A superhydrophobic and porous polymer adsorbent with large surface area†
Li
Gong
abc,
Wenhao
Wu
abc,
Daohui
Lin
 abc and
Kun
Yang
abc and
Kun
Yang
 *abcd
*abcd
aDepartment of Environmental Science, Zhejiang University, Hangzhou 310058, China. E-mail: kyang@zju.edu.cn; Fax: +86-571-88982590; Tel: +86-571-88982589
bKey Laboratory of Environmental Pollution and Ecological Health of Ministry of Education, Hangzhou 310058, China
cZhejiang Provincial Key Laboratory of Organic Pollution Process and Control, Hangzhou 310058, China
dZhejiang University-Hangzhou Global Scientific and Technological Innovation Center, Hangzhou 311200, China
First published on 23rd November 2020
Abstract
A superhydrophobic hypercrosslinked microporous polymer adsorbent, SHMP-1, with a water contact angle of 167° and an oil contact angle < 3°, was synthesized. SHMP-1 has a specific surface area of up to 2100 m2 g−1, higher than the largest one (i.e. 1618 m2 g−1) reported for superhydrophobic materials. It is an amorphous polymer with a highly cross-linked structure of monomers linked by methylene, and consists of packed amorphous porous cells with pore channel widths of 5–19 Å. SHMP-1 can be stable up to 380 °C, and in organic solvents (e.g. ethanol, acetone, carbon tetrachloride, tetrahydrofuran and n-hexane), HCl (5 mol L−1) and NaOH (5 mol L−1) solution. This polymer could be a promising adsorbent for absorption of organic solvents with capacities ranging from 4–25 times its own weight and capturing CO2 with an adsorption capacity of up to 6.02 mmol g−1 (273 K, 1 bar). Moreover, the CO2 adsorption capacity of SHMP-1 will not be decreased significantly (loss < 2.03%) by moisture even at a relative humidity of up to 90%, because of the surface superhydrophobicity.
Superhydrophobic materials, with a water contact angle (WCA) larger than 150°,1 have received substantial attention due to their potential applications in areas such as self-cleaning,2 anti-fouling,3 microdroplet transportation,4 and separating organic compounds from air and water.5,6 Superhydrophobic materials with large surface area are expected to be efficient adsorbents, especially for separation and removal of organic contaminants or oil from water7–9 and for capture of CO2 (ref. 10) or volatile organic compounds11 by suppressing the competition of water molecules on their surface. For example, Zhang et al.12 reported that a superhydrophobic nanoporous polymer with a surface area of 702 m2 g−1 displays larger adsorption capacity (8–15 g g−1) for organic solvents (i.e., nitrobenzene, benzene, kerosene and n-heptane) than traditional adsorbents like active carbon or XAD-4. Moreover, Li et al.13 reported that microporous polymer HCMP-1 is an excellent adsorbent for organic solvents (e.g., 1,2-dichlorobenzene, nitrobenzene and chloroform) and oils (e.g., pump-oil and vegetable-oil), due to its surface superhydrophobicity (WCA = 167°) and large surface area (955 m2 g−1). Therefore, developing superhydrophobic adsorbents with larger surface area is of special significance but remains a great challenge.
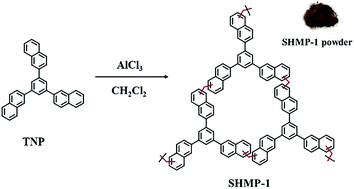
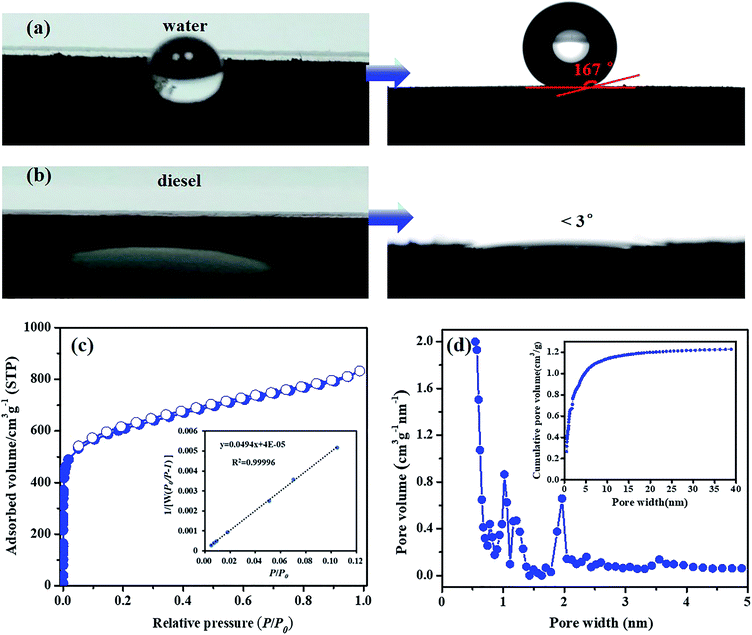
Among the reported superhydrophobic materials, including metal oxides or non-metal oxides,14 carbon materials,15,16 polymers,12,13,17 metal–organic frameworks18 and other composite materials,19,20 the largest specific surface area, 1618 m2 g−1, was observed for the octadecylphosphonic acid modified metal–organic framework PCN-222.21 In this study, a superhydrophobic hypercrosslinked microporous polymer, SHMP-1, a dark brown powder, was synthesized from 1,3,5-trinaphthylbenzene (TNP) using CH2Cl2 as the linker and anhydrous AlCl3 as the catalyst based on the Friedel–Crafts alkylation reaction (Fig. 1). The detailed synthesis of SHMP-1 is described in the ESI. The yield is 123%, calculated from the mass of added TNP using the eqn (S1) listed in the ESI.† This superhydrophobic polymer is an amorphous material (identified using the XRD pattern in Fig. S1†) with a WCA of 167° (Fig. 2a), diesel contact angle < 3° (Fig. 2b), specific surface area of up to 2100 m2 g−1 (Fig. 2c), and a micropore volume of 0.830 cm3 g−1 (Fig. 2d). The specific surface area of SHMP-1 is higher than the largest one reported for superhydrophobic materials (Table S1†), i.e., 1618 m2 g−1.21
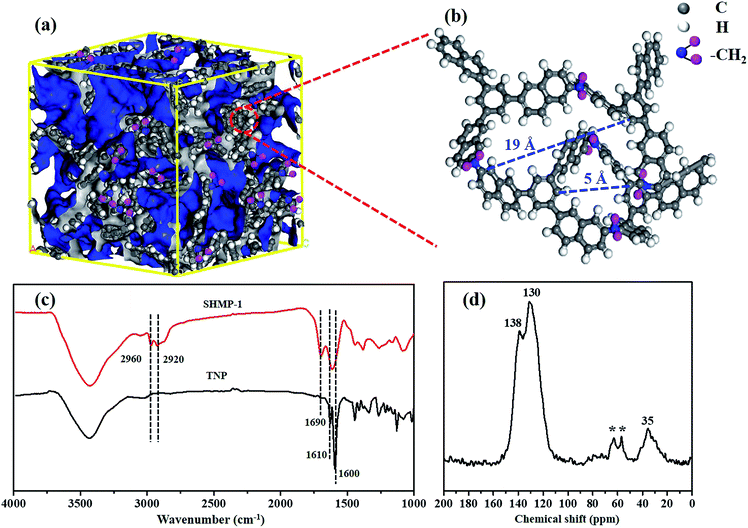
The structure of SHMP-1, simulated by Material Studio v.8.0 software, is a highly cross-linked structure (Fig. 3a) with the molecular formula as (C147H84)n and consists of packed porous cells with a major pore channel ranging from 5 Å to 19 Å (Fig. 3b), where the monomers are cross-linked by methylene. The simulated pore channel widths of SHMP-1 are largely consistent with the pore sizes calculated from the N2 adsorption isotherm (at 77 K) by the NLDFT method (i.e., about 0.5, 1.0 and 2.0 nm in Fig. 2d) and that calculated from the CO2 adsorption isotherm (at 273 K) by the GCMS method for micropores (i.e., about 0.5 and 0.8–0.9 nm Fig. S2†). The appearance of a peak at about 1690 cm−1 and the disappearance of the peak at 1610 cm−1 in the FTIR spectra of SHMP-1 as compared with that of TNP (Fig. 3c), indicating the changes in the vibrations of the conjugated aromatic ring of SHMP-1 compared to TNP,22 is due to the crosslinking of the naphthyl group of TNP with carbon of CH2Cl2. This can be also indicated by the C–H stretching vibrations of –CH2 (ref. 23) at 2920 cm−1 and 2960 cm−1 which appeared in the FTIR spectra of SHMP-1 (Fig. 3c). Moreover, the resonance peak around 35 ppm in the solid-state 13C CP/MAS NMR spectrum of SHMP-1 (Fig. 3d), indicates that the carbon of the liquid methylene cross-linkers has been crosslinked in the solid SHMP-1.24 In the NMR spectrum of SHMP-1 (Fig. 3d), the peaks with the * symbol and the resonance peaks around 138 or 130 ppm are attributed to the spinning side-bands,25 and the substituted or non-substituted aromatic carbon.22
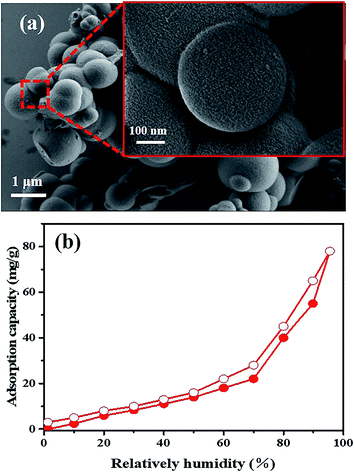
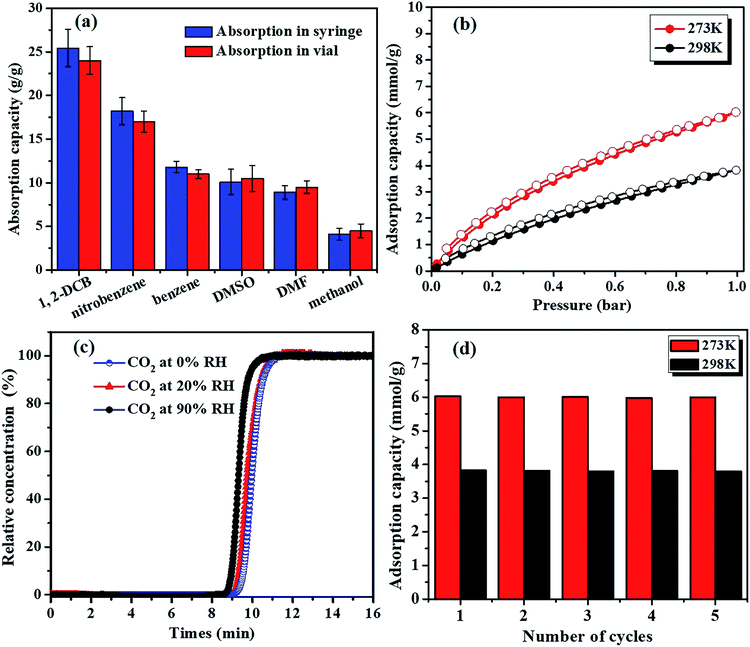
SHMP-1 is agglomerated spheres having nano structure roughness on the sphere surface (Fig. 4a). The cooperation of roughness on the surface and the hydrophobic framework (i.e., hydrophobic conjugated aromatic rings and alkyls) is the key point responsible for the superhydrophobicity of SHMP-1.8,13,26 Surface superhydrophobicity of SHMP-1 is also identified by the large WCA (i.e., 167° in Fig. 2a) and the low adsorption of water vapor (i.e., less than 7.8% of the SHMP-1 weight even at a relative humidity (RH) of up to 90% in Fig. 4b). The surface area normalized water adsorption capacity of SHMP-1 at 90% RH is 0.037 mg m−2, much less than that of the reported superhydrophobic polymer PDVB-[C1vim][SO3CF3] (0.138 mg m−2, WCA = 150°)27 and IISERP-COF2 (0.115 mg m−2, WCA = 160°).8 Therefore, SHMP-1, with larger surface area than the reported superhydrophobic materials, can take up polar and non-polar organic solvents, e.g., 1,2-dichlorobenzene (1,2-DCB), nitrobenzene, benzene, dimethyl sulfoxide (DMSO), N,N-dimethylformamide (DMF) and methanol (Fig. 5a), with larger capacity than that of the reported superhydrophobic materials (Table S2†). The largest uptake capacity, 25 g g−1 in Fig. 5a (i.e., 170 mmol g−1 and 19.48 mL g−1 in Fig. S3a and b,† respectively), observed for 1,2-DCB, is up to 25 times the SHMP-1 weight. The ultrahigh uptake of organic solvents will result in significant swelling of SHMP-1 (Fig. S4†). In addition, a linear relationship between the uptake of organic solvents on SHMP-1 and their density was observed, which accounts for the largest uptake of 1,2-DCB among these organic solvents because of the highest density of 1,2-DCB (Fig. S4†).
With large surface area and surface superhydrophobicity, SHMP-1 has a large adsorption capacity for CO2, 6.02 mmol g−1 at 273 K and 1 bar (Fig. 5b) as an example, which is comparable to the highest one (i.e., 8.51 mmol g−1 at 273 K and 1 bar) reported for porous polymer TrzPOP-3,28 and higher than that of other materials like porous polymers,29 MOFs30 and porous carbons.31 Moreover, the CO2 adsorption capacity of SHMP-1 cannot be decreased significantly by moisture even at a RH of up to 90% (Fig. 5c).
For example, the calculated loss of CO2 adsorption capacity is less than 2.03% at 90% RH (Fig. 5c). Therefore, SHMP-1 is an excellent adsorbent expected for CO2 as well as volatile organic compounds in wet environments because the effects of competitive sorption by water molecules on the SHMP-1 surface can be largely suppressed.8,10,11 In addition, there is almost no loss in the CO2 adsorption capacity after 5 regenerations (Fig. 5d), indicating that SHMP-1 can be cycled and re-used for adsorption at least 5 times.
Thermal analysis (Fig. S5†) shows that SHMP-1 possesses good thermal stability in an O2 atmosphere up to 380 °C, indicating the highly cross-linked structure nature of SHMP-1.32 Moreover, SHMP-1 can be stable in 5 mol L−1 HCl and 5 mol L−1 NaOH solution, showing no significant decrease in specific surface area (1970 m2 g−1 and 1900 m2 g−1 in Fig. S6†) and WCA (160° and 157° in Fig. S7†) after immersion in HCl and NaOH for 2 days. Moreover, SHMP-1 does not dissolve in common organic solvents (e.g., ethanol, acetone, carbon tetrachloride, tetrahydrofuran and n-hexane), showing no changes in its FTIR spectra (Fig. S8†) and almost no mass losses (Table S3†) after immersion in these solvents for 2 days. Therefore, SHMP-1 can be used to remove oils, CO2 and organic compounds from harsh wastewater33 or exhaust gas at high temperatures.
In conclusion, the novel polymer SHMP-1, synthesized in this study, is a superhydrophobic porous material with a WCA of 167° and a large specific surface area of up to 2100 m2 g−1. The specific surface area of SHMP-1 is the largest one for superhydrophobic materials ever reported. Moreover, this polymer has excellent chemical stability in organic solvents, and in 5 mol L−1 HCl and NaOH solution, and can be stable up to 380 °C. SHMP-1 has superior absorption capacities for polar and non-polar organic solvents, up to 25 times its own weight, but limited adsorption for water (i.e., less than 7.8% of its own weight at a RH of up to 90%). The CO2 adsorption capacity of SHMP-1 is up to 6.02 mmol g−1 at 273 K and 1 bar, which is near to the largest one reported for CO2 (i.e., TrzPOP-3 of 8.51 mmol g−1 at 273 K and 1 bar) and cannot be decreased significantly by moisture (e.g., loss < 2.03% at a RH of up to 90%) due to the surface superhydrophobicity. Therefore, SHMP-1 could be a promising adsorbent for capturing of CO2 in flue gas, oil/water separation and sorptive removal of organic contaminants from air and water under harsh conditions and at high temperatures.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2017YFC0211803), the National Natural Science Foundation of China (21621005), and the Key Research and Development Program of Zhejiang Province (2019C03105).Notes and references
- X. J. Feng and L. Jiang, Adv. Mater., 2006, 18, 3063–3078 CrossRef CAS.
- Y. Lu, S. Sathasivam, J. L. Song, C. R. Crick, C. J. Carmalt and I. P. Parkin, Science, 2015, 347, 1132–1135 CrossRef CAS PubMed.
- S. Jung, M. K. Tiwari, N. V. Doan and D. Poulikakos, Nat. Commun., 2012, 3, 1630–1636 Search PubMed.
- X. Hong, X. F. Gao and L. Jiang, J. Am. Chem. Soc., 2007, 129, 1478–1479 CrossRef CAS PubMed.
- F. Z. Zhang, L. L. Zhao, H. Y. Chen, S. L. Xu, D. G. Evans and X. Duan, Angew. Chem., Int. Ed., 2008, 47, 2466–2469 CrossRef CAS PubMed.
- A. Pakdel, C. Y. Zhi, Y. Bando, T. Nakayama and D. Golberg, ACS Nano, 2011, 5, 6507–6515 CrossRef CAS PubMed.
- X. J. Zeng, L. Qian, X. X. Yuan, C. L. Zhou, Z. W. Li, J. Cheng, S. P. Xu, S. F. Wang, P. H. Pi and X. F. Wen, ACS Nano, 2017, 11, 760–769 CrossRef CAS PubMed.
- D. Mullangi, S. Shalini, S. Nandi, B. Choksi and R. Vaidhyanathan, J. Mater. Chem. A, 2017, 5, 8376–8384 RSC.
- H. G. Zhu, D. Y. Chen, W. An, N. J. Li, Q. F. Xu, H. Li, J. H. He and J. M. Lu, Small, 2015, 11, 5222–5229 CrossRef CAS PubMed.
- R. Dawson, L. A. Stevens, T. C. Drage, C. E. Snape, M. W. Smith, D. J. Adams and A. I. Cooper, J. Am. Chem. Soc., 2012, 134, 10741–10744 CrossRef CAS PubMed.
- T. Jafari, I. Noshadi, N. Khakpash and S. L. Suib, J. Mater. Chem. A, 2015, 3, 5023–5030 RSC.
- Y. L. Zhang, S. Wei, F. J. Liu, Y. C. Du, S. Liu, Y. Y. Ji, T. Yokoi, T. Tatsumi and F. S. Xiao, Nano Today, 2009, 4, 135–142 CrossRef CAS.
- A. Li, H. X. Sun, D. Z. Tan, W. J. Fan, S. H. Wen, X. J. Qing, G. X. Li, S. Y. Li and W. Q. Deng, Energy Environ. Sci., 2011, 4, 2062–2065 RSC.
- H. Ye, L. Q. Zhu, W. P. Li, H. C. Liu and H. N. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 858–867 CrossRef CAS PubMed.
- G. Y. Li, G. Hong, D. P. Dong, W. H. Song and X. T. Zhang, Adv. Mater., 2018, 30, 1801754–1801761 CrossRef PubMed.
- D. L. Sivadas, R. Narasimman, R. Rajeev, K. Prabhakaran and K. N. Ninan, J. Mater. Chem. A, 2015, 3, 16213–16221 RSC.
- H. Wei, F. Wang, H. Sun, Z. Zhu, C. Xiao, W. Liang, B. Yang, L. Chen and A. Li, J. Mater. Chem. A, 2018, 6, 8633–8642 RSC.
- C. Yang, U. Kaipa, Q. Z. Mather, X. P. Wang, V. Nesterov, A. F. Venero and M. A. Omary, J. Am. Chem. Soc., 2011, 133, 18094–18097 CrossRef CAS PubMed.
- K. Jayaramulu, F. Geyer, M. Petr, R. Zboril, D. Vollmer and R. A. Fischer, Adv. Mater., 2017, 29, 1605307–1605312 CrossRef PubMed.
- J. He, X. L. Li, D. Su, H. M. Ji, X. Zhang and W. Zhang, J. Mater. Chem. A, 2016, 4, 5632–5638 RSC.
- Y. X. Sun, Q. Sun, H. L. Huang, B. Aguila, Z. Niu, J. A. Perman and S. Q. Ma, J. Mater. Chem. A, 2017, 5, 18770–18776 RSC.
- Z. A. Qiao, S. H. Chai, K. Nelson, Z. H. Bi, J. H. Chen, S. M. Mahurin, X. Zhu and S. Dai, Nat. Commun., 2014, 5, 3705–3712 CrossRef PubMed.
- J. H. Zhu, Q. Chen, Z. Y. Sui, L. Pan, J. G. Yu and B. H. Han, J. Mater. Chem. A, 2014, 2, 16181–16189 RSC.
- K. J. Msayib and N. B. Mckeown, J. Mater. Chem. A, 2016, 4, 10110–10113 RSC.
- G. L. Liu, Y. X. Wang, C. J. Shen, Z. F. Ju and D. Q. Yuan, J. Mater. Chem. A, 2015, 3, 3051–3058 RSC.
- S. Kim, D. Thirion, T. S. Nguyen, B. Kim, N. A. Dogan and C. T. Yavuz, Chem. Mater., 2019, 31, 5206–5213 CrossRef CAS.
- F. J. Liu, L. Wang, Q. Sun, L. F. Zhu, X. J. Meng and F. S. Xiao, J. Am. Chem. Soc., 2012, 134, 16948–16950 CrossRef CAS PubMed.
- S. K. Das, P. Bhanja, S. K. Kundu, S. Mondal and A. Bhaumik, ACS Appl. Mater. Interfaces, 2018, 10, 23813–23824 CrossRef CAS PubMed.
- M. X. Shan, X. L. Liu, X. R. Wang, I. Yarulina, B. Seoane, F. Kapteijn and J. Gascon, Sci. Adv., 2018, 4, 1698–1703 CrossRef PubMed.
- A. Aijaz, N. Fujiwara and Q. Xu, J. Am. Chem. Soc., 2014, 136, 6790–6793 CrossRef CAS PubMed.
- J. M. Lee, M. E. Briggs, T. Hasell and A. I. Cooper, Adv. Mater., 2016, 28, 9804–9810 CrossRef CAS PubMed.
- Q. Chen, M. Luo, P. Hammershoj, D. Zhou, Y. Han, B. W. Laursen, C. G. Yan and B. H. Han, J. Am. Chem. Soc., 2012, 134, 6084–6087 CrossRef CAS.
- N. Han, Z. X. Zhang, H. K. Gao, Y. Q. Qian, L. L. Tan, C. Yang, H. R. Zhang, Z. Y. Cui, W. Li and X. X. Zhang, ACS Appl. Mater. Interfaces, 2020, 12(2), 2926–2934 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and supplementary figures and tables. See DOI: 10.1039/d0ta07944h |
| This journal is © The Royal Society of Chemistry 2021 |