Open Access Article
Open Access ArticleThe effect of headgroup methylation on polymorphic phase behaviour in hydrated N-methylated phosphoethanolamine:palmitic acid membranes†
Matthew E.
Allen
 a,
Yuval
Elani
a,
Yuval
Elani
 b,
Nicholas J.
Brooks
b,
Nicholas J.
Brooks
 a and
John M.
Seddon
a and
John M.
Seddon
 *a
*a
aDepartment of Chemistry, Imperial College London, W12 7SL, UK. E-mail: j.seddon@imperial.ac.uk
bDepartment of Chemical Engineering, Imperial College London, SW7 2AZ, UK
First published on 17th May 2021
Abstract
Mixtures of fatty acids and phospholipids can form hexagonal (HII) and inverse bicontinuous cubic phases, the latter of which are implicated in various cellular processes and have wide-ranging biotechnological applications in protein crystallisation and drug delivery systems. Therefore, it is vitally important to understand the formation conditions of inverse bicontinuous cubic phases and how their properties can be tuned. We have used differential scanning calorimetry and synchrotron-based small angle and wide angle X-ray scattering (SAXS/WAXS) to investigate the polymorphic phase behaviour of palmitic acid/partially-methylated phospholipid mixtures, and how headgroup methylation impacts on inverse bicontinuous cubic phase formation. We find that upon partial methylation of the phospholipid headgroup (1 or 2 methyl substituents) inverse bicontinuous cubic phases are formed (of the Im3m spacegroup), which is not the case with 0 or 3 methyl substituents. This shows how important headgroup methylation is for controlling phase behaviour and how a change in headgroup methylation can be used to controllably tune various inverse bicontinuous phase features such as their lattice parameter and the temperature range of their stability.
Introduction
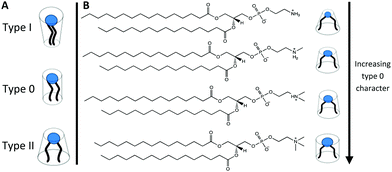
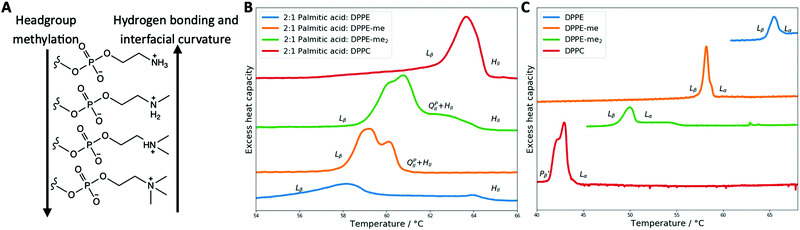
Lipids and other amphiphilic molecules can self-assemble to form a wide variety of liquid-crystalline phases1 depending on their chemical structure and interaction properties. Amphiphilic molecules can be broadly classified into 3 types (type 0, I or II amphiphiles). Type 0 amphiphiles are bilayer forming as the headgroup and the hydrocarbon tail exert similar lateral pressures. On the other hand, type I or II amphiphiles are non-bilayer forming as the headgroup and hydrocarbon tail exert different lateral pressures, thus leading to a tendency for the polar/nonpolar interface to curve either towards the tail or headgroup regions respectively.2Due to the different self-assembly properties of amphiphiles, structures that include crystalline, gel and fluid lamellar phases (LC, Lβ and Lα respectively) and non-lamellar phases can be formed from bilayer forming and non-bilayer forming amphiphiles respectively (Fig. 1). Particularly important non-lamellar phases include the inverse hexagonal phase (HII) where the lipids are arranged into inverse cylinders, with water on the inside, that pack onto a 2-D hexagonal lattice,3 and inverse bicontinuous cubic phases (QDII, QGII, QPII), where a continuous lipid bilayer is draped onto a D, G or P infinite minimal periodic surface, giving rise to cubic phases of Pn3m, Ia3d and Im3m crystallographic space groups respectively.4
 | ||
| Fig. 1 Schematic of the lamellar phase, an inverse bicontinuous cubic phase (Im3m spacegroup) and the inverse hexagonal phase and how they are related by interfacial curvature. | ||
Non-lamellar phases, due to their interfacial curvature, are important in vivo as they have important biological functions5 and affect biological membrane organisation.6 Inverse bicontinuous cubic phases are known to appear within mammalian cells7,8 and are associated and formed within cells that have experienced stress,9 starvation10 and infection by viruses.11 For example the SARS coronavirus causes formation of a Ia3d gyroid phase12 within cells infected by this virus.13
In vitro inverse bicontinuous cubic phases have attracted significant attention due to their unique properties;14 they are soft biocompatible structures that have a large surface area due to their interconnected water pore network. Therefore, inverse bicontinuous cubic structures have found uses as drug delivery platforms15,16 and protein crystallisation systems17,18 where amphiphile composition,19 stereochemical purity,20 water content,21 charge of the system,22 solvents,23 pH,24 pressure and temperature25 can be altered to achieve a particular bicontinuous cubic spacegroup or bicontinuous cubic lattice parameter.
A particularly interesting set of biological amphiphiles that can induce inverse bicontinuous cubic structures are long chain fatty acids. These molecules have been observed to be important in membrane fusion events26,27 and can influence biological functions by regulating ion channel activity,28 membrane fluidity29 and bending rigidity30 in addition to affecting cell signalling behaviour.31 Specifically oleic acid can reduce blood pressure through controlling G-protein mediated signalling by promoting inverse hexagonal phase behaviour at high contents.32 Additionally, palmitic acid, the most common saturated fatty acid in the body33 can impair insulin signalling and increase hepatocyte apoptosis.34 Therefore, it is critically important to gain an understanding of how lipids and fatty acids combine to influence membrane phase behaviour and function. This will enable exploitation of these mixtures properties to design biologically relevant and useful inverse bicontinuous cubic systems.
Previous work has looked at how saturated fatty acid composition affects the phase behaviour of the natural bilayer forming phosphatidylcholine (PC) phospholipids.35 It was observed that the incorporation of fatty acids shifts the gel–fluid transition to higher temperatures36 and at significant molar fatty acid fractions (0.67 mol fraction) the Lβ–Lα gel–fluid transition present in pure phosphatidylcholine systems shifts towards a Lβ–HII transition.37 Thus, the fluid bilayer Lα phase is completely suppressed, due to the fatty acid affecting the membrane lateral pressure profile. This leads to the favouring of non-lamellar phase formation.38,39 Furthermore, at this stoichiometry sharp phase transitions occur due to formation of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 fatty acid
1 fatty acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phospholipid complex, and azeotropic behaviour is observed at this point.40 Moreover for short chain fatty acid:phosphatidylcholine mixtures (lauric acid:DLPC and myristic acid:DMPC) inverse bicontinuous cubic phase formation is exhibited at the 2
phospholipid complex, and azeotropic behaviour is observed at this point.40 Moreover for short chain fatty acid:phosphatidylcholine mixtures (lauric acid:DLPC and myristic acid:DMPC) inverse bicontinuous cubic phase formation is exhibited at the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 fatty acid
1 fatty acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phospholipid ratio.21 However, at longer chain lengths (palmitic acid:DPPC) there is no evidence of inverse bicontinuous cubic phase formation for this composition.
phospholipid ratio.21 However, at longer chain lengths (palmitic acid:DPPC) there is no evidence of inverse bicontinuous cubic phase formation for this composition.
As headgroup methylation impacts the uptake of important biological molecules such as L-arginine41 and affects cell signalling pathways,42 the influence of headgroup methylation within membranes is an important topic to explore. Phosphatidylethanolamines (PE), phospholipids that have no headgroup methylation, and readily form non-lamellar phases such as HII and bicontinuous cubic phases at elevated temperatures,43,44 have a more significant non-bilayer forming characteristic than PC phospholipids. Furthermore, phospholipids with a partially methylated headgroup structure, i.e. one or two methylations of the headgroup nitrogen (Fig. 2) also exhibit different phase behaviour to that of PC or PE phospholipids45–47 and have bilayer forming tendencies that are in between those of PC and PE phospholipids. In particular single N-methylated DOPE (DOPE-me) shows inverse bicontinuous cubic and HII phase formation.48,49 Therefore, by altering the hydrogen bonding network and the headgroup size (spontaneous radius of curvature50) through changing the nitrogen methylation of the phospholipid headgroup, inverse bicontinuous cubic phase formation can be suppressed or encouraged.
In this work we have investigated how palmitic acid (PA) interacts with 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-methyl (DPPE-me) and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N,N-dimethyl (DPPE-me2) phospholipids. These phospholipids along with DPPE and DPPC ordinarily show gel–fluid lamellar transitions between 40 and 70 °C.46 We have seen that by altering the headgroup methylation that inverse bicontinuous cubic phases can be formed in these longer chain fatty acid:phospholipids mixtures. Moreover 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 palmitic acid
1 palmitic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phospholipid samples were analysed across a range of headgroup methylations (DPPE, DPPE-me, DPPE-me2 and DPPC) to rationalise the effect of headgroup methylation on phase behaviour. Demonstrating that these essential biological molecules can form inverse bicontinuous cubic phases and properties such as phase formation temperature and lattice parameters can be adjusted by headgroup methylation allows exploitation of this knowledge to design new biologically and therapeutically important systems. Furthermore, the rationale behind the impact of headgroup methylation within these systems can be applied to existing formulations51 to further enhance their potential use.
phospholipid samples were analysed across a range of headgroup methylations (DPPE, DPPE-me, DPPE-me2 and DPPC) to rationalise the effect of headgroup methylation on phase behaviour. Demonstrating that these essential biological molecules can form inverse bicontinuous cubic phases and properties such as phase formation temperature and lattice parameters can be adjusted by headgroup methylation allows exploitation of this knowledge to design new biologically and therapeutically important systems. Furthermore, the rationale behind the impact of headgroup methylation within these systems can be applied to existing formulations51 to further enhance their potential use.
Experimental
Materials
The phospholipids DPPE (1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine), DPPE-me (1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-methyl), DPPE-me2 (1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N,N-dimethyl) and DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine) were purchased from Sigma Aldrich (Gillingham, UK) as powders. Palmitic acid was also purchased from Sigma Aldrich (Gillingham, UK) as a powder. All of the chemicals had a purity of >99% and were used without subsequent purification.Sample preparation
Samples were prepared by co-dissolving appropriate amounts of the phospholipid and palmitic acid in chloroform (Sigma Aldrich, Gillingham, UK). The solutions were then vortexed to ensure mixing before the chloroform was removed under a stream of N2 gas. The resultant films were lyophilised for at least 12 hours before being hydrated with ultrapure water (18.2 mΩ) to a 70% water weight fraction in order to ensure the samples were under excess hydration conditions.DSC preparation and measurements
A known weight of the hydrated phospholipid:palmitic acid mixture was placed into a hermetically sealed DSC pan. A PerkinElmer Diamond DSC was then used to analyse the samples; before any data were recorded the samples underwent at least 2 heating/cooling cycles on the DSC to ensure homogenous mixing without any water loss. A scan rate of 1 °C was used to obtain thermographs of the samples from which the transition temperatures and phase transition behaviour were obtained.X-Ray preparation and measurements
The hydrated phospholipid:palmitic acid samples were placed into 1.8 mm inner diameter polycarbonate capillaries that were sealed with epoxy resin. The sealed capillaries were heat cycled to ensure homogenous mixing while minimising water loss. Small and wide-angle X-ray diffraction data were obtained for the mixed samples using beamline I22 at Diamond Light Source. Diffraction patterns were collected using an X-ray energy of 18 keV (wavelength 0.689 Å) and a sample to detector distance of 2 m. The raw data were corrected using the procedure developed by the beamline technicians at Diamond.52 The processed data were analysed by fitting pseudo-Voigt functions to Bragg peaks in order to extract peak maxima values that were used to calculate the d-spacings, and hence the lattice parameters once the lipid mesophases had been correctly identified from indexing of the Bragg peaks (see ESI,† Fig. S6 or S10 for more detail).Results and discussion
The phase behaviour of DPPE-me:palmitic acid complexes
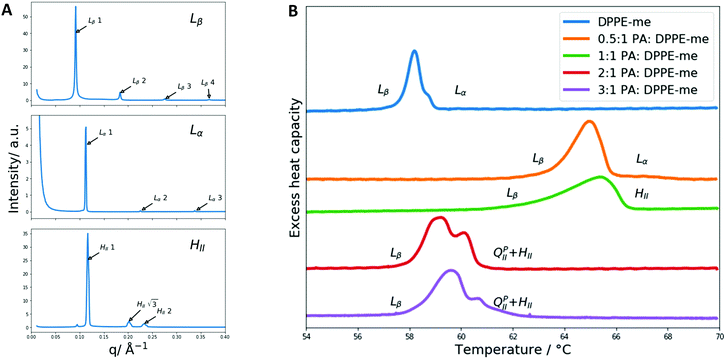
Initially DSC and X-ray diffraction experiments were used to characterise the phase behaviour of PA:DPPE-me mixtures in excess water. The experimental results are summarised in Fig. 3. At low molar concentrations of palmitic acid (0.33 mol fraction) the phases identified through SAXS (the Lβ and Lα phases) were the same as those seen within pure DPPE-me. This matches previous observations in PA:DPPC samples where small amounts of palmitic acid did not alter the phase behaviour.39 However, upon adding a significant amount of palmitic acid (0.5 mol fraction) a HII phase was seen instead of the Lα phase. This occurs as palmitic acid is a non-bilayer forming amphiphile,53 hence at a high enough concentration of palmitic acid the phase behaviour shifts to favour non-lamellar structures to reduce the increased curvature frustration energy within the phospholipid bilayer.54 Through using DSC, the addition of palmitic acid led to a broadening and increase in the transition temperature of the gel–fluid phase transition peak. This again has been observed within PA:DPPC samples.39 DSC also verified that at higher palmitic acid concentrations (0.5 mol fraction) the main gel–fluid phase transition occurred between the Lβ and HII phases.However, the molar fraction of palmitic acid needed to induce the Lβ–HII transition is lower than the amount reported in PA:DPPC mixtures. This occurs as DPPE-me has a smaller headgroup than DPPC and thus has a larger degree of non-bilayer forming character (characterised by a smaller spontaneous radius of curvature50). This allows the HII phase to be formed more readily.
At higher concentrations of palmitic acid (0.67 and 0.75 mol fractions), the gel–fluid transition still corresponds to Lβ–HII, although the transition temperature has decreased significantly. This arises from the increased presence of non-bilayer forming amphiphiles increasing the curvature frustration energy within the bilayer thus favouring the formation of the HII phase at a lower temperature.
The phase transition peak for the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of palmitic acid
1 ratio of palmitic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPE-me is sharper than the 3
DPPE-me is sharper than the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, because the azeotropic point of PA
1 ratio, because the azeotropic point of PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPE-me occurs at the former composition. This matches previous work where FA:PC mixtures exhibit a sharp DSC transition and an azeotropic point at this molar ratio.35 This further enhances the argument that PA
DPPE-me occurs at the former composition. This matches previous work where FA:PC mixtures exhibit a sharp DSC transition and an azeotropic point at this molar ratio.35 This further enhances the argument that PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPE-me mixtures also experience the formation of a 2
DPPE-me mixtures also experience the formation of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex and shows the 2
1 complex and shows the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 fatty acid
1 fatty acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phospholipid complex formation is common among phospholipid
phospholipid complex formation is common among phospholipid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fatty acid mixtures.
fatty acid mixtures.
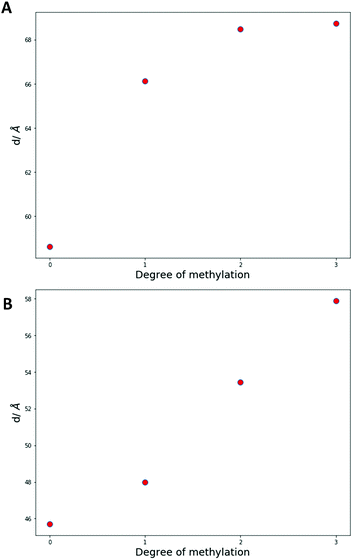
Furthermore, at high concentrations of palmitic acid (0.67 and 0.75 mol fractions) a double humped transition is present indicating that more complex phase behaviour is occurring than what was present with lower palmitic acid concentrations and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 palmitic acid
1 palmitic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
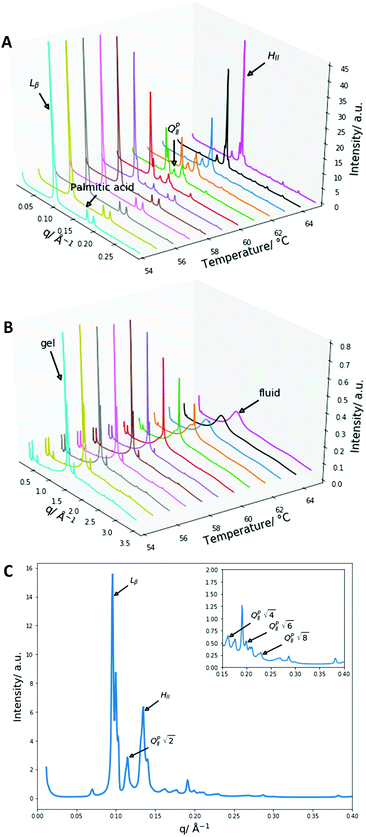
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPC mixtures. As a result, we collected SAXS and WAXS measurements at temperatures from 54 to 65 °C to investigate the phase behaviour more closely. The data are summarised in Fig. 4.
DPPC mixtures. As a result, we collected SAXS and WAXS measurements at temperatures from 54 to 65 °C to investigate the phase behaviour more closely. The data are summarised in Fig. 4.
Once again, the SAXS and WAXS spectra demonstrate the main phase change occurring is a Lβ–HII transition shown by the 1, 2, 3, 4 reflections at 54 °C for the Lβ phase and the 1, √3, 2, √7 reflections at 65 °C for the HII phase. This transition was also accompanied by an increase in fluidity shown by the broadening of the sharp peak in the WAXS spectra. Furthermore, at lower temperatures a peak at 0.175 Å−1 that matched crystalline palmitic acid (ESI,† Fig. S4) was present. This indicates that ‘excess’ palmitic acid crystallises out of the membrane and forms its own crystalline phase at high molar fractions (demonstrated within ESI,† Fig. S5) which matches previous observations.39 At higher temperatures the palmitic acid peaks disappear which is indicative of palmitic acid melting into an isotropic liquid.
From the onset of the gel–fluid transition (59 °C) to 65 °C another set of peaks appear alongside the HII phase peaks. These peaks have been assigned the Im3m cubic space group (QPII), with the first 4 reflections 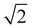 ,
, 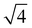 ,
, 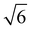 ,
, 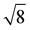 present and indicated (Fig. 4C). Furthermore, earlier studies looking at inverse bicontinuous cubic phase formation in the shorter chain 2
present and indicated (Fig. 4C). Furthermore, earlier studies looking at inverse bicontinuous cubic phase formation in the shorter chain 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 myristic acid
1 myristic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMPC mixtures gave evidence of HII and QPII coexistence21 at excess water weight fractions. As this system is very similar to the system being investigated this gives additional weighting to the Im3m assignment.
DMPC mixtures gave evidence of HII and QPII coexistence21 at excess water weight fractions. As this system is very similar to the system being investigated this gives additional weighting to the Im3m assignment.
Other unassigned peaks indicate other structures are present in the mixture. However, these peaks cannot be assigned unambiguously due to the lack of reflections present. The weak yet prominent peak present at 0.070 Å−1 does not align with any of the already assigned peaks or agree with theoretical Bonnet ratios55that would have indicated another inverse bicontinuous cubic phase was present alongside the already assigned Im3m phase.
The Im3m inverse bicontinuous cubic phase identified has a lattice parameter of 77.08 Å (ESI,† Fig. S6) at 61 °C which is small when compared to Im3m lattice parameters in other fatty acid:phospholipid mixtures.21,53 This is rationalised by DPPE-me containing more non-bilayer forming character than PC phospholipids thus leading to a more curved Im3m phase with a smaller lattice parameter.
The coexistence between the QPII and HII phases is explained by their formation occurring through a common intermediate. Upon a lamellar to non-lamellar phase transition, the lamellar phase bilayers form trans monolayer contacts.56 This structure can then produce the HII phase through a series of precursor structures or inverse bicontinuous cubic phases if enough interlamellar attachments are formed.57 Partially methylated phosphatidylethanolamines (DPPE-me) have been shown to form interlamellar attachments around phase transition temperatures49 while phosphatidylcholines (DPPC) do not form such interlamellar attachments due to their large spontaneous radius of curvature.45 This results in QII phase formation within PA:DPPE-me systems but not in PA:DPPC systems.35
More recently the formation of interlamellar attachments has been linked to the Gaussian curvature elastic modulus of monolayers.58 This value is dependent on the amphiphile composition, hence QII phase formation will be dependent on the molar concentration of palmitic acid hereby explaining QPII phase formation occurring only at the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PA
1 PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPE-me stoichiometries.
DPPE-me stoichiometries.
The phase behaviour of DPPE-me2:palmitic acid mixtures
Next, we explored the impact of one additional headgroup methyl group. Thus, PA:DPPE-me2 mixtures in excess water had their phase behaviour characterised through DSC and X-ray diffraction experiments. The results from these experiments are summarised in Fig. 5.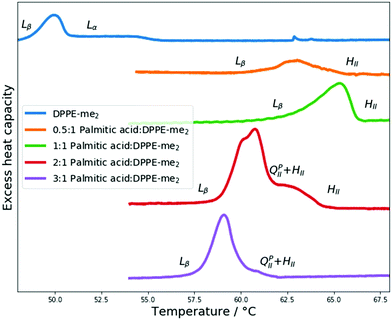 | ||
| Fig. 5 DSC scans of PA:DPPE-me2 mixtures and pure DPPE-me2, with their respective phase transitions indicated. | ||
Once again through SAXS the Lα phases seen within pure DPPE-me2 were replaced with HII phases upon addition of palmitic acid. Moreover, using DSC, palmitic acid addition again broadened the phase transitions; however, the gel–fluid transition temperature increase was more significant than with DPPE-me. Furthermore, the onset of the Lβ–HII phase transition occurred at a lower molar palmitic acid content (0.33 mol fraction) than for PA:DPPE-me and PA:DPPC.39 Therefore, palmitic acid is again inducing the formation of non-lamellar phases, although the earlier onset of this transition compared to the more intrinsically curved PA:DPPE-me mixtures45 that contain a smaller headgroup (DPPE-me) indicates that the induction of the HII phase is more complex than just increasing the spontaneous curvature in the complex.
Another parameter affecting HII phase formation is hydrogen bonding between the headgroups within the lipid membrane and the surrounding water. Upon increased headgroup methylation of the phospholipid headgroup the ability to interact with neighbouring headgroups and both the external water through hydrogen bonding decreases due to the sequential loss of first one, then two hydrogen atoms which act as hydrogen bond donors.59 Hence within PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phospholipid mixtures changing the methylation of the headgroup and increasing the amount of palmitic acid both affect the hydrogen bonding network of the mixture. Within PA:DPPE-me mixtures increasing the palmitic acid content reduces hydrogen bonding through reducing the number of H bond donors, this has been argued to alter the intermolecular forces within the bilayer and between bilayers.60 This leads to an increase in the area per molecule within the bilayer61 and thus increases the spontaneous radius of curvature which leads to the disfavouring of HII phases. However, in PA:DPPE-me2 mixtures palmitic acid addition alters the hydrogen bonding network very little leading to no phase formation favouring/disfavouring due to hydrogen bonding. The above potentially rationalises why PA:DPPE-me2 forms HII phases at a lower palmitic acid content than PA:DPPE-me. Furthermore, within PA:DPPC mixtures hydrogen bonding is increased upon palmitic acid addition due to the availability of more hydrogen bonding donors. Using the same argument as above the spontaneous radius of curvature will be decreased favouring the formation of the HII phase. This also further rationalises why DPPC, a bilayer forming phospholipids, can form non-lamellar phases when mixed with fatty acids.
phospholipid mixtures changing the methylation of the headgroup and increasing the amount of palmitic acid both affect the hydrogen bonding network of the mixture. Within PA:DPPE-me mixtures increasing the palmitic acid content reduces hydrogen bonding through reducing the number of H bond donors, this has been argued to alter the intermolecular forces within the bilayer and between bilayers.60 This leads to an increase in the area per molecule within the bilayer61 and thus increases the spontaneous radius of curvature which leads to the disfavouring of HII phases. However, in PA:DPPE-me2 mixtures palmitic acid addition alters the hydrogen bonding network very little leading to no phase formation favouring/disfavouring due to hydrogen bonding. The above potentially rationalises why PA:DPPE-me2 forms HII phases at a lower palmitic acid content than PA:DPPE-me. Furthermore, within PA:DPPC mixtures hydrogen bonding is increased upon palmitic acid addition due to the availability of more hydrogen bonding donors. Using the same argument as above the spontaneous radius of curvature will be decreased favouring the formation of the HII phase. This also further rationalises why DPPC, a bilayer forming phospholipids, can form non-lamellar phases when mixed with fatty acids.
At higher palmitic acid concentrations (0.67 and 0.75 mol fractions) a decrease in the Lβ–HII transition temperature is once again seen. This matches observations in PA:DPPE-me bilayers (Fig. 3) that the significantly increased presence of palmitic acid increases the curvature frustration energy and favours lower temperature HII phase formation. As before, the phase transitions around the 0.67 mol fraction are more distinct and sharper giving further credence to the idea that fatty acids and phospholipids form 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes35 and that this complex formation is not limited to PC based phospholipids.
1 complexes35 and that this complex formation is not limited to PC based phospholipids.
The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PA
1 PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
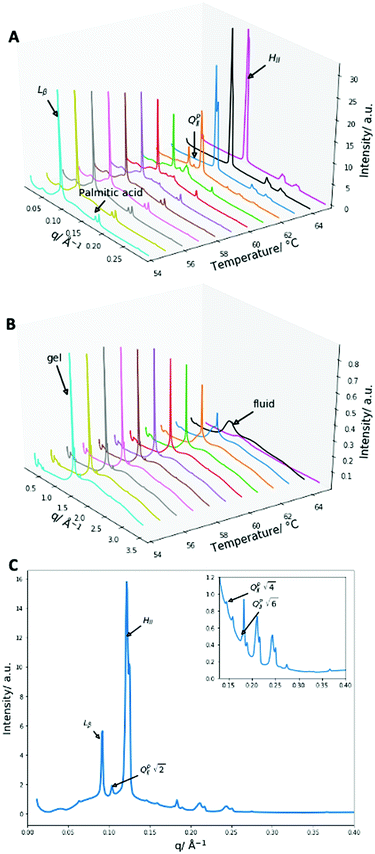
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPE-me2 mixture also exhibits a double humped transition. To verify that inverse bicontinuous cubic phase formation was again behind this complex phase behaviour SAXS and WAXS measurements were collected between 54 and 65 °C. The data are summarised in Fig. 6.
DPPE-me2 mixture also exhibits a double humped transition. To verify that inverse bicontinuous cubic phase formation was again behind this complex phase behaviour SAXS and WAXS measurements were collected between 54 and 65 °C. The data are summarised in Fig. 6.
Once more, as with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PA
1 PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPE-me the main transition occurring is the Lβ–HII transition shown by the presence of the 1, 2, 3, 4 reflections at 54 °C for the Lβ phase and the 1, √3, 2 reflections at 65 °C for the HII phase. The gel peak in the WAXS spectra once again broadened at the phase transition temperature confirming that a gel–fluid transition was occurring. The peak at 0.175 Å−1 again matched crystalline palmitic acid (ESI,† Fig. S4) and matches previous work shown within this paper and other papers39 on the phase separation of palmitic acid from the membrane at high molar contents, giving further weight to the idea that this occurs across a range of different membranes.
DPPE-me the main transition occurring is the Lβ–HII transition shown by the presence of the 1, 2, 3, 4 reflections at 54 °C for the Lβ phase and the 1, √3, 2 reflections at 65 °C for the HII phase. The gel peak in the WAXS spectra once again broadened at the phase transition temperature confirming that a gel–fluid transition was occurring. The peak at 0.175 Å−1 again matched crystalline palmitic acid (ESI,† Fig. S4) and matches previous work shown within this paper and other papers39 on the phase separation of palmitic acid from the membrane at high molar contents, giving further weight to the idea that this occurs across a range of different membranes.
At the onset of the gel–fluid transition (60 °C) a set of peaks coexist with the HII phase peaks over a very narrow temperature range (59–62 °C). Again, some of these peaks can be assigned to the Im3m inverse bicontinuous cubic space group (QPII) with the first 3 reflections 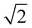 ,
, 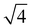 ,
, 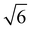 (which is very faint) present and indicated in Fig. 4C. As this system is very similar to the better characterised Im3m phase in the 2
(which is very faint) present and indicated in Fig. 4C. As this system is very similar to the better characterised Im3m phase in the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PA
1 PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPE-me system and previous systems that showed Im3m phases at excess water,21 the Im3m phase can be assigned.
DPPE-me system and previous systems that showed Im3m phases at excess water,21 the Im3m phase can be assigned.
As with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PA
1 PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPE-me, in PA
DPPE-me, in PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPE-me2 mixtures there are unassigned peaks present which cannot be assigned unambiguously due to the lack of reflections present or agreement with theoretical Bonnet ratios.55 However, these unassigned peaks appear in the same regions of the PA:DPPE-me and PA:DPPE-me2 patterns and at similar positions in relation to the QPII reflections. The appearance of these peaks in the same positions across a range of headgroup methylations suggests that the peaks are related in some way to non-lamellar phase formation.
DPPE-me2 mixtures there are unassigned peaks present which cannot be assigned unambiguously due to the lack of reflections present or agreement with theoretical Bonnet ratios.55 However, these unassigned peaks appear in the same regions of the PA:DPPE-me and PA:DPPE-me2 patterns and at similar positions in relation to the QPII reflections. The appearance of these peaks in the same positions across a range of headgroup methylations suggests that the peaks are related in some way to non-lamellar phase formation.
The lattice parameter of the Im3m inverse bicontinuous cubic phase was 86.0 Å at 62 °C (ESI,† Fig. S10), this value is significantly larger than the lattice parameter values of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PA
1 PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPE-me at 61 and 62 °C (77.1 and 74.1 Å respectively) indicating that the larger lattice parameter occurs due to DPPE-me2 having a larger headgroup than DPPE-me thus leading to a less curved Im3m phase.
DPPE-me at 61 and 62 °C (77.1 and 74.1 Å respectively) indicating that the larger lattice parameter occurs due to DPPE-me2 having a larger headgroup than DPPE-me thus leading to a less curved Im3m phase.
The ratio of the Im3m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HII peak intensities within the 2
HII peak intensities within the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PA
1 PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPE-me2 sample is smaller than the same ratio within the 2
DPPE-me2 sample is smaller than the same ratio within the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PA
1 PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPE-me sample. This indicates that along with the small range of Im3m stability in the 2
DPPE-me sample. This indicates that along with the small range of Im3m stability in the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PA
1 PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPE-me2 sample, that the HII phase is increasingly favoured over the Im3m phase upon increasing headgroup methylation at this stoichiometry. This follows previous rationale suggesting that DPPE-me2, due to its larger headgroup, will have a larger spontaneous radius of curvature, thus forming fewer interlamellar attachments during the lamellar–non lamellar transition.45 This will reduce the prevalence of the QII phases as seen in the SAXS data. Furthermore, the stability of the QII phases is related to the temperature dependent Gaussian curvature elastic modulus,58 thus an increase in temperature leads to a change in this value which causes the destabilisation of the QII phases and favouring of the HII phase.
DPPE-me2 sample, that the HII phase is increasingly favoured over the Im3m phase upon increasing headgroup methylation at this stoichiometry. This follows previous rationale suggesting that DPPE-me2, due to its larger headgroup, will have a larger spontaneous radius of curvature, thus forming fewer interlamellar attachments during the lamellar–non lamellar transition.45 This will reduce the prevalence of the QII phases as seen in the SAXS data. Furthermore, the stability of the QII phases is related to the temperature dependent Gaussian curvature elastic modulus,58 thus an increase in temperature leads to a change in this value which causes the destabilisation of the QII phases and favouring of the HII phase.
With other stoichiometries of PA:DPPE-me2Im3m spacegroups are also present. Evidence for this is again seen in the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture, which despite a single melting peak in the DSC scan exhibits remarkably similar phase behaviour to the 2
1 mixture, which despite a single melting peak in the DSC scan exhibits remarkably similar phase behaviour to the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PA
1 PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPE-me2 sample. However, one key difference is the stability of the QPII phase,
DPPE-me2 sample. However, one key difference is the stability of the QPII phase,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) in the 3
in the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sample the QPII phase is stable across a wider temperature range (ESI,† Fig. S7) potentially indicating that the slightly increased concentration of palmitic acid helps stabilise this phase relative to the HII phase. Furthermore, within the 1
1 sample the QPII phase is stable across a wider temperature range (ESI,† Fig. S7) potentially indicating that the slightly increased concentration of palmitic acid helps stabilise this phase relative to the HII phase. Furthermore, within the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry there are two unassigned peaks at high temperatures that correlate to
1 stoichiometry there are two unassigned peaks at high temperatures that correlate to 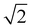 and
and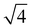 Im3m reflections (ESI,† Fig. S8) although lack of any further peaks means that an assignment cannot be completed with confidence. More work needs to be done to further identify the region of QII phase formation in both PA:DPPE-me and PA:DPPE-me2 systems.
Im3m reflections (ESI,† Fig. S8) although lack of any further peaks means that an assignment cannot be completed with confidence. More work needs to be done to further identify the region of QII phase formation in both PA:DPPE-me and PA:DPPE-me2 systems.
The effect of headgroup methylation on phase behaviour
After characterising the phase behaviour of PA:DPPE-me and PA:DPPE-me2 mixtures and gaining an understanding of what factors affect inverse bicontinuous cubic phase formation in these samples, we investigated the effect of headgroup methylation across the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PA
1 PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phospholipid mixtures and in the pure phospholipid samples in excess water. This was done in order to further understand how both lamellar and non-lamellar phase behaviour is affected by the degree of methylation. The results from this are summarised in Fig. 7.
phospholipid mixtures and in the pure phospholipid samples in excess water. This was done in order to further understand how both lamellar and non-lamellar phase behaviour is affected by the degree of methylation. The results from this are summarised in Fig. 7.
For the pure phospholipid samples an increase in headgroup methylation decreases the Lβ–Lα transition temperature (or in the case of DPPC the ripple gel Pβ′–Lα transition temperature), a trend that has been observed by others.46 This is in part due to the decrease in hydrogen bonding promoting lateral expansion within the bilayer leading to a lower temperature where the bilayer fluidises.62
Conversely, for the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 samples, an increase in phospholipid headgroup methylation increases the transition temperature. As the transitions are all lamellar–non lamellar, a different set of energetics govern these transitions when compared to the pure phospholipids. As discussed previously an increase in headgroup methylation increases the spontaneous radius of curvature of the phospholipids. As a larger spontaneous radius of curvature disfavours HII phase formation,45 a higher Lβ–HII transition temperature will be seen for the larger headgroup phospholipids. In addition, increasing headgroup methylation will decrease hydrogen bonding and thus alter the intermolecular forces within and between bilayers.60 As discussed before this leads to an increase in the area per molecule within the bilayer,61 increasing the spontaneous radius of curvature, and disfavouring HII phase formation. The 2
1 samples, an increase in phospholipid headgroup methylation increases the transition temperature. As the transitions are all lamellar–non lamellar, a different set of energetics govern these transitions when compared to the pure phospholipids. As discussed previously an increase in headgroup methylation increases the spontaneous radius of curvature of the phospholipids. As a larger spontaneous radius of curvature disfavours HII phase formation,45 a higher Lβ–HII transition temperature will be seen for the larger headgroup phospholipids. In addition, increasing headgroup methylation will decrease hydrogen bonding and thus alter the intermolecular forces within and between bilayers.60 As discussed before this leads to an increase in the area per molecule within the bilayer,61 increasing the spontaneous radius of curvature, and disfavouring HII phase formation. The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 samples also clearly show that QPII phase formation is favoured at intermediate degrees of methylation which supports previous work in the field.45,58
1 samples also clearly show that QPII phase formation is favoured at intermediate degrees of methylation which supports previous work in the field.45,58
When comparing the pure phospholipid samples to the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture samples it is clear that headgroup methylation influences phospholipid phase behaviour in greatly different ways depending on the transition involved. This shows a simple change to phospholipid structure can lead to complex changes of self-assembly properties of the system.
1 mixture samples it is clear that headgroup methylation influences phospholipid phase behaviour in greatly different ways depending on the transition involved. This shows a simple change to phospholipid structure can lead to complex changes of self-assembly properties of the system.
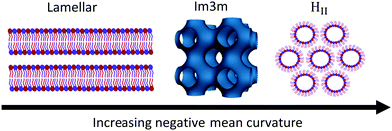
In addition to investigating the effects of headgroup methylation on phase behaviour, the effects of headgroup methylation on the d spacings of the Lβ phase at 50 °C and the HII phase at 65 °C in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PA
1 PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phospholipid samples were analysed. The results are summarised in Fig. 8.
phospholipid samples were analysed. The results are summarised in Fig. 8.
Throughout both the phases an increase in methylation increases the d spacing values. However, within the Lβ phase consecutive increases in methylation have less effect on the d spacing values while within the HII phase the increase is more linear.
For the Lβ phase an initial large increase upon methylation is attributed to a single methyl group having a greater degree of steric interference compared to a hydrogen atom. This interferes with the ability of the headgroup to interact with other headgroups between bilayers,63 which will increase the d spacing. Due to long axis rotation within the bilayers, subsequent methylation will impact the ability of the phospholipids to hydrogen bond far more than affect the sterics of the system. In addition DPPC has been seen to tilt to fill in void space within the hydrocarbon tail region64 and reduce the steric impact of having a large headgroup. Therefore, the slight increases after the initial methylation are due to a reduction in interactions between bilayers predominantly caused by hydrogen bonding. These results for the Lβ phase align closely with results for pure phospholipid systems where a large increase in d spacing was evident upon the initial methylation and subsequent methylations led to little change in this parameter.45
In the more fluid HII phase, due to the larger volume available for the molecules to occupy, the effect of sterics on screening interactions between molecules is reduced significantly. Thus, the increase in d spacing upon methylation can be attributed to decreasing interaction strengths predominantly through hydrogen bonding between molecules. This fits with the largely linear data that suggests a near constant change in forces is occurring to increase the d spacing. Increasing methylation removes one hydrogen bonding equivalent which would lead to a similar change in forces upon each methylation.
Conclusions
In this study we have demonstrated that inverse bicontinuous cubic phases of spacegroup Im3m can be formed in palmitic acid:phospholipid mixtures by altering the headgroup methylation. We also showed both palmitic acid content and the methylation of the phospholipid headgroup play vital roles in phase formation. Furthermore, the impact of phospholipid methylation within fatty acid systems has been shown to impact on d-spacings as well as phase formation. Showing that longer chain saturated fatty acids systems can form inverse bicontinuous cubic phases and how headgroup methylation affects these systems will hopefully enable the production of biological system mimics involving these components. In addition, the increased understanding of the effect of headgroup methylation will improve our ability to tune inverse bicontinuous cubic systems and further enhance uses in different fields such as drug delivery and protein crystallisation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge Diamond Light Source for time on beamline I22 under proposal SM20789 and would like to thank Dr Andy Smith for his support and assistance in using the beamline. This work was supported by EPSRC Programme grant EP/J017566/1 and a UKRI Future Leaders Fellowship, grant reference number MR/S031537/1.Notes and references
- V. Luzzati and F. Husson, J. Cell Biol., 1962, 12, 207–219 CrossRef CAS.
- G. C. Shearman, O. Ces, R. H. Templer and J. M. Seddon, J. Phys.: Condens. Matter, 2006, 18, S1105–S1124 CrossRef CAS PubMed.
- J. M. Seddon, Biochim. Biophys. Acta, Biomembr., 1990, 1031, 1–69 CrossRef CAS.
- G. C. Shearman, A. I. I. Tyler, N. J. Brooks, R. H. Templer, O. Ces, R. V. Law and J. M. Seddon, Liq. Cryst., 2010, 37, 679–694 CrossRef CAS.
- O. Vögler, J. Casas, D. Capó, T. Nagy, G. Borchert, G. Martorell and P. V. Escribá, J. Biol. Chem., 2004, 279, 36540–36545 CrossRef PubMed.
- J. Jouhet, Front. Plant Sci., 2013, 4 Search PubMed.
- T. Landh, FEBS Lett., 1995, 369, 13–17 CrossRef CAS PubMed.
- Z. A. Almsherqi, S. D. Kohlwein and Y. Deng, J. Cell Biol., 2006, 173, 839–844 CrossRef CAS PubMed.
- Y. Deng and Z. A. Almsherqi, Interface Focus, 2015, 5 Search PubMed.
- E. W. Daniels and E. P. Breyer, Zeitschrift für Zellforsch. und Mikroskopische Anat., 1968, 91, 159–169 CrossRef CAS PubMed.
- Y. Deng, Z. A. Almsherqi, M. M. L. Ng and S. D. Kohlwein, Trends Cell Biol., 2010, 20, 371–379 CrossRef CAS PubMed.
- Z. A. Almsherqi, C. S. McLachlan, P. Mossop, K. Knoops and Y. Deng, Redox Rep., 2005, 10, 167–171 CrossRef CAS PubMed.
- C. S. Goldsmith, K. M. Tatti, T. G. Ksiazek, P. E. Rollin, J. A. Comer, W. W. Lee, P. A. Rota, B. Bankamp, W. J. Bellini and S. R. Zaki, Emerging Infect. Dis., 2004, 10, 320–326 CrossRef PubMed.
- J. Zhai, S. Sarkar, C. E. Conn and C. J. Drummond, Mol. Syst. Des. Eng., 2020, 5, 1354–1375 RSC.
- R. Negrini, A. Sánchez-Ferrer and R. Mezzenga, Langmuir, 2014, 30, 4280–4288 CrossRef CAS PubMed.
- B. Fan, J. Wan, J. Zhai, X. Chen and S. H. Thang, ACS Nano, 2021, 15, 16 Search PubMed.
- P. Nogly, D. James, D. Wang, T. A. White, N. Zatsepin, A. Shilova, G. Nelson, H. Liu, L. Johansson, M. Heymann, K. Jaeger, M. Metz, C. Wickstrand, W. Wu, P. Båth, P. Berntsen, D. Oberthuer, V. Panneels, V. Cherezov, H. Chapman, G. Schertler, R. Neutze, J. Spence, I. Moraes, M. Burghammer, J. Standfuss and U. Weierstall, IUCrJ, 2015, 2, 168–176 CrossRef CAS PubMed.
- L. Van Dalsen, L. Smithers, C. Boland, D. Weichert and M. Caffrey, Cryst. Growth Des., 2020, 21, 500 Search PubMed.
- A. Angelova, B. Angelov, M. Drechsler, T. Bizien, Y. E. Gorshkova and Y. Deng, Front. Cell Dev. Biol., 2021, 9 Search PubMed.
- J. J. Vallooran, M. Duss, P. Ansorge, R. Mezzenga and E. M. Landau, Langmuir, 2020, 36, 9132–9141 CrossRef CAS PubMed.
- R. H. Templer, J. M. Seddon, N. A. Warrender, A. Syrykh, Z. Huang, R. Winter and J. Erbes, J. Phys. Chem. B, 1998, 102, 7251–7261 CrossRef CAS.
- A. I. I. Tyler, H. M. G. Barriga, E. S. Parsons, N. L. C. McCarthy, O. Ces, R. V. Law, J. M. Seddon and N. J. Brooks, Soft Matter, 2015, 11, 3279–3286 RSC.
- M. C. G. Lotierzo, B. R. Casadei, R. D. de Castro, B. Malheiros and L. R. S. Barbosa, Drug Delivery Transl. Res., 2020, 10, 1571–1583 CrossRef CAS PubMed.
- S. Rajesh, J. Zhai, C. J. Drummond and N. Tran, J. Colloid Interface Sci., 2021, 589, 85–95 CrossRef CAS PubMed.
- H. M. G. Barriga, A. I. I. Tyler, N. L. C. McCarthy, E. S. Parsons, O. Ces, R. V. Law, J. M. Seddon and N. J. Brooks, Soft Matter, 2015, 11, 600–607 RSC.
- H. L. Kantor and J. H. Prestegard, Biochemistry, 1978, 17, 3592–3597 CrossRef CAS PubMed.
- A. Angelova, B. Angelov, V. M. Garamus and M. Drechsler, J. Mol. Liq., 2019, 279, 518–523 CrossRef CAS.
- R. W. Ordway, J. J. Singer and J. V. Walsh, Trends Neurosci., 1991, 14, 96–100 CrossRef CAS PubMed.
- G. Maulucci, O. Cohen, B. Daniel, A. Sansone, P. I. Petropoulou, S. Filou, A. Spyridonidis, G. Pani, M. De Spirito, C. Chatgilialoglu, C. Ferreri, K. E. Kypreos and S. Sasson, Free Radic. Res., 2016, 50, S40–S50 CrossRef CAS PubMed.
- A. I. I. Tyler, J. L. Greenfield, J. M. Seddon, N. J. Brooks and S. Purushothaman, Front. Cell Dev. Biol., 2019, 7, 187 CrossRef PubMed.
- M. Ibarguren, D. J. López and P. V. Escribá, Biochim. Biophys. Acta, Biomembr., 1838, 2014, 1518–1528 Search PubMed.
- S. Terés, G. Barceló-Coblijn, M. Benet, R. Álvarez, R. Bressani, J. E. Halver and P. V. Escribá, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 13811–13816 CrossRef PubMed.
- G. Carta, E. Murru, S. Banni and C. Manca, Front. Physiol., 2017, 8, 902 CrossRef PubMed.
- M. Ricchi, M. R. Odoardi, L. Carulli, C. Anzivino, S. Ballestri, A. Pinetti, L. I. Fantoni, F. Marra, M. Bertolotti, S. Banni, A. Lonardo, N. Carulli and P. Loria, J. Gastroenterol. Hepatol., 2009, 24, 830–840 CrossRef CAS PubMed.
- J. M. Seddon, R. H. Templer, N. A. Warrender, Z. Huang, G. Cevc and D. Marsh, Biochim. Biophys. Acta, Biomembr., 1997, 1327, 131–147 CrossRef CAS.
- S. Mabrey and J. M. Sturtevant, Biochim. Biophys. Acta, Lipids Lipid Metab., 1977, 486, 444–450 CrossRef CAS.
- D. Marsh and J. M. Seddon, Biochim. Biophys. Acta, Biomembr., 1982, 690, 117–123 CrossRef CAS.
- J. M. Seddon, A. M. Squires, C. E. Conn, O. Ces, A. J. Heron, X. Mulet, G. C. Shearman, R. H. Templer, H. F. Gleeson, V. Percec, S. T. Lagerwall, P. Palffy-Muhoray and C. R. Safinya, Philos. Trans. R. Soc., A, 2006, 364, 2635–2655 CrossRef CAS PubMed.
- R. D. Koynova, B. G. Tenchovl, P. J. Quinn and P. Laggner, Chem. Phys. Lipids, 1988, 48, 205–214 CrossRef CAS PubMed.
- R. D. Koynova, A. I. Boyanov and B. G. Tenchov, Biochim. Biophys. Acta, Biomembr., 1987, 903, 186–196 CrossRef CAS.
- A. Bouchet, F. Lairion and A. Disalvo, Biochim. Biophys. Acta, Biomembr., 2012, 1818, 1395–1401 CrossRef CAS PubMed.
- F. Hirata, J. F. Tallman, R. C. Henneberry, P. Mallorga, W. J. Strittmatter and J. Axelrod, Prog. Clin. Biol. Res., 1981, 63, 383–388 CAS.
- J. M. Seddon, G. Cevc, R. D. Kaye and D. Marsh, Biochemistry, 1984, 23, 2634–2644 CrossRef CAS PubMed.
- R. Koynova and B. Tenchov, Data Br., 2018, 18, 501–505 CrossRef PubMed.
- S. M. Gruner, M. W. Tate, G. L. Kirk, P. T. C. So, D. C. Turner, D. T. Keane, C. P. S. Tilcock and P. R. Cullis, Biochemistry, 1988, 27, 2853–2866 CrossRef CAS PubMed.
- M. Kusube, H. Matsuki and S. Kaneshina, Biochim. Biophys. Acta, Biomembr., 1668, 2005, 25–32 Search PubMed.
- M. Kusube, M. Goto, N. Tamai, H. Matsuki and S. Kaneshina, Chem. Phys. Lipids, 2006, 142, 94–102 CrossRef CAS PubMed.
- D. P. Siegel and J. L. Banschbach, Biochemistry, 1990, 29, 5975–5981 CrossRef CAS PubMed.
- V. Cherezov, D. P. Siegel, W. Shaw, S. W. Burgess and M. Caffrey, J. Membr. Biol., 2003, 195, 165–182 CrossRef CAS PubMed.
- G. L. Kirk, S. M. Gruner and D. L. Stein, Biochemistry, 1984, 23, 1093–1102 CrossRef CAS.
- M. Fornasier, R. Pireddu, A. Del Giudice, C. Sinico, T. Nylander, K. Schillén, L. Galantini and S. Murgia, Colloids Surf., B, 2021, 199, 111564 CrossRef CAS PubMed.
- B. R. Pauw, A. J. Smith, T. Snow, N. J. Terrill and A. F. Thünemann, J. Appl. Crystallogr., 2017, 50, 1800–1811 CrossRef CAS PubMed.
- R. Koynova, B. Tenchov and G. Rapp, Chem. Phys. Lipids, 1997, 88, 45–61 CrossRef CAS.
- G. Pabst, N. Kučerka, M.-P. Nieh and J. Katsaras, Liposomes, Lipid Bilayers and Model Membranes: From Basic Research to Application, CRC Press, 2014 Search PubMed.
- R. H. Templer, J. M. Seddon and N. A. Warrender, Biophys. Chem., 1994, 49, 1–12 CrossRef CAS.
- D. P. Siegel, Biophys. J., 1993, 65, 2124–2140 CrossRef CAS PubMed.
- D. P. Siegel, Biophys. J., 1999, 76, 291–313 CrossRef CAS PubMed.
- D. P. Siegel and M. M. Kozlov, Biophys. J., 2004, 87, 366–374 CrossRef CAS PubMed.
- J. T. Mason and T. J. O’Leary, Biophys. J., 1990, 58, 277–281 CrossRef CAS PubMed.
- T. J. McIntosh, Chem. Phys. Lipids, 1996, 81, 117–131 CrossRef CAS PubMed.
- P. M. Brown, J. Steers, S. W. Hui, P. L. Yeagle and J. R. Silvius, Biochemistry, 1986, 25, 4259–4267 CrossRef CAS PubMed.
- H. L. Casal and H. H. Mantsch, Biochim. Biophys. Acta, Biomembr., 1983, 735, 387–396 CrossRef CAS.
- T. J. McIntosh and S. A. Simon, Biochemistry, 1986, 25, 8474 Search PubMed.
- T. J. McIntosh, Biophys. J., 1980, 29, 237–245 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sm00178g |
| This journal is © The Royal Society of Chemistry 2021 |