amines, several experiments were conducted, in which DEAPTES@SBA-15 was loaded with a specific water vapor partial pressure (0.7, 1.3, and 2.7 kPa) prior to 13CO2 adsorption (2.7, 13, 100–103 kPa).
The experimental strategy designed in this work is significantly different from that of other reported studies where 13C NMR signals for either physisorbed or chemisorbed CO2 were not detected when the solid sorbent (SBA15 functionalised with N,N-dimethyl-3-aminopropyltrimethoxysilane) is mixed with water, forming a slurry.40 In contrast, exposure of our amine-functionalized sorbents (dried under high vacuum at 150 °C for 3 h) to a water/13CO2 mixture in the gas phase enabled the observation of moisture-induced CO2 species even for small water partial pressures (e.g., 0.7 kPa). Therefore, we avoided the use of liquid water slurries, which may induce the formation of CO2 species dissolved in the excess of water, particularly due to the high pH produced by the presence of amines.
The discussion regarding CO2 speciation in the presence of moisture has been categorized by amine type, primary (Section 3.4) and tertiary (Section 3.5), in an attempt to rationalize the observed significant differences in CO2 speciation as confirmed by their 1H/13C NMR spectra. The role of water in enhancing CO2 sorption capacity on primary amines due to the formation of bicarbonate species has been suggested based on the hypothesis that the CO2/amine ratio increases with moisture, but molecular-level insight into this mechanism remains elusive and a rather complex issue, as will be discussed in Section 3.4. The following sections aim at shedding light into this matter.
3.4. Water influence on CO2 speciation for sorbents modified with primary amines
The 13C NMR spectra show that, in the presence of water, the resonance associated with alkylammonium carbamate species (denoted as species C in Fig. 2a) is the most abundant and also the most stable (see Table 1). Under strictly dry conditions, species A, B and C (Fig. 2b) are present in the NMR spectrum of CO2-loaded SBA15-APTES (Fig. 2a). Henceforth, a prime symbol (′) following the letter label (e.g., A′, B′, C′) will be used to denote 13C resonances in the NMR spectra of “wet” samples to avoid confusion. Upon water uptake (2.7 kPa of H2O), the 13C NMR spectrum changes considerably; species A (detected in dry samples) disappears, as expected from our previous studies,4 and another resonance emerges in moist samples, here denoted as resonance A′ (160.9 ppm), which is considerably narrower than resonance B (160.4 ppm) only observed in dry SBA15-APTES (cf.Fig. 2a top and bottom). A′ is also slightly shifted by ∼0.5 ppm with respect to resonance B. Therefore, NMR data seem to provide strong indications that B and A′ are not the same species.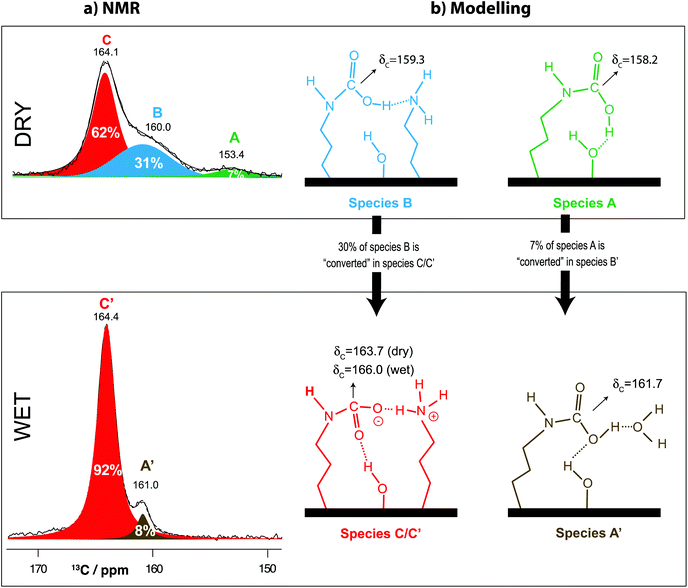 | ||
| Fig. 2 (a) 13C single-pulse NMR spectra recorded at 12 kHz MAS on a 16.4 T spectrometer of samples containing APTES@SBA-15 loaded with 103 kPa of 13CO2 (top, solid line) and 2.7 kPa of water followed by 100 kPa of 13CO2 (bottom, solid line). Deconvolution of each spectrum is given in the figure (overall fit as a dashed line). Species B was fitted with a Lorentzian function whereas A′ had to be fitted using a purely Gaussian function to minimize errors; (b) A, B, and C denote the three resonances attributed to the chemisorbed CO2 species, according to our previous work.4,9,20 Spectra were normalised with respect to their methylene 13C peak areas thus enabling direct comparison of their peak areas. Note that all spectra were recorded using the exact same acquisition and processing parameters and refer to the same batch sample. | ||
| Species | ΔG (kJ mol−1) |
|---|---|
| Without H 2 O | |
| Adsorbed CO2 | 43 |
| Ammonium carbamate | 4 |
| Carbamic acid | 0 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| With H 2 O | |
| Adsorbed CO2 and H2O | 68 |
| Bicarbonate/carbonic acid | 45 |
| Carbamic acid | 29 |
| Ammonium carbamate | 0 |
The observed chemical shift changes in 13C NMR spectra under dry and moist APTES@SBA-15 (cf.Fig. 2a) provide an excellent opportunity to study the effect of water on CO2 speciation. A quantitative analysis of the 13C NMR spectra reveals that about 31% of carbamic acid species (B), observed under dry conditions, vanishes. The resonance associated with this species (B) seems to have been converted into alkylammonium carbamate ion pairs (C′), in the presence of moisture (62% of C + 31% of B are converted into 92% of C′, Fig. 2a). This observation clearly demonstrates that CO2 adsorption under humid conditions favours the formation of alkylammonium carbamate ion pairs (C) instead of bicarbonate species as suggested in the literature.28,39 This finding complies with previous studies claiming that the amount of carbamic acid (B) is significantly reduced for CO2 adsorption in a moist environment as compared to dry conditions. Bacsik et al.28 suggest that this difference might be due to water mediated formation of ammonium carbamates from carbamic acids being H-bonded with –NH2 groups (as in the case of species B, Fig. 2b). Molecular modeling results reflect this phenomenon extremely well showing that water induces the proton transfer from carbamic acid to neighbouring amines, reflecting a water-induced transformation of carbamic acid (B) into ammonium carbamate (C′) (Fig. 2b). Our molecular modeling study shows that only when the water molecule interacts with isolated carbamic acid species (A) it is possible to stabilize the carbamic acid species labeled A′ (Fig. 2b). The final relative stability of the different species (represented in Table 1) shows that ammonium carbamate is substantially more stable than carbamic acid (or bicarbonate) in the presence of water (unlike under dry conditions), supporting the idea that carbamic acid (B) might be converted into ammonium carbamate (C′) upon water uptake.
Our previous work showed how sensitive resonance A is to very low H2O partial pressures. i.e., this resonance (∼155 ppm) vanishes as soon as water interacts with the CO2 binding site.4 One may question if water induces desorption of A or if this species is converted to another CO2 species. Simulation results (shown in Tables 1 and S2†) seem to indicate that adsorbed H2O and CO2 are always less stable than the formation of chemisorbed CO2 species, thus strongly hinting at the latter hypothesis. Due to their similar peak areas, the initial 7% of species A present in the dry APTES@SBA-15 sample (Fig. 2a, top) seems to be converted into species A′ (8%, Fig. 2a, bottom), which might be assigned either to putative bicarbonate (presenting the same 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CO2/N stoichiometry as species A, Fig. 2b) or carbamic acid. If we consider that species A′ is ascribed to bicarbonate, the amount observed (8%) would not justify what some authors report as an increase in CO2 uptake under moist conditions.1,28,34,35,64 That was not confirmed in our material under the conditions of pressure and temperature of adsorption (Fig. 1). Table 1 presents a comparison of the calculated Gibbs energies of the different species, which may exist when two amines closely interact with each other, under dry and humid conditions. Under dry conditions, the Gibbs energy values for carbamic acid and carbamate species are very similar, with the latter being only 4 kJ mol−1 less stable than the former. When water is introduced into the theoretical system, there is a radical shift in relative stabilities; alkylammonium carbamate stability increases by 29 kJ mol−1 compared to carbamic acid. This supports the idea that the experimentally observed shift from species B to species C is likely due to the conversion of carbamic acid into ammonium carbamate. It seems that the intricate network of hydrogen bonded water molecules aids in the proton transfer from the carbamate group to the amino group. Moreover, under humid conditions, the bicarbonate species is even less stable than carbamic acid (45 kJ mol−1 less stable than ammonium carbamate, Table 1), which does not vouch for a dominant presence of bicarbonate species.
1 CO2/N stoichiometry as species A, Fig. 2b) or carbamic acid. If we consider that species A′ is ascribed to bicarbonate, the amount observed (8%) would not justify what some authors report as an increase in CO2 uptake under moist conditions.1,28,34,35,64 That was not confirmed in our material under the conditions of pressure and temperature of adsorption (Fig. 1). Table 1 presents a comparison of the calculated Gibbs energies of the different species, which may exist when two amines closely interact with each other, under dry and humid conditions. Under dry conditions, the Gibbs energy values for carbamic acid and carbamate species are very similar, with the latter being only 4 kJ mol−1 less stable than the former. When water is introduced into the theoretical system, there is a radical shift in relative stabilities; alkylammonium carbamate stability increases by 29 kJ mol−1 compared to carbamic acid. This supports the idea that the experimentally observed shift from species B to species C is likely due to the conversion of carbamic acid into ammonium carbamate. It seems that the intricate network of hydrogen bonded water molecules aids in the proton transfer from the carbamate group to the amino group. Moreover, under humid conditions, the bicarbonate species is even less stable than carbamic acid (45 kJ mol−1 less stable than ammonium carbamate, Table 1), which does not vouch for a dominant presence of bicarbonate species.
Successive experimental and theoretical results3,4,9,20,38,43,44,65 indicate that both carbamic acid and bicarbonate resonate at 159–162 ppm, thus making it particularly difficult to distinguish between these two species on the basis of NMR spectra alone. Considering the computational results and the 13C NMR quantitative analysis shown previously, we assign the CO2 species A′ to a non-paired (isolated) carbamic acid species interacting with water molecules (with or without neighbouring silanols), the same that generates resonance A under dry conditions (Fig. 2). Indeed, the addition of water molecules into the simulation clusters systematically shifts the 13C resonances attributed to carbamic acid, towards higher CS values (Table S3†), i.e., in the direction A → A′.
With the strategy described in this work, it was possible to evaluate the influence of water in the CO2 chemisorption process on primary amines. To unequivocally identify the formation of bicarbonate species, a complementary study using tertiary amine-grafted materials is also pursued in Section 3.5 as carbamic acid formation is not favoured by bulkier amines, thus providing additional structural insight. Moreover, studies on CO2 speciation involving silica materials modified with tertiary amines under rigorous control over partial pressures of adsorbed gases were not reported so far.
3.5. Water influence on CO2 speciation for sorbents modified with tertiary amines
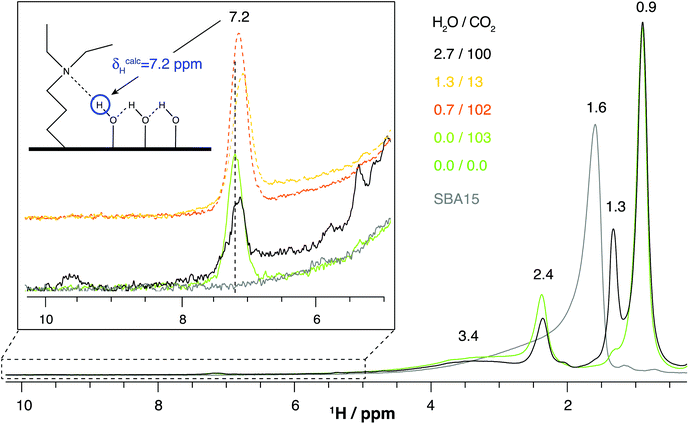 | ||
| Fig. 3 1H Hahn-echo NMR spectra of pristine SBA-15, DEAPTES@SBA-15 and the latter exposed to distinct combinations of water and CO2 partial pressures (in kPa), recorded at 16.4 T with a spinning rate of 15 kHz. Additional parameters are provided in the experimental section. The structure model exhibiting the 1H resonance at 7.2 ppm is shown in Fig. S6.† | ||
 | ||
| Fig. 4 (a) 13C CPMAS NMR spectra of DEAPTES@SBA-15 materials, recorded at 16.4 T with a spinning rate of 12 kHz, after exposure to a given partial pressure of H2O followed by exposure to 13CO2. Specific partial pressures are given in the figure, in kPa. (b) Two-dimensional representation of the optimised structures for bicarbonate and carbamic acid-like species (the corresponding 3D structure models are depicted in Fig. 5 and 6). Spectra were normalised with respect to the intensity of the propylamine carbons to obtain a fair comparison between NMR spectra at varying CO2 and H2O partial pressures. | ||
For comparison purposes, an additional sample was prepared, in which pure 13CO2 was adsorbed under dry conditions (103 kPa of 13CO2). In this case, a weak resonance appears at 154.8 ppm (Fig. 4), ascribed to carbamic acid-like species as supported by the structure models presented in Fig. 6a and c. The two models depicted in Fig. 6a–d exhibit combinations of possible structures resulting from the reaction between 3ary amines and CO2. These models are all compatible with CO2-adducts giving rise to a 13C chemical shift lower than 156 ppm, supporting the fact that the activation of CO2 by 3ary amines is possible under dry conditions albeit to a very small extent given the low intensity of the resonance. Notice that a hypothetical CO2 zwitterion (Fig. 6b and d) was also computed to check whether such species could be compatible with our experimental 13C CS (154.8 ppm). A calculated 13C CS of 141.6 ppm was obtained for the zwitterion, clearly far from this resonance observed at 154.8 ppm, presumably associated with carbamic acid species (Fig. 6a and c) generated by proton transfer from surrounding silanol groups to the N–COO- group. Such silanol groups are likely to possess stronger Brønsted acidity, induced by framework defects, thus facilitating the proton extraction by –+N–COO− zwitterion species. Thus, the number of amines that can bind CO2 under dry conditions is limited to those that have Brönsted acid groups in the vicinity, explaining in this way the lower intensity of the 154.8 ppm peak (Fig. 4).
The presence of this 13C resonance at 154.8 ppm is typical of chemisorbed CO2-adducts of the type A (carbamic acid), as found in 1ary amines (Fig. 2). This may seem counterintuitive for 3ary amines as their bulkiness and the absence of NH protons are believed to hinder the formation of carbamic acid species. Hence, the presence of this resonance in the 13C MAS NMR spectrum strongly supports the fact that 3ary amines can act as a nucleophilic agent and react with the CO2 Lewis acid. Molecular models containing 3ary amines bonded to CO2, also support this fact as the resulting carbonyl carbon exhibits a theoretical 13C CS of 155.6 ppm (Fig. 6), typical of carbamic acid functional groups. Note that this resonance vanishes by just exposing the sample at very low water vapor pressure (0.7 kPa), resembling the behaviour of CO2 species A in primary amines as reported in our previous work.4,9,20 We emphasize that the disappearance of this resonance depends on the order in which CO2 and H2O are adsorbed and in their relative partial pressures (Fig. 7). Notice that only when a significant difference between the partial pressures of both gases is used, the resulting 13C NMR spectrum is altered depending on whether water is added first or if both gases are added simultaneously (Fig. 7). For example, the sample loaded with 1.3 kPa of water vapor and 101 kPa of 13CO2 gives rise to a single 13C resonance at 161 ppm, when the material is exposed to water vapour prior to CO2 adsorption. On the other hand, on exposing the material simultaneously to a binary mixture containing both water vapor and 13CO2 at similar partial pressures, a peak emerges at 154.8 ppm (Fig. 7, bottom), resembling the spectrum of pure CO2 loaded materials. Although, in both cases described earlier, the water vapor pressure is kept constant (1.3 kPa), the order in which water is mixed with CO2 will favour the formation of either carbamic acid or bicarbonate species (Fig. 7 and 4b). The formation of the former species (under moist conditions) can be explained by the much lower accessibility of water to the amine binding sites when CO2 concentration is overwhelmingly superior to that of water when both gases are adsorbed simultaneously.
Focusing on samples with a similar amount of CO2 partial pressure (ca. 100 kPa), namely samples with the following H2O/CO2 content: 2.7/100, 1.3/101 and 0.7/102 (black, red and orange lines in Fig. 4a, respectively; units in kPa), it is possible to observe that as the partial pressure of water vapor increases, the intensity of the peak also increases and the centre of the main peak shifts (from 160.1 ppm) towards higher chemical shift values, up to the final value of 161.1 ppm. The same observation is valid for the sample with the same partial pressure of CO2 and H2O (2.7/2.7, dark green line in Fig. 4a), in which the peak centre is slightly shifted to lower chemical shift values (ca. 160.5 ppm). In this case, although the amount of water is the same as for sample 2.7/100 (black line, Fig. 4a), the amount of CO2 available (2.7 kPa) is not sufficient to generate as much bicarbonate (centred at 161.1 ppm) as in the 2.7/100 sample. It is worth mentioning that 2.7 kPa is the highest water vapor pressure we were able to admit, at room temperature. For the sample loaded with 0.7 kPa of water vapor (orange line in Fig. 4a), the spectrum is more complex, showing at least three distinct chemical environments in the region between 161 and 164 ppm, associated with the formation of moisture-induced CO2 species (see dashed lines in Fig. 4a).
To obtain a deeper insight into the intermolecular interactions associated with the formation of bicarbonate species, computer models comprising this species engaged in multiple H-bonding modes were investigated. Fig. 5 depicts the lowest-energy optimised structure model for the bicarbonate ion interacting with a tertiary ammonium ion, surrounded by silanol groups and a water molecule with the corresponding calculated and experimental 1H and 13C CS. This model was the most compatible with the NMR data; i.e., calculated 13C and 1H CS values obtained from this model (159.9 and 8.1 ppm, respectively) are in close agreement with the experimental CS (cf.Fig. 4 and 5).
To better support the proposed model for the bicarbonate species (presented in Fig. 5), we have explored 1H NMR-based techniques, namely two-dimensional (2D) 1H–13C (Fig. 8) and 1H–29Si (Fig. S7†) HETCOR NMR experiments that rely on through-space dipole–dipole interactions to selectively detect 1H⋯13C and 1H⋯29Si nuclear proximities, respectively. The 2D 1H–13C HETCOR spectrum (Fig. 8) of DEAPTES@SBA-15, after exposure to 2.7 kPa of water and 100 kPa 13CO2, shows 1H–13C cross-peaks arising from the alkylamine carbons (resonating at 9.9, 20.2, 46.6 and 55.7 ppm) interacting with the different methylene (CH2) and methyl (CH3) protons (between 0 and 5 ppm) from the amine moiety (see assignment in Fig. 8).
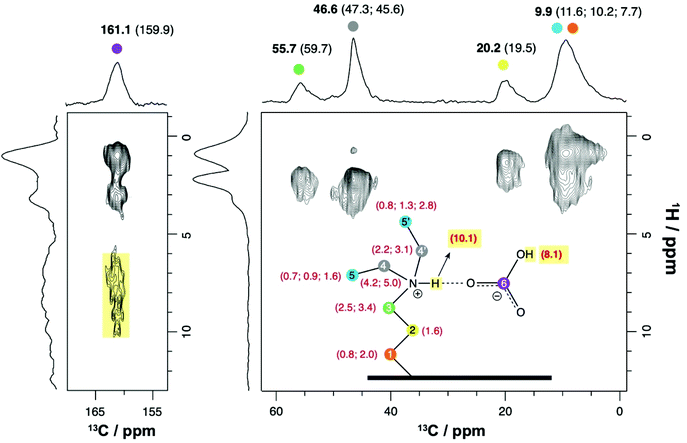 | ||
| Fig. 8 1H–13C CPMAS HETCOR NMR spectrum of DEAPTES@SBA-15 loaded with 2.7 kPa of water and 100 kPa of 13CO2, recorded at 16.4 T with a spinning rate of 12 kHz and using a contact time of 2 ms. Additional parameters are provided in the experimental section. Numbers in black and bold depict experimental 13C CSs (in ppm) and numbers in parenthesis (red for 1H, black for 13C) refer to calculated CSs from the structure model displayed in Fig. 5. The calculated 1H CSs for N–H and bicarbonate protons are highlighted in yellow. | ||
The bicarbonate 13C resonance at 161.1 ppm presents two strong correlations with 1H resonances at 1.0 and 3.2 ppm. The former is assigned to both Si–CH2 and CH3 groups, whose signals overlap, while the latter is associated with two 13C resonances: the methylene carbon adjacent to the methyl group at 46.6 ppm (marked grey in Fig. 8) and the bicarbonate carbon. This seems to suggest that the CH2 protons at 3.2 ppm are in close proximity to the bicarbonate molecule as demonstrated in the model shown in Fig. 5.
Even though the low signal-to-noise ratio presented in this spectrum, in the 1H CS region between 6 and 10 ppm (Fig. 8, 4 days acquisition time), does not allow us to distinguish among the different 1H environments interacting with the bicarbonate molecule (δC = 161.1 ppm, Fig. 5), a 13C–1H cross-peak is clearly visible in that region centered at ca. 7–8 ppm. Calculated 1H and 13C CSs (shown in Fig. 8, close to the respective atoms) from the proposed lowest-energy DEAPTES-bicarbonate structure model (Fig. 5) exhibit protons that are strongly deshielded compared to the calculated 1H CS for isolated DEAPTES (Fig. S8†) due to the polarization effect of bicarbonate molecules in their proximity. The DEAPTES-bicarbonate structure model shows that specific 1H CSs are deshielded with respect to isolated DEAPTES i.e., 1H CSs from C(1)H2 and C(5′)H3 protons are shifted from 0.8 to 2.0 and from 1.6 to 2.8 ppm, respectively, due to their interaction with the bicarbonate oxygen atom through CH⋯O weak H-bonds (Fig. 5). The 2D 1H–13C HETCOR spectrum of DEAPTES@SBA-15 seems to agree with this observation as both these functional groups contribute to the cross-peaks appearing at δ(1H) ≥ 2 ppm. The calculated 1H CS of the DEAPTES +NH group appears at 10.1 ppm upon H-bonding with bicarbonate, which is difficult to ascertain through comparison with the experimental data. Nevertheless, in addition to the peak at ca. 7.1 ppm, observed in the 1H NMR spectra (Fig. 3), a weak resonance at 9.6 ppm is also visible. Although this peak (9.6 ppm, Fig. 3) matches appreciably well with the calculated 10.1 ppm for the bicarbonate model (N–H proton), we prefer to remain conservative by not proposing an unequivocal assignment of this resonance.
The bicarbonate molecular pair model exhibits an OH proton at 8.1 ppm, correlated with the bicarbonate 13C resonance at 159.9 ppm (Fig. 5), which is in good agreement with the experimental NMR data displaying a cross-peak at similar 1H and 13C CS values, at 7–8 ppm and ca. 161 ppm, respectively (Fig. 8).
4. Conclusions
The CO2 speciation on primary and tertiary amine-modified mesoporous silicas under dry and wet conditions has been studied combining solid-state NMR and computer modelling. Adsorption of 13CO2 and water vapor under controlled partial pressures, followed by the application of solid-state NMR methods was essential to gather molecular-level information on the moisture-induced CO2 species formed in such sorbent materials.Our study shows that CO2 adsorbed to primary amines, under humid conditions favours the formation of alkylammonium carbamate ion pairs instead of bicarbonate species. Molecular modeling and theoretical Gibbs energy results agree with a water-induced transformation of carbamic acid into alkylammonium carbamate, supported by the fact that the latter species is energetically much more stable than carbamic acid (or bicarbonate) in the presence of water, as opposed to dry conditions. Adsorption data of APTES@SBA-15 with pure CO2 and mixed CO2 + H2O show that water does not seem to increase the CO2 adsorption up to ∼1000 kPa.
In contrast, for the case of tertiary amines, the CO2 adsorbed amounts were significantly enhanced with the presence of minor amounts of water at the surface (0.140 mmol g−1 of water leads to a 0.24 mmol g−1 increase in adsorbed CO2). Special focus was given to two chemisorbed CO2 species identified, by NMR and DFT calculations, from distinct carbonyl environments resonating at δC ∼161 and 155 ppm, assigned to bicarbonate and a carbamic acid-like species, respectively. Unlike previous studies in the literature, that detect the formation of bicarbonate species only when slurries are formed, in this study bicarbonate is detected for DEAPTES@SBA-15 exposed to water vapor pressures from 0.7 kPa up to values close to water vapor saturation pressure at ambient temperature (2.7 kPa).
The presence of a 13C resonance at ∼155 ppm and the agreement with the calculated 13C CS extracted from structure models (<156 ppm) support the fact that tertiary amines may directly activate CO2 to form carbamic acid-like species in the vicinity of Brönsted protons covering the silica surface. This species was observed under dry conditions, as well as under the adsorption of a CO2/H2O binary mixture with partial pressures of 101 and 1.3 kPa, respectively. Atomic scale studies of CO2 speciation upon gas adsorption in solid surfaces are still scarce; this work attempts to shorten this gap by shedding light on how the chemisorbed CO2 species are formed and interconverted, under moist conditions, using amine residues presenting distinct bulkiness.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was developed in the scope of project CICECO-Aveiro Institute of Materials from the University of Aveiro ref. UIDB/50011/2020 & UIDP/50011/2020, project CERENA ref. UIDB/04028/2020 & UIDP/04028/2020. We also acknowledge funding from projects ref. PTDC/QUI-QFI/28747/2017 (GAS2MAT-DNPSENS - POCI-01-0145-FEDER-028747), PTDC/QUI-QFI/31002/2017 (SILVIA - CENTRO-01-0145-FEDER-31002), PTDC/QEQ-QAN/6373/2014 and Smart Green Homes POCI-01-0247-FEDER-007678, a co-promotion between Bosch Termotecnologia S.A. and the University of Aveiro. These projects are financed through FCT/MEC and co-financed by FEDER under the PT2020 Partnership Agreement. The NMR spectrometers are part of the National NMR Network (PTNMR) and are partially supported by Infrastructure Project 022161 (co-financed by FEDER through COMPETE 2020, POCI and PORL and FCT through PIDDAC). This work has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement no. 865974).References
- E. S. Sanz-Pérez, C. R. Murdock, S. A. Didas and C. W. Jones, Chem. Rev., 2016, 116, 11840–11876 CrossRef.
- P. M. MacQueen, R. A. Bach, C. T. P. MacLean and S. L. MacQuarrie, J. Phys. Chem. C, 2014, 118, 5239–5242 CrossRef CAS.
- M. L. Pinto, L. L. Mafra, J. M. Guil, J. Pires and J. Rocha, Chem. Mater., 2011, 23, 1387–1395 CrossRef CAS.
- L. Mafra, T. Čendak, S. Schneider, P. V. Wiper, J. Pires, J. R. B. Gomes and M. L. Pinto, J. Am. Chem. Soc., 2017, 139, 389–408 CrossRef CAS.
- D. Reichle, J. Houghton, S. Benson, J. Clarke, R. Dahlman, D. G. Hendrey, H. Herzog, J. Hunter-Cevera, G. Jacobs, R. Judkins, B. Kane, J. Ekmann, J. Ogden, A. Palmisano, D. R. Socolow, J. Stringer, T. Surles, A. Wolsky, N. Woodward, D. M. York and D. Ii, Carbon Sequestration – State of the Science, Washington DC, 1999 Search PubMed.
- B. A. Oyenekan and G. T. Rochelle, AIChE J., 2007, 53, 3144–3154 CrossRef CAS.
- A. S. Bhown and B. C. Freeman, Environ. Sci. Technol., 2011, 45, 8624–8632 CrossRef CAS.
- X. Xu, C. Song, B. G. Miller and A. W. Scaroni, Ind. Eng. Chem. Res., 2005, 44, 8113–8119 CrossRef CAS.
- R. Afonso, M. Sardo, L. Mafra and J. R. B. Gomes, Environ. Sci. Technol., 2019, 53, 2758–2767 CrossRef CAS.
- S. Nandi, S. Collins, D. Chakraborty, D. Banerjee, P. K. Thallapally, T. K. Woo and R. Vaidhyanathan, J. Am. Chem. Soc., 2017, 139, 1734–1737 CrossRef CAS.
- P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Ma, B. Space, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, Nature, 2013, 495, 80–84 CrossRef CAS.
- A. Asghar, N. Iqbal, L. Aftab, T. Noor, B. M. Kariuki, L. Kidwell and T. L. Easun, R. Soc. Open Sci., 2020, 7, 191934 CrossRef CAS.
- Y. Chen, Z. Qiao, J. Huang, H. Wu, J. Xiao, Q. Xia, H. Xi, J. Hu, J. Zhou and Z. Li, ACS Appl. Mater. Interfaces, 2018, 10, 38638–38647 CrossRef CAS.
- S. Chand, A. Pal and M. C. Das, Chem.–Eur. J., 2018, 24, 5982–5986 CrossRef CAS.
- J. Zhu, L. Wu, Z. Bu, S. Jie and B.-G. Li, ACS Omega, 2019, 4, 3188–3197 CrossRef CAS.
- Y. Wang, Z. Hu, T. Kundu, Y. Cheng, J. Dong, Y. Qian, L. Zhai and D. Zhao, ACS Sustainable Chem. Eng., 2018, 6, 11904–11912 CrossRef CAS.
- D. Andirova, Y. Lei, X. Zhao and S. Choi, ChemSusChem, 2015, 8, 3405–3409 CrossRef CAS.
- I. Yanase, K. Sato, H. Kobayashi, T. Doe and T. Naka, Chem. Eng. J., 2019, 356, 81–90 CrossRef CAS.
- K. Essaki, K. Nakagawa, M. Kato and H. Uemoto, J. Chem. Eng., 2004, 37, 772–777 CrossRef CAS.
- T. Čendak, L. Sequeira, M. Sardo, A. Valente, M. L. Pinto and L. Mafra, Chem.–Eur. J., 2018, 24, 10136–10145 CrossRef.
- A. Sayari and Y. Belmabkhout, J. Am. Chem. Soc., 2010, 132, 6312–6314 CrossRef CAS.
- E. E. Ünveren, B. Ö. Monkul, Ş. Sarıoğlan, N. Karademir and E. Alper, Petroleum, 2017, 3, 37–50 CrossRef.
- J. Yu and S. S. C. Chuang, Ind. Eng. Chem. Res., 2017, 56, 6337–6347 CrossRef CAS.
- H. Zhang, A. Goeppert, G. A. Olah and G. K. S. Prakash, J. CO2 Util., 2017, 19, 91–99 CrossRef CAS.
- N. Hiyoshi, K. Yogo and T. Yashima, Microporous Mesoporous Mater., 2005, 84, 357–365 CrossRef CAS.
- N. Hiyoshi, K. Yogo and T. Yashima, Stud. Surf. Sci. Catal., 2004, 153, 417–422 CrossRef CAS.
- N. Hiyoshi, K. Yogo and T. Yashima, Chem. Lett., 2004, 33, 510–511 CrossRef CAS.
- Z. Bacsik, N. Ahlsten, A. Ziadi, G. Zhao, A. E. Garcia-Bennett, B. Martín-Matute and N. Hedin, Langmuir, 2011, 27, 11118–11128 CrossRef CAS.
- G. P. Knowles, S. W. Delaney and A. L. Chaffee, Ind. Eng. Chem. Res., 2006, 45, 2626–2633 CrossRef CAS.
- G. P. Knowles, J. V. Graham, S. W. Delaney and A. L. Chaffee, Fuel Process. Technol., 2005, 86, 1435–1448 CrossRef CAS.
- T. L. Donaldson and Y. N. Nguyen, Ind. Eng. Chem. Fundam., 1980, 19, 260–266 CrossRef CAS.
- S. Choi, J. H. Drese and C. W. Jones, ChemSusChem, 2009, 2, 796–854 CrossRef CAS.
- P. Bollini, S. a. Didas and C. W. Jones, J. Mater. Chem., 2011, 21, 15100–15120 RSC.
- M. W. Hahn, M. Steib, A. Jentys and J. A. Lercher, J. Phys. Chem. C, 2015, 119, 4126–4135 CrossRef CAS.
- K. Li, J. D. Kress and D. S. Mebane, J. Phys. Chem. C, 2016, 120, 23683–23691 CrossRef CAS.
- R. Zhang, Z. Liang, H. Liu, W. Rongwong, X. Luo, R. Idem and Q. Yang, Ind. Eng. Chem. Res., 2016, 55, 3710–3717 CrossRef CAS.
- J. P. Jakobsen, J. Krane and H. F. Svendsen, Ind. Eng. Chem. Res., 2005, 44, 9894–9903 CrossRef.
- G. S. Foo, J. J. Lee, C.-H. Chen, S. E. Hayes, C. Sievers and C. W. Jones, ChemSusChem, 2017, 10, 266–276 CrossRef CAS.
- S. A. Didas, M. A. Sakwa-novak, G. S. Foo, C. Sievers and C. W. Jones, J. Phys. Chem. Lett., 2014, 5, 4194–4200 CrossRef CAS.
- C.-H. Chen, D. Shimon, J. J. Lee, F. Mentink-Vigier, I. Hung, C. Sievers, C. W. Jones and S. E. Hayes, J. Am. Chem. Soc., 2018, 140, 8648–8651 CrossRef CAS.
- K. Gottschling, L. Stegbauer, G. Savasci, N. A. Prisco, Z. J. Berkson, C. Ochsenfeld, B. F. Chmelka and B. V. Lotsch, Chem. Mater., 2019, 31, 1946–1955 CrossRef CAS.
- J. K. Moore, M. A. Sakwa-Novak, W. Chaikittisilp, A. K. Mehta, M. S. Conradi, C. W. Jones and S. E. Hayes, Environ. Sci. Technol., 2015, 49, 13684–13691 CrossRef CAS.
- C.-H. Chen, D. Shimon, J. J. Lee, S. A. Didas, A. K. Mehta, C. Sievers, C. W. Jones and S. E. Hayes, Environ. Sci. Technol., 2017, 51, 6553–6559 CrossRef CAS.
- J. J. Lee, C.-H. Chen, D. Shimon, S. E. Hayes, C. Sievers and C. W. Jones, J. Phys. Chem. C, 2017, 121, 23480–23487 CrossRef CAS.
- X. Li, E. Hagaman, C. Tsouris and J. W. Lee, Energy Fuels, 2003, 17, 69–74 CrossRef CAS.
- L. Mafra, T. Čendak, S. Schneider, P. V. Wiper, J. Pires, J. R. B. Gomes and M. L. Pinto, Chem. Eng. J., 2018, 336, 612–621 CrossRef CAS.
- S. M. Antao, I. Hassan, J. Wang, P. L. Lee and B. H. Toby, Can. Mineral., 2008, 46, 1501–1509 CrossRef CAS.
- J. R. B. Gomes, M. N. D. S. Cordeiro and M. Jorge, Geochim. Cosmochim. Acta, 2008, 72, 4421–4439 CrossRef CAS.
- J. R. Gomes, F. Illas and B. Silvi, Chem. Phys. Lett., 2004, 388, 132–138 CrossRef CAS.
- N. Lopez, F. Illas and G. Pacchioni, J. Am. Chem. Soc., 1999, 121, 813–821 CrossRef CAS.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2007, 120, 215–241 Search PubMed.
- Y. Zhao and D. G. Truhlar, J. Phys. Chem. A, 2006, 110, 5121–5129 CrossRef CAS.
- P. C. Hariharan and J. A. Pople, Theor. Chim. Acta, 1973, 28, 213–222 CrossRef CAS.
- M. M. Francl, W. J. Pietro, W. J. Hehre, J. S. Binkley, M. S. Gordon, D. J. DeFrees and J. A. Pople, J. Chem. Phys., 1982, 77, 3654–3665 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision B.01, Gaussian, Inc., Wallingford CT, 2010 Search PubMed.
- K. Wolinski, J. F. Hinton and P. Pulay, J. Am. Chem. Soc., 1990, 112, 8251–8260 CrossRef CAS.
- J. R. Cheeseman, G. W. Trucks, T. A. Keith and M. J. Frisch, J. Chem. Phys., 1996, 104, 5497–5509 CrossRef CAS.
- M. W. Lodewyk, M. R. Siebert and D. J. Tantillo, Chem. Rev., 2012, 112, 1839–1862 CrossRef CAS.
- D. Flaig, M. Maurer, M. Hanni, K. Braunger, L. Kick, M. Thubauville and C. Ochsenfeld, J. Chem. Theory Comput., 2014, 10, 572–578 CrossRef CAS.
- D. Massiot, F. Fayon, M. Capron, I. King, S. Le Calvé, B. Alonso, J.-O. Durand, B. Bujoli, Z. Gan and G. Hoatson, Magn. Reson. Chem., 2002, 40, 70–76 CrossRef CAS.
- K. Robinson, A. McCluskey and M. I. Attalla, ChemPhysChem, 2011, 12, 1088–1099 CrossRef CAS.
- P. D. Vaidya and E. Y. Kenig, Chem. Eng. Technol., 2007, 30, 1467–1474 CrossRef CAS.
- J. H. Drese, S. Choi, R. P. Lively, W. J. Koros, D. J. Fauth, M. L. Gray and C. W. Jones, Adv. Funct. Mater., 2009, 19, 3821–3832 CrossRef CAS.
- R. Serna-Guerrero, E. Da’na and A. Sayari, Ind. Eng. Chem. Res., 2008, 47, 9406–9412 CrossRef CAS.
- D. Shimon, C.-H. Chen, J. J. Lee, S. A. Didas, C. Sievers, C. W. Jones and S. E. Hayes, Environ. Sci. Technol., 2018, 52, 1488–1495 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ta09808f |
| This journal is © The Royal Society of Chemistry 2021 |



