Open Access Article
Open Access ArticleMolecular rotors as intracellular probes of red blood cell stiffness
Alice
Briole
 *a,
Thomas
Podgorski
*a,
Thomas
Podgorski
 b and
Bérengère
Abou
a
b and
Bérengère
Abou
a
aLaboratoire Matière et Systèmes Complexes, UMR 7057 CNRS - Université de Paris, 75013 Paris, France. E-mail: alice.briole@univ-paris-diderot.fr; berengere.abou@univ-paris-diderot.fr
bLaboratoire Rhéologie et Procédés, UMR 5520 CNRS-UGA-G.INP - Domaine Universitaire, BP 53 38041 Grenoble Cedex 9, France. E-mail: thomas.podgorski@univ-grenoble-alpes.fr
First published on 6th April 2021
Abstract
The deformability of red blood cells is an essential parameter that controls the rheology of blood as well as its circulation in the body. Characterizing the rigidity of the cells and their heterogeneity in a blood sample is thus a key point in the understanding of occlusive phenomena, particularly in the case of erythrocytic diseases in which healthy cells coexist with pathological cells. However, measuring intracellular rheology in small biological compartments requires the development of specific techniques. We propose a technique based on molecular rotors - viscosity-sensitive fluorescent probes - to evaluate the above key point. DASPI molecular rotor has been identified with spectral fluorescence properties decoupled from those of hemoglobin, the main component of the cytosol. After validation of the rotor as a viscosity probe in model fluids, we showed by confocal microscopy that, in addition to binding to the membrane, the rotor penetrates spontaneously and uniformly into red blood cells. Experiments on red blood cells whose rigidity is varied with temperature, show that molecular rotors can detect variations in their overall rigidity. A simple model allowed us to separate the contribution of the cytosol from that of the membrane, allowing a qualitative determination of the variation of cytosol viscosity with temperature, consistent with independent measurements of the viscosity of hemoglobin solutions. Our experiments show that the rotor can be used to study the intracellular rheology of red blood cells at the cellular level, as well as the heterogeneity of this stiffness in a blood sample. This opens up new possibilities for biomedical applications, diagnosis and disease monitoring.
1 Introduction
Red blood cells (RBCs) are the main cellular component of blood and perform the essential function of carrying oxygen through the body. These cells essentially consist of a deformable membrane containing a hemoglobin solution considered as a Newtonian fluid1 in the healthy case where the mean corpuscular hemoglobin concentration (MCHC) is approximately 32 g dL−1. The physical properties of the components – membrane bending and shear resistance, cytosol viscosity – condition the deformability of the red blood cells and their flow dynamics. This is crucial for the proper functioning of the circulatory system, particularly for microcirculation where RBCs have to squeeze through complex networks of narrow capillaries.The physical properties of red blood cells vary over their lifetime (∼100–120 days). Cells become denser due to dehydration, resulting in a 25–30% increase in the concentration of corpuscular hemoglobin between the more and less dense fractions.2,3 Since the viscosity of hemoglobin solutions varies sharply between concentrations of 30 and 40 g dL−1,4,5 the densification of red blood cells leads to an increase in their internal viscosity from about 5 to 20 mPa s, resulting in significant heterogeneity of blood samples.6,7 This is not without consequences. It is well documented in the literature on erythrocyte suspension flows and rheology that the dynamics of cells (or more generally capsules and vesicles) in simple and complex flows strongly depends on the viscosity ratio between the internal fluid (cytosol) and the suspending fluid (plasma or buffer).8–13 In addition, the reduced deformability of senescent cells affects their elimination by mechanical filtration through the spleen. Several pathological conditions also lead to alterations in the deformability of red blood cells. This is the case of sickle cell disease, where red blood cells become rigid under the effect of deoxygenation, with serious consequences on the microcirculation in small capillaries, which can lead to occlusion phenomena and vaso-occlusive crises.
All this indicates that the deformability and stiffness of red blood cells is a key haemorheological parameter for diagnosis.14,15 It is likely that jamming and occlusion phenomena are strongly influenced by the presence and quantity of rigid cells rather than by the average rigidity of the sample: a small fraction of rigid red blood cells (i.e. irreversibly sickled cells in the case of sickle cell disease) may be sufficient to nucleate occlusions despite an apparently low average rigidity value. The details of the distribution of mechanical properties of RBCs – which highlights the heterogeneity of a given blood sample – is therefore an information that could be useful in assessing the risk of occlusion.
A number of techniques have been proposed to quantify the deformability of red blood cells. Atomic force microscopy (AFM) has been used to characterize the stiffness of RBCs at the cellular level, in healthy and pathological cases.16,17 The technique can reveal significant variations in cell elasticity in a research context, but requires heavy equipment, and is not suitable for statistical measurements of large-scale samples in a clinical practice. Ektacytometry, developed in the 1980s, is based on light diffraction through a blood sample in shear flow, to measure a RBC elongation index,18 with the intrinsic limitation of providing only the average deformability of the red blood cells in the sample and challenging interpretation of the results.19–22 In recent years, microfluidic techniques have been proposed to characterize deformability at the cellular level.23,24 Although they partly answer the question of the dispersion of RBC properties in a sample, they require heavy image processing and shape analysis giving indirect information on the mechanical properties. Other techniques have focused specifically on membrane shear and bending moduli, including pipette aspiration,25 electric field deformation,26 optical tweezers,27,28 and optical microrheology.29–31 It appears that there is currently a need for sub-cellular rheology techniques allowing quantitative measurements of the mechanical properties of different parts of the cell and operating on large samples. As further evidence, in the particular case of RBCs, measurements of the viscosity of the internal hemoglobin solution have mostly been performed by extracting the cell's hemoglobin content by lysis followed by conventional rheology techniques on relatively large samples.5,32,33
Molecular rotors (MRs) have recently proven their usefulness in measuring the rheological properties of intracellular media for the purpose of characterization and diagnostics.34–36 This is a group of fluorescent molecules, some of which have the ability to form twisted intra-molecular charge transfer (TICT) states, upon photoexcitation. Relaxation from the TICT conformation occurs either by emission of a red-shifted emission band or by non-radiative relaxation, with a lower excited-state/ground-state energy gap than the planar, locally excited (LE) state.37 For viscosity measurements, molecular rotors with a single emission band are most preferred because emission from the planar (non-twisted) LE conformation is highly sensitive towards viscosity,37–39 and relatively insensitive towards the solvent polarity.40–42 It has been demonstrated in a wide range of viscosities and in both polar and non polar fluids, that the quantum yield ϕ of the LE peak increases with viscosity η, according the so-called Förster–Hoffmann equation, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ϕ = C + b
ϕ = C + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η, where C and b are solvent and dye dependent constants.38,39,42–45 These rheology dependent properties have been used for real-time monitoring of aggregation and polymerization processes,46 to report aggregation and protein conformational changes,47,48 and study phospholipid bilayers and cell membranes.41,49,50
η, where C and b are solvent and dye dependent constants.38,39,42–45 These rheology dependent properties have been used for real-time monitoring of aggregation and polymerization processes,46 to report aggregation and protein conformational changes,47,48 and study phospholipid bilayers and cell membranes.41,49,50
Here we study the ability of a selected molecular rotor to quantify the heterogeneity of a blood sample, and to characterize the rigidity and intracellular rheology of red blood cells. After characterizing its spectral properties, which should not interfere with those of hemoglobin, and its effectiveness as a viscosity probe in simple fluids, we demonstrate that, in addition to attaching to the membrane, the rotor penetrates spontaneously and uniformly into the red blood cells. We show that it is possible to distinguish red blood cells with different levels of deformability in a blood sample, and to quantify the heterogeneity of this sample. The evolution of the average stiffness of a blood sample and the distribution of stiffness in the sample are measured by varying the temperature. The fluorescence of the cytosol alone is estimated at different temperatures using a simple geometrical model of RBC allowing the separation of cytosol and membrane contributions. The estimated variation of cytosol viscosity with temperature is consistent with independent measurements of the viscosity of hemoglobin solutions. This opens up opportunities for the development of rapid and inexpensive hematological tests for diagnosis and clinical monitoring of patients.
2 Materials and methods
2.1 Red blood cell samples and hemoglobin solutions
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g for 15 min. Hemoglobin solutions of various concentrations were obtained by dilution in PBS or by concentration using centrifugal ultrafiltration units (Vivaspin 2 PES 30 kDa from Sartorius). The hemoglobin concentration of the final solutions was measured by colorimetry (MAK 115 Hemoglobin Assay Kit from Sigma-Aldrich). The pH of hemoglobin solutions was of constant value 7 ± 0.2 for all concentrations.
000×g for 15 min. Hemoglobin solutions of various concentrations were obtained by dilution in PBS or by concentration using centrifugal ultrafiltration units (Vivaspin 2 PES 30 kDa from Sartorius). The hemoglobin concentration of the final solutions was measured by colorimetry (MAK 115 Hemoglobin Assay Kit from Sigma-Aldrich). The pH of hemoglobin solutions was of constant value 7 ± 0.2 for all concentrations.
2.2 Sample temperature control
For the microrheology and fluorescence microscopy experiments (Sections 3.1.2, 3.2 and 3.3.2), the samples were observed on an inverted Leica DMI 8 microscope. The temperature of the samples was controlled and varied by an objective heater through the immersion oil in contact with the sample (Bioptechs Inc., Butler, PA, USA) with an accuracy of ±0.1 °C.2.3 Particle tracking microrheology
The viscosity of the solutions (water/glycerol, glycerol/ethylene glycol and hemoglobin) was measured by particle tracking microrheology. In experiments described in Section 3.1.2, the viscosity of the solutions was measured in situ with microrheology experiments, before proceeding with the fluorescence measurements. The technique is based on the Brownian motion of microscopic probe particles immersed in the fluid. Microrheology experiments are generally carried out on small volumes of about 1 μL. The technique is interesting in biological samples where minute volumes can be studied,51,52 and also in heterogeneous samples where the technique gives access to the amplitude of the heterogeneities in the sample.53 Calibrated polystyrene beads (0.994 μm in diameter) were dispersed in the solutions to be studied at a volume fraction of less than 1%. The thermal motion of the tracers was recorded for 20 s at a rate of 100 Hz with an OrcaFlash v2+ fast sCMOS camera. This camera is mounted on a Leica inverted bright field microscope DMI 8, including an oil immersion objective with 100× magnification (NA = 1.3, depth of field: ∼200 nm). Custom image analysis software allows us to track the x and y positions of any bead close to the objective focus plane. For a reliable analysis, special attention was brought to record only the beads far from the glass slide surfaces.For each tracer, the time-averaged mean-squared displacement (MSD) was calculated as follows: 〈Δr2(t)〉 = 〈(x(t + t′) − x(t′))2 + (y(t + t′) − y(t′))2〉t′. For a purely viscous fluid, the ensemble-averaged MSD increases linearly with the lag time t, according to: 〈Δr2(t)〉 = 4Dt (in two dimensions), where D is the diffusion coefficient. In this case, the viscosity η can be estimated with the Stokes–Einstein relation, D = kT/6πRη, with R the diameter of the bead and kT the thermal energy.54 Glycerol/water and ethylene glycol/glycerol solutions studied in Section 3.1.2, were homogeneous and purely viscous, allowing for a simple determination of the sample viscosity.
2.4 Fluorescence microscopy and segmentation of red blood cells
For fluorescence measurements of the DASPI molecular rotor in the solutions (water/glycerol, glycerol/ethylene glycol and hemoglobin) and RBC suspensions, samples were placed between a glass slide and a coverslip separated by a 250 μm thick spacer. For RBCs, the glass slides and coverslips were pre-treated with a plasma cleaner to prevent the formation of echinocytes.Observation of the samples was carried out by fluorescence microscopy (Leica DMI 8, Leica Microsystems GmbH, Wetzlar, Germany) at 100× magnification with excitation between 460–500 nm and detection between 565–625 nm. The images were recorded with a fast sCMOS camera (OrcaFlash4.0 v2+, Hamamatsu Photonics France S.A.R.L., Massy, France). The intensity measurements of the fluorescence level of the images were performed using ImageJ software (ImageJ, Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/, 1997–2018). A macro program allows us to detect and segment RBCs on an image, measure their average fluorescence intensity, their surface area and their circularity. The circularity parameter is adjusted to select RBCs according to their orientation (front or side view) and to exclude echinocytes. In the experiments described in Section 3.3.2, the segmentation is designed to measure the intensity on an area slightly smaller than the actual RBC surface area, in order to eliminate membranes contributions at the edges. During the analysis, the background fluorescence level is subtracted from the total image. This normalization allows us to obtain the signal actually produced by the red blood cells and to compare the realizations obtained when the temperature is varied.
The statistical analysis of the temperature dependence on RBC fluorescence intensity was performed with the fitlm function from Matlab software, which provides, from a scatter plot, the correlation coefficient of the linear regression r and the associated P value under the null hypothesis of zero slope. The tests were considered significant for P < 0.05.
2.5 Confocal microscopy
The penetration and localization of the DASPI molecular rotor in red blood cells was characterized with a Leica TCS SP8 confocal microscope equipped with a 40× oil-immersion objective (1.3 numerical aperture). Diluted red blood cell samples (hematocrit 0.5%), incubated with DASPI, were placed in a PDMS microchannel on the microscope stage. Confocal slices were acquired with a 0.5 μm step in the z-direction to measure the fluorescence signal at different levels in RBCs. The excitation wavelength was 488 nm and the depth of field was 600 nm for a pinhole size of 63.5 μm. The laser power settings and detection gain were kept constant between experiments for comparison purposes. Image processing allowed us to extract fluorescence intensity profiles, averaged over a region of interest in the (x,y) plane, for different z.2.6 Spectrophotometry
Fluorescence Excitation Emission Matrices (EEMs) of DASPI in various solvents (DMSO, glycerol, ethylene glycol) and hemoglobin solutions were measured with a spectrophotometer at room temperature. The excitation wavelength was set between 350 nm and 650 nm and the emission spectrum was scanned between 450 nm and 750 nm. The excitation and emission slits were 5 nm, the scan speed was 600 nm min−1 and the voltage of the PMT detector was 600 V. Absorption spectra were measured with a Varian Cary 50 UV-Vis spectrophotometer (Agilent) at room temperature. All experiments were performed in 2 mL PS spectro cells (LP ITALIANA SPA). Hemoglobin was highly diluted to form a solution of reduced absorbance (1000× dilution ∼33 mg dL−1 concentration). DASPI concentration was set to 3.3 μM and the solutions were prepared with an 8 mM DASPI stock solution.3 Results
3.1 DASPI molecular rotor properties
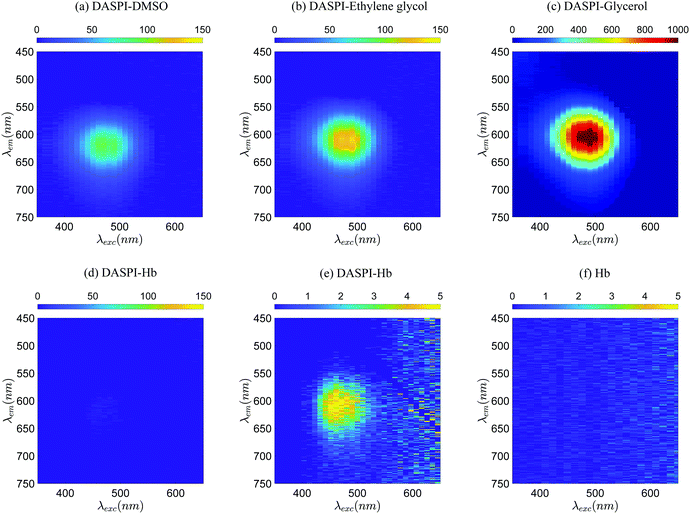
Fluorescence Excitation Emission Matrices of DASPI were measured in four selected solvents, at room temperature: DMSO, ethylene glycol, glycerol and hemoglobin/PBS solutions (dilution 1000×). Fig. 1(a, b, c and e) shows that the fluorescence spectra of DASPI are similar from one solvent to another. In the investigated range, DASPI is a single band emission molecular rotor, with fluorescence excitation and emission peaks respectively around 480 nm and 600 nm. More importantly, the excitation–emission spectrum of DASPI in hemoglobin (Fig. 1(d and e)) is outside the intrinsic fluorescent spectrum of hemoglobin known to be in the ultraviolet with excitation maxima below 400 nm and emission below 460 nm.56 The fluorescence EEM of hemoglobin alone was also measured to demonstrate that there is no significant contribution of hemoglobin in the DASPI spectral range (spectrum (f)).
From the EEM spectra (a–c) in Fig. 1, we can observe that the rotor has a fluorescence emission intensity which increases with viscosity. In hemoglobin solutions however (spectra (d and e)), the emission peak value is lower than in DMSO (spectrum (a)) for similar viscosities due to the high absorbance of hemoglobin and the behaviour of the rotor in an aqueous protein medium. This absorbance remains non-negligible even if the emission of DASPI is around a minimum of local absorption of hemoglobin as shown in Fig. 2 around ∼600 nm. The absorbance spectra of Fig. 2 also attest to (i) the presence of compounds (Hb, DASPI) that have been highly diluted to minimize the absorbance of hemoglobin, (ii) the influence of these compounds in the spectral range of DASPI.
The emission intensity of DASPI was measured by fluorescence microscopy (excitation at 480 ± 20 nm, emission at 595 ± 30 nm) in ethylene glycol/glycerol solutions and aqueous glycerol solutions whose viscosity was measured in situ by microrheology, just before the fluorescence intensity measurements. We measured the emission intensity of DASPI at different temperatures between 16 and 20 °C and at different rotor concentrations: 0.01, 0.1 and 1 mM (Fig. 3(a and b)). The relative concentration of the two solvents in each solution was modified to extend the achievable viscosity range, typically between 2–200 mPa s. Ethylene glycol/glycerol solutions were chosen as one of the study systems because their polarity remains approximately constant when the relative concentration between the solvents and therefore the viscosity varies, as the polarities of the two liquids – glycerol and ethylene glycol – are similar.42 In aqueous solutions of glycerol however, the polarity of the solvent decreases with increasing viscosity, based on the dielectric constant values of water and glycerol (water: 80.1 F m−1, glycerol: 42.5 F m−1, ethylene glycol: 37.7 F m−1).42
Fig. 3 shows that the rotor's emission intensity increases with the local viscosity and follows a Förster–Hoffmann equation, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ϕ = C + b
ϕ = C + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η in both solutions, with ϕ the quantum yield directly proportional to the emission intensity, and b and C two solvent-dependent parameters. The C parameter increases significantly with the DASPI concentration as shown in the insets in the Fig. 3. The exponent b was similar in the two solutions and close to 0.7. (water/glycerol: C = 74, 344, 1590 and b = 0.70 ± 0.01; ethylene glycol/glycerol solutions: C = 94, 642, 2206 and b = 0.69 ± 0.01). From our measurements, we deduce that temperature has little influence on the C parameter, and thus on the signal emitted by DASPI, as highlighted specifically at the 0.01 mM rotor concentration where all data points coming from different temperatures are on the same Förster–Hoffmann power-law curve, in consistency with a previous study.57 Similarly, polarity has little effect on rotor response in aqueous glycerol solutions, in the sense that the Förster–Hoffmann equation remains valid although polarity varies with concentration, which is also consistent with previous studies where molecular rotors with a single emission band showed an emission intensity strongly dependent on viscosity but not on solvent polarity.40–42 At low viscosity around 1–2 mPa s in the aqueous glycerol solutions, a deviation is observed from the Förster–Hoffmann law as already observed in previous studies.42,55
η in both solutions, with ϕ the quantum yield directly proportional to the emission intensity, and b and C two solvent-dependent parameters. The C parameter increases significantly with the DASPI concentration as shown in the insets in the Fig. 3. The exponent b was similar in the two solutions and close to 0.7. (water/glycerol: C = 74, 344, 1590 and b = 0.70 ± 0.01; ethylene glycol/glycerol solutions: C = 94, 642, 2206 and b = 0.69 ± 0.01). From our measurements, we deduce that temperature has little influence on the C parameter, and thus on the signal emitted by DASPI, as highlighted specifically at the 0.01 mM rotor concentration where all data points coming from different temperatures are on the same Förster–Hoffmann power-law curve, in consistency with a previous study.57 Similarly, polarity has little effect on rotor response in aqueous glycerol solutions, in the sense that the Förster–Hoffmann equation remains valid although polarity varies with concentration, which is also consistent with previous studies where molecular rotors with a single emission band showed an emission intensity strongly dependent on viscosity but not on solvent polarity.40–42 At low viscosity around 1–2 mPa s in the aqueous glycerol solutions, a deviation is observed from the Förster–Hoffmann law as already observed in previous studies.42,55
3.2 Viscosity of hemoglobin solutions with temperature
Here we present independent measurements of the viscosity of solutions of hemoglobin, major component of the cytosol inside RBCs, as a function of temperature (Fig. 4). In the following, we use temperature as a parameter to vary the stiffness of RBCs (Section 3.3.2) and qualitatively compare these hemoglobin viscosity variations with those observed on the studied RBCs with the DASPI molecular rotor (Section 3.4).Viscosity was measured by microrheology in hemoglobin solutions with concentrations between 25.5 g dL−1 and 38.5 g dL−1. Our experiments show that hemoglobin behaves like a purely viscous fluid (not shown), that the solutions are homogeneous (not shown) and that the viscosity decreases with temperature at fixed concentration, and increases with concentration at fixed temperature. For a given concentration range, viscosity values extend over a wider range at low temperature than at high temperature. This evolution of viscosity with temperature is consistent with the literature.61,62
3.3 Molecular rotor DASPI in red blood cells
Fig. 6 shows images of red blood cells at different temperatures (Fig. 6a) as well as the intensity profiles of the cells labelled with a star (Fig. 6b). The intensity profiles all show homogeneous backgrounds, sharp peaks at the edges and a plateau inside the cell. Both the background (i.e. PBS solution) and the RBC profiles increase as the temperature decreases. Since DASPI signal does not depend on temperature in the studied range (Section 3.1.2), this increase in the peak and plateau fluorescence when temperature decreases demonstrates that the fluorescence signal of DASPI is sensitive to, and increases with, the rigidity of RBCs. The observed peaks on the profiles are further evidence, along with confocal microscopy observations, that the membrane-DASPI interactions result in a stronger fluorescence response than that of cytosol-DASPI. A closer look at the plateaus (for example clearly visible here at 30 °C) reveals small height variations that reflect the cell geometry and suggest that the cytosol contribution is proportional to the local thickness of the cell. The viscosity–intensity dependence of the background was determined at each temperature using microrheology in the PBS surrounding the RBCs. Fig. 6c shows that the rotor emission increases with the PBS viscosity and that the data can be adjusted with a Förster–Hoffmann power law of exponent x = 0.7.†
To ensure a statistically relevant sampling, we studied blood samples from five different donors with mean corpuscular hemoglobin concentrations (MCHC) between 30 g dL−1 and 35 g dL−1. About four hundred cells were imaged at each temperature, and the average intensity was measured on each cell (see Section 2.4). Fig. 7a gathers the data together within a boxplot. As the temperature decreases, the median fluorescence intensity increases (by 35% in 1 mM DASPI solutions and 26% in 0.1 mM DASPI solutions) and the collected data are more scattered. Statistical analysis provides a negative correlation between fluorescence intensity and temperature in 0.01 mM DASPI solution (r = −0.48, P < 0.0001) and in 1 mM DASPI solution (r = −0.55, P < 0.0001), which means that temperature, as a parameter controlling cell stiffness, does have a significant impact on DASPI fluorescence. The same pattern is observed when considering RBCs coming from a single donor (Fig. 7b): the fluorescence intensity distribution of RBCs shifts towards high intensities and becomes wider at low temperatures. This dispersion of red blood cell fluorescence intensities, measured at a given temperature, reflects the variability of their properties: variation in the mean corpuscular hemoglobin concentration from one donor to another (Fig. 7a) and dispersion due to the age of the cells within a sample (Fig. 7a and b). Variations in the physico-chemical properties of membranes may also play a role in the dispersion of the data, the separation of the two contributions will be discussed in the following section. However, these experiments prove that the DASPI molecular rotor can quantify the rigidity of RBCs as well as the dispersion, or heterogeneity, of the mechanical properties of red blood cells in a sample.
3.4 Estimation of cytosol fluorescence using a simple model of RBC
In fluorescence microscopy experiments (Section 3.3.2), both the membrane and the cytosol contribute to the signal measured on RBCs. We propose here a simplified RBC model to separate the two contributions and to find the evolution of the cytosol fluorescence with temperature (i.e. stiffness) (Fig. 7a and b). We show how geometrical parameters can influence this estimation and that we can in principle relate fluorescence measurements to the mechanical properties of the cytosol, i.e. the internal viscosity of hemoglobin.The relevant parameters are as follows:
• geometrical parameters:
- hm: thickness of the membrane;
- Hi: thickness of the cytosol along the z axis in the central part;
- R: average radius of curvature at the edges of the RBC;
• Fluorescence intensities:
- ϕm (m−3): fluorescence intensity per unit volume of the membrane;
- ϕi (m−3): fluorescence intensity per unit volume of the cytosol.
Cytosol and plateau intensities (C and P) are related to the terms defined above as follows:
 | (1) |
As hp is large compared to the membrane thickness 40 nm ≤ hm ≤ 100 nm,66–68 the peak signal M is composed of the signal of the portions of the curved membrane and cytosol over the width hp (zoom in Fig. 8c), so that:
| M = hmhpSΦm + hpAΦi |
•  : curvilinear length of the membrane, in red on Fig. 8c-zoom;
: curvilinear length of the membrane, in red on Fig. 8c-zoom;
•  : area of cytosol, in orange on Fig. 8c-zoom.
: area of cytosol, in orange on Fig. 8c-zoom.
This leads to the following formula for the peaks intensity:
 | (2) |
Finally, the cytosol intensity reads:
 | (3) |
In practical cases, hp ≪ Hi so that the denominator of eqn (3) cannot be zero. Eqn (3) indicates that C depends on the geometrical parameters R and Hi and the values M and P which can be measured on the intensity profiles (Fig. 8). C can also be expressed, using eqn (1), with the ratio of elementary intensities Φm/Φi:
 | (4) |
In the following, we will focus on C and C/P which represents the contribution of cytosol to the plateau intensity P, measured in fluorescence microscopy experiments (Section 3.3.2).
A RBC is classically described in the literature as biconcave, with a maximum height at the edges of 2–3 μm, a radius of curvature of about half this maximum height, and a minimum height at the center of 0.8–1 μm.69 Therefore, in our model, R is chosen in the range 1 μm ≤ R ≤ 1.5 μm and Hi in the range 1.3 μm ≤ Hi ≤ 1.9 μm as an average between the maximum and minimum heights. By varying R and Hi within these ranges, we cover a large number of realistic shapes for a RBC. We note G1 (R = 1.5 μm, Hi = 1.3 μm) and G2 (R = 1 μm, Hi = 1.9 μm) the geometries leading to extreme values of C/P for a given M/P (see eqn (3)). G1 corresponds to a thin RBC with sharp edges, and G2 to a thick RBC with rounded edges. We also note G3 (R = 1.25 μm, Hi = 1.6 μm) the geometry corresponding to the mean value of the range considered. G1, G2 and G3 are shown in Fig. 9. In this section, we consider that for a given RBC, R and Hi do not vary with temperature. This hypothesis is supported by recent observations by Jaferzadeh et al.70 showing that the shape of the edges does not vary significantly with temperature, and that although fluctuations in the central part are more important at higher temperatures, the mean thickness does not vary.
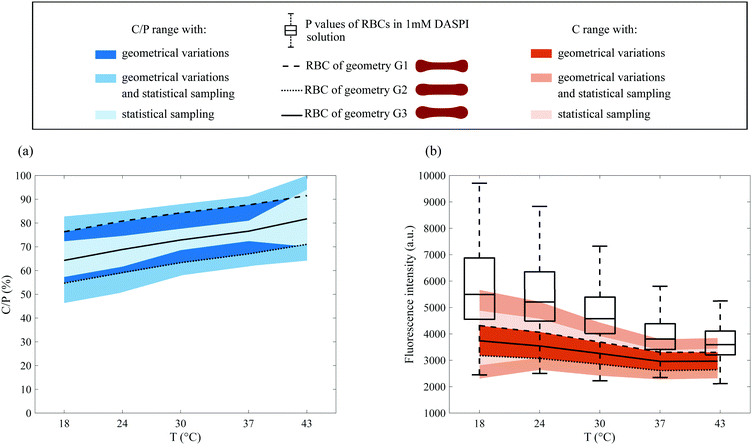 | ||
| Fig. 9 Cytosol to plateau C/P (a) and cytosol C (b) contributions with temperature, for RBCs in a 1 mM DASPI solution. The fluorescence intensities of RBCs with temperature from Fig. 7a (boxplot, corresponding to P) are reported in (b). The light-colored zones delineate an area corresponding to the statistical sampling effect. The continuous lines within these light color zones are obtained by considering the median values calculated with the G3 geometry (R = 1.25 μm, Hi = 1.6 μm). The dark bands quantify the dispersion of C/P and C for all possible geometries in the ranges 1 μm < R < 1.5 μm and 1.3 μm < Hi < 1.9 μm. The dashed lines correspond to the median values of C and C/P obtained for the extreme geometries G1 and G2. The intermediate color bands gather both the effects of statistical sampling and the effects of possible geometries. | ||
We evaluated the C/P and C values, separating or not the effects of RBC geometry and statistical sampling. As indicated above, approximately 100 RBCs per temperature were considered and the corresponding pairs (M, P) were measured on each of their intensity profiles. In Fig. 9b, the P values from Section 3.3.2 were added for comparison. The first way of processing the data was to visualise the effect of the statistical sampling alone, when the geometry is set at its mean value G3. It delineates light-colored zones corresponding to the data dispersion of C/P and C (Fig. 9(a and b)). The continuous lines within these zones correspond to their median values. A second way of processing data was to quantify the effect of the possible geometries only. To do this, the geometry was set at the G1 or G2 extremes. Next, the resulting median values of C/P and C were calculated and are represented by dashed lines. These dashed lines define dark bands that quantify the dispersion due to possible geometries. Finally, both effects, statistical sampling and geometry, were combined, and are shown in the intermediate color bands (all possible geometries for 100 RBCs).
In all cases, the bands corresponding to C/P values increase roughly linearly with temperature. In parallel, the ones corresponding to C decrease with temperature, as evidenced in particular in Fig. 9b, when the geometry is fixed at the extremes or its mean value (dashed and solid lines). This decrease in the cytosol signal with temperature, caused by the sharp decrease of P, is consistent with the decrease of the viscosity of hemoglobin solutions measured in Fig. 5.
We focus now specifically on the dispersion of C and C/P with temperature. The dispersion of C/P remains constant when considering the combined effects of geometry and statistical sampling (intermediate color band). Conversely, the one of C decreases with temperature. Since its light band is larger than the dark band which is quasi constant in width, this decrease is mainly due to the effect of statistical sampling alone. It thus reflects the intrinsic heterogeneity of a blood sample and in particular the dispersion of internal viscosity. This is quite consistent with the decrease in the dispersion of hemoglobin viscosity values (independent measurements in Section 3.2).
While more information on the cell geometry would be needed to reduce uncertainties in the determination of C, this simple model shows that it is in principle possible to separate the contributions of the cytosol and membrane from simple fluorescence profile measurements, and that the derived values are consistent with independent viscosity measurements on hemoglobin solutions. The same study could be devoted to the membrane by considering (P–C)/2 = hp2hmΦm which represents the intensity of a membrane element of surface area hp2 and thickness hm (eqn (1)).‡ We can however note that the increase in C/P reflects a decrease in the Φm/Φi ratio (eqn (4)), which means that the contribution of the membrane decreases much faster than that of the cytosol when temperature increases, a feature that remains to be explored in terms of rotor-membrane interactions.
4 Discussion and conclusion
In this paper, we study the ability of molecular rotors to characterize the intracellular rheology of red blood cells. We identified a molecular rotor (DASPI: trans-4-[4-(dimethylamino)-styryl]-1-methylpyridinium iodide), that is suitable for the study of hemoglobin and intracellular medium of red blood cells. For this purpose, the excitation–emission spectra of DASPI were measured in selected solutions: DMSO, ethylene glycol, glycerol and hemoglobin. In all fluids, DASPI presented a fluorescence excitation peak around 480 nm and emission peak around 600 nm. We have thus shown that the excitation–emission spectrum of DASPI does not overlap with the intrinsic fluorescent spectrum of hemoglobin in the ultraviolet domain.56 Moreover, its emission spectrum is in the range where the absorbance of hemoglobin is lowest (∼600 nm, Fig. 2), which allows it to be used at moderate concentrations.Tested in simple solutions such as ethylene glycol/glycerol and glycerol/water solutions, DASPI shows sensitivity to the local viscosity η, and exhibits a Förster–Hoffmann dependency in a range covering two decades, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ϕ = C + b
ϕ = C + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η, with ϕ the LE-state quantum yield and b and C two solvent and dye-dependent parameters. The exponent b, which is a measure of the efficiency of the molecular rotor in the solvent, was found to be around 0.7 in both solutions, which is close to the predicted theoretical values of 2/343 or 0.6.38 The parameter C increases by about one and a half decade when the concentration of DASPI increases by two decades, which allows to adjust the dye concentration to the sensitivity of the intensity reading device in potential applications. Experiments performed in glycerol/water solutions with variable polarity confirm the result previously obtained by Haidekker and colleagues42 that there is no impact of polarity variation on obtaining a Förster–Hoffmann equation between quantum yield and viscosity. In addition, our data show that temperature has little influence on the C parameter, which allows us to use it as a RBC stiffening control parameter in the Section 3.3.2.
η, with ϕ the LE-state quantum yield and b and C two solvent and dye-dependent parameters. The exponent b, which is a measure of the efficiency of the molecular rotor in the solvent, was found to be around 0.7 in both solutions, which is close to the predicted theoretical values of 2/343 or 0.6.38 The parameter C increases by about one and a half decade when the concentration of DASPI increases by two decades, which allows to adjust the dye concentration to the sensitivity of the intensity reading device in potential applications. Experiments performed in glycerol/water solutions with variable polarity confirm the result previously obtained by Haidekker and colleagues42 that there is no impact of polarity variation on obtaining a Förster–Hoffmann equation between quantum yield and viscosity. In addition, our data show that temperature has little influence on the C parameter, which allows us to use it as a RBC stiffening control parameter in the Section 3.3.2.
The penetration of the molecular rotor (DASPI) has been studied in healthy red blood cells. We showed using confocal microscopy that the molecular rotor spontaneously penetrates into red blood cells. With a depth of field of approximately 600 nm, confocal microscopy allows us to unambiguously separate the fluorescence response of the cytosol and the lower and upper membranes as soon as the focal plane is far enough away from the membrane. We therefore revealed that the molecular rotor is uniformly distributed throughout the volume of RBCs, giving evidence that it is a suitable probe for characterizing their intracellular rheology. As fluorescence profiles include a contribution from the membrane with a locally strong signal, the specific interactions between DASPI and the cell membrane should receive particular attention in future studies in order to reach quantitative understanding of the influence of structural and rheological properties of membranes on MR fluorescence.
In order to highlight and characterize the sensitivity of DASPI to RBC rheological properties, we varied temperature as a way to control cytosol and membrane properties, as a decrease in temperature leads to an overall stiffening of RBCs. Results show that for a given healthy blood sample, the measured fluorescence intensity at the whole cell level varies nearly by a factor 2 between 18 °C and 43 °C in correlation with variations of cytosol viscosity and membrane elasticity. We showed that by making reasonable assumptions about the RBC geometry, it is possible to separate and quantify the respective contributions of the cytosol and the membrane to the overall fluorescence signal and thus to derive information on the modifications of both components due to temperature change. We show in particular that the estimated contribution from the cytosol reproduces well the intrinsic heterogeneity of the RBC sample, and is consistent with independent measurements on hemoglobin solutions. Finally, by simply varying temperature for healthy cells, we demonstrate by this example that in principle the DASPI molecular rotor can be used to derive measurements of intracellular viscosity and quantify sample heterogeneity.
For a more quantitative analysis, allowing the rotor intensity to be related to a viscosity value, the partial absorption of both incident and emitted light by hemoglobin will have to be modeled and taken into account. Besides, independent experiments show that the sensitivity of the rotor to viscosity is non-trivially dependent on the protein concentration, which does not allow us to classify the samples according to their MCHC. A quantitative determination of the viscosity will require resolving the issues about hemoglobin absorbance and protein concentration. The use of temperature as a control parameter for stiffness allows us to work at constant protein concentration and hemoglobin absorbance on a sample, and to evaluate the evolution of the sample with temperature. In the future, these results should be generalized to other causes of rigidification including pathological situations where the structure of hemoglobin may be altered.
Our results are a proof of concept that MRs can be used as nanoscale rheological probes in small compartments such as red blood cells. Their use in this context of evaluation of mechanical properties at the cellular level is a promising prospect, in particular because it would make it possible to measure the distribution of cell properties over large samples, unlike other techniques that only allow the measurement of average values over the entire sample. The alteration and dispersion of cell properties in pathological cases can indeed be critical for clinical aspects and such a technique opens new perspectives for medical diagnosis purposes by providing more detailed and quantitative information through a rather simple and direct measurement of fluorescence.
Author contributions
A. B., T. P. and B. A. wrote the manuscript. A. B. performed the experiments and processed the experimental data. A. B., T. P. and B. A. discussed the results and implications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors wish to thank C. Makowski and B. Polack from CHU Grenoble-Alpes for helpful discussions on blood and hemoglobin preparation. F. Molina (Sys2Diag Montpellier) and A. Pasturel (MSC Paris & Sys2Diag Montpellier) are acknowledged for fruitful discussions. The authors thank O. Stefan (LIPhy Grenoble) for help with hemoglobin purification. M. Seigneuret (MSC Paris) and M. Garrigos (I2BC CEA-Saclay) are acknowledged for their help with the hemoglobin concentration protocol. Funding of the confocal microscopy platform at UGA-LIPhy by LabEx Tec21 (Investissement d'Avenir – grant agreement no. ANR-11-LABX-0030) is acknowledged. T. P. acknowledges support of CNES for investigations on blood rheology and flows.References
- G. R. Cokelet and H. J. Meiselman, Science, 1968, 162, 275–277 CrossRef CAS PubMed.
- F. H. Bosch, J. M. Werre, B. Roerdinkholder-Stoelwinder, T. H. Huls, F. L. Willekens and M. R. Halie, Blood, 1992, 79, 254–260 CrossRef CAS.
- F. H. Bosch, J. M. Werre, L. Schipper, B. Roerdinkholder-Stoelwinder, T. Huls, F. Willekens, G. Wichers and M. R. Halie, Eur. J. Haematol., 1994, 52, 35–41 CrossRef CAS PubMed.
- P. D. Ross and A. P. Minton, Biochem. Biophys. Res. Commun., 1977, 76, 971–976 CrossRef CAS.
- S. Chien, S. Usami and J. F. Bertles, J. Clin. Invest., 1970, 49, 623–634 CrossRef CAS PubMed.
- R. Waugh, N. Mohandas, C. Jackson, T. Mueller, T. Suzuki and G. Dale, Am. Soc. Hem., 1996, 79, 1351–1358 Search PubMed.
- R. S. Franco, M. E. Puchulu-Campanella, L. A. Barber, M. B. Palascak, C. H. Joiner, P. S. Low and R. M. Cohen, Am. J. Hematol., 2013, 88, 44–51 CrossRef CAS PubMed.
- J. Dupire, M. Socol and A. Viallat, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 20808–20813 CrossRef CAS.
- T. Fischer and R. Korzeniewski, J. Fluid Mech., 2013, 736, 351 CrossRef.
- J. Mauer, S. Mendez, L. Lanotte, F. Nicoud, M. Abkarian, G. Gompper and D. A. Fedosov, Phys. Rev. Lett., 2018, 121, 118103 CrossRef CAS PubMed.
- C. Minetti, V. Audemar, T. Podgorski and G. Coupier, J. Fluid Mech., 2019, 864, 408–448 CrossRef CAS.
- S. Losserand, G. Coupier and T. Podgorski, Microvasc. Res., 2019, 124, 30–36 CrossRef CAS PubMed.
- Z. Shen, G. Coupier, B. Kaoui, B. Polack, J. Harting, C. Misbah and T. Podgorski, Microvasc. Res., 2016, 105, 40–46 CrossRef PubMed.
- R. Banerjee, K. Nageshwari and R. Puniyani, Clin. Hemorheol. Microcirc., 1998, 19, 21–24 CAS.
- E. Du, M. Diez-Silva, G. J. Kato, M. Dao and S. Suresh, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 1422–1427 CrossRef CAS PubMed.
- J. L. Maciaszek, B. Andemariam and G. Lykotrafitis, J. Strain Anal., 2011, 46, 368–379 CrossRef.
- J. L. Maciaszek and G. Lykotrafitis, J. Biomech., 2011, 44, 657–661 CrossRef PubMed.
- M. Bessis, N. Mohandas and C. Feo, Blood Cells, 1980, 6, 315–327 CAS.
- M. Rabai, J. A. Detterich, R. B. Wenby, T. M. Hernandez, K. Toth, H. J. Meiselman and J. C. Wood, Biorheology, 2014, 51, 159–170 Search PubMed.
- S. Y. Nikitin and Y. S. Yurchuk, Quantum Electron., 2015, 45, 776–780 CrossRef CAS.
- J. M. Sosa, N. D. Nielsen, S. M. Vignes, T. G. Chen and S. S. Shevkoplyas, Clin. Hemorheol. Microcirc., 2014, 57, 275–289 CAS.
- C. Renoux, N. Parrow, C. Faes, P. Joly, M. Hardeman, J. Tisdale, M. Levine, N. Garnier, Y. Bertrand, K. Kebaili, D. Cuzzubbo, G. Cannas, C. Martin and P. Connes, Clin. Hemorheol. Microcirc., 2016, 62, 173–179 CAS.
- Q. Guo, S. P. Duffy, K. Matthews, A. T. Santoso, M. D. Scott and H. Ma, J. Biomech., 2014, 47, 1767–1776 CrossRef PubMed.
- J. Kim, H. Lee and S. Shin, J. Cellular Biotechnol., 2015, 1, 63–79 Search PubMed.
- D. E. Discher, N. Mohandas and E. A. Evans, Science, 1994, 206, 1032–1035 CrossRef PubMed.
- H. Engelhardt, H. Gaub and E. Sackmann, Nature, 1984, 307, 378–380 CrossRef CAS PubMed.
- J. Sleep, D. Wilson, R. Simmons and W. Gratzer, Biophys. J., 1999, 77, 3085–3095 CrossRef CAS PubMed.
- M. Dao, C. T. Lim and S. Suresh, J. Mech. Phys. Solids, 2003, 51, 2259–2280 CrossRef.
- M. Puig-de Morales, K. T. Turner, J. P. Butler, J. J. Fredberg and S. Suresh, J. Appl. Physiol., 2007, 293, 597–605 Search PubMed.
- M. S. Amin, Y. K. Park, N. Lue, R. R. Dasari, K. Badizadegan, M. S. Feld and G. Popescu, Opt. Express, 2007, 15, 17001–17009 CrossRef CAS PubMed.
- R. Wang, H. Ding, M. Mir, K. Tangella and G. Popescu, Biomed. Opt. Express, 2011, 2, 485–490 CrossRef PubMed.
- K. Monkos, Int. J. Biol. Macromol., 1994, 16, 31–35 CrossRef CAS PubMed.
- M. Iino and Y. Okuda, Jpn. J. Appl. Phys., 1997, 36, 3786–3790 CrossRef CAS.
- M. K. Kuimova, G. Yahioglu, J. A. Levitt and K. Suhling, J. Am. Chem. Soc., 2008, 130, 6672–6673 CrossRef CAS PubMed.
- M. K. Kuimova, S. W. Botchway, A. W. Parker, M. Balaz, H. A. Collins, H. L. Anderson, K. Suhling and P. R. Ogilby, Nat. Chem., 2009, 1, 69–73 CrossRef CAS PubMed.
- E. M. Woodcock, P. Girvan, J. Eckert, I. Lopez-Duarte, M. Kubànkovà, J. van Loon, N. J. Brooks and M. K. Kuimova, Biophys. J., 2019, 116, 1984–1993 CrossRef CAS PubMed.
- M. A. Haidekker, M. Nipper, A. Mustafic, D. Lichlyter, M. Dakanali and E. A. Theodorakis, in Dyes with Segmental Mobility: Molecular Rotors, ed. A. P. Demchenko, Springer Berlin Heidelberg, Berlin, Heidelberg, 2010, pp. 267–308 Search PubMed.
- R. O. Loutfy and A. A. Bradley, J. Phys. Chem., 1982, 86, 4205–4211 CrossRef CAS.
- K. Law, Chem. Phys. Lett., 1980, 75, 545–549 CrossRef CAS.
- B. Allen, A. C. Benniston, A. Harriman, S. A. Rostron and C. Yua, Phys. Chem. Chem. Phys., 2005, 7, 3035–3040 RSC.
- C. E. Kung and J. K. Reed, Biochemistry, 1986, 25, 6114–6121 CrossRef CAS.
- M. A. Haidekker, T. Brady, D. Lichlyter and E. Theodorakis, Bioorg. Chem., 2005, 33, 415–425 CrossRef CAS PubMed.
- T. Förster and G. Hoffmann, Z. Phys. Chem., 1971, 75, 63–76 CrossRef.
- T. Iwaki, C. Torigoe, M. Noji and M. Nakanishi, Biochemistry, 1993, 32, 7589–7592 CrossRef CAS PubMed.
- W. Wang, K. Shi, J. Jia, S. Chen and H. Ma, Talanta, 2009, 77, 1795–1799 CrossRef PubMed.
- R. O. Loutfy and D. M. Teegarden, Macromolecules, 1983, 16, 452–456 CrossRef CAS.
- A. Hawe, V. Filipe and W. Jiskoot, Pharm. Res., 2010, 27, 314–326 CrossRef CAS PubMed.
- C. E. Kung and J. K. Reed, Biochemistry, 1989, 28, 6678–6686 CrossRef CAS PubMed.
- T. Furuno, R. Isoda, K. Inagaki, T. Iwaki, M. Noji and M. Nakanishi, Immunol. Lett., 1992, 33, 285–288 CrossRef CAS PubMed.
- M. L. Viriot, M. C. Carre, C. Geoffroy-Chapotot, A. Brembilla, S. Muller and J. F. Stoltz, Clin. Hemorheol. Microcirc., 1998, 19, 151–160 CAS.
- B. Abou, C. Gay, B. Laurent, O. Cardoso, H. Voigt, D. Peisker and S. Gorb, J. R. Soc., Interface, 2010, 7, 1745–1752 CrossRef PubMed.
- F. Menzel, S. Morsbach, J. Martens, P. Räder, S. Hadjaje, M. Poizat and B. Abou, J. Exp. Biol., 2019, 222, jeb210807 CrossRef PubMed.
- R. Colin, A. M. Alsayed, J.-C. Castaing, R. Goyal, L. Hough and B. Abou, Soft Matter, 2011, 11, 4504 RSC.
- A. Einstein, Ann. Phys., 1905, 18, 549–560 CrossRef.
- M. A. Haidekker and E. A. Theodorakis, J. Biol. Eng., 2010, 4, 1–14 CrossRef PubMed.
- M. R. Waterman, Methods Enzymol., 1978, 52, 456–463 CAS.
- S. Howell, M. Dakanali, E. A. Theodorakis and M. A. Haidekker, J. Fluoresc., 2012, 22, 457–465 CrossRef CAS.
- M. Haidekker, N. LHeureux and J. Frangos, Am. J. Physiol., 2000, 278, H1401 CAS.
- A. Vyšniauskas, M. Qurashi, N. Gallop, M. Balaz, H. L. Anderson and M. K. Kuimova, Chem. Sci., 2015, 6, 5773–5778 RSC.
- A. Vyšniauskas, I. López-Duarte, N. Duchemin, T.-T. Vu, Y. Wu, E. M. Budynina, Y. A. Volkova, E. Peña Cabrera, D. E. Ramírez-Ornelas and M. K. Kuimova, Phys. Chem. Chem. Phys., 2017, 19, 25252–25259 RSC.
- G. Artmann, C. Kelemen, D. Porst, G. Büldt and S. Chien, Biophys. J., 1998, 75, 3179–3183 CrossRef CAS PubMed.
- C. Kelemen, S. Chien and G. Artmann, Biophys. J., 2001, 80, 2622–2630 CrossRef CAS.
- T. Lecklin, S. Egginton and G. Nash, Eur. J. Physiol., 1996, 432, 753–759 CrossRef CAS.
- Z. Xu, Y. Zheng, X. Wang, N. Shehata, C. Wang and Y. Sun, Microsyst. Nanoeng., 2018, 4, 17103 CrossRef CAS.
- R. Waugh and E. Evans, Biophys. J., 1979, 26, 115–131 CrossRef CAS.
- V. Heinrich, K. Ritchie, N. Mohandas and E. Evans, Biophys. J., 2001, 81, 1452–1463 CrossRef CAS PubMed.
- A. S. Smith, R. B. Nowak, S. Zhou, M. Giannetto, D. S. Gokhin, J. Papoin, I. C. Ghiran, L. Blanc, J. Wan and V. M. Fowler, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, E4377–E4385 CrossRef CAS PubMed.
- S. E. Lux, Blood, 2016, 127, 187–199 CrossRef CAS PubMed.
- E. Evans and Y.-C. Fung, Microvasc. Res., 1972, 4, 335–347 CrossRef CAS PubMed.
- K. Jaferzadeh, M. Sim, N. Kim and I. Moon, Sci. Rep., 2019, 9, 14062 CrossRef PubMed.
Footnotes |
| † Data can be fitted with a Förster–Hoffmann relation even at low viscosity were a deviation from the law at higher viscosity was observed (Section 3.1.2). |
| ‡ We observe that the signal from the membrane and its dispersion due to statistical sampling increases with decreasing temperature (not shown). |
| This journal is © The Royal Society of Chemistry 2021 |