 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stabilisation of hollow colloidal TiO2 particles by partial coating with evenly distributed lobes†
Bo
Peng
 *ab,
Yanyan
Liu
a,
Dirk G. A. L.
Aarts
a and
Roel P. A.
Dullens
*a
*ab,
Yanyan
Liu
a,
Dirk G. A. L.
Aarts
a and
Roel P. A.
Dullens
*a
aDepartment of Chemistry, Physical and Theoretical Chemistry Laboratory, University of Oxford, South Parks Road, Oxford OX1 3QZ, UK. E-mail: roel.dullens@chem.ox.ac.uk
bDepartment of Applied Physics, Aalto University, Espoo FI-00076, Finland. E-mail: pengbo006@gmail.com
First published on 13th January 2021
Abstract
Photo-catalytically active crystalline TiO2 has attracted special attention due to its relevance for renewable energy and is typically obtained by the calcination of amorphous TiO2. However, stabilising hollow colloidal TiO2 particles against aggregation during calcination without compromising their photocatalytic activity poses two conflicting demands: to be stable their surface needs to be coated, while efficient photocatalysis requires an exposed TiO2 surface. Here, this incompatibility is resolved by partially coating TiO2 shells with evenly distributed 3-trimethoxysilyl propyl methacrylate (TPM) lobes. These lobes act both as steric barriers and surface charge enhancers that efficiently stabilise the TiO2 shells against aggregation during calcination. The morphology of the TPM lobes and their coverage, and the associated particle stability during the calcination-induced TiO2 crystallization, can be controlled by the pH and the contact angle between TPM and TiO2. The crystal structure and the grain size of the coated TiO2 shells are controlled by varying the calcination temperature, which allows tuning their photocatalytic activity. Finally, the durable photocatalytic activity over many usage cycles of the coated TiO2 compared to uncoated shells is demonstrated in a simple way by measuring the photo-degradation of a fluorescent dye. Our approach offers a general strategy for stabilising colloidal materials, without compromising access to their active surfaces.
1 Introduction
Titanium dioxide (TiO2) has been one of the most intensely studied semiconductors over the past few decades due to its wide variety of applications across many different fields,1–4 including energy harvesting and storage, photocatalysis, electrochromic devices, sensors, bio-separation, pigment and photonic crystals.1,2,4–7 In particular, its application as a photocatalyst in for example water splitting attracted special attention due to its relevance for renewable energy and environmental pollution.2,4–9 The photocatalytic activity of TiO2 relies on its crystal structure, which sensitively depends on the synthetic strategy adopted.1,6 Crystalline hollow colloidal TiO2 particles are preferable due to their large active surface area and are conventionally prepared by combining sol–gel, template-sacrifice and calcination approaches.10–18 During calcination, the photocatalytically inactive amorphous TiO2 is transformed into photoactive crystalline TiO2, where the exact crystal form depends on the calcination temperature.1 However, calcination induces simultaneous structural rearrangement and grain growth in the colloidal TiO2 particles, which makes aggregation between them often unavoidable.13,19,20Recently, a ‘wrap-bake-peel’ strategy has been proposed to overcome these problems, which involves growing a homogeneous silica (SiO2) shell around the material of interest to prevent particle aggregation during calcination, and then removing this SiO2 shell to expose the ‘active’ surface.21 Further progress has been made by selecting different materials for the core and the protective shells.19,22,23 However, in all of these approaches the homogeneous shells had to be removed after calcination to expose the ‘active’ surface of the core material. Unfortunately, the removal of the shells compromises the stability of crystalline TiO2 shells in a practical environment such as wastewater, where the presence of ions and polymers screens the electric double layer24 and induces depletion attractions,25 respectively. The stability of these particles in such contaminated aqueous media is further deteriorated by the relatively strong van der Waals attractions between the high refractive index TiO2 shells.26–29 Thus, the preparation of stable photocatalytic TiO2 shells is a serious challenge because photocatalysis requires an exposed active surface, while a protective coating is needed for their stabilization during calcination and subsequent usage as a photocatalyst in aqueous environments.
Here, we overcome this challenge by partially coating colloidal TiO2 shells with evenly distributed lobes that stabilize the shells during their calcination and their subsequent use as photocatalysts. To this end, the TiO2 shells are covered by evenly distributed TPM (3-trimethoxysilyl propyl methacrylate) droplets, which are polymerized into solid lobes.30,31 Conveniently, the morphology of these protective lobes on the TiO2 shells can be tuned by varying the pH and the contact angle between TPM and TiO2. In addition, the crystal structure and the grain size of the crystalline TiO2 shells can be controlled by the calcination temperature. Finally, we demonstrate the photocatalytic activity of our partially coated colloidal TiO2 shells in a simple way by measuring the decomposition of a fluorescent dye in water and show that they exhibit photocatalytic activity over many usage cycles compared to uncoated TiO2 shells.
2 Materials and methods
2.1 Materials
All chemicals are used as received from Sigma-Aldrich unless mentioned otherwise. For the synthesis of cationic polystyrene (PS) particles, styrene and 2-(methacryloyl)ethyltrimethylammonium chloride (MTC, 80 wt% in H2O) are used as the monomers with the initiator azobisisobutyronitrile (AIBN), recrystallized from ethanol. Polyvinylpyrrolidone (PVP-40) and ethanol serve as the stabilizer and co-solvent, respectively. Titanium(IV) tetraisopropoxide (TTIP, 97%) and 3-(trimethoxysilyl)propyl methacrylate (TPM, 98%) are the precursors for synthesising TiO2 and TPM shells, respectively. Ammonia (25% in H2O) is used as the catalyst during TPM coating. Rhodamine b isothiocyanate (RITC) is employed for the photo-catalytic evaluation experiments. De-ionized water is used throughout the experiments and obtained from a Millipore Direct-Q UV 3 reverse osmosis filter apparatus.2.2 Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in mass) consisting of PVP (2.5 g), styrene (5 g) and AIBN (0.25 g) was fed into a 250 mL, three-necked flask equipped with a gas supply, a condenser, and Teflon-coated magnetic stir bar. This mixture was homogenized with a magnetic stir at 200 rpm and deoxygenized with nitrogen for 30 min prior to heating up to 70 °C to commence the polymerization. In order to not disturb the nucleation stage, a mixture comprising of ethanol (10 g), water (2.5 g), styrene (5 g) and MTC (0.3 g) was continually fed into the flask within 2 h, starting from at the point at which the polymerization had been running for 1 h.33,34 The reaction was considered complete in 24 h, and resulted in monodisperse PS cores with a mean diameter of 1.07 μm (see Fig. 1b and Fig. S1a, ESI†). PS cores were washed by repeated centrifugation and redispersion (three times) with ethanol and stored in ethanol with a mass content of 11.1%.
1 in mass) consisting of PVP (2.5 g), styrene (5 g) and AIBN (0.25 g) was fed into a 250 mL, three-necked flask equipped with a gas supply, a condenser, and Teflon-coated magnetic stir bar. This mixture was homogenized with a magnetic stir at 200 rpm and deoxygenized with nitrogen for 30 min prior to heating up to 70 °C to commence the polymerization. In order to not disturb the nucleation stage, a mixture comprising of ethanol (10 g), water (2.5 g), styrene (5 g) and MTC (0.3 g) was continually fed into the flask within 2 h, starting from at the point at which the polymerization had been running for 1 h.33,34 The reaction was considered complete in 24 h, and resulted in monodisperse PS cores with a mean diameter of 1.07 μm (see Fig. 1b and Fig. S1a, ESI†). PS cores were washed by repeated centrifugation and redispersion (three times) with ethanol and stored in ethanol with a mass content of 11.1%.
The crystalline structures of the samples were evaluated with a PANalytical X’Pert Pro X-ray diffractometer (XRD) equipped with a monochromatized Cu Kα X-ray source (λ = 1.5403 Å). The grain size of the samples was estimated with Debye–Scherrer equation, τ = Kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, where τ is the mean size of the grain; K the dimensionless shape factor, in this case, 0.89; λ the wavelength of the X-ray source; β the width of the XRD peak at the half-peak height in radians, and θ the Bragg angle. The thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were performed on a Mettler Toledo TGA/DSC 1 system by heating up the sample from room temperature to 1000 °C with a rate of 5 °C/min in air. The Zeta potential of the samples was measured with a Malvern Zetasizer Nano.
θ, where τ is the mean size of the grain; K the dimensionless shape factor, in this case, 0.89; λ the wavelength of the X-ray source; β the width of the XRD peak at the half-peak height in radians, and θ the Bragg angle. The thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were performed on a Mettler Toledo TGA/DSC 1 system by heating up the sample from room temperature to 1000 °C with a rate of 5 °C/min in air. The Zeta potential of the samples was measured with a Malvern Zetasizer Nano.
3. Results and discussion
The three-step preparation of the partially coated colloidal TiO2 shells is schematically shown in Fig. 1a. First, monodisperse cationic polystyrene (PS) particles with a mean diameter of 1.07 μm (see Fig. 1b, Fig. S1a, and Table S1, ESI†) are synthesized by dispersion polymerization32,34 (for details see Section 2.2.1 and Fig. S1, Table S1, ESI†). Next, the cationic PS particles are coated with an anionic TiO2 shell of 50 nm14,35 (step I in Fig. 1a, see also Fig. 1c and Fig. S1b, Table S1, ESI†). Then, the PS–TiO2 particles are mixed with pre-hydrolysed TPM in alkaline water in the presence of the stabilizer polyvinylpyrrolidone (PVP), which results in raspberry-like PS–TiO2–TPM particles, as shown in Fig. 1d (step II, which can be scaled up in volume, see Fig. S2, ESI†). Finally, the PS–TiO2–TPM particles are calcinated at a temperature within a range of 500–1000 °C (step III). Importantly, during calcination the PS core is removed, the TPM is converted into SiO2 and the initially amorphous TiO2 crystallizes, which eventually results in hollow colloidal TiO2 particles partially coated with evenly distributed SiO2 lobes. In the following we will denote these particles as crystalline-TiO2–SiO2, or in short as c-TiO2–SiO2 as shown in Fig. 1e. The composition of the particles before (PS–TiO2–TPM) and after (c-TiO2–SiO2) calcination is characterized by energy dispersive X-ray spectroscopy with elemental line-scans and map spectra as displayed in Fig. 1f and g. Further characterization with transmission electron microscopy and energy dispersive X-ray spectroscopy with elemental analysis in a selected area, and electron diffraction is shown in Fig. S3 (ESI†). These analyses indeed confirm the presence of TiO2 and its crystallization, the conversion of the TPM lobes into silica, and the thermal decomposition of the PS cores.The surface morphology of the evenly distributed TPM lobes that partially coat the TiO2 shell, is of crucial importance for the particle stability during the calcination and its subsequent application as a photocatalyst (see Fig. 1a, final panel). The morphology of the TPM lobes on the TiO2 surface can be quantified using the following three geometric parameters: the number of lobes (N), the contact angle between the TPM lobe and the TiO2 surface (θ) and the lobe size (r) as defined in Fig. S4 (ESI†). Conveniently, all these parameters can be tuned by varying the experimental conditions. In particular, increasing the PVP concentration (CPVP) increases both N and θ, as shown in Fig. 2a. As the contact angle increases, the coalescence of liquid TPM droplets on the TiO2 surface is suppressed, resulting in an increasing fraction of particles with a larger number of lobes (N). At very high PVP concentrations (CPVP > 7.5 wt%), however, the attachment of TPM lobes becomes very unfavourable as is evident from the small fraction of particles with N > 0 (Fig. 2a-9). This, in fact, is reminiscent of grime-removal with detergents at colloidal scales, where PVP acts as the detergent, breaking up and removing TPM lobes from PS–TiO2 colloids. Based on these observations, we have used a CPVP of 5 wt% in the following experiments (Fig. 2b).
 | ||
| Fig. 2 Controlling the surface morphology of TPM lobes. SEM images of TPM coated TiO2 particles at increasing PVP concentration (CPVP): (a-1) 0, (a-2) 2, (a-3) 3, (a-4) 4, (a-5) 5, (a-6) 7.5, (a-7) 10, to (a-8) 15 wt%, respectively; (a-9) a graphic summary of CPVP effect on the number of TPM lobes (N) and the contact angle (θ). Controlling the surface roughness by varying the ammonia concentration (CNH3) at a CPVP of 5 wt%: (b-1) 0.338, (b-2) 0.676, (b-3) 1.014, (b-4) 1.69, (b-5) 3.38, (b-6) 6.78, to (b-7) 16.9 mM, respectively; (b-8) The lobe size (2r) and N as a function of CNH3, where the error bars indicate the polydispersity (for details see ESI,† Section S1.2.7). Note that the data corresponding to (b-7) are not included in (b-8) because the lobe size in (b-7) is too small to be reliably measured. N is measured from SEM images. Scale bars are 1 μm and error bars are standard deviation. | ||
The surface morphology of the TPM lobes can be further tuned by varying the concentration of the catalyst NH3 (CNH3). As illustrated in Fig. 2b-1–b-7, the lobe radius r decreases while the number of lobes N increases with increasing CNH3, i.e., increasing pH. Ammonia catalyses the condensation of pre-hydrolysed TPM into droplets. Hence, increasing CNH3 leads to the precipitation of many small-sized TPM lobes on the TiO2 surface, as demonstrated in Fig. 2b-8. Another efficient way to tune the TPM surface morphology is to alter the amount of pre-hydrolysed TPM, as shown in Fig. S5 and S6 (ESI†). Here it is observed that the lobe size increases with the amount of added hydrolysed TPM. We roughly estimate the maximum surface coverage to be about 60%. The fact that N decreases at the same time, suggests that the lobes may be coalescing.
Calcination is one of the key process in the production of photo-catalytically active TiO2, as this transforms amorphous TiO2, which is commonly regarded as being photo-catalytically inert, into crystalline and photo-catalytically active TiO2 phases.2,15 Here, both TiO2 shells coated with TPM lobes and uncoated TiO2 shells were exposed to calcination temperatures between 500–1000 °C. As shown in Fig. 3 and Fig. S7, Table S2 (ESI†), for the uncoated TiO2 shells the rutile phase appears at ∼700 °C. In contrast, for the coated TiO2 shells the anatase phase5,20 emerges at ∼500–600 °C, while the photo-catalytically less active rutile phase only appears at ∼900 °C. This is also verified by differential scanning calorimetry (see Fig. S8, ESI†), as well as transmission electron microscopy and electron diffraction of a selected area (see Fig. S3e–g, ESI†). Coating the TiO2 shells with TPM lobes thus widens the thermal stability range of the photo-catalytically more active anatase phase.
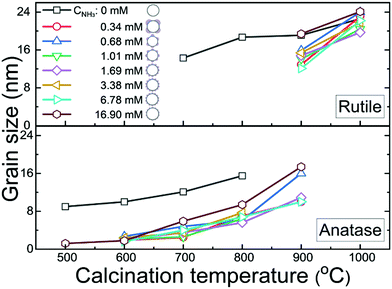 | ||
| Fig. 3 The grain size dependence on the calcination temperature for the anatase and rutile TiO2 crystal phases, as determined from X-ray diffraction spectra (detailed in Fig. S7 and Table S2, ESI†), for different ammonia concentrations, CNH3, used during the synthesis (corresponding to the samples shown in Fig. 2b-1–b-8). Note that the samples with CNH3 = 0 are uncoated pristine TiO2 shells and the other samples are c-TiO2–SiO2 shells. | ||
Coating the TiO2 shells with TPM lobes also affects the grain size and stability during calcination. As seen in Fig. 3, the grain size increases with increasing calcination temperature for both the coated and uncoated TiO2 shells (Fig. S7 and Table S2, ESI†), though it is smaller for coated shells. We speculate that this is due to the water-soluble silicate being absorbed in the small pores of the TiO2 shell, which would limit the grain growth of TiO2 during calcination20 The overlap of the data for different ammonia concentrations (CNH3) in Fig. 3 suggests that the grain size and the relative stability of the anatase and rutile phases are not affected by the surface morphology of the TPM lobes, which is controlled by the CNH3 during the synthesis in step II (see Fig. 2b). Importantly, however, the presence of the TPM lobes does significantly enhance the stability of the TiO2 shells during calcination. While the hollow morphology of the coated TiO2 shells and their stability are retained during calcination (see Fig. S9–S15, ESI†), the uncoated TiO2 shells collapse and aggregate, especially for calcination temperatures above 600 °C (see Fig. S16, ESI†). In particular, TiO2 shells coated with evenly distributed and moderately sized (∼100 nm) TPM lobes (Fig. S14, ESI† and Fig. 2b-6) survive the calcination up to 1000 °C. TiO2 shells that are either sparsely coated with TPM lobes (Fig. S9–S13, ESI,† and Fig. 2b-1–b-5) or with very small lobes (Fig. S15, ESI† and Fig. 2b-7) aggregate during calcination.
We next compare the photocatalytic activities of the TPM coated TiO2 shells, and the uncoated TiO2 shells, at different calcination temperatures by measuring the photo-degradation of rhodamine b isothiocyanate (RITC) under UV irradiation36 (for details see Section 2.2.6, and Fig. S17, ESI†). RITC is a common dye widely used in polymer and colloidal experiments.33,37,38 The catalytic degradation of a dye is a simple, yet convenient experiment for demonstrating the particles’ stability, and we note that the study of the photodegradation mechanism is beyond the focus of this work. In particular, we compare the photo-catalytic activity of TiO2 shells coated with evenly distributed lobes (Fig. 4a and b) with that of uncoated TiO2 shells (Fig. 4c and d). Note that the uncoated TiO2 shells (Fig. S18 and S19, ESI†) used for these experiments were obtained by removing the SiO2 lobes of already coated and calcinated shells (c-TiO2–SiO2 in Fig. S14b–f, ESI†) using NaOH (see Materials and methods, Section 2.2.5). Clearly, the photo-catalytic activities of TiO2 shells both depend non-monotonically on the calcination temperature: their catalytic activities exhibit a maximum at 800 °C, with the activity between 600–700 °C being larger than that between 900–1000 °C. Correlating this with the data in Fig. 3, shows that the anatase TiO2 is catalytically more active than its rutile form.5,20
 | ||
| Fig. 4 The photocatalytic activity and stability of c-TiO2–SiO2 and uncoated TiO2 shells during the degradation of rhodamine b isothiocyanate (RITC) under UV irradiation measured at the wavelength of maximum absorbance of RITC. (a) The relative absorbance (C/C0) as a function of the irradiation time using c-TiO2–SiO2 shells (see Fig. 2b-6 and Fig. S14b–f, ESI†) calcinated at temperatures between 600 to 1000 °C; (b) the corresponding fits to the data in (a) to obtain the effective first order reaction rate constants (k); (c and d) the same quantities as in (a and b) for the uncoated shells, which are obtained by removing the SiO2 lobes of the coated shells using NaOH. (e) A recycling comparison in catalytic efficiency of c-TiO2–SiO2 (sample in a) and TiO2 (sample in c) shells. (f) The zeta potential as a function of time of c-TiO2–SiO2 and uncoated TiO2 shells during the photocatalysis. | ||
Although the rate constants (k) quantifying the kinetics of the photo-degradation of RITC are ∼20% smaller for the coated TiO2 shells, their recycling stability is superior to that of the uncoated TiO2, as shown in Fig. 4e. While the catalytic efficiency of coated TiO2 shells remains around 90%, only about 10% of the initial catalytic efficiency is retained for uncoated TiO2 shells after 8 cycles of re-usage, which we attribute to the significantly increased stability of the coated TiO2 shells during their usage as photo-catalyst. This is corroborated by the fact that the uncoated TiO2 shells initially are weakly positively charged after NaOH treatment, and their zeta-potential decreases and even changes sign during the catalysis (see Fig. 4f). This decrease and sign-reversal of the surface charge will destabilize and lead to aggregation of the uncoated TiO2 shells (Fig. S20, ESI†). The underlying mechanism of the surface charge reversal may be complex with many factors contributing to it including for instance the pH and the different products of the RITC degradation, and should be interesting for further studies. In contrast, the coated TiO2 shells are strongly negatively charged, and their zeta-potential initially becomes even more negative during the catalysis before it plateaus, thereby ensuring their stability as the photo-catalysis progresses. As a reference, we also characterized the photo-catalytic activity of pristine TiO2 shells, which tend to aggregate during calcination (Fig. S16, ESI†) and exhibit significantly lower photo-activity than both the TPM coated and uncoated TiO2 shells calcinated at the same temperature (Fig. S21, ESI†). This corroborates that partially coating the TiO2 shells with evenly distributed TPM lobes is an effective way to stabilize colloidal photocatalysts without compromising their photocatalytic activity.
4 Conclusions
In summary, we have developed colloidal TiO2 shells that are partially covered with evenly distributed TPM lobes, which stabilize the shells during their calcination and their use as photocatalysts. The coverage and morphology of these lobes on the TiO2 shells can be tuned by varying the pH and the contact angle between TPM and TiO2. In addition, both the grain size and the crystal structure of the crystalline TiO2 shells can be tuned via the calcination temperature. We have demonstrated the photocatalytic activity of the partially coated colloidal TiO2 shells by quantifying the decomposition of a fluorescent dye in water and the TPM coated TiO2 shells exhibit photocatalytic activity over many usage cycles compared to uncoated TiO2 shells. Our work thus not only represents a new route for synthesising partially coated colloidal TiO2 shells with an enhanced and durable stability, but it also provides a general strategy to stabilize colloidal materials while maintaining access to their active surfaces.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Carla Fernandez-Rico for useful discussions. This work partially made use of Aalto University Bioeconomy Facilities. The ERC (ERC Starting Grant No. 279541 – IMCOLMAT) and the EPSRC (Grant No. EP/J001902/1) are acknowledged for financial support. B. P. thanks financial support from the Academy of Finland (No. 321443 and No. 328942).Notes and references
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS.
- M. Dahl, Y. Liu and Y. Yin, Chem. Rev., 2014, 114, 9853–9889 CrossRef CAS.
- U. Diebold, Surf. Sci. Rep., 2003, 48, 53–229 CrossRef CAS.
- K. Hashimoto, H. Irie and A. Fujishima, Jpn. J. Appl. Phys., 2005, 44, 8269–8285 CrossRef CAS.
- A. L. Linsebigler, G. Lu and J. T. Yates, Chem. Rev., 1995, 95, 735–758 CrossRef CAS.
- A. Fujishima, T. N. Rao and D. A. Tryk, J. Photochem. Photobiol., C, 2000, 1, 1–21 CrossRef CAS.
- W. Li, Z. Wu, J. Wang, A. A. Elzatahry and D. Zhao, Chem. Mater., 2014, 26, 287–298 CrossRef CAS.
- Z. Wang, L. Zhou and X. W. Lou, Adv. Mater., 2012, 24, 1903–1911 CrossRef CAS.
- W. Sun, S. Zhou, B. You and L. Wu, Chem. Mater., 2012, 24, 3800–3810 CrossRef CAS.
- X. W. Lou, L. A. Archer and Z. Yang, Adv. Mater., 2008, 20, 3987–4019 CrossRef CAS.
- J. Hu, M. Chen, X. Fang and L. Wu, Chem. Soc. Rev., 2011, 40, 5472–5491 RSC.
- X. Wang, J. Feng, Y. Bai, Q. Zhang and Y. Yin, Chem. Rev., 2016, 116, 10983–11060 CrossRef CAS.
- A. F. Demirörs, A. van Blaaderen and A. Imhof, Langmuir, 2010, 26, 9297–9303 CrossRef.
- A. Imhof, Langmuir, 2001, 17, 3579–3585 CrossRef CAS.
- T. H. Kim, K. H. Lee and Y. K. Kwon, J. Colloid Interface Sci., 2006, 304, 370–377 CrossRef CAS.
- J. Cai, X. Wu, F. Zheng, S. Li, Y. Wu, Y. Lin, L. Lin, B. Liu, Q. Chen and L. Lin, J. Colloid Interface Sci., 2017, 490, 37–45 CrossRef CAS.
- X. Zhang, X. Yao, X. Wang, L. Feng, J. Qu and P. Liu, Soft Matter, 2014, 10, 873–881 RSC.
- X. Guo, Y. Chen, D. Li, G. Li, M. Xin, M. Zhao, C. Yang, C. Hao and Q. Lei, Soft Matter, 2016, 12, 546–554 RSC.
- J. B. Joo, M. Dahl, N. Li, F. Zaera and Y. Yin, Energy Environ. Sci., 2013, 6, 2082 RSC.
- J. B. Joo, Q. Zhang, I. Lee, M. Dahl, F. Zaera and Y. Yin, Adv. Funct. Mater., 2012, 22, 166–174 CrossRef CAS.
- Y. Piao, J. Kim, H. Bin Na, D. Kim, J. S. Baek, M. K. Ko, J. H. Lee, M. Shokouhimehr and T. Hyeon, Nat. Mater., 2008, 7, 242–247 CrossRef CAS.
- Q. Zhang, I. Lee, J. B. Joo, F. Zaera and Y. Yin, Acc. Chem. Res., 2013, 46, 1816–1824 CrossRef CAS.
- H. Liu, J. B. Joo, M. Dahl, L. Fu, Z. Zeng and Y. Yin, Energy Environ. Sci., 2015, 8, 286–296 RSC.
- E. J. W. Verwey and J. T. G. Overbeek, Theory of the stability of lyophobic colloids, Elsevier Pub. Co., New York, 1948 Search PubMed.
- H. N. W. Lekkerkerker and R. Tuinier, Colloids and the Depletion Interaction, Springer Science + Business Media B.V., 2011 Search PubMed.
- H. C. Hamaker, Physica, 1937, 4, 1058–1072 CrossRef CAS.
- J. N. Israelachvili, Intermolecular and Surface Forces, Academic Press, Inc., 3rd edn, 2011 Search PubMed.
- A. Jannasch, A. F. Demirörs, P. D. J. van Oostrum, A. van Blaaderen and E. Schäffer, Nat. Photonics, 2012, 6, 469–473 CrossRef CAS.
- A. F. Demirörs, A. Jannasch, P. D. J. van Oostrum, E. Schäffer, A. Imhof and A. van Blaaderen, Langmuir, 2011, 27, 1626–1634 CrossRef.
- S. Sacanna, M. Korpics, K. Rodriguez, L. Colón-Meléndez, S.-H. Kim, D. J. Pine and G.-R. Yi, Nat. Commun., 2013, 4, 1688 CrossRef.
- Y. Liu, K. V. Edmond, A. Curran, C. Bryant, B. Peng, D. G. A. L. Aarts, S. Sacanna and R. P. A. Dullens, Adv. Mater., 2016, 28, 8001–8006 CrossRef CAS.
- J. Song and M. A. Winnik, J. Am. Chem. Soc., 2004, 126, 6562–6563 CrossRef CAS.
- B. Peng, E. van der Wee, A. Imhof and A. van Blaaderen, Langmuir, 2012, 28, 6776–6785 CrossRef CAS.
- B. Peng and A. Imhof, Soft Matter, 2015, 11, 3589–3598 RSC.
- X. Cheng, M. Chen, L. Wu and G. Gu, Langmuir, 2006, 22, 3858–3863 CrossRef CAS.
- V. K. Gupta, R. Jain, A. Mittal, M. Mathur and S. Sikarwar, J. Colloid Interface Sci., 2007, 309, 464–469 CrossRef CAS.
- B. Peng, A. van Blaaderen and A. Imhof, ACS Appl. Mater. Interfaces, 2013, 5, 4277–4284 CrossRef CAS.
- G. Bosma, C. Pathmamanoharan, E. H. A. De Hoog, W. K. Kegel, A. Van Blaaderen and H. N. W. Lekkerkerker, J. Colloid Interface Sci., 2002, 245, 292–300 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization and analysis of the particles. See DOI: 10.1039/d0sm02100h |
| This journal is © The Royal Society of Chemistry 2021 |

