 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: An auxiliary electrode mediated membrane-free redox electrochemical cell for energy storage
Senthil Velan
Venkatesan
a,
Kunal
Karan
b,
Stephen R.
Larter
c and
Venkataraman
Thangadurai
*a
aDepartment of Chemistry, University of Calgary, 2500 University Drive NW, Calgary, Canada. E-mail: vthangad@ucalgary.ca
bDepartment of Chemical and Petroleum Engineering, University of Calgary, 2500 University Drive NW, Calgary, Canada
cDepartment of Geoscience, University of Calgary, 2500 University Drive NW, Calgary, Canada
First published on 14th May 2021
Abstract
Correction for ‘An auxiliary electrode mediated membrane-free redox electrochemical cell for energy storage’ by Senthil Velan Venkatesan et al., Sustainable Energy Fuels, 2020, 4, 2149–2152, DOI: 10.1039/c9se00734b.
The authors would like to clarify that in the original paper, the open-circuit voltage (OCV) value of 0.5 V was estimated (page 2150) using the standard reduction potentials. The reported value [(−0.25 − 0.14) + (1 − 0.14) = ∼0.5 V] is incorrect, and the estimated theoretical OCV, using the standard reduction potentials,1 should be read correctly as [0.15 − (−0.25)] + (1 − 0.15) = 1.25 V, as shown in the updated Fig. 1(a) below.
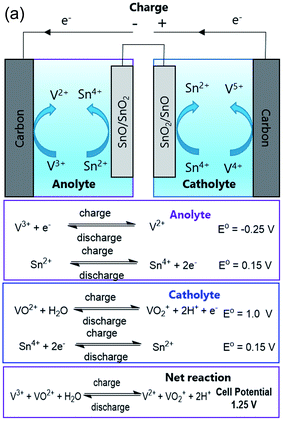 | ||
| Fig. 1 (a) Schematic depiction of the auxiliary electrode mediated membrane-free redox electrochemical cell (AEM2RC) with half-cell and net reactions. The standard reduction potentials of the involved elements are presented from Petr Vanysek.1 | ||
It is also noted that based on strict thermodynamic considerations, the OCV of the vanadium flow redox battery will be a function of the activity or concentration ratio of the pertinent vanadium ions in the catholyte and anolyte. Thus, it is well known that the OCV in redox flow batteries changes with the state of charge (SOC), which alters the ion concentration ratio.2 In other words, the ionic species are not necessarily in their standard states. Thus, the reported experimental OCV of 0.5 V at non-standard conditions should be different from the theoretical open circuit potential determined by assuming that the reactants and products are at their standard state. The SOC, electrode and electrolyte equilibrium, and the lower concentration of electrolyte used (100 mM VOSO4 in 1 M H2SO4) could explain the observed low OCV value. Furthermore, eqn (4), which was shown as a discharge reaction in the original paper, should be read as a charging reaction, as below.
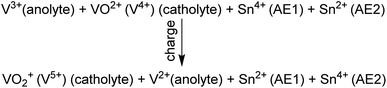 | (4) |
The authors regret any confusion that may have been caused due to the erroneous theoretical OCV and/or the typographical error in mischaracterizing the charging reaction as a discharge reaction. The corrected half-cell reactions are presented in the updated Fig. 1(a). These additions and corrections/explanations do not affect the experimental results or key conclusions of the original paper.
The authors thank Prathap Iyapazham Vaigunda Suba for pointing out this voltage discrepancy between the conventional flow battery and present auxiliary-electrode based vanadium redox cell, and his help with the preparation of the figure in this correction.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- P. Vanysek, CRC Handbook of Chemistry and Physics, ed. D. R. Lide, CRC Press LLC, 2004 Search PubMed.
- M. Skyllas-Kazacos, L. Cao, M. Kazacos, N. Kausar and A. Mousa, ChemSusChem, 2016, 9(13), 1521–1543 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |
