Instantaneous splicing and excision of inteins to synthesize polyproteins on a substrate with tunable linkers†
Abstract
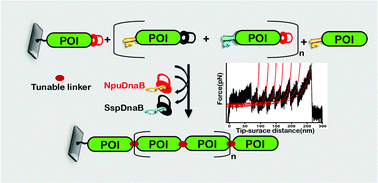
Nature has adapted chimeric polyproteins to achieve superior and multiplexed functionality in a single protein. However, the hurdles in in vitro synthesis have restricted the biomimicry of and subsequent fundamental studies on the structure–function relationship of polyproteins. Recombinant expression of polyproteins and the synthesis of polyproteins via the enzyme-mediated repetitive digestion and ligation of individual protein domains have been widely practiced. However, recombinant expression often suffers from an in vitro refolding process, whereas enzyme-assisted peptide conjugation results in heterogeneous products, primarily due to enzymatic re-digestion, and prolonged and multistep reactions. Moreover, both methods incorporate enzyme-recognition residues of varying lengths as artifacts at interdomain linkers. The linkers, although tiny, regulate the spatiotemporal conformations of the polyproteins differentially and tune the folding dynamics, stability, and functions of the constituent protein. In an attempt to leave no string behind at the interdomain junctions, here, we develop a ‘splice and excise’ synthetic route for polyproteins on a substrate using two orthogonal split inteins. Inteins self-excise and conjugate the protein units covalently and instantaneously, without any cofactors, and incorporate a single cysteine or serine residue at the interdomain junctions.


 Please wait while we load your content...
Please wait while we load your content...