Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Complex fluids in animal survival strategies
Patrick A.
Rühs
 a,
Jotam
Bergfreund
a,
Jotam
Bergfreund
 b,
Pascal
Bertsch
b,
Pascal
Bertsch
 b,
Stefan J.
Gstöhl
b,
Stefan J.
Gstöhl
 b and
Peter
Fischer
b and
Peter
Fischer
 *b
*b
aDepartment of Bioengineering, University of California, 218 Hearst Memorial Mining Building, Berkeley, CA 94704, USA
bDepartment of Health, Science, and Technology, Institute of Food Nutrition and Health, ETH Zurich, Schmelzbergstrasse 7, 8092 Zurich, Switzerland. E-mail: peter.fischer@hest.ethz.ch
First published on 8th March 2021
Abstract
Animals have evolved distinctive survival strategies in response to constant selective pressure. In this review, we highlight how animals exploit flow phenomena by manipulating their habitat (exogenous) or by secreting (endogenous) complex fluids. Ubiquitous endogenous complex fluids such as mucus demonstrate rheological versatility and are therefore involved in many animal behavioral traits ranging from sexual reproduction to protection against predators. Exogenous complex fluids such as sand can be used either for movement or for predation. In all cases, time-dependent rheological properties of complex fluids are decisive for the fate of the biological behavior and vice versa. To exploit these rheological properties, it is essential that the animal is able to sense the rheology of their surrounding complex fluids in a timely fashion. As timing is key in nature, such rheological materials often have clearly defined action windows matching the time frame of their direct biological behavior. As many rheological properties of these biological materials remain poorly studied, we demonstrate with this review that rheology and material science might provide an interesting quantitative approach to study these biological materials in particular in context towards ethology and bio-mimicking material design.
Introduction
Animals are under constant selective pressure to adapt themselves to their surrounding environment. Countless survival strategies have evolved and enabled animals to survive, colonize, and reproduce successfully in the most diverse habitats on earth.1 From penguin species climate-adapted size differences2 to the food-adapted Darwin finches,3 an overwhelming array of examples suggests that selective pressure continues to drive natural selection and, if successful, results in well-adapted animals that integrate into an ever-changing environment.4 Selective pressure derives from the entirety of environmental factors acting on animals that can be biotic or abiotic. Biotic factors include all organisms in an individual's environment, the individual itself, and the resulting consequences, such as competition for food supply or physical and chemical changes in the environment. Abiotic factors are of chemical or physical nature; they are non-living factors such as temperature, humidity, light, nutrients, and the mechanical properties of the surrounding materials. The animals' relationship with the surrounding materials strongly influences their behavior, e.g., locomotion, while also providing them with food and shelter. These intriguing material-animal relationships featuring optimized mechanisms, unprecedented material properties,5–7 and matching biological behavior are bio-inspiring a large community.8 In addition, understanding of the soft material–animal interaction might offer a new quantitative perspective to animal behavior in the context of evolution.Animal–material interaction can be distinguished as of endogenous or exogenous origin.9 Endogenous abiotic material originates from an animal itself (endogenous), however, it is not formed by a biological process (abiotic), e.g., humpback whales blowing a “net” of air bubbles to trap fish.10 In contrast, exogenous biotic material is formed by living organisms but not by the individual that utilizes it. Insects, for example, feed on and inhabit cow dung, as it provides shelter and nutrients for their larvae.11 Endogenous biotic material is formed by a biological process and originates from the organism that utilizes it, such as saliva for lubrication and digestion. The material and its property are often linked directly or indirectly to an adaptive behavioral trait, relieving a species from selective pressure.
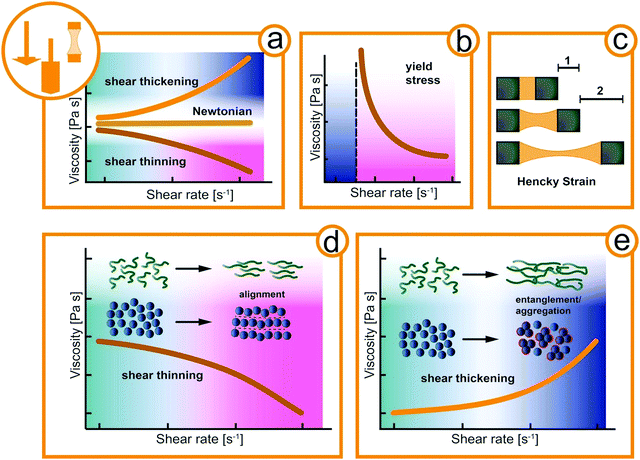
Materials can be classified by their mechanical properties into hard and soft materials.12 Hard (i.e., solid) materials of exogenous origin generally do not change over time or as a function of applied stress and offer little physical response to be exploited by animals. In contrast, fluids are easily affected by external forces and often have fascinating material properties as a function of time, stress, and temperature.13 Such complex fluids are macromolecular or multiphase systems consisting of molecular, colloidal, and non-colloidal components in liquid, solid, and gaseous state. The simplest way of describing the mechanical properties of fluid is by its viscosity, a measure of the internal friction. For Newtonian fluids, the viscosity is constant regardless of the external acting forces as shown in Fig. 1a. Complex fluids, as the name suggests, exhibit a more complex, non-Newtonian flow behavior.14 For example, the viscosity of a complex fluid may increase (shear thickening) or decrease (shear thinning) under applied mechanical deformation such as the shear rate (Fig. 1a). Both shear thickening and shear thinning originate in the flow-induced orientation and alignment of the structural elements of the fluid, e.g. macromolecular assemblies in mucus or suspended particles as depicted in Fig. 1d and e. At rest, the structural elements of complex fluids can also arrange in a solid-like structure that needs to be destroyed before flow sets it.15 The necessary stress to initial flow is called yield stress and a typical flow curve is depicted in Fig. 1b. Also, many complex fluids exhibit elastic (solid-like energy storage) and viscous (liquid-like energy dissipation) properties at the same time, and are thus defined as viscoelastic.16–18 In extensional flow such as in filament stretching or capillary breakup experiments the elongation viscosity is obtained. The transient deformation is reflected in the Hencky strain accounting for the deformation rate and filament thinning (Fig. 1c). This broad spectrum of flow behavior of soft materials offers a variety of mechanical properties to be exploited by animals.
In the following we reviewed how animals passively and actively utilize the rheological properties of exogenous or endogenous, biotic or abiotic complex fluids toward a competitive advantage for movement, prey, defense, and reproduction (Fig. 2). For example, it is shown how animals manipulate their habitat, e.g., sandfish and crabs that exploit the granular rheology of sand (exogenous abiotic) and showcase exotic animals that produce unique bio-fluids (endogenous biotic) such as mucus from deep-sea hagfishes to predatory velvet worms or spider silk.19–21 For unknown and uncharted flow phenomena, the so far untested rheological properties of the involved complex fluids are proposed as hypothesis (i.e., in Fig. 4a, c, 5a and 6e). This review aims to assess complex flow behavior used by animals in nature, compare and reveal the effect of material properties on biological behavior, and demonstrate the importance of rheology in nature.
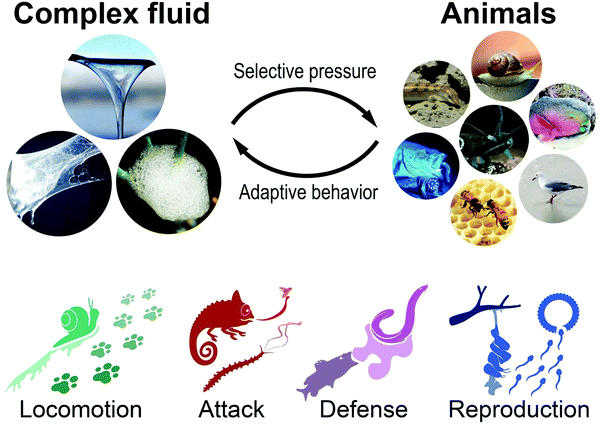 | ||
| Fig. 2 Materials link to an adaptive behavioral trait to relieve a species from selective pressure. Animals utilize or manipulate endogen or exogen, biotic or abiotic materials for competitive advantage in locomotion, attack, defense, and reproduction (Rights and permission: Spittlebug: David Iliff (CC BY-SA 3.0) and Dave (CC BY-SA 3.0). Snail: UC3M Department of Thermal and Fluids Engineering, Carlos III of the University of Madrid. Sandfish: Wilfried Berns – Wilfried Berns/Tiermotive.de (CC BY-SA 2.0). Velvet worm: Adapted with permission from Baer et al.22 (Copyright 2019 American Chemistry Society). Hagfish (Eptatretus stoutii): Adapted with permission from Zintzen et al.23 (Springer Nature, CC BY-NC-ND 3.0). Parrot fish: Igor Cristino Silva Cruz (CC BY-SA 4.0)). | ||
Rheology for locomotion
Since the first animals set foot (or fin) on land 400 Mio years ago, animals colonized and adapted to all possible habitats.24,25 Different surrounding media (water, soil, air) wherein or whereon animals are moving has resulted in various specialized locomotion. For instance, in mammals, the same initial set of appendicular bones evolved into specialized limbs like fins, wings, hoofs, hands, and feet.26 With this ‘mechanical’ toolset, animals can act on their surroundings to propel themselves forward. Simple fluids, such as air or water, provide a strain-rate-independent viscosity (Newtonian). Elsewhere, solidified soils respond linearly to stress or strain, resembling a linear spring mechanically (Hooke's law) until they yield. In contrast, complex fluids like sand or mucus exhibit strain and time dependent flow behavior and we show how gastropods secrete their endogenous viscoelastic locomotion aid and lizards exploit granular rheology to “swim” in sand (Fig. 3).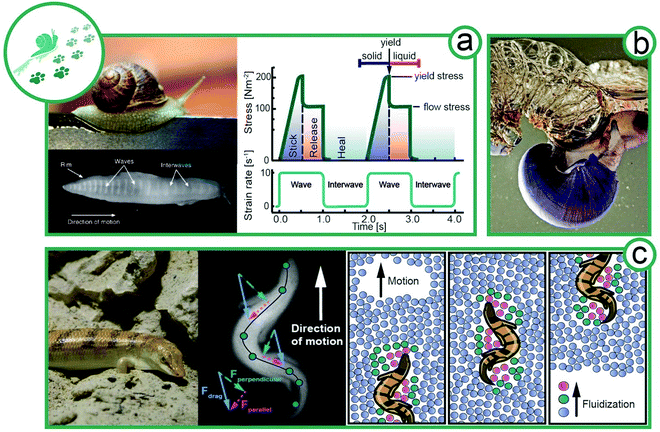 | ||
| Fig. 3 Locomotion of animals exploiting complex flow behavior. (a) Facilitated locomotion via mucus viscoelasticity by slugs (animal size ∼8 cm). Plan view of the ventral surface of the foot of a slug moving over a glass surface from left to right.32 Rheological properties of pedal mucus of Ariolimax columbia during locomotion (re-drawn from Denny et al.29). (b) The violet sea snail (Janthina janthina, animal size 2–4 cm) uses complex fluids to form a foam raft for movement.33 (c) A sandfish (Scincus scincus, animal size 18–20 cm) on sand; an X-ray image of sandfish undulatory movement in glass beads; and a schematic illustration of the fluidization of sand particles surrounding the sandfish. The sandfish opposes strong transverse forces to his longitudinal axis to create a net forward thrust by relying on interparticle Coulomb friction (Rights and permission: (a) snail image: UC3M Department of Thermal and Fluids Engineering, Carlos III of the University of Madrid. Bottom view of snail: adapted with permission from Lai et al.32 (Copyright 2019 Company of Biologists Ltd). (b) Picture of violet sea snail kindly provided by Dimitris Poursandinis. (c) Wilfried Berns – Wilfried Berns/Tiermotive.de (CC BY-SA 2.0), X-ray image of sandfish kindly provided by Daniel Goldman). | ||
Movement by viscoelasticity
Terrestrial gastropods, such as slugs and snails, crawl with a single foot by a mechanism called adhesive locomotion. This propulsion is powered by muscular waves that propagate along the ventral surface of the foot from tail to head.27,28 These periodic contraction–relaxation waves (depicted in Fig. 3a) are transmitted to the ground by a thin layer of viscoelastic mucus secreted by the animal.29 The nonlinear rheology of the mucus enables the gastropods to propel without fully detaching from the solid ground by a stick-and-release mechanism. The mucus further provides adhesion to climb walls and crawl across ceilings.30–32Gastropod mucus consists mainly of a mucin-like, protein–polysaccharide complex, similar to glycoproteins and glycosaminoglycans in vertebrates, which forms a physically crosslinked gel responsible for mucus viscoelasticity.34 From a rheological point of view, mucus requires (i) a solid-like elasticity at low stresses, (ii) a high and sharp yield point with a transition to a highly viscous and shear-thinning liquid and (iii) a fast thixotropic recovery of network structure after stress release. Thus, a movement cycle, as visualized in Fig. 3a, starts with elastic solid-like mucus. The approaching muscular wave shears the mucus, thereby increasing the stress. At the yield point, the mucus structure breaks and liquefies. During the interwave (no shear), the mucus network recovers its elastic, solid-like properties.29–31 In comparison the synthetic polymer,35 the recovery might be maintained by a small but constant addition of fresh mucus from slime glands. The movement cycle generates an asymmetric shear force under the foot with a net forward component that propels the animal.32 Adhesive locomotion is the most energy-intensive form of movement, approximately 12 times more costly than running. Surprisingly, the largest fraction of the energy is required for the production of mucus and not for muscle activity.36 The energy input is well invested, as the rheological behavior beneficially enables snails to adhere to surfaces, for example, to climb up trees and overhangs. At the same time, mucus production and adhesion might limit the speed of snails. This well-defined balance between stick and slip demonstrates that the tailored rheology of mucus is optimized for gastropod propulsion.37 A passive and a particularly low energy form of locomotion is passive flotation in aquatic environments. Violet sea snails (Janthina janthina) use mucus to form a foam raft for movement, as depicted in Fig. 3b.33 The sea snail secretes mucus, entraps air in the mucus, and holds the air bubbles with its foot. The amphiphilic mucus adsorbed at the air–water interface decreasing the surface tension and forms a presumably viscoelastic or gel-like mucus layer that is crucial for foam stability.
Movement by granular rheology
An exceptional way of locomotion is observed for some snakes and lizards, which show swimming-like movement despite living at the driest places on earth. Granular materials like sand can flow like a fluid, giving rise to this unique way of locomotion (Fig. 3c). Animals inhabiting granular media have adapted the ability to fluidize locally the granular material surrounding them. For certain species, digging has proven to be a favorable escape strategy, while others bury their eggs in the sand to protect them from predators and changing climate.38–40 We focus here on the locomotion of sandfish (Scincus scincus). The mechanical properties of granular media can change dramatically with differing water contents from dry to saturated conditions.41 A popular way to describe granular flow is frictional rheology.42,43 In dry conditions, the macroscopic friction is characterized by a dimensionless inertial number, while in wet conditions, it is replaced with a dimensionless viscous number.44 The dimensionless inertial or viscous number is then only a function of the local solid packing fraction.At dry conditions, sand can be locally fluidized by undulating stresses, and temporarily, behave like a fluid. Sandfish exploit this principle to “swim” in sand (exogenous abiotic) in a self-generated localized frictional fluid. Hosoi and Goldman38 identified four different regimes of locomotion in granular media depending on the dimensionless digger size and inertial number, in which sandfish belong to the large and fast digging organisms. In analogy to low Reynolds number swimmers, the sandfish achieves a net forward displacement by undulating motion. The scallop theorem postulates that at low Reynolds numbers a simple symmetrical back and forth motion is not sufficient for locomotion.45 However, Qiu et al. were able to show that a back-and-forth movement can cause a net forward displacement, provided the swimmer is immersed in a non-Newtonian liquid.46 The viscosity in the front and rear part of the swimmer varies, thus enabling the forward movement. This means that, in addition to breaking the symmetry by undulating movements, the sandfish also achieves net forward movement by lateral forces that are stronger than the longitudinal forces when moving through granular material (Fig. 3c). Movement at a 30–45° angle of attack to the longitudinal axis of the sandfish contributes to the forward thrust by Coulomb friction in the surrounding medium. The locomotor's behavior remains the same even at changing packing density.38 Drag increases linearly with depth, limiting the habitable zone for the sandfish. In contrast, drag increases non-linearly with moisture content due to suction forces, limiting sand-swimming animals to arid regions.47
Rheology for attack
The manipulation of fluid flow for prey is omnipresent in aquatic environments, e.g. for the suction feeding of predatory fish48 or flow-controlling jellyfish49 and copepods,50 whereas land-living animals generally rely on endogenous biotic complex fluids. In this section it is outlined how complex fluids can be used to capture prey exploiting viscoelasticity and the flow properties of granular media (Fig. 4).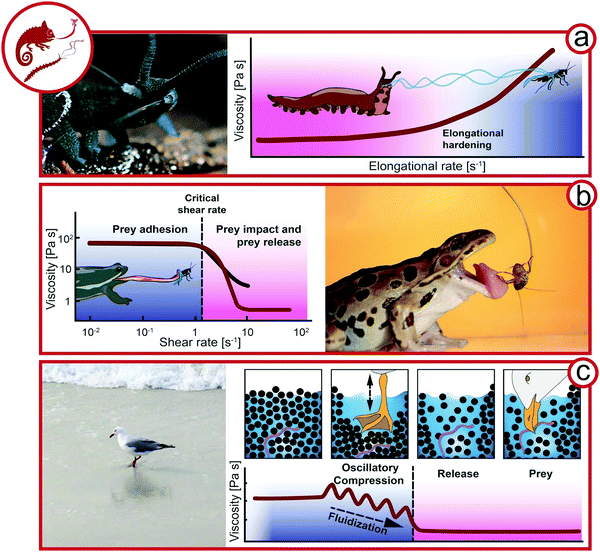 | ||
| Fig. 4 Exploiting complex fluids for catching prey. (a) Slime ejection from slime papillae of a velvet worm (Euperipatoides rowelli, Onychophora, Peripatopsidae, animal size 3–5 cm) from Baer et al.22 Hypothesis of the viscosity change during the elongation ejection of velvet worm slime. (b) Viscosity as a function of the shear rate (re-drawn from Noel et al.57). Frog (Anura, animal size ∼10 cm) capturing an insect. (c) Seagull (Laridae, animal size 20–25 cm) feeding on worms by fluidizing sand. Proposed rheological behavior of wet sand under oscillatory compression by a seagull preying on worms (Rights and permission: velvet worm (a) adapted with permission from Baer et al.22 (Copyright 2019 American Chemistry Society) and frog (b) adapted with permission from Noel et al.63 (Copyright 2019 Royal Society)). | ||
Preying with endogenous complex fluids
One strategy is the immobilization of prey by endogenous complex fluids that are strain-hardening and adhesive, as applied by velvet worms (Onychophora). The 0.5–20 cm long, many-legged velvet worms inhabit humid regions of the tropical and temperate zone.51,52 Velvet worms sneak up on their prey and eject a sticky slime from two slime papillae flanking their mouth as depicted in Fig. 4a. The slime ejection induces uncontrolled papillae oscillations at 30–60 Hz, which favor the crossing of threads mid-air leading to an entangled slime network immobilizing the prey.53,54 The velvet worm then injects its hydrolytic, enzyme-containing saliva, killing the prey and inducing liquefaction for later ingestion.52,55 In contrast to other secreted adhesive fibers like spider or silkworm silk, which are solid upon excretion,56 velvet worm slime is truly a remarkable showcase of a biological complex fluid. Fluid-like at rest, it only develops cohesiveness and mechanical strength upon agitation, such as the elongational flow upon slime ejection.22,57 The main functional components of the slime, which contains 90% water, are protein–lipid nanoglobules. Proteins and lipids contribute 50 and 1% of the non-aqueous slime components, respectively, and reflect the role of additives in biological and bio-mimicing composite materials. The nanoglobules are approximately 150 nm in diameter with narrow size distribution and are probably formed by the complex coacervation of anionic and cationic protein moieties.58 Baer et al. could show that the nanoglobules dissociate upon salt-induced charge screening, supporting the theory that they are stabilized by attractive electrostatic interactions within proteins.59 The proteins have a high molecular weight and charge density, which may indeed favor intra- and intermolecular electrostatic interactions. The proteins were long thought to lack any secondary structure, but were recently reported to contain portions of β-sheets.60–62The transition of fluid-like crude slime to elastic fibers upon extrusion outside the animal's body suggests that elongational rate thickening is crucial for slime functionality, i.e., the viscosity of the slime increases upon elongation ex vivo during fiber drawing. This so far untested hypothesis is depicted in Fig. 4a where the increase of the viscosity is plotted as a function of the elongation rate, i.e., during the stretching of the slime filament. During elongational flow, the protein–lipid nanoglobules assemble into stretched protein nano- and microfibers immobilizing the pray.22 The fibers have a coating layer which has a higher lipid content compared to the protein-rich core. Whereas the protein fibers are probably responsible for the toughness of the final slime, the role of the lipid phase is not fully clear. It was argued that the lipids may be crucial to stabilize the protein–lipid nanoglobules and prevent their assembly prior to elongation. The final macroscopic slime consists of thin, elastic slime threads with adhesive droplets distributed along the threads. Upon drying, the threads’ stiffness increases and reaches 4 GPa for the fully dried fibers due to a glass transition. Stunningly, the initial protein–lipid nanoglobules are reformed upon rehydration of the dried slime, and new fibers can be drawn from regenerated slime.22,57,62 This underlines that non-covalent electrostatic interactions are responsible for slime formation, and the liquid–liquid phase separation into dispersed protein–lipid nanoglobules corresponds to the slime's equilibrium state. Hence, the protein–lipid nanoglobules in velvet worm slime are the mechanoresponsive, reversible building-blocks of velvet worm slime.22
Viscous adhesion by frogs and chameleons
Chameleons (Chamaeleonidae) and frogs (Anura) use specialized saliva for attack (Fig. 4b). Chameleon saliva has a viscosity 400 times higher than human saliva. As a consequence, prey adheres to chameleon tongues by viscous adhesion.64 For this attack strategy, the viscous adhesion to the tongue needs to exceed prey inertia. However, due to the high viscosity of chameleon saliva, the prey size is not limited by viscous adhesion.65 Similarly, frogs possess a viscoelastic tongue coupled with high viscosity and shear-thinning saliva (Fig. 3b). Upon tongue impact, a critical shear rate is exceeded, which decreases the viscosity and ensures the spreading of saliva. The viscoelastic tongue acts as a shock-absorber and adapts to prey shape, further increasing the tongue-prey contact area. A lower shear rate is applied by tongue retraction, resulting in a higher saliva viscosity.63 Hence, the saliva must recover sufficiently fast to provide viscous adhesion.Exploiting granular rheology for prey
A specialized technique exploiting granular rheology can be observed for seagulls (Laridae) in tidal zones in the form of two-footed pedaling. Various glovers and gulls pedal the sand to loosen its structure around worms and ease their prey.66 It was shown that on solid dry ground, the vibrations mimic approaching moles and promote the escape of worms.67 On wet ground, the two-footed pedaling has been shown to promote the release of small animals by the fluidization of the wet sand.68,69 Here we propose the following mechanism based on granular rheology as working hypothesis for future research (see Fig. 4c). Defined rheologically, the sand in tidal zones is randomly close-packed and completely wetted with a glossy surface. Upon pedaling, the sand is spatially rearranged and the water table is lowered temporarily; the sand surface appears matte. When the surrounding water refills the void, the sand is diluted and can flow with far less resistance due to its lower solid volume fraction. The seagull is then able to pick the prey from the diluted suspension rather than from densely packed sand with high resistance to deformation. Hence, the pedaling, representing oscillatory compression, results in a more dilute sand structure (positive dilatancy70).Rheology for reproduction & parental care
Complex fluids play a crucial role in animal reproduction. While it is well-known that the rheology of cervical mucus is vital for sexual reproduction in humans,71 many animals use external complex fluids for reproduction and parental care (Fig. 5).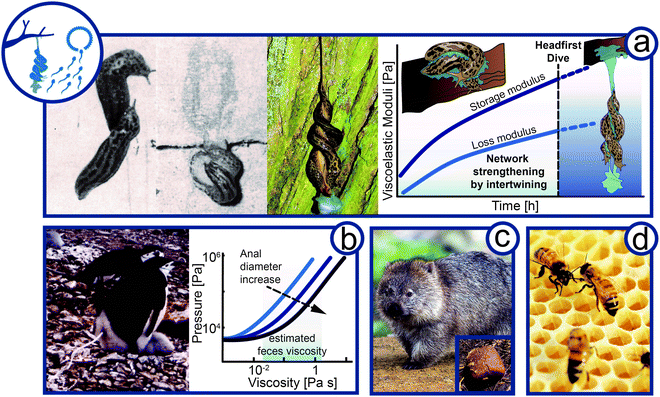 | ||
| Fig. 5 Flow of complex material in reproduction and parental care. (a) Viscoelasticity in courtship of the leopard slug (Limax maximus, animal size 10–20 cm). Slugs meet mating partner, intertwining of mucus, and hanging intercourse after headfirst dive. Hypothesis of the change in viscoelasticity expressed by the storage and loss modulus of Limax maximus mucin over time during courtship and mating. (b) Feces rheology of penguin (Pygoscelis, animal size 70–95 cm) housekeeping. Graph is re-drawn from Meyer-Rochow et al.74 (c) Wombat (Vombatidae, animal size ∼100 cm) cubic feces to mark territory (d) Bees (Apis, animal size 1–2 cm) constructing hexagonal shaped comb in a hive (Rights and permission: (a) The Ohio State University and The Ohio Academy of Science and T. Hiddessen (CC BY-SA 3.0), (b) kindly provided and with permission from V. B. Meyer-Rochow, (c) Wombat and wombat feces: Bjørn Christian Tørrissen (CC BY-SA 3.0) and Sheba Also (CC BY-SA 2.0)). | ||
Viscoelasticity in courtship
Probably the most spectacular example of complex fluids for reproduction is the eerie, but beautiful, mating ritual of leopard slugs (Limax maximus). The twosome of leopard slugs uses a mucus thread to suspend themselves mid-air to perform their circus-like sexual act (Fig. 5a).72 The mucus is usually used for adhesive locomotion by the slugs as addressed above. Once the twosome reaches the desired location, preferably the bottom side of a tree branch, the slugs intertwine and form a thick mucus layer around themselves. The slugs rub their secreted mucus against each other for up to one hour until a viscoelastic to gel-like mucus quality is achieved.73 The slugs then lower themselves from the branch, dangling mid-air by this mucus.Although it is known that slugs may produce mucus with altering composition and rheological properties depending on its use, we suggest that for leopard slug mating the time-dependent change in rheological properties is crucial.75 As generally observed for salivary, nose, and slug mucus, the yield stress and viscosity increase upon drying. Mucus viscosity was shown to increase with mucin concentration to the power of three.76 The shear stresses induced by the constant intertwining may further promote mucus elasticity by the formation of elastic threads. Shear forces are known to cause aggregation of mucin molecules into fiber-like structures. Thus, the slugs must react to the changing material properties by drying and intertwining to achieve ideal viscoelasticity before performing a slow-motion head dive together. The slugs have to time their suspension with perfection. Too early and the slug thread will not hold the weight of two slugs. Wait too long and the mucus will dry out and lose its viscoelastic or gel-like properties and becomes solid. In both cases, the slugs would not be able to exploit the viscoelasticity to lower themselves from the tree branch. As shown in Fig. 5a, we propose the so far untested hypothesis that both storage and loss moduli increase during the prelude until a gel-like constancy supporting the headfirst dive is reached. A mid-air position allows full extension of male genitalia, a feat difficult to perform without being suspended. The suspending mucus thread can be up to 50 cm in length.73 The mucus thread will experience a strong extensional force as it holds two fully-grown leopard slugs, estimated to weigh around 1 to 8 g each.77 This gravitational force has to be balanced by the elasticity of the mucus thread. At the end of the sexual encounter, the thread may either rupture before the slugs leave or eat up their mucus.73
High pressure extrusion for defecation
Penguins (Spheniscidae) often lay eggs at low temperatures that do not permit leaving the egg unprotected. Penguins incubate in shifts of several days allowing the other parent to feed.78 To avoid exposure of the egg or newborn and prevent contamination, chinstrap (Pygoscelis antarctica) and Adélie (Pygoscelis adeliae) penguins propel their feces radially from the nests (Fig. 5b), which are no closer than 60 cm even at high population density.79,80 With an approximate firing distance of 40 cm, the feces cannot soil neighboring nests. The required expulsion pressure at the orificium venti was approximated by Meyer-Rochow and Gal from the density and viscosity of penguin feces, firing range, shape, aperture, and height of the anus.74 The precise determination of feces viscosity was impaired by small sample volumes and remains of crustacean cuticles and fish bones. Nevertheless, the viscosity could be approximated to a range from a few tens of mPa s (glycol) up to several hundred mPa s (olive oil to glycerol) depending on food source. The velocity of the feces is at 2 m s−1 with an average volume of 20 mL and a firing time of 0.4 s. Summing up the pressure required to accelerate the fluid volume and the pressure needed to overcome the viscous friction (Hagen–Poiseuille equation) leads to an expulsion pressure of 10 to 60 kPa (i.e., 1000 mmHg), which is about four or eight times the blood pressure of giraffes or humans, respectively. The pressure curves are shown for three cloacal apertures (Rockhopper (Eudyptes chrysocome) = 4.2 mm, Adélie = 8.0 mm, and Gentoo (Pygoscelis papua) = 13.8 mm). It was noted that despite the high pressure no energetically unfavorable turbulent flow occurs. Tajima and Fujisawa recently corrected the original calculations by air resistance of propelled feces, yielding higher pressures.81 Owed to the experimental difficulties to determine the feces viscosity, the feces was assumed Newtonian in the original work. However, animal defecation generally comprises non-Newtonian fluid behavior82 and it is suggested that penguin feces are non-Newtonian, most likely shear-thinning, and may thus require lower pressures to be expelled. On the other hand, one could argue that a high solid content following a crustacean rich diet may induce an apparent yield stress, potentially increasing the required pressure to accelerate the feces. Hence, the true rectal pressure may be under- or overestimated depending on the non-Newtonian flow properties of the penguin feces and could vary depending on diet. Future research in the area thus should address a comprehensive rheological analysis of penguin feces.Cubic feces for communication
Another curious fecal secretion is observed for wombats (Vombatidae) (Fig. 5c). Of all animals, wombats are the only ones that secrete excrement in a cubic form.82,83 It is suggested, that wombats use their feces as a communication tool to mark their territory and to communicate with each other by scent. The cubic shape of wombat feces could prevent it from rolling off the stones and cliffs, habitats where wombats often live. The feces are produced through alternating stresses along the gastrointestinal tract. As the interior intestinal wall is less compliant than the exterior, the exterior wall is stretched more and feces are formed in squares. The excretion of cubic poo is only possible for feces that exhibit a Bingham or more complex Herschel–Bulkley flow behavior. The feces need to have a yield stress to retain their cubic shape, yet they need to flow beyond the yield stress to allow their shaping in the intestine.Foam for spawn protection and aeration
Fish and amphibia reproduce by spawning, i.e., the release or deposition of eggs in an aquatic environment. A subfamily of armored catfish (Callichthyinae) and a family of frogs (Rhacophoridae) further protect their spawn by the formation of protective foam. Foams are characterized by a high interfacial contact area of air and water. Hence, foam formation requires a surface-active substance that adsorbs at the energetic unfavorable air–water interface and decreases the surface tension. In analogy to 3D bulk materials, 2D interfacial layers can also exhibit complex flow behavior on their own. Proteins that adsorb at air–water interfaces form viscoelastic interfacial layers that significantly enhance foam stability, as well known from culinary uses as in whipped cream.84–86 The catfish form a protective foam by swimming in circles belly-up close to the water surface and pumping water through their gills.87 In the gills, the water is enriched with amphiphilic mucin that acts as surface-active agent and stabilizes the foam. The same mechanism of foam formation is exploited by the violet sea snail for passive flotation, as previously discussed. Callichthyinae are thought to have evolved this strategy to improve the aeration of their spawn as they often inhabit oxygen-deprived waters in the tropics.88 Rhacophoridae, on the other hand use a specialized protein, ranaspumin, as stabilizer for their protective foam. The female secretes the amphiphilic ranaspumins, which are whipped into a stable foam using their legs, similar to whipping cream. The ranaspumins allow the production of foam that is stable enough to withstand environmental conditions for at least 10 days. The foam protects the eggs from predation and climate variations while providing sufficient oxygen.89Flow in the hive
Honeybees (Apis) live communally and care for their offspring cooperatively. In their nest, honeybees construct an elaborate system of highly structured, hexagonal, prismatic cells known as honeycomb (Fig. 5d). Inside the cells, the larvae are raised and honey and pollen are stored. The formation mechanism and the precise repetitive geometry of the honeycombs intrigued natural philosophers and scientists for centuries and continues unabated.90–94 The bees construct the comb cells by secreting endogenous biotic thermoplastic wax. Beeswax is a viscoelastic building material that contains more than 300 different chemical components.95 As for other fatty systems, beeswax does not have a sharp melting point. With increasing temperature wax liquefies, i.e., viscous properties increase and the elasticity decreases.96 The transition from fully elastic crystalline wax to the viscoelastic amorphous structure is not continuous and takes place in two steps at about 25 and 40 °C. Bees can raise their body temperature to more than 43 °C, allowing them to change the malleability of the wax.95 In the early stage of comb construction, bees use their bodies as templates to build cylindrical cells around themselves.92,97,98 This shape remains stable for many weeks after their construction. The final hexagonal shape is formed when the bees heat the wax to 37–40 °C and it becomes amorphous and viscoelastic. The decreased viscosity enables the wax to flow into its energetic most favorable shape due to surface tension. The flow into the triple plateau border between adjacent walls results in straight walls and 120° angles relative to one another, i.e., the well-known hexagons. The same process is also observed when foam bubbles get into contact.92,97,98 Hence, bees shape the hexagonal cell by controlling the viscoelasticity of the endogenous wax over temperature.Rheology for defense and protection
Animals have found a variety of defensive protection strategies exploiting complex fluids such as viscoelastic slime or foams as well as granular material (Fig. 6).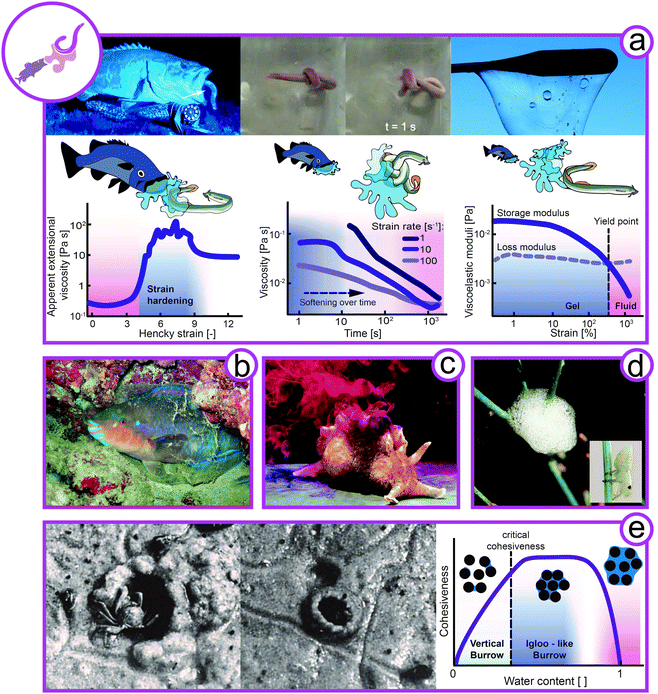 | ||
| Fig. 6 Rheology of complex flow in defense. (a) Hagfish (left image: Eptatretus stoutii, animal size 40–65 cm, middle images: Myxine glutinosa, animal size 25–40 cm) slime formation for defense and knot formation to escape its slime. Apparent extensional viscoelasticity of hagfish mucin (left), viscosity of hagfish slime under shear (middle), and viscoelasticity of hagfish slime (right) (redrawn from Böni et al.100). (b) Mucus sleeping bag of the parrot fish (Chlorurus sordidus, animal size 30–40 cm). (c) Inking sea hare as a defensive slime (Aplysia californica, animal size 30–75 cm). (d) Protein-stabilized foam for protection from predators by spittlebug (Cercopidae, animal size ∼7 mm) nymphs. (e) Sand crab (Dotilla) building an igloo or a hole depending on the water content in the sand. Hypothesis to link the borrow's design with the cohesiveness of sand with increasing water content (Rights and permissions: (a) from Zintzen et al.23 and re-drawn from Böni et al.100 (Springer Nature CC BY-NC-ND 3.0). (b) From Igor Cristino Silva Cruz (CC BY-SA 4.0), (c) with kind permission from Genevieve Anderson, (d) David Iliff (CC BY-SA 3.0) and Dave (CC BY-SA 3.0), (e) adapted with permission from Takeda et al.108 (Copyright 2019 Elsevier)). | ||
Viscoelastic gel to avoid predation
One particularly striking example of animal defense using complex fluids is the notorious hagfish (Myxine glutinosa, Eptatretus stoutii) (Fig. 6a). Hagfish are crucial for aquatic ecosystems as their burrowing and feeding activities have a significant impact on substrate turnover and ocean cleanup by feeding on carcasses that sink to the sea bed.99 When hagfish are attacked by predators such as sharks or suction feeding fish, they form vast amounts of slime in less than a second. This remarkable defensive slime formation is triggered by the release of hagfish exudate from ventral pores into the surrounding seawater. Once in contact with water, the exudate rapidly forms a fibrous hydrogel that clogs the mouth and gills of the predator.23,100–102 The crude exudate is composed of coiled-up skeins and mucin vesicles, both crucial components for slime formation. Hagfish skeins are keratin-like protein threads coiled up into a microscopic ball of yarn with dimensions of 50 by 150 μm.103,104 When hagfish skeins come in contact with water, the skeins unravel into long protein threads with a length of up to 15 cm.102,105,106 The mucin vesicles swell in contact with water and eventually burst, releasing water-absorbing mucin molecules into the slime network.107 As opposed to conventional hydrogel formers, mucins gel without additional energy input and swell immediately.Hagfish slime forms a very soft, yet elastic hydrogel with higher water content than any other known biological hydrogel. A functional slime can only be formed when both components are present in their natural environment. The long skein threads are crucial for cohesion and viscoelasticity of the slime, whereas the mucins facilitate water entrapment.23,100–102 The hagfish slime is unique because it gels large amounts of cold water within seconds. As only very little exudate (approximately 0.02 wt% solids in final slime), and no further energy input is required, the slime is an economical yet efficient defense mechanism. Due to the low solid content the slime is short-lived, mechanically unstable, and prone to wash out. However, as rapid gelling is more important than longevity to avoid seizure, the slime seems perfectly timed to the defense mechanism of the hagfish.23,100 The rheology of hagfish slime is fine-tuned for the desired functionality (Fig. 6a). In extensional flow, mimicking the suction flow of suction feeding fish, the mucin extensional viscosity increases by two orders of magnitude in just a second, efficiently deterring suction feeding predators. On the other hand, the slime is shear thinning and the hagfish can easily wipe slime off itself by the formation of a knot that is sheared down its body (depicted in the top middle image of Fig. 6a).100,109 This mechanism allows the hagfish to escape its trap after successfully deterring the predator. This is also supported by the deformation dependent decrease of the storage and loss moduli, i.e., a gel-like behavior at small deformation (dominant storage modulus) and a fluid-like behavior at larger deformation above the yield point (dominant loss modulus).
Slimy stars, viscoelastic sleeping bags, distracting taste, and foam for protection
The hagfish is not the only sea creature that uses slime to deter its predators. The slime star (Pteraster tesselatus) uses respiratory water flow and production of mucus to produce large quantities of slime when molested.110,111 Water is pressed through mucus channels, rapidly forming a slime body, engulfing the sea star. There is currently no rheological data available on this slime, but we suggest that the slime shows viscoelasticity and transient rheological properties similar to those of the hagfish. The parrotfish (Chlorurus sordidus) secretes mucus at night to form a gelatinous, protective sleeping bag around its body to protect itself from ectoparasitic gnathiid isopods (Fig. 6b). In contrast to the instantly formed but short-lived hagfish slime, the parrotfish mucus is produced within one hour and its protective function remains for 4.5 hours.112 Although no rheological characterization of parrotfish mucus has been reported, it was described as gel or solid-like. We thus assume that it is more elastic and has a higher solid content than hagfish slime Other protective secretions are made by sea hares (Aplysia californica). Sea hares are not on the dietary plan of most marine animals due to two unpalatable secretions, ink and opaline, which sea hares squirt at approaching predators (Fig. 6c). Both secretions have a high viscosity in order to stick to the antenna and mouth of predators. Opaline further sticks to the chemosensors and physically blocks the perception of food odors. The high viscosity mimics the feedings stimulus and the concentrated amino acids in the secretion induce an overstimulation of the chemosensors. These combined mechanisms result in attacker retreat.113,114 The spittlebug (Cercopidae) nymphs produce foam for protection from predators, moisture loss, UV-radiation, and temperature variations, as shown in Fig. 6d.115–117 The spittlebug nymphs secrete proteins that stabilize the foam by a reduction of surface tension and formation of a viscoelastic interfacial layer. As the ancestor of the spittlebug lived underground, it is believed that the foam could have enabled the spittlebug to adopt a lifestyle above ground.117 The foam production of spittlebug nymphs is further linked to their feeding on xylem sap.Snail mucus for defense
It was previously discussed that snail mucus plays a crucial role for locomotion and reproduction. It has further been evidenced that the red triangle slug (Triboniophorus graeffei) produces large amounts of mucus for defense, facilitating to immobilize predators as large as frogs.118Tikoconus costarricanus has been observed to use mucus from their caudal gland to hang upside down from the underside of leaves, thereby reducing the risk of dehydration and predation.119Sand igloos
Sand-dwelling crabs of the genus Dotilla have been observed to create vertical burrows or igloo-like structures depending on the water content of sand (Fig. 6e).108,120,121 As discussed above for sandfish locomotion, dry sand behaves like a fluid and can be described only by the friction between particles. At increasing water content, the cohesiveness of sand increases due to arising suction forces. Eventually, at further increasing water content, the sand-water suspension has a more fluid-like character again. The crabs thus adapt their construction designs depending on the water content. When sand is well-drained and cohesive, the crabs create vertical burrows. Under unstable and semi-fluid conditions, they adapt their behavior and create igloo-like structures. As demonstrated by Takeda et al.,108 the sand has to be semi-fluid for the igloo to hold its shape. Under these semi-fluid conditions, the sand would not allow for the construction of a vertical burrow. Hence, crabs exploit the water-induced suction forces within sand particles that provide cohesiveness and allow the construction of self-standing architectures. In Fig. 6e the link between the burrow design with the cohesiveness of sand as hypothesis for future investigations.Discussion and perspectives
In this work, we highlight examples of animals using passively and actively complex fluids as part of their survival strategy. Focus is laid on how animals manipulate their surrounding liquid and granular materials or have evolutionary evolved endogenous biotic materials. In the following, a framework of the relevant time scales of complex fluids for animals, the evolution of the most common involved polymer mucin, and the ability of animals to sense environmental rheological conditions is proposed. As many of the presented fluid systems are barely charted both experimental and theoretical approaches originating from soft matter science are expected to boost the understand the origin of the discussed phenomena.Relevance of ambient conditions and workarounds taken by animals
Animals generally have no to little influence on the ambient conditions at which they exploit complex flow phenomena. As a consequence, some of the presented survival strategies are only possible in specific habitats. For instance, the “swimming” locomotion in sand is only possible in arid regions where sand can be locally fluidized by the animal, whereas the construction of complex sand formations is only possible in tidal zones where the increased water content makes sand cohesive. Some animals even adapt their behavior depending on the given material properties. For example, sand crabs adapt their burrow shape to the water content of the sand, thereby reacting to changes in material properties due to a shift between suction and frictional forces (a skill that human offspring often lack in their attempts at building complex sand structures). On the other hand, many animals such as the velvet worm, leopard slug, or the hagfish have evolved endogenous complex fluids that are less dependent on ambient conditions.59,100 These endogenous fluids often exploit mucin as versatile ingredient for fluids with properties that are targeted at the relevant ambient conditions and fluid use, as discussed further in the next paragraph. An extreme example is hagfish slime, which manages to gel vast amounts of water instantly despite low water temperatures by a combination of shear- and ion-sensitive mucin vesicles and proteins skeins.122,123 Also, crude velvet worm slime is merely a fluid with dispersed protein–lipid nanoglobules, and it is only upon elongational flow that the nanoglobules assemble into strong protein fibers.59 In summary, hagfish and velvet worm slime behavior are determined by the chemical composition of the endogenous materials in close connecting to the exerted flow profile that the material is experiencing during its employment.7 Beside the mentioned use of mucus-based composites, other animals exploit mechano- or chemoresponsive properties to achieve desired flow properties.Relevant timescales
The relevant timescales at which the presented fluids need to alter or retain their properties range from milliseconds to days. It appears that in cases where an instantaneous change in fluid properties is required, animals have evolved materials that allow a rapid change in fluid structure and properties as e.g., in hagfish or velvet worm slime. The longevity of excreted endogenous fluids may be altered by their solid content or chemical composition. For instance, hagfish slime is well-known as one of the hydrogels with the lowest solid content, but it is also short-lived and dissolves rapidly in seawater. In contrast, the mucus-based sleeping bag of parrotfish remain stable for several hours in seawater to prevent infestation with parasitic gnathiids during sleep, which probably derives from its higher solid content. A remarkable example demonstrating the importance of right timing is the mating ritual of leopard slugs. As proposed previously, the slug twosome seems to alter the rheology of their mucus by drying and shear-induced fibrillation and needs to time their head dive perfectly in order to complete their mating process.Mucin and sand: material with universal applications for animals
Both mucin and sand as endogenous and exogenous complex materials are widely used by animals as outlined in the previous sections. Mucins are glycoproteins, i.e., proteins with covalently bound sugar residues and are present in all mucosa and involved in many vital functions of animals like nutrient uptake in the gastro-intestinal system or oxygen transport in lungs. Mucus appeared early in metazoan evolution, and probably evolved multiple times independently.124,125 Thus, mucus is a collective term for substances with similar composition and properties and is used for various specialized complex fluids. Mucus can fulfill these versatile tasks due to the broad toolbox of proteins and sugars that can be combined. Depending on the structure and composition of mucins they can provide suitable rheology for adhesive locomotion or the adhesive force of a chameleon tongue, act as amphiphilic substances to stabilize foams for passive flotation or protection, facilitate incorporation of water in fluids like hagfish slime, or allow the spectacular mating ritual of leopard slugs. On the other hand, sand as exogenous material serves also multiple purposes for animal survival, from housing and protection as seen for sand dwelling crabs, to dilatancy supported prey and motion in dry granular materials. The governing equation to describe the flow properties of sand spans from frictional rheology (captured by either the inertial number or viscous number) for a self-generated localized frictional fluid in case of the sand fish to more classical suspension rheology with positive dilatancy in case of the hunting seagull.As outlined in the previous sections, the rheological and structural properties of the involved endogenous and exogenous complex materials are largely unknown with the notable exception of velvet worm and hagfish slime as well as the motion of the sandfish in granular media. For example, the difference in mucus viscosity and stickiness, the role of minor additions of protein or lipids to composite materials have not been discussed from a soft matter point of view and thus also not taken further into the design of high-performance bio-mimicking materials. In another example, the motion of slugs shows that endogenous biotic material can rapidly cycle between a yielding or fracture state into a healing process. The rapid adaptation of these materials to the environmental condition suggests that phase transitions or concentration fluctuations govern the structural changes and the subsequent performance of the material. During evolution, animals found a way to use the rheology and structure of complex fluids to gain advantage and increase their Darwinian fitness. These design concepts studied by soft matter science, material science, and rheology can help to understand animal behavior, provide a quantitative approach towards ethology, and also might yield new insights to bio-material mimicking.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the Swiss National Science Foundation for funding, project No. P300P2_171233 and 200021-175994, and Caroline Giacomin for proof-reading.References
- J. M. Smith and S. J. Maynard, The theory of evolution, Cambridge University Press, Cambridge, 1993 Search PubMed.
- C. Bergmann, Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Größe, Göttinger Studien, 1847, 3, 595–708 Search PubMed.
- S. Lamichhaney, J. Berglund, M. Sällman Almén, K. Maqbool, M. Grabherr, A. Martinez-Barrio, M. Promerová, C.-J. Rubin, C. Wang, N. Zamani, B. R. Grant, P. R. Grant, M. T. Webster and L. Andersson, Evolution of Darwin's finches and their beaks revealed by genome sequencing, Nature, 2015, 518, 371 CrossRef CAS PubMed.
- C. Darwin, On the origin of species by means of natural selection, or preservation of favoured races in the struggle for life, John Murray, London, 1859 Search PubMed.
- P. Fratzl and F. G. Barth, Biomaterial systems for mechanosensing and actuation, Nature, 2009, 462, 441–448 CrossRef PubMed.
- P. Fratzl and R. Weinkamer, Nature's hierarchical materials, Prog. Mater. Sci., 2007, 52, 1263–1334 CrossRef CAS.
- M. A. Meyers, P.-Y. Chen, M. I. Lopez, Y. Seki and A. Y. M. Lin, Biological materials: A materials science approach, J. Mech. Behav. Biomed. Mater., 2011, 4, 626–657 CrossRef PubMed.
- R. A. Campbell and M. N. Dean, Adaptation and evolution of biological materials, Integr. Comp. Biol., 2019, 59, 1629–1635 CrossRef PubMed.
- A. V. Furano and J. P. Green, Differences in the disposition of endogenous and exogenous substances by cells, Nature, 1963, 199, 380–381 CrossRef CAS PubMed.
- A. Friedlaender, A. Bocconcelli, D. Wiley, D. Cholewiak, C. Ware, M. Weinrich and M. Thompson, Underwater components of humpback whale bubble-net feeding behaviour, Behaviour, 2011, 148, 575–602 Search PubMed.
- P. Holter, Effect of dung-beetles (Aphodius spp.) and earthworms on the disappearance of cattle dung, Oikos, 1979, 393–402 CrossRef.
- M. Kleman and O. D. Laverntovich, Soft matter physics: An introduction, Springer, 2007 Search PubMed.
- P.-G. de Gennes, Soft matter, Angew. Chem., Int. Ed. Engl., 1992, 31, 842–845 CrossRef.
- R. G. Larson, The structure and rheology of complex fluids, Oxford University Press, New York, 1999 Search PubMed.
- M. M. Cross, Rheology of non-Newtonian fluids: A new flow equation for pseudoplastic systems, J. Colloid Sci., 1965, 20, 417–437 CrossRef CAS.
- R. H. Ewoldt, A. E. Hosoi and G. H. McKinley, Nonlinear viscoelastic biomaterials: meaningful characterization and engineering inspiration, Integr. Comp. Biol., 2009, 49, 40–50 CrossRef PubMed.
- J. Mewis and N. J. Wagner, Colloidal Suspension Rheology, Cambridge University Press, Cambridge, 2012 Search PubMed.
- M. Rubinstein and R. H. Colby, Polymer Physics, Oxford University Press, Oxford, 2003 Search PubMed.
- N. Kojic, J. Bico, C. Clasen and G. H. McKinley, Ex vivo rheology of spider silk, J. Exp. Biol., 2006, 209, 4335–4362 CrossRef PubMed.
- P. R. Laity, S. E. Gilks and C. Holland, Rheological behaviour of native silk feedstocks, Polymer, 2015, 67, 28–39 CrossRef CAS.
- P. R. Laity and C. Holland, The rheology behind stress-induced solidification in native silk feedstocks, Int. J. Mol. Sci., 2016, 17, 1812 CrossRef PubMed.
- A. Baer, S. Schmidt, S. Haensch, M. Eder, G. Mayer and M. J. Harrington, Mechanoresponsive lipid-protein nanoglobules facilitate reversible fibre formation in velvet worm slime, Nat. Commun., 2017, 8, 974 CrossRef PubMed.
- V. Zintzen, C. D. Roberts, M. J. Anderson, A. L. Stewart, C. D. Struthers and E. S. Harvey, Hagfish predatory behaviour and slime defence mechanism, Sci. Rep., 2011, 1, 131 CrossRef CAS PubMed.
- S. E. Suarez, M. E. Brookfield, E. J. Catlos and D. F. Stöckli, A U-Pb zircon age constraint on the oldest-recorded air-breathing land animal, PLoS One, 2017, 12, e0179262 CrossRef PubMed.
- Z.-Q. Zhang, Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness, Zootaxa, 2011, 3148, 7–237 CrossRef.
- A. A. Abbasi, Evolution of vertebrate appendicular structures: Insight from genetic and palaeontological data, Dev. Dyn., 2011, 240, 1005–1016 CrossRef PubMed.
- H. W. Lissmann, The mechanism of locomotion in gastropod molluscs, II Kinetics, J. Exp. Biol., 1945, 22, 37–50 CrossRef CAS.
- G. H. Parker, The mechanism of locomotion in gastropods, J. Morphol., 1911, 22, 155–170 CrossRef.
- M. W. Denny, The role of gastropod pedal mucus in locomotion, Nature, 1980, 285, 160–161 CrossRef.
- R. H. Ewoldt, C. Clasen, A. E. Hosoi and G. H. McKinley, Rheological fingerprinting of gastropod pedal mucus and synthetic complex fluids for biomimicking adhesive locomotion, Soft Matter, 2007, 3, 634 RSC.
- M. Iwamoto, D. Ueyama and R. Kobayashi, The advantage of mucus for adhesive locomotion in gastropods, J. Theor. Biol., 2014, 353, 133–141 CrossRef PubMed.
- J. H. Lai, J. C. del Alamo, J. Rodríguez-Rodríguez and J. C. Lasheras, The mechanics of the adhesive locomotion of terrestrial gastropods, J. Exp. Biol., 2010, 213, 3920–3933 CrossRef PubMed.
- C. K. C. Churchill, D. Ó. Foighil and E. E. Strong, and G. A, Females floated first in bubble-rafting snails, Curr. Biol., 2011, 21, R802–R803 CrossRef CAS PubMed.
- M. W. Denny, Molecular biomechanics of molluscan mucoussecretions, Metabolic biochemistry and molecular biomechanics, Academic Press, New York, 1983 Search PubMed.
- R. A. Stratton and A. F. Butcher, Stress relaxation upon cessation of steady flow and the overshoot effect of polymer solutions, J. Polym. Sci., Polym. Phys. Ed., 1973, 11, 1747–1758 CAS.
- M. W. Denny, Locomotion: The cost of gastropod crawling, Science, 1980, 208, 1288–1290 CrossRef CAS PubMed.
- E. Lauga and A. E. Hosoi, Tuning gastropod locomotion: Modeling the influence of mucus rheology on the cost of crawling, Phys. Fluids, 2006, 18, 113102 CrossRef.
- A. E. Hosoi and D. I. Goldman, Beneath our feet: Strategies for locomotion in granular media, Annu. Rev. Fluid Mech., 2015, 47, 431–453 CrossRef.
- R. D. Maladen, Y. Ding, C. Li and D. I. Goldman, Undulatory swimming in sand: Subsurface locomotion of the sandfish lizard, Science, 2009, 325, 314–318 CrossRef CAS PubMed.
- R. D. Maladen, Y. Ding, P. B. Umbanhowar, A. Kamor and D. I. Goldman, Mechanical models of sandfish locomotion reveal principles of high performance subsurface sand-swimming, J. R. Soc., Interface, 2011, 8, 1332–1345 CrossRef PubMed.
- C. Ness, R. Mari and M. E. Cates, Shaken and stirred: Random organization reduces viscosity and dissipation in granular suspensions, Sci. Adv., 2018, 4, eaar3296 CrossRef PubMed.
- Y. Forterre and O. Pouliquen, Flows of dense granular media, Annu. Rev. Fluid Mech., 2008, 40, 1–24 CrossRef.
- P. Jop, Y. Forterre and O. Pouliquen, A constitutive law for dense granular flows, Nature, 2006, 441, 727–730 CrossRef CAS PubMed.
- F. Boyer, É. Guazzelli and O. Pouliquen, Unifying suspension and granular rheology, Phys. Rev. Lett., 2011, 107, 188301 CrossRef PubMed.
- E. M. Purcell, Life at low Reynolds number, Am. J. Phys., 1977, 45, 3–11 CrossRef.
- T. Qiu, T.-C. Lee, A. G. Mark, K. I. Morozov, R. Münster, O. Mierka, S. Turek, A. M. Leshansky and P. Fischer, Swimming by reciprocal motion at low Reynolds number, Nat. Commun., 2014, 5, 5119 CrossRef CAS PubMed.
- S. S. Sharpe, R. Kuckuk and D. I. Goldman, Controlled preparation of wet granular media reveals limits to lizard burial ability, Phys. Biol., 2015, 12, 46009 CrossRef PubMed.
- M. Muller and J. W. M. Osse, Hydrodynamics of suction feeding in fish, Trans. Zool. Soc. London, 1984, 37, 51–135 CrossRef.
- J. Peng and J. O. Dabiri, Transport of inertial particles by Lagrangian coherent structures: Application to predator-prey interaction in jellyfish feeding, J. Fluid Mech., 2009, 623, 75–84 CrossRef.
- D. M. Fields and J. Yen, Implications of the feeding current structure of Euchaeta rimana, a carnivorous pelagic copepod, on the spatial orientation of their prey, J. Plankton Res., 1997, 19, 79–95 CrossRef.
- G. Mayer, I. S. Oliveira, A. Baer, J. U. Hammel, J. Gallant and R. Hochberg, Capture of prey, feeding, and functional anatomy of the jaws in velvet worms (Onychophora), Integr. Comp. Biol., 2015, 55, 217–227 CrossRef PubMed.
- V. M. S. J. Read and R. N. Hughes, Feeding behaviour and prey choice in Macroperipatus torquatus (Onychophora), Proc. R. Soc. London, Ser. B, 1987, 230, 483–506 Search PubMed.
- A. Concha, P. Mellado, B. Morera-Brenes, C. Sampaio Costa, L. Mahadevan and J. Monge-Nájera, Oscillation of the velvet worm slime jet by passive hydrodynamic instability, Nat. Commun., 2015, 6, 6292 CrossRef CAS PubMed.
- B. Morera-Brenes and J. Monge-Nájera, A new giant species of placented worm and the mechanism by which onychophorans weave their nets (Onychophora: Peripatidae), Rev. Biol. Trop., 2010, 58, 1127–1142 Search PubMed.
- G. Mayer, F. A. Franke, S. Treffkorn, V. Gross and I. de Sena Oliveira, in Ecdysozoa I: Non-Tetraconata, ed. A. Wanninger, Springer, Vienna, 2015 Search PubMed.
- M. Heim, D. Keerl and T. Scheibel, Spider silk: From soluble protein to extraordinary fiber, Angew. Chem., Int. Ed., 2009, 48, 3584–3596 CrossRef CAS PubMed.
- S. M. Manton and N. Y. Heatley, Studies on the Onychophora II – The feeding, digestion, excretion and food storage of Peripatopsis, Philos. Trans. R. Soc., B, 1937, 227, 411–464 Search PubMed.
- A. Baer, S. Schmidt, G. Mayer and M. J. Harrington, Fibers on the fly: Multiscale mechanisms of fiber formation in the capture slime of velvet worms, Integr. Comp. Biol., 2019, 59, 1690–1699 CrossRef PubMed.
- A. Baer, S. Hänsch, G. Mayer, M. J. Harrington and S. Schmidt, Reversible supramolecular assembly of velvet worm adhesive fibers via electrostatic interactions of charged phosphoproteins, Biomacromolecules, 2018, 19, 4034–4043 CrossRef CAS PubMed.
- A. Baer, N. Horbelt, M. Nijemeisland, S. J. Garcia, P. Fratzl, S. Schmid, G. Mayer and M. J. Harrington, Shear-induced β-crystallite unfolding in condensed phase nanodroplets promotes fiber formation in a biological adhesive, ACS Nano, 2019, 13, 4992–5001 CrossRef CAS PubMed.
- K. Benkendorff, K. Beardmore, A. A. Gooley, N. H. Packer and N. N. Tait, Characterisation of the slime gland secretion from the peripatus, Euperipatoides kanangrensis (Onychophora: Peripatopsidae), Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 1999, 124, 457–465 CrossRef.
- V. S. Haritos, A. Niranjane, S. Weisman, H. E. Trueman, A. Sriskantha and T. D. Sutherland, Harnessing disorder: Onychophorans use highly unstructured proteins, not silks, for prey capture, Proc. R. Soc. B, 2010, 277, 3255–3263 CrossRef CAS PubMed.
- A. C. Noel, H.-Y. Guo, M. L. Mandica and D. L. Hu, Frogs use a viscoelastic tongue and non-Newtonian saliva to catch prey, J. R. Soc., Interface, 2017, 14, 20160764 CrossRef PubMed.
- M. Houze and P. Damman, Predation with the tongue through viscous adhesion, a scaling approach, Soft Matter, 2017, 13, 2120–2124 RSC.
- F. Brau, D. Lanterbecq, L.-N. Zghikh, V. Bels and P. Damman, Dynamics of prey prehension by chameleons through viscous adhesion, Nat. Phys., 2016, 12, 931 Search PubMed.
- K. E. L. Simmons, Foot-movements in plovers and other birds, Br. Birds, 1961, 54, 418–422 Search PubMed.
- K. C. Catania, Worm grunting, fiddling, and charming—Humans unknowingly mimic a predator to harvest bait, PLoS One, 2008, 3, e3472 CrossRef PubMed.
- P. A. Buckley, Foot-paddling in four American gulls, with comments on its possible function and stimulation, Z. Tierpsychol., 1966, 23, 395–402 CAS.
- J. H. Sparks, The relationship between food-movements and feeding in shore birds, Br. Birds, 1961, 54, 337–340 Search PubMed.
- E. Brown and H. M. Jaeger, Shear thickening in concentrated suspensions: phenomenology, mechanisms and relations to jamming, Rep. Prog. Phys., 2014, 77, 46602 CrossRef PubMed.
- A. F. Clift, Observations on certain rheological properties of human cervical secretion, Proc. R. Soc. Med., 1945, 39, 1–9 Search PubMed.
- L. E. Adams, Observations on the pairing of Limax maximus, J. Conchol., 1898, 9, 92–95 Search PubMed.
- T. H. Langlois, The conjugal behavior of the introduced European giant garden slug, Limax Maximus L. as observed on South Bass Island, Lake Erie, Ohio J. Sci., 1965, 298–304 Search PubMed.
- V. B. Meyer-Rochow and J. Gal, Pressures produced when penguins pooh – calculations on avian defaecation, Polar Biol., 2003, 27, 56–58 CrossRef.
- A. M. Smith, Biological Adhesive Systems, Springer, Berlin, 2010 Search PubMed.
- D. P. Wolf, L. Blasco, M. A. Khan and M. Litt, Human cervical mucus. I. Rheologic characteristics, Fertil. Steril., 1977, 28, 41–46 CrossRef CAS.
- G. M. Barker and R. A. McGhie, The biology of introduced slugs (Pulmonata) in New Zealand 1. Introduction and notes on Limax maximus, New Zeal. Entomol., 1984, 8, 106–111 CrossRef.
- M. Numata, L. S. Davis and M. Renner, Prolonged foraging trips and egg desertion in little penguins (Eudyptula minor), New Zeal. J. Zool., 2000, 27, 277–289 CrossRef.
- M. Rietkerk and J. van de Koppel, Regular pattern formation in real ecosystems, Trends Ecol. Evol., 2008, 23, 169–175 CrossRef PubMed.
- T. D. Wiliams, The penguins, Spheniscidae, Oxford University Press, Oxford, 1991 Search PubMed.
- H. Tajima and F. Fujisawa, Projectile trajectory of Penguin's faeces and rectal pressure revisited, 2020, arXiv:2007.00926.
- P. J. Yang, M. LaMarca, C. Kaminski, D. I. Chuc and D. L. Hu, Hydrodynamics of defecation, Soft Matter, 2017, 13, 4960–4970 RSC.
- P. J. Yang, A. B. Lee, M. Chan, M. Kowalski, K. Qiu, C. Waid, G. Cervantes, B. Magondu, M. Biagioni, L. Vogelnest, A. Martin, A. Edwards, S. Carver and D. L. Hu, Intestines of non-uniform stiffness mold the corners of wombat feces, Soft Matter, 2021, 17, 475–488 RSC.
- P. Erni, Deformation modes of complex fluid interfaces, Soft Matter, 2011, 7, 7586–7600 RSC.
- P. Erni, E. J. Windhab and P. Fischer, Emulsion drops with complex interfaces: Globular versus flexible proteins, Macromol. Mater. Eng., 2011, 296, 249–262 CrossRef CAS.
- R. Mezzenga and P. Fischer, The self-assembly, aggregation and phase transitions of food protein systems in one, two and three dimensions, Rep. Prog. Phys., 2013, 76, 046601 CrossRef PubMed.
- D. V. Andrade and A. S. Abe, Foam nest production in the armoured catfish, J. Fish Biol., 1997, 50, 665–667 CrossRef.
- J. H. A. Mol, in The Freshwater Ecosystems of Suriname, ed. P. E. Ouboter, Springer, Berlin, 1993 Search PubMed.
- A. Cooper, M. W. Kennedy, R. I. Fleming, E. H. Wilson, H. Videler, D. L. Wokosin, T. Su, R. J. Green and J. R. Lu, Adsorption of frog foam nest proteins at the air-water interface, Biophys. J., 2005, 88, 2114–2125 CrossRef CAS.
- J. Hunter, Observations on bees, Philos. Trans. R. Soc. London, 1792, 82, 128–195 CrossRef.
- T. Narumi, K. Uemichi, H. Honda and K. Osaki, Self-organization at the first stage of honeycomb construction: Analysis of an attachment-excavation model, PLoS One, 2018, 13, e0205353 CrossRef PubMed.
- C. W. W. Pirk, H. R. Hepburn, S. E. Radloff and J. Tautz, Honeybee combs: Construction through a liquid equilibrium process?, Naturwissenschaften, 2004, 91, 350–353 CrossRef CAS PubMed.
- G. R. I. Waterhouse, On the formation of the cells of bees and wasps, Trans. R. Entomol. Soc. London, 1864, 12, 115–129 CrossRef.
- K. Zhang, H. Duan, B. L. Karihaloo and J. Wang, Hierarchical, multilayered cell walls reinforced by recycled silk cocoons enhance the structural integrity of honeybee combs, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 9502–9506 CrossRef CAS.
- J. Tautz, The buzz about bees, Springer, Berlin, 2008 Search PubMed.
- T. H. Shellhammer, T. R. Rumsey and J. M. Krochta, Viscoelastic properties of edible lipids, J. Food Eng., 1997, 33, 305–320 CrossRef.
- B. L. Karihaloo, K. Zhang and J. Wang, Honeybee combs: how the circular cells transform into rounded hexagons, J. R. Soc., Interface, 2013, 10, 20130299 CrossRef CAS PubMed.
- F. Nazzi, The hexagonal shape of the honeycomb cells depends on the construction behavior of bees, Sci. Rep., 2016, 6, 28341 CrossRef CAS PubMed.
- F. H. Martini, in The biology of hagfishes, ed. J. M. Jorgensen, J. P. Lomholt, R. E. Weber and H. Malte, Springer, Berlin, 1998 Search PubMed.
- L. Böni, P. Fischer, L. Böcker, S. Kuster and P. A. Rühs, Hagfish slime and mucin flow properties and their implications for defense, Sci. Rep., 2016, 6, 30371 CrossRef.
- R. H. Ewoldt, T. M. Winegard and D. S. Fudge, Non-linear viscoelasticity of hagfish slime, Int. J. Non Linear Mech., 2011, 46, 627–636 CrossRef.
- D. S. Fudge, N. Levy, S. Chiu and J. M. Gosline, Composition, morphology and mechanics of hagfish slime, J. Exp. Biol., 2005, 208, 4613–4625 CrossRef CAS.
- J. Fu, P. A. Guerette and A. Miserez, Self-assembly of recombinant hagfish thread keratins amenable to a strain-induced α-helix to β-sheet transition, Biomacromolecules, 2015, 16, 2327–2339 CrossRef CAS PubMed.
- D. S. Fudge, K. H. Gardner, V. T. Forsyth, C. Riekel and J. M. Gosline, The mechanical properties of hydrated intermediate filaments: insights from hagfish slime threads, Biophys. J., 2003, 85, 2015–2027 CrossRef CAS PubMed.
- S. W. Downing, R. H. Spitzer, E. A. Koch and W. L. Salo, The hagfish slime gland thread cell. I. A unique cellular system for the study of intermediate filaments and intermediate filament-microtubule interactions, J. Cell Biol., 1984, 98, 653–669 CrossRef CAS PubMed.
- B. Fernholm, Thread cells from the slime glands of hagfish (Myxinidae), Acto Zool., 1981, 62, 137–145 CrossRef.
- J. E. Herr, T. M. Winegard, M. J. O’Donnell, P. H. Yancey and D. S. Fudge, Stabilization and swelling of hagfish slime mucin vesicles, J. Exp. Biol., 2010, 213, 1092–1099 CrossRef CAS PubMed.
- S. Takeda, M. Matsumasa, H.-S. Yong and M. Murai, “Igloo” construction by the ocypodid crab, Dotilla myctiroides (Milne-Edwards) (Crustacea; Brachyura): The role of an air chamber when burrowing in a saturated sandy substratum, J. Exp. Mar. Biol. Ecol., 1996, 198, 237–247 CrossRef.
- H. Adam, Different types of body movement in the hagfish, Myxine glutinosa, Nature, 1960, 188, 595–596 CrossRef.
- J. M. Nance, Respiratory water flow and production of mucus in the cushion star, Pteraster tesselatus Ives (Echinodermata: Asteroidea), J. Exp. Mar. Biol. Ecol., 1981, 50, 21–31 CrossRef.
- J. M. Nance and L. F. Braithwaite, The function of mucous secretions in the cushion star Pteraster tesselatus Ives, J. Exp. Mar. Biol. Ecol., 1979, 40, 259–266 CrossRef.
- A. S. Grutter, J. G. Rumney, T. Sinclair-Taylor, P. Waldie and C. E. Franklin, Fish mucous cocoons: The ‘mosquito nets’ of the sea, Biol. Lett., 2011, 7, 292–294 CrossRef PubMed.
- C. E. Kicklighter, S. Shabani, P. M. Johnson and C. D. Derby, Sea hares use novel antipredatory chemical defenses, Curr. Biol., 2005, 15, 549–554 CrossRef CAS PubMed.
- T. Love-Chezem, J. F. Aggio and C. D. Derby, Defense through sensory inactivation: sea hare ink reduces sensory and motor responses of spiny lobsters to food odors, J. Exp. Biol., 2013, 2016, 1364–4372 CrossRef.
- X. U. Chen, V. B. Meyer-Rochow, A. Fereres, M. Morente and A.-P. Liang, The role of biofoam in shielding spittlebug nymphs (Insecta, Hemiptera, Cercopidae) against bright light, Ecol. Entomol., 2018, 43, 273–281 CrossRef.
- A. T. Marshall, Protein synthesis and secretion by the Malpighian tubules of cercopoid larvae (Homoptera), J. Insect Physiol., 1973, 19, 2317–2326 CrossRef CAS.
- M. Tonelli, G. Gomes, W. D. Silva, N. T. C. Magri, D. M. Vieira, C. L. Aguiar and B. J. M. S. Spittlebugs, produce foam as a thermoregulatory adaptation, Sci. Rep., 2018, 8, 4729 CrossRef PubMed.
- J. Gould, J. W. Valdez and R. Upton, Adhesive defence mucus secretions in the red triangle slug (Triboniophorus graeffei) can incapacitate adult frogs, Ethology, 2019, 125, 587–591 Search PubMed.
- Z. Barrientos, A new aestivation strategy for land molluscs: hanging upside down like bats, UNED Res. J., 2020, 12, e2802 CrossRef.
- R. G. Hartnoll, Factors affecting the distribution and behaviour of the crab Dotilla fenestrata on East African shores, Estuarine Coastal Mar. Sci., 1973, 1, 137–152 CrossRef.
- S. Takeda and Y. Kurihara, The distribution and abundance of Helice tridens (De Haan) burrows and substratum conditions in a northeastern Japan salt marsh (Crustacea: Brachyura), Exp. Mar. Biol. Ecol., 1987, 107, 9–19 CrossRef.
- L. J. Böni, R. Zurflüh, M. E. Baumgartner, E. J. Windhab, P. Fischer, S. Kuster and P. A. Rühs, Effect of ionic strength and seawater cations on hagfish slime formation, Sci. Rep., 2018, 8, 9867 CrossRef PubMed.
- K. Rementzi, L. J. Böni, J. Adamcik, P. Fischer and D. Vlassopoulos, Structure and dynamics of hagfish mucin in different saline environments, Soft Matter, 2019, 15, 8627–8637 RSC.
- T. Lang, G. C. Hansson and T. Samuelsson, Gel-forming mucins appeared early in metazoan evolution, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 16209–16214 CrossRef CAS PubMed.
- T. Lang, S. Klasson, E. Larsson, M. E. V. Johansson, G. C. Hansson and T. Samuelsson, Searching the evolutionary origin of epithelial mucus protein components: Mucins and FCGBP, Mol. Biol. Evol., 2016, 33, 1921–1936 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |