Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Techno-economic and environmental assessment of BECCS in fuel generation for FT-fuel, bioSNG and OMEx†
Oluchi
Emenike
,
Stavros
Michailos
 *,
Kevin J.
Hughes
,
Derek
Ingham
and
Mohamed
Pourkashanian
*,
Kevin J.
Hughes
,
Derek
Ingham
and
Mohamed
Pourkashanian
Energy 2050, Department of Mechanical Engineering, Faculty of Engineering, University of Sheffield, Sheffield, S3 7RD, UK. E-mail: s.michailos@sheffield.ac.uk
First published on 10th June 2021
Abstract
This study focuses on bioenergy with carbon capture and storage (BECCS) in fuel generation and assesses the potential of biofuel generation to decarbonise the fuel economy by reducing CO2 emissions to the atmosphere. The research investigates the technical, economic, and environmental performances of three biofuel production routes, namely Fischer–Tropsch synthesis (FTS), bio-synthetic natural gas (bioSNG) and oxymethylene ethers (OMEx) synthesis using flowsheets developed in Aspen Plus. It constitutes the first attempt to holistically evaluate both the techno-economic performance and the environmental benefits of employing BECCS in fuel generation. For an input of 1020 dry tonnes per day of woody biomass, the FTS route yields 275 t d−1, the bioSNG route yields 238 t d−1 and the OMEx route yields 635 t d−1 of fuel and the energy efficiency is in the range of 44.9% to59.7% without CCS and 44.0% to 58.2% with CCS. In addition, negative emissions can be achieved for all routes with CCS in the range of 301![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 519
000 to 519![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tCO2 per year. For economic viability, the minimum selling price for FT-fuels, bioSNG, and OMEx production with CCS have been calculated as £23.4 per GJ, £14.5 per GJ and £26.5 per GJ, respectively. However, competition with conventional fossil-derived fuels is not possible without the combination of existing financial incentives and a proposed carbon pricing. With carbon credit as the only financial incentive, carbon pricing in the range of £48 to £86 per tCO2 needs to be applied to achieve feasibility. Also, more negative emissions need to be generated to decrease the value of this range and reasonably phase out dependence on fossil-derived fuels. Parametric studies identified as crucial parameters to be improved the fuel output, CAPEX, operating hours and feedstock cost.
000 tCO2 per year. For economic viability, the minimum selling price for FT-fuels, bioSNG, and OMEx production with CCS have been calculated as £23.4 per GJ, £14.5 per GJ and £26.5 per GJ, respectively. However, competition with conventional fossil-derived fuels is not possible without the combination of existing financial incentives and a proposed carbon pricing. With carbon credit as the only financial incentive, carbon pricing in the range of £48 to £86 per tCO2 needs to be applied to achieve feasibility. Also, more negative emissions need to be generated to decrease the value of this range and reasonably phase out dependence on fossil-derived fuels. Parametric studies identified as crucial parameters to be improved the fuel output, CAPEX, operating hours and feedstock cost.
1 Introduction
The concentration of greenhouse gases in the atmosphere is on the increase and as human civilisation relies heavily on energy, it is of utmost importance that the drastic effects of these gases in the atmosphere should be avoided. A proposed solution to mitigate emissions is the employment of negative emissions technologies (NETs).1 The contribution of NETs in meeting the 2050 targets, set by the Paris Agreement in 2015, needs to be better understood.2 One such technology is bioenergy with carbon capture and storage (BECCS). Based on the concept of negative emissions, BECCS can play a significant role in limiting global warming when deployed under the appropriate conditions. Previous and current BECCS research has focused on its role in power generation neglecting the possible benefits in fuel generation. Compared to BECCS in power generation, carbon capture is intrinsic in the fuel generation plant, thus creating an initial economic advantage. BECCS In fuel generation can assist the decarbonisation of the transport and heating sectors.To this end, the current study examines the technical, economical, and environmental feasibility of three fuel production routes using a second-generation biomass, i.e., lignocellulosic woody biomass, as a potential BECCS technology. As many economies move towards decarbonisation, conventional fuels cannot be displaced due to their importance, instead they can be replaced by alternatives that can help meet the goal. Suitable alternatives involve conversion of biomass by various routes. Biomass is an important fuel in a low-carbon economy because of its composition and similar processes to fossil fuels that it can undergo. Also, it should be noted that in this work, since carbon capture is an integral part of each production process, carbon capture and storage (CCS) refers to the captured CO2 compression, transportation, and storage. The use of second-generation biomass in this study is rooted in the fact that the production of this class of biomass does not compete with food production and also that the technology for these routes are readily available but not developed to a commercial scale.3–5 Previous studies6–10 on the biofuels production from second-generation biofuels has solely focused on the need to increase biofuel production to displace fossil-fuels but the economic and environmental impact of employing CCS has not been fully considered.
Biomass is considered carbon-neutral because the CO2 released during combustion is absorbed to regenerate more biomass. This is assuming that no CO2 emissions are given off during the process but in reality, emissions are given off during the planting, harvesting, transporting and processing of biomass so the use of biomass results in many cases in net positive emissions.11 Hence, while biofuels have the potential to remove CO2 emissions from the atmosphere, a less than optimal route could result in net positive emissions to the atmosphere. This study focuses on the use of biomass in addition to CCS to produce biofuels while removing CO2 from the atmosphere. In addition to the technical and economic feasibility of the routes examined, the environmental impact is determined in terms of the mitigation potential. The routes considered are the common Fischer–Tropsch synthesis (FTS) to produce hydrocarbons, methanation to produce bio-synthetic natural gas (bioSNG) and oxymethylene ethers synthesis to produce OMEx. All three production routes are thermochemical processes and selected based on a common gasification process. Fischer–Tropsch synthesis fuel (FT-fuel) is chosen because it is an alternative for the most common liquid fuels in transportation – gasoline and diesel. BioSNG is chosen as the alternative for the most common gaseous fuel – natural gas and OMEx is chosen because it is a relatively novel biofuel which is derived from methanol and it has shown potential in diesel engines; one of which is its ability to serve as an almost carbon-neutral component when 24% (wt) is blended with diesel.12
The FTS is not a new process and has been in existence since the 1920s and it has been commercially used to produce liquid transportation fuels from coal or natural gas.13 The process involves reacting hydrogen (H2) and carbon monoxide (CO) over a catalyst to form a range of hydrocarbon chains of varying length. The most common ones are the South African SASOL coal-to-liquid (CTL) technology and the Shell gas-to-liquid (GTL) technology.14 The use of biomass in FTS has since been researched and a commercial scale plant producing 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of biofuel per annum was established by CHOREN. However, it requires producing a large volume of 100
000 tonnes of biofuel per annum was established by CHOREN. However, it requires producing a large volume of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes for profitability.15 Methanation is the conversion of CO and carbon dioxide (CO2) to methane (CH4) and water via hydrogenation. The UK has commissioned a commercial bioSNG plant using up to 175
000 tonnes for profitability.15 Methanation is the conversion of CO and carbon dioxide (CO2) to methane (CH4) and water via hydrogenation. The UK has commissioned a commercial bioSNG plant using up to 175![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of bio-resources, including unrecyclable wood and refuse-derived fuel (RDF).16 The Swedish GoBiGas project which produced 20 MW of bioSNG demonstrated good results but plans to move to large-scale production were terminated.17 Oxymethylene ethers (OMEx) synthesis, is an emerging technology and interest into this has increased over the past decade.18 While OME1 studies as fuel is more common, interest in higher OMEs (OME3–OME5) are being considered due to their behaviour in diesel engines. The main precursor for OMEx is methanol which can be produced from syngas. While methanol itself is a useful fuel, its low flammability makes it unsuitable in compression engines and its high toxicity increases operating expenditure.19 The production process includes methanol synthesis and formaldehyde synthesis which are established processes.
000 tonnes of bio-resources, including unrecyclable wood and refuse-derived fuel (RDF).16 The Swedish GoBiGas project which produced 20 MW of bioSNG demonstrated good results but plans to move to large-scale production were terminated.17 Oxymethylene ethers (OMEx) synthesis, is an emerging technology and interest into this has increased over the past decade.18 While OME1 studies as fuel is more common, interest in higher OMEs (OME3–OME5) are being considered due to their behaviour in diesel engines. The main precursor for OMEx is methanol which can be produced from syngas. While methanol itself is a useful fuel, its low flammability makes it unsuitable in compression engines and its high toxicity increases operating expenditure.19 The production process includes methanol synthesis and formaldehyde synthesis which are established processes.
Techno-economic assessments provide information to support the deployment of technologies and identify areas of improvement. Most of the existing literature related to BECCS deals with the power generation sector.20–25 Some of the literature consider the addition of CCS In fuel production, albeit without much information on the environmental impact and the required policy framework necessary for the biofuel routes to be competitive with fossil fuels. Tagomori et al.26 performed a techno-economic assessment of FT-diesel production from eucalyptus and pine residues with the CCS case increasing production costs by 1%. Song et al.27 carried out an assessment on four different agriculture residues to produce methane and the addition of CCS increased the production cost in the range of 3–4%. Michailos et al.28 investigated the feasibility of coupling CCS with syngas biomethanation and concluded that the addition of CCS increases the production costs by roughly 17%. De Álamo et al.29 studied the techno-economic feasibility of adding CCS to FTS and bioSNG and estimated that production costs increase by 10% and 14%, respectively. The current study updates the work in the literature and adds much more information on the economic and environmental impact of deploying BECCS in the investigated fuel production routes. The results of this study answer the following questions:
• Is biofuel generation suitable to create substantial negative emissions?
• Are there opportunities present in current processes to maximise carbon removal?
• Are the biofuels produced competitive with conventional fossil fuels in transportation and what tools can help in transitioning to a low-carbon economy?
2 Methodology
2.1 Process simulation and description
All the examined processes were modelled using the sequential-modular approach in the Aspen Plus V10 software. Aspen Plus is capable of simulating large complex processes even those involving solids and non-ideal components such as biomass. Thermodynamic models were used to solve the mass and energy balances in the system. Generally, the Peng Robinson equation of state with Boston Mathias alpha function (PR-BM) was chosen to estimate phase equilibria and properties, and steam tables for the power generation section.30 The enthalpy of formation, specific heat capacity and density of the non-conventional components (biomass and ash) were estimated using the HCOALGEN and DCOALGIT property methods.31 Modelling techniques and approaches applied in this study along with respective process flow diagrams and process conditions are detailed in the ESI.†In Aspen Plus, the gasification process can either be equilibrium modelled or kinetic modelled. The complexity of the involved reactions prevents the simulation of the gasification process as a single unit operation model in Aspen Plus, so the process is simulated using various reactor models. In this work, equilibrium modelling32,33 is applied across all processes due to its suitability for providing good estimates as is the scope of this work and create a solid foundation for techno-economic assessments. The acid gas removal (AGR) section with MEA is not modelled in Aspen Plus but instead the mass and energy requirements are calculated using equations provided in the gas processors suppliers association (GPSA) Engineering Databook.34 There is little information on OMEx modelling which is mostly based on kinetics so as to simplify the OMEx reactor, mass yield fractions are implemented based on the work of Schmitz et al.35 In the gasification section, two types of gasifiers are considered based on the final product. An entrained flow gasifier (EFG) is used in FTS and OMEx synthesis while a dual fluidised bed (DFB) gasifier is used in methanation. This is because a DFB gasifier promotes the formation of methane thereby maximising the final output (bioSNG) while the entrained flow gasifier destroys hydrocarbons such as methane formed during the gasification process. The cryogenic air separation unit for the EFG is not modelled in this work. Instead it is represented by an MCOMPR block and the power requirements are calculated based on the report in ref. 36 that a cryogenic air separation system consumes about 260 to 340 kW h of energy per tonne of oxygen produced with 90% used by the main compressor depending on the plant capacity.
| White wood pellet | wt% |
|---|---|
| Proximate analysis | |
| Moisture (wb) | 15 |
| Volatile matter (db) | 83.7 |
| Fixed carbon (db) | 15.55 |
| Ash (db) | 0.75 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Ultimate analysis (db) | |
| C | 51.89 |
| H | 6.79 |
| N | 0.16 |
| S | 0.02 |
| Cl | 0.01 |
| O | 40.38 |
| Ash | 0.75 |
| LHV (MJ kg−1) | 16.28 |
| Price (£ per tonne) | 50 |
In the preparation section of the plant, wood is dried and milled to 1 mm for EFG and 6 mm for the DFB gasifier. For the entrained flow gasifier, a chopper is first employed to reduce the size of the wood to 12 mm and then a grinder is used to further reduce the size to 1 mm. The electrical requirement for the chopper is scaled from Swanson et al.8 while the electrical requirement of the grinder is calculated using the regressions from Mani et al.43
The chopped wood is sent to the drier to reduce the moisture level to 10%. This is achieved by steam drying and the reaction is modelled in an RSTOIC reactor based on the equation:
| Wood (wet) → 0.0555 H2O | (1) |
A calculator block using FORTRAN statements defined in Aspen Plus determines the amount of steam to the reactor and the extent of drying (the final moisture content is 10%).
The gasifying media could be air, oxygen (O2), steam (H2O) or carbon dioxide (CO2). However, the choice of gasifying agent affects the heating value of the product gas. Air is the poorest choice as it results in the lowest heating value range of 4–7 MJ Nm−3 (ref. 44) because the high amount of nitrogen present in the product gas dilutes the quality of the product gas. Steam is a better gasifying agent which gives a product gas with a heating value in the range of 10–18 MJ Nm−3; this higher heating value is because of the increased H/C ratio. Pure oxygen results in a product gas with a heating value in the range of 12–28 MJ Nm−3 (ref. 44) however, caution needs to be exercised when using oxygen; Ghassemi and Shahsavan-Markadeh45 observed that while increasing the O/C content would increase the cold gas efficiency and heating value of the product gas, there is a threshold beyond which the effect becomes negative.
Gasification occurs in different types of gasifiers – fixed or moving bed, fluidised, and entrained flow bed. The choice of gasifier is dependent on the range of application and desired outcome. Fixed or moving bed gasifiers include the updraft gasifier, downdraft gasifier and crossdraft gasifier, which are suitable for applications in the range of 10 kW to 10 MW but it experiences problems such as tar production and entrainment, and difficulty maintaining uniform temperature due to poor mixing. The fluidised bed gasifier includes the bubbling fluidised bed and circulating fluidised bed gasifiers which are capable of perfect mixing and maintaining a uniform temperature. Fluidised bed gasifiers operate at moderately high temperatures (1000 °C) but experiences char entrainment in the product gas; a cyclone is used to solve this problem. The entrained flow gasifier (EFG) is commonly used in large scale operations and basically acts like a plug flow reactor. Conditions in the gasifier are such that the residence time of biomass particles in the reactor is short, carbon conversion is up to 99.5% and the product gas contains very little or even no tar. These conditions include high temperatures of up to 1600 °C and high pressure, up to 80 bar, and finely reduced biomass below 75 μm.44
The gasification module consists of a combination of unit operations. An RYIELD reactor present in both gasifiers decomposes the non-conventional biomass into conventional components based on the ultimate analysis. The entrained flow gasifier is modelled after the GE gasifier which is approximately at equilibrium using an RGIBBS reactor, which estimates the composition of the syngas product by minimising the Gibbs free energy. The option to restrict equilibrium by specifying the temperature of individual reactions is employed and applied to the water-gas shift reaction, see (eqn (4)). The equations modelled in the gasifier are given as follows:
| C + 2H2 → CH4 | (2) |
| 2C + 1.5O2 → CO + CO2 | (3) |
| CO + H2O → CO2 + H2 | (4) |
| 2CO + O2 → 2CO2 | (5) |
| H2 + S → H2S | (6) |
| 0.5N2 + 1.5H2 → NH3 | (7) |
| CO + H2S → COS + H2 | (8) |
| H2 + Cl2 → 2HCl | (9) |
Steam and oxygen are used as the gasifying agents. The oxygen to carbon mole ratio is set at 0.25 while the steam to carbon mole ratio is set at 0.75 based on the stoichiometric equation for syngas formation.46
| 1.34C + 0.34O2 + H2O → 0.34CO2 + CO + H2 | (10) |
Oxygen at 95% purity from an air separation unit is fed in at 149 °C and 28 bar while steam generated on the plant is fed in at 120 °C and 28 bar. Before the dried biomass goes into the Gibbs reactor, it is pressurised in a lock hopper using CO2, an inert gas, produced from the AGR section at 0.09 kg kg−1 dry biomass as reported by Higman and van der Burgt47 for gasifiers at 25 bar. The EFG operates at 1300 °C and 28 bar.
The DFB gasifier is based on the Göteborg biomass gasification project (GoBiGas) plant in Sweden that produced bioSNG from woody biomass gasification.48 In the DFB gasifier, gasification occurs in one fluidised bed and the heat for gasification is generated in the other fluidised bed. In this gasifier, since air is introduced in a different zone, the syngas is practically nitrogen free. The gasifier model is validated against experimental data from Alamia et al.49 (see ESI, S.2.1†). While thermodynamic modelling is suitable for first estimates and determining the limits of the system, it underestimates hydrocarbons (C1, C2, tars) production and overestimates hydrogen production from gasification.32,50,51 In this model, to reduce the inaccuracies from thermodynamic modelling, non-ideal corrections are applied to account for the formation of hydrocarbons especially methane and tar. This is based on similar equilibrium modelling techniques in ref. 52–54.
After the RYIELD reactor, a separator is used to remove a portion of the char (assuming incomplete conversion of carbon) that is burnt in a combustor to provide heat for the gasifier. The rest of the biomass goes to an RSTOIC reactor where hydrocarbon formation is simulated. The hydrocarbons considered are C2H2, C2H4, C2H6, C6H6, C7H8 and C10H8 (tar). The fractional carbon conversion of each reaction is set to match the experimental data in Alamia et al.55 After this reactor, the hydrocarbons formed are separated from the biomass stream to prevent destruction in the Gibbs reactor which is where the rest of the stream is directed to form the remaining components of the resulting syngas – H2, CO, CO2, CH4, NH3, HCl, H2S and H2O. Steam is introduced to the gasifier for steam to biomass (dry ash free) mass ratio of 0.5. The DFB gasifier operates at 1.24 bar and 870 °C. The syngas composition for both gasifiers are presented in Table 2.
| EF gasifier | DFB gasifier | |
|---|---|---|
| H2 | 47.5 | 45.4 |
| CO | 40.8 | 23.1 |
| CO2 | 8.34 | 16.5 |
| N2 | 3.35 | 0.09 |
| H2O (vol% wet) | 21.5 | 4.37 |
| CH4 | 1.42 × 10−2 | 8.24 |
| NH3 | 6.75 × 10−7 | 2.85 × 10−8 |
| HCl | 6.4 × 10−7 | 5.14 × 10−7 |
| H2S | 3.54 × 10−7 | 0.01 |
| C2H2 | — | 0.12 |
| C2H4 | — | 1.98 |
| C2H6 | — | 0.18 |
In this study, the cold gas cleanup method is applied as it is the most common method across fuel production processes. For the EFG, the syngas is cooled by direct-contact water quench to 203 °C and then slag from melted ash is removed from the syngas using a separator. Wet scrubbing using water is applied in a flash unit to simulate removal of impurities such as NH3, HCl and Cl2. In FTS, the H2/CO ratio is 1.17 which quite low. To adjust the ratio to the optimal value of 2.1 for FTS, a sour water-gas shift (SWGS) reactor is used. This reaction is modelled in an REQUIL reactor. For suitable WGS activity, and to reduce the volume of the SWGS reactor, only a portion of the syngas undergoes the reaction. To achieve the optimal ratio, a design specification to set the temperature of the reactor and a calculator block to set the steam flow rate to the reactor at 3 times the flow rate of CO in syngas to the reactor are used. In OMEx synthesis, the H2/CO ratio is adjusted to 3 for methanol synthesis in an SWGS reactor. This is achieved in an REQUIL reactor and a design specification where the flow rate of steam is varied.
The adjusted syngas is further cooled, and water removed in three flash units before the acid gas removal (AGR) section. In the AGR section, CO2 and H2S are removed using 30 wt% MEA. This is not modelled in Aspen Plus but the reboiler duty, cooling duty and pump requirements are calculated based on the equations in the Engineering Date Book34 and results are presented in Table 3. In OMEx synthesis, the amount of CO2 removed is set by the stoichiometric ratio of (H2 − CO2)/(CO + CO2). This is set at 2.1 as the optimal value should be greater than 2.40 Also, this keeps the CO2 composition in the syngas at ≤7% in order to improve the methanol productivity.58 In FTS, CO2 and H2S removal are set at 96% and 99%, respectively.
| Acid gas parameter | FT synthesis | Methanation | OMEx synthesis |
|---|---|---|---|
| Circulation rate (m3 h−1) | 537.4 | 382.5 | 634.7 |
| Absorber pressure (bar) | 22.1 | 15.6 | 24.8 |
| Stripper pressure (bar) | 1.01 | 1.01 | 1.01 |
| Reboiler duty (MW) | 49.9 | 35.5 | 59.0 |
| Condenser duty (MW) | 20.8 | 14.8 | 24.6 |
| Amine cooler duty (MW) | 10.4 | 7.4 | 12.3 |
In methanation, after the dual fluidised bed gasifier, tar is condensed from the raw syngas in a three-phase flash unit and recycled to the combustor to be burnt alongside char for heat generation.
After this, the cyclic hydrocarbons–benzene and toluene–are removed from the syngas and also recycled to be burnt with tar and char. This occurs when the raw syngas is passed through an activated carbon bed modelled by a separator unit. Before removing the rest of the impurities, the syngas is compressed to the methanation pressure of 16 bar then cooled to 40 °C for acid gas removal using 30 wt% MEA. CO2 and H2S removal are set as the same in FTS. A guard bed located after the AGR section and before the water-gas shift (WGS) reactor absorbs impurities to prevent possible contamination of catalysts upstream. In the WGS reactor, a design specification to maintain a desired H2/CO ratio of 3.5 in the clean syngas is used. This is due to the optimal value of the methane synthesis being greater than 3 as seen in the equation:
| 3H2 + CO → CH4 + H2O | (11) |
2.1.4.1 Fischer–Tropsch synthesis. In the FTS, syngas is compressed to 25 bar and heated to 200 °C before passing through a zinc-oxide guard bed modelled using a separator unit to reduce H2S to 200 ppb/50 ppb to avoid catalyst poisoning in the FTS reactor. Before the FTS reactor, a pressure swing absorber (PSA) isolates a stream of hydrogen from a fraction of the syngas for hydroprocessing of wax downstream of the FTS reactor. The FTS reactor operates at 25 bar and 200 °C using a cobalt-based catalyst8 and is modelled as a RSTOIC reactor. The product distribution was estimated by the Anderson–Schluz–Flory (ASF) model (described in Song et al.59) with a chain growth factor of 0.9 and carbon monoxide per-pass conversion of 40%.8 This was implemented using a calculator block with FORTRAN statements. The output of the FTS reactor consisted of unconverted syngas, light gases and hydrocarbons. The hydrocarbons produced were all alkanes from C1–C20 and C30. Alkanes from C5 to C10 were grouped as naphtha, C11 to C20 were grouped as diesel and C20 to C30 was wax. The products were separated in a series of flash units and vacuum distillation (RADFRAC) columns. Wax was further cracked to naphtha and diesel using the hydrogen stream isolated earlier on in a RSTOIC reactor operating at 370 °C and 0 bar. The unconverted syngas was recycled to the process with one stream going from product recovery to the FTS reactor, and another stream going to the syngas cleaning section. A small portion of the unconverted syngas stream was sent to the power generation to prevent accumulation in the FTS reactor and generate power for the plant. The mass fraction that returns to the FTS reactor is 0.75; this figure is not optimised and it was selected in order for the FTS case to be a near zero net electricity scenario.
2.1.4.2 Methanation. The methanation process is modelled after the GoBiGas setup.49 In a premethanation reactor, hydrocarbons present in the syngas are cracked using steam while some of the CO and CO2 present are converted to methane. The steam to hydrocarbon ratio is set at 0.5.60 Syngas goes into methanation at 16 bar and 300 °C. Methanation takes place in a series of four reactors without recycle based on the Topsøe Recycle Energy-efficient Methanation Process (TREMP) using the MCR catalyst.61 Coolers are situated between reactors due to the exothermic nature of the reaction and to maintain catalyst activity. The heat removed is recovered later in the process to generate steam for parts of the plant. Steam is added to the first reactor to prevent the formation of carbon on the catalyst. The methanation reactors are modelled as RGIBBS reactors with a pressure drop of 0.5 bar in each reactor. BioSNG is upgraded to recover unconverted H2 and reduce CO2 in the final product.
2.1.4.3 Oxymethylene ethers synthesis. OMEx is synthesised from methanol and formaldehyde so the first step in this process is the conversion of syngas to methanol. Methanol synthesis was modelled after the ICI Synetix Methanol process available from the Aspen Plus database. Syngas is compressed to 80 bar in two stages, and then cooled to 230 °C before the reactor. In the ICI Synetix process, the methanol reactor is modelled as four RPLUG reactors in series with fresh syngas introduced between each reactor for cooling. In this work, the reactor is modelled as a single REQUIL reactor operating at 250 °C and 80 bar (see the ESI, S.6†). The reactions occurring in the reactor include the WGS reaction in eqn (4) and the following:
| 3H2 + CO2 → CH3OH + H2O | (12) |
| 2H2 + CO → CH3OH | (13) |
Crude methanol is cooled to 38 °C and flashed where most of the methanol leaves in one stream. The other stream containing unconverted syngas and some methanol is compressed and recycled to the reactor. However, a fraction of this purged and sent to power generation to prevent build up. The methanol stream is sent to methanol recovery where a couple of flash units and distillation (RADFRAC) columns are used to separate methanol from CO2 and H2O. The CO2 recovered in this part of the plant is sent to compression in the CCS plant while H2O is sent to wastewater treatment.
In an RSTOIC reactor operating at 200 °C and 3 bar, methanol (MeOH) is converted to formaldehyde (FA) using air at a conversion rate of 87%. Based on the stoichiometric reaction and required conversion, the air flow to the reactor is determined by a calculator block. The product contains 37 wt% FA. In the OMEx reactor, H2O, FA and MeOH go through a series of reactions forming hemiacetals and glycols, which in turn react with FA and MeOH to form OMEx. The reaction pathways are detailed in Schmitz.62 In this work, this is modelled as an RYIELD reactor operating at 96 °C and 3.04 bar over Amberlyst-36 catalyst based on mass fractions calculated from the mass balance in the model by Ai.63 Ai63 modelled the OMEx synthesis and this consisted of a CSTR and distillation columns to separate the products and recycle OME1–2,4–8, FA, and MeOH. The energy requirements in distillation of the products (modelled as separator units) were calculated from this work at 44.7 MJ kg−1 of OMEx produced for the heating duty and 47.1 MJ kg−1 for the cooling duty. The final OMEx product is a mix of OME3–OME5.
2.2 Economic assessment
An economic model was built to determine the feasibility of production of each product as well as monitor the effect of certain parameters on the price. Discounted cash flow rate of return (DCFROR) analysis, net present value (NPV) break-even analysis to estimate the minimum selling price (MSP) of the biofuels. The major economic assumptions are listed in Table 4. These assumptions are well aligned with previous studies8,41,66 while a discount rate of 10% constitutes a conventional and legitimate market assumption. Also any salvage value is assumed to be fully offset by decommissioning costs.| Parameter | Value |
|---|---|
| Location | United Kingdom |
| Currency | GBP |
| Base year | 2018 |
| Project lifetime (years) | 20 |
| Construction period (years) | 2.5 |
| Start-up time (years) | 0.5 |
| Capacity factor (%) | 85 |
| Tax rate (%) | 30 |
| Equity/Debt (%/%) | 100/0 |
| Discount rate (%) | 10 |
| Depreciation | Straight-line |
| Depreciation period (yr) | 10 |
| Salvage value (£) | 0 |
| Parameter | Method |
|---|---|
| Total purchased equipment cost (TPEC) | Aspen process economic analyzer, open literature |
| Total installed cost (TIC) | TPEC installation factor |
| Non-installed direct costs (NDC) | 47% of TPEC |
| Indirect costs (IC) | 89% of TPEC |
| Contingency (CC) | 20% of TPEC |
| Fixed capital investment (FCI) | TIC + CC + NDC + IC |
| Working capital (WC) | 15% of FCI |
| Total capital investment (TCI) | FCI + WC |
In this work, the TCI for each plant was calculated using the factorial estimation method and the costs for the major components were taken from the open literature. Other unit operations that are not available in the open literature were estimated using the Aspen Process Economic Analyzer (APEA). However, due to the installation factors by APEA being generally low, an overall installation factor of 3.02 was used as suggested by Peters et al.67 for solid–liquid plants. Equipment costs were calculated using eqn (14).
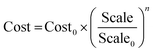 | (14) |
The operating and maintenance (OPEX) costs (summarised in Table 12 and Table 13 in the Appendix) covers every other cost for the day-to-day running of the plant. This includes labour costs, maintenance, insurance and purchase of raw materials. For this study, the fixed costs were estimated using guidance from Peters et al., and Sinnott and Towler.67,68 The labour costs were estimated using data from Glassdoor and Payscale.70,71 The number of employees and shifts were derived from Phillips et al.72 In FTS and methanation, there are 8 maintenance technicians and 20 shift operators while in OMEx synthesis, there is an extra section for methanol synthesis, increasing the number of maintenance technicians and shift operators to 12 and 30, respectively. The cost of CO2 trasport and storage is taken as variable cost and equal to £19/tCO2.90
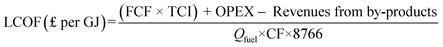 | (15) |
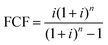 | (16) |
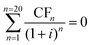 | (17) |
| CFn = P(1 − t) + Dt | (18) |
2.2.4 CO2 avoidance cost
The CO2 avoidance cost represents the minimum carbon tax to be paid when the CCS plant is compared to a similar plant without CCS. In comparison to a similar plant, the cost is calculated on the basis of transportation and storage costs as capture is included in all scenarios. The avoidance cost is calculated as follows: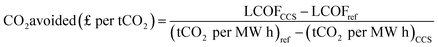 | (19) |
2.2.5 Financial tools
In the UK, there are several laws and policies that have been adopted to fight climate change. Most of these laws and policies put financial incentives in place to support the roll out of energy efficient and greenhouse gas reduction technologies. Two of these policies are considered in this study for transport fuels. The first one is the Renewables Transport Fuel Obligation (RTFO)73 which was introduced to support the supply of transport fuels from renewable energy sources in to support the UK's government policy to reduce GHG emissions from vehicles and drive the supply of renewable fuels. Under this scheme, suppliers that meet the eligibility criteria are issued Renewable Transport Fuel Certificates (RTFCs) for each litre or kg of biofuel produced. The second one is the non-domestic Renewable Heat Incentive74 which provides financial support for generating renewable heat for non-domestic purposes. Another financial tool considered in this study is carbon pricing which is commonly used in emissions trading schemes.2.3 Process performance indicators
The performance of each plant can be quantified by several indicators. These performance indicators determine how efficient a production process is. The performance of the gasifier is determined by the cold gas efficiency which is calculated as the energy content of the resulting syngas against the energy content of dry ash free biomass sent to the gasifier.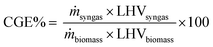 | (20) |
Another important technical parameter considered is the overall energy conversion efficiency and it represents the amount of biomass energy present in the fuel generated. This value also considers the electricity input and output on the plant.
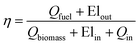 | (21) |
3 Results and discussion
This section covers the technical, environmental, and economic results and interpretation of the three production routes. For each route, with and without CCS scenarios are investigated. Then the results are compared to the conventional fossil-derived fuel counterparts. In addition, financial analysis is presented for all cases and a sensitivity analysis has been carried out for the CCS cases.3.1 Mass balance
The diagrams in Fig. 1 show the mass balance on the plant based on the carbon mole flows. In both FTS cases, 43.9% of carbon in the feedstock to plant is stored in the resulting FT-fuels. In the FTS + CCS case, 46.8% of carbon is captured, and 9.3% is vented in the flue gas from the power generation while 0.1% is lost in wastewater; in the FTS case, 56.1% is vented to the atmosphere. In both bioSNG cases, 32.6% of carbon is stored in bioSNG; in the bioSNG + CCS case 32.5% is captured and 34.9% is vented in the flue gas from the power generation and gasification; without CCS, 67.4% is vented to the atmosphere. In both OMEx synthesis cases, 52.9% of carbon to process is stored in OMEx. In OMEx + CCS, 45.4% of carbon is captured and 1.7% is vented to the atmosphere from waste streams; without CCS, 47.1% of carbon is vented to the atmosphere. The system with the least CO2 venting is in the OMEx production while the most venting occurs in bioSNG production due to the flue gas generated from the dual fluidised gasifier.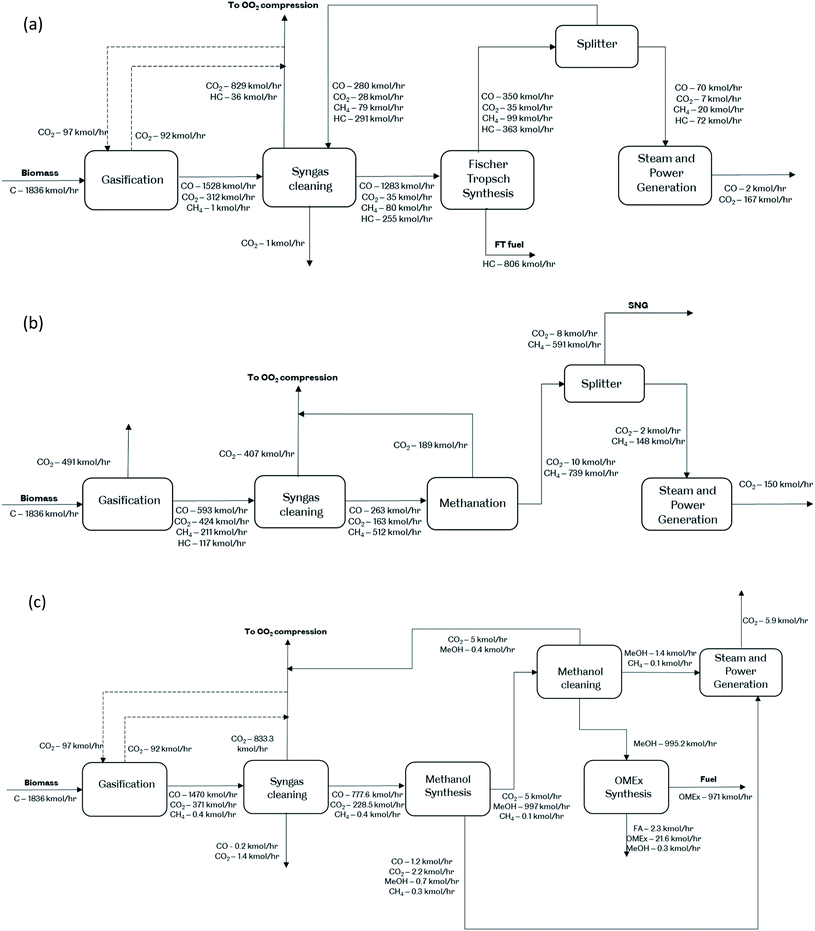 | ||
| Fig. 1 Carbon balance investigated for the production routes (a) Fischer–Tropsch synthesis, (b) methanation and (c) oxymethylene ether synthesis. | ||
3.2 Energy balance
In all production routes, the common sources of heating demand include the distillation columns, gasifier, dryers, intermediate heaters, and power generation unit. The sources of cooling demand include distillation columns and intermediate coolers. Suitable heat integration was performed to recover useful heat from different streams and minimise heat waste on the plant. This allowed manufacture of low pressure to high pressure steam where necessary for use in different locations in each plant. Where further heating was required, fired heat from natural gas was used. Further cooling was achieved with air, cooling water, and refrigerants. Electricity was imported from the grid to meet power requirements in the case that a plant is not self-sustaining. Table 6 shows a breakdown of the power usage and generation for each production route.| Power (MW) | Fischer–Tropsch | Methanation | Oxymethylene ethers | |||
|---|---|---|---|---|---|---|
| CCS | CCS | CCS | ||||
| a *negative net electricity signifies that electricity is imported. | ||||||
| USAGE | ||||||
| Chopper | 0.26 | 0.26 | 0.26 | 0.26 | 0.26 | 0.26 |
| Grinder | 1.36 | 1.36 | 0.32 | 0.32 | 1.36 | 1.36 |
| Lock hopper system | 0.10 | 0.10 | — | — | 0.10 | 0.10 |
| AGR pumps | 1.02 | 1.02 | 0.78 | 0.78 | 1.47 | 1.47 |
| Syngas booster compressor | 0.87 | 0.87 | 7.72 | 7.72 | — | — |
| PSA compressor | 0.08 | 0.08 | — | — | — | — |
| Methanation compressor | — | — | 0.18 | 0.18 | — | — |
| BioSNG compressor | — | — | 1.01 | 1.01 | — | — |
| Naphtha pump | 3.30 × 10−4 | 3.30 × 10−4 | — | — | — | — |
| Diesel pump | 7.10 × 10−4 | 7.10 × 10−4 | — | — | — | — |
| MeOH synthesis compressors | — | — | — | — | 4.86 | 4.86 |
| MeOH cleaning pumps | — | — | — | — | 2.20 × 10−3 | 2.20 × 10−3 |
| OMEx pump | — | — | — | — | 3.10 × 10−3 | 3.10 × 10−3 |
| Air compressor (OMEx) | — | — | — | — | 1.48 | 1.48 |
| Hydroprocessing | 0.21 | 0.21 | — | — | — | — |
| Air compressor (GT) | 2.82 | 2.82 | 7.88 | 7.88 | — | — |
| Air separation unit | 5.00 | 5.00 | — | — | 5.00 | 5.00 |
| Oxygen compressor (ASU) | 2.38 | 2.38 | — | — | 2.38 | 2.38 |
| Water pumps (steam generation) | 0.05 | 0.05 | 0.02 | 0.02 | 0.31 | 0.31 |
| CO2 compression | 0.00 | 5.00 | 0.00 | 3.60 | 0.00 | 4.68 |
| Refrigeration | 1.82 | 1.82 | 0.00 | 0.00 | 0.00 | 0.00 |
| Total | 16.2 | 21.2 | 18.2 | 21.8 | 17.7 | 22.4 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Generation | ||||||
| Gas turbine | 10.7 | 10.7 | 17.6 | 17.6 | 4.8 | 4.8 |
| Steam turbine | 4.6 | 4.6 | 3.7 | 3.7 | 0.7 | 0.7 |
| Net electricity | −1.0 | −6.0 | 3.18 | −0.43 | −12.3 | −16.9 |
3.3 Plant efficiency
The cold gas efficiency, given in eqn (20) is a technical parameter that represents the efficiency of the EFG and DFB gasifiers. A heat loss from the reactor is assumed as 1% of the biomass energy input. The efficiency of the EFG was calculated from Aspen at 87.2% and that of the DFB gasifier was calculated at 70.1%. The difference in these efficiencies lies in the operation of the gasifiers. In the DFG gasifier, further efficiency is lost due to char and tar formation when compared to the EFG which operates near equilibrium and destroys tar and char formed during the gasification process. The summary of the results from the process simulation are presented in Table 7.| FTS | FTS + CCS | BioSNG | BioSNG + CCS | OMEx | OMEx + CCS | |
|---|---|---|---|---|---|---|
| Product yield (%) | 22.9 | 22.9 | 19.8 | 19.8 | 52.9 | 52.9 |
| Energy produced (MW) | 111.1 | 111.1 | 131.8 | 131.8 | 140.4 | 140.4 |
| Energy conversion (%) | 44.9 | 44.0 | 59.7 | 58.2 | 48.5 | 47.7 |
| Net heat input (MW) | 20.5 | 20.5 | 0 | 0 | 51.3 | 51.3 |
| Electricity produced (MW) | 15.1 | 15.1 | 21.3 | 21.3 | 5.5 | 5.5 |
| Electricity demand (MW) | 16.2 | 21.2 | 18.2 | 21.8 | 17.7 | 22.4 |
| Net electricity (MW) | −1.0 | −6.0 | 3.18 | −0.43 | −12.3 | −16.9 |
For all the biofuels considered, without CCS, the bioSNG route has the highest energy efficiency at 59.7% while the FT-fuel route has the lowest at 44.9%. With the CCS route, it is the same with bioSNG at 58.2% and the FT-fuel route at 44%. Across all three cases, the addition of CCS results in no more than a 1.5-percentage point drop. Energy efficiency loss in routes using the EFG (FTS and OMEx synthesis) is due to the cooling method of the raw syngas. Quench cooling is used and, in this way, sensible heat that would otherwise be recovered for heat integration is lost. Cool gas cleanup is used with the EFG and this induces energy penalties on the plant but switching to hot gas cleanup can reduce waste streams and costs while the overall plant efficiency is improved when compared to cold gas cleanup. To improve the efficiency, radiant cooling is more suitable, but the capital costs will increase. For the bioSNG route, the product yield decreases due to a portion being sent off to generate power for the plant demand; this in turn reduces the energy conversion efficiency.
In terms of mass yield, the OMEx route has the highest product yield at 53%. Due to the recycling in OMEx synthesis to optimise the process, the OMEx yield from methanol increases from 38% reported in Zhang et al.,75 to 83%, as proposed by Ai et al.63 This 83% mass yield corresponds to a methanol conversion of 99.9%. Also, the syngas conversion to methanol process is 95%. Therefore, the methanol yield from biomass is compared with previous studies. In the present work, the stoichiometric ratio of (H2 − CO2)/(CO + CO2) in the syngas is set at 2.1 in this work as advised by E4Tech40 and this gives methanol yield of 67% (from biomass). In the models on OMEx production by Zhang et al.75 and Oyedun et al.66 this ratio is set between 0.29 and 0.55 resulting in methanol yields between 29% and 52%. Also, in Phillips et al.58 a stoichiometric ratio of 1 results in a methanol yield of 49%.
The FT-fuel yield of 22.9% is comparable to values reported in the literature; Tagomori et al.26 reports a yield of up to 15% for FT-liquids from forestry residue while Dimitriou et al.41 reports a yield of 20.4% using an entrained flow gasifier and woody biomass to produce FT-fuel. In FTS, the single pass conversion is 40%; by recycling unconverted syngas, this goes up to 72.7%. The initial yield of bioSNG at 25% is consistent with the value of bioSNG yield of 24% obtained using a gasifier operating at 1 bar as reported by Vitasari et al.76
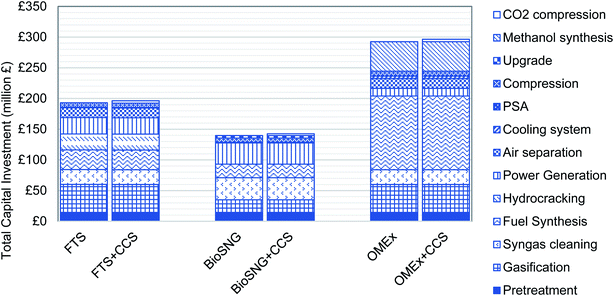
3.4 Capital, operating and maintenance expenditures
The total capital investment breakdown for all cases is presented in Fig. 2. The capital cost is in the range of £140 to £296 million. The only contribution costs of CCS to the capital cost is associated with CO2 compression as a capture unit is present in all cases. Likewise, the contribution costs of CCS to the operating and maintaining expenditure is associated with transport and storage. The highest capital cost is encountered in OMEx production. In this process, the major contribution to the capital cost is the fuel synthesis which is 41% of the entire cost. This fuel synthesis includes OMEx production from methanol and trioxane. In essence, this includes 4 synthesis units – formaldehyde, trioxane, methylal and OMEx syntheses.For the FTS, gasification is the most significant contributor to the capital cost accounting for 24% of the total investment. In bioSNG production, syngas cleaning and the power generation section are the biggest contributors to the capital cost, each accounting for 25% of the overall cost. In bioSNG without CCS, excess electricity from burning a fraction of the product to meet plant demand is sold to generate revenue. In OMEx production, the fuel synthesis section accounts for 41% of the overall cost as a result of the additional synthesis steps required in the production process.
The cost of the entrained gasification units used in FTS and OMEx synthesis is noticeably higher than the cost of the dual fluidised gasifier used in methanation. The operating conditions of the gasifiers influence the gasification unit cost. The EFG operates at a significantly higher pressure of 28 bar than the DFB gasifier operating at atmospheric pressure. Also, the EFG operates at 1300 °C while the DFB operates at 870 °C. These are the conditions considered when designing the pressure vessel for the gasifier as these factors determine the thickness of the vessel and amount of materials required to build the gasifiers. In all cases, the cost of additional CO2 compression is between 1% and 2% of the total investment, thus indicating that the additional investment for CCS is minimal.
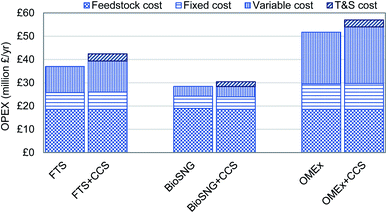
The annual operating cost and maintenance costs are presented in Fig. 3. The feedstock cost is the same across all processes as the feed input is fixed at 1020 dt d−1. In terms of the fixed cost which includes labour, insurance, and rent; the methanation process has the lowest annual cost while OMEx has the highest cost.
This is the same trend as the total capital investment as the fixed costs are a fraction of the fixed capital investment. The feedstock cost is the major contributor to the operating costs in all cases except OMEx production where the variable cost dominates. The addition of transport & storage minimally affects the overall OPEX contributing to between 5.3% and 7.3% of this cost. While the variable cost is constant in FTS and methanation with the addition of CCS, the variable cost in OMEx production increases due to an increase in electricity demand in the OMEx plant which is not self-sufficient. The OMEx production clearly is the most expensive to run. This is due to several reasons such as the daily cost of electricity; the use of fired heat compared to the other plants to vaporise water for steam generation; and also, daily wastewater treatment and cooling water for heat exchange on the plant is significantly higher than the other plants due to more synthesis units required.
3.5 Levelised cost of fuel (LCOF)
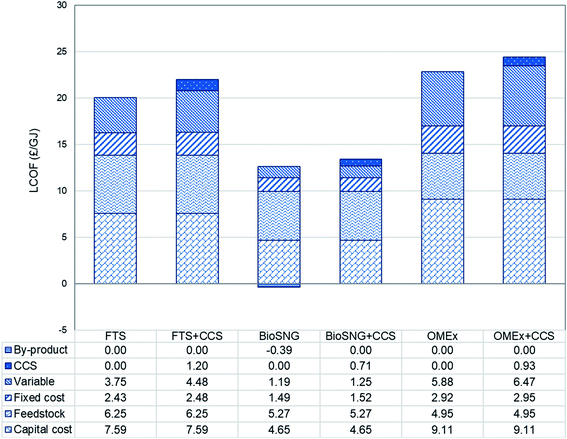
The levelised cost of fuel production is presented in Fig. 4. It represents the costs – capital, operating and maintenance – required to produce one unit (GJ) of fuel based on the lower heating value. The cost of production of these biofuels is in the range of £12.2 to £22.9 per GJ without CCS and £13.4 to £24.4 per GJ with CCS.For all routes, the increase in the production costs of the CCS cases is associated with storing CO2; this includes the cost of additional compressors to prepare CO2 for transportation, pipeline costs and storage costs. In FT-fuel, an increase value of approximaly 10% is observed (from £20.03 per GJ to £22.01 per GJ), 9.7% in bioSNG production (from £12.21 per GJ to £13.40 per GJ), and 6.8% in OMEx (from £22.86 per GJ to £24.42 per GJ). The bio-SNG production, even without by-product (electricity) credits in the CCS case, it still has the least production costs in both the with and without CCS pathways while the OMEx route has the highest production cost. The production cost is a function of the capital cost, fixed charge factor, by-products and fuel output as presented in eqn (15).
Based on the trend of the CAPEX and OPEX, it would be expected that the highest production cost would be for OMEx while the least would be for bioSNG and this is what is observed. Further, the results are in good agreement with the literature for all routes. Brown et al.77 have reported bioSNG and FT-fuel production costs that lie in the range of £15.3 to £27.5 per GJ and £18.3 to £35.3 per GJ, respectively. Comparing the OMEx production cost, Oyedun et al.66 reported a value of £1.24 per L when processing 500 tonnes per day of woody biomass day. While Schmitz et al.35 reported a value £0.41 per L (from $614.8 tonnes assuming OMEx density of 1097 kg m−3 and exchange range values for 2014) when producing OMEx at 1 million tonnes per year. For the present work, the values are £0.47–£0.50 per L when processing 1020 dt d−1 for an output of 0.2 million tonnes per year of OMEx.
3.6 Greenhouse gas (GHG) emissions
Greenhouse gas (GHG) emissions are a source of environmental concern when establishing a production plant. In this sub-section, the GHG emissions for each production route are characterised by emission factors. The emission factor estimates how much greenhouse gases in CO2 equivalent is released per unit of energy output; this also measures the potential for negative emissions. This emission factor includes the biomass harvesting and processing emissions, natural gas for fired heat on the plant, electricity imported to the plant, and CH4 and N2O emissions in the resulting biofuels. It basically covers the lifetime emissions of biomass use but excludes emissions from transportation and distribution of the biofuels.The emission factors used in this study are GHG conversion factors based on the outline from the UK Department of Business, Energy and Industrial Strategy (BEIS).78 The BEIS framework categorises the emissions based on activities into three scopes – scope 1, scope 2, and scope 3. Scope 1 covers direct emissions from the plant itself such as fuel combustion which is zero for CO2 emissions in the case of biomass and biofuels due to its status as CO2 neutral. However, N2O and CH4 released are not absorbed in biomass regeneration so scope 1 covers these gases. Scope 2 covers indirect emissions from the plant such as imported electricity and heat purchased to cover internal needs. Scope 3 covers indirect emissions from sources not owned or controlled by the plant such as the emissions due to harvesting, refining, and transporting biomass. The values used in this study are presented in Table 8. For the wood pellets and the natural gas, the summation of scope 1 and scope 3 were used.
| Fuel | Scope 1 (kg CO2e kW h−1) | Scope 2 (kg CO2e kW h−1) | Scope 3 (kg CO2e kW h−1) | Overall emission factor (kg CO2e kW h−1) |
|---|---|---|---|---|
| Wood pellets | 0.01563 | — | 0.03744 | 0.05307 |
| Natural gas | 0.20428 | — | 0.02657 | 0.23085 |
| Electricity | — | 0.2556 | — | 0.2556 |
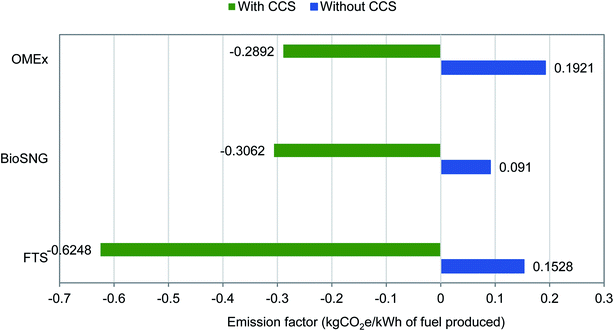
The calculated emission factors for the three routes investigated with and without CCS are presented in Fig. 5. Without CCS, all the production routes are net positive for GHG emissions. With CCS, there is a significant decrease of over 250% in the emission factors. While adding CCS is minimal to production costs (economics) – as seen in previous section – the effect on the environment is significant in terms of huge emission savings.
Adding CCS to FT-fuel production results in negative emissions equivalent to a GHG mitigation potential of 519![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tCO2 per year; for bioSNG production, there exists a mitigation potential equivalent to 301
000 tCO2 per year; for bioSNG production, there exists a mitigation potential equivalent to 301![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tCO2 per year; and for the OMEx route, there is a mitigation potential equal to 303
000 tCO2 per year; and for the OMEx route, there is a mitigation potential equal to 303![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tCO2 per year. The OMEx route without CCS has the highest emission potential at 201
000 tCO2 per year. The OMEx route without CCS has the highest emission potential at 201![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tCO2 per year and this is mainly due to the indirect emissions from the purchased electriciy. The OMEx route purchases the most electricity from the grid. This also limits the emission saving potential with CCS applied. The bioSNG route has the least emission savings potential with CCS. This is mainly due to the amount of captured and stored carbon; looking at the carbon balance in Fig. 1, the most amount of carbon is vented within this process due to the required gasification technology and power generation. While the OMEx route imports much electricity which contributes to emissions, a higher energy output and CO2 capture helps in reducing the emission factor. The emission factor is also a function of energy output, so to decrease emissions and improve environmental performance, improving the plant efficiency, which results in a higher output is one way. Other ways include decreasing the amount of CO2 vented to the atmosphere (especially with the bioSNG route) and limiting electricity imports from the grid by coupling to renewable energy power generation.
000 tCO2 per year and this is mainly due to the indirect emissions from the purchased electriciy. The OMEx route purchases the most electricity from the grid. This also limits the emission saving potential with CCS applied. The bioSNG route has the least emission savings potential with CCS. This is mainly due to the amount of captured and stored carbon; looking at the carbon balance in Fig. 1, the most amount of carbon is vented within this process due to the required gasification technology and power generation. While the OMEx route imports much electricity which contributes to emissions, a higher energy output and CO2 capture helps in reducing the emission factor. The emission factor is also a function of energy output, so to decrease emissions and improve environmental performance, improving the plant efficiency, which results in a higher output is one way. Other ways include decreasing the amount of CO2 vented to the atmosphere (especially with the bioSNG route) and limiting electricity imports from the grid by coupling to renewable energy power generation.
3.7 CO2 avoidance cost
The CO2 avoidance cost (in the CCS cases) is presented in Table 9. In this study, this value is calculated based on two reference plants. Plant A refers to a conventional fossil fuel plant (natural gas for bioSNG, and diesel for FT-fuel and OMEx) while plant B refers to the biomass-based plant without CCS. These values represent the minimal carbon tax to be paid on CO2 emissions. With the conventional plant, the values are much higher due to the lower cost of fuel production using fossil fuels. A maximum avoidance cost of £119 per tonne CO2 is reported in this study and it is comparable to the 2011 values reported for the UK. Charles et al.79 reported an abatement cost of biofuels of £115 per tonne CO2 for bioethanol and £154 per tonne CO2 for used cooking oil diesel.| Plant A | Plant B | |
|---|---|---|
| FTS | 75 | 21.7 |
| BioSNG | 91 | 22.4 |
| OMEx | 119 | 25.7 |
Fuel switching to biomass resources results in a reduced avoidance cost as seen in the case of plant B. Across the three routes, production of OMEx generally has the highest cost due to its relatively low emission factor as more emissions to the atmosphere needs to be paid for. In the bioSNG route, although a higher percentage of carbon is vented in the process, minimal electricity is purchased and there is no heat or steam purchase to indirectly increasing the CO2 emissions from the process. The FT-fuel route has the lowest avoidance cost even with electricity and heat imports; however, the amount of emissions from these imports are offset by a higher amount of CO2 capture in the process. Note that electricity and heat imports are less than half of what is required in the OMEx route. This is because the avoidance cost is a function of the emission factor and the more negative emissions achieved by a plant, the lower is the cost. Alternatively, increasing the price of conventional fossil-derived fuels will lower the avoidance cost. Finally, including BECCS in carbon trading systems will further result in reduced avoidance costs.
3.8 Comparison with fossil-derived fuels – diesel and natural gas
In this section, the minimum selling price (MSP) of each biofuel is compared to the market price of conventional fossil fuels. Since OMEx is a diesel additive, its production route will also be compared to the diesel production cost. Using a DCFROR and NPV break-even analysis, the MSP was determined for all routes and presented in Table 10.| Without CCS (£ per GJ) | With CCS (£ per GJ) | |
|---|---|---|
| FTS | 21.8 | 23.4 |
| BioSNG | 13.4 | 14.5 |
| OMEx | 24.6 | 26.5 |
The average 2018 UK wholesale price of natural gas was £5.5 per GJ (ref. 80) while that of diesel excluding UK fuel duty was £13.23 per GJ (ref. 81) (for a diesel energy density of 32 MJ L−1). The MSP for bioSNG with and without CCS is at least 2.5 times the cost of producing natural gas while the MSP of FT-fuels and OMEx is up to two times the price of conventional diesel price. This means that the three routes investigated cannot compete with natural gas and diesel without process improvements to decrease costs and incentives such as policy schemes.
To encourage the production of these biofuels to compete with and phase out the dependence on fossil-derived fuels, policy instruments such as subsidies and carbon taxes are required. The next section covers the existing policy schemes within the UK that support the production of biofuels and suggest a possible scheme to assist with decarbonisation.
3.9 Financial tools analysis
The UK government introduced the Renewable Transport Fuel Obligation (RTFO) in 2008 with the aim of reducing GHG emissions from fuel used for transport purposes. It lays out an obligation for fuel suppliers to meet a target of renewable fuel in each obligation period. Renewable Transport Fuel Certificates (RTFCs) are issued for each litre or kg of biofuel produced. For certain biofuel sources such as waste, double RTFCs are issued. As such, these RTFCs can be traded and the price is dependent on demand and supply. In the past, the trade prices have varied between £0.09 and £0.20 per certificate.82,83 For this analysis, an RTFC price of £0.145 per RTFC will be used. Based on the RTFO guidelines, for each litre of biofuel produced, 1 RTFC has been issued.The Renewable Heat Incentive (RHI) established in 2009, provides financial support for renewable heat technologies. A tariff is paid to the renewable heat generator for each unit of energy generated. Currently, for biomethane produced and injected to the grid, the current tariff is an average of £22.16 per MW h;84 this is the value assumed for the plant's lifetime. BioSNG is also eligible for RTFCs at 1.9 RTFCs for each kilogram of biomethane, using feedstocks that are wastes or residues or dedicated energy crops doubles the RTFCs to 3.8 RTFCs kg−1. In this case, biomass feedstock is assumed to be woody biomass and thus qualifies for the 1.9 RTFCs kg−1 and with preliminary calculations, the RHI provides more savings. The RHI is applied to bioSNG while the RTFO is applied to FT-fuels and OMEx.
Fig. 6 depicts the effect of the RTFCs and the RHI on biofuel production. While the application of the RTFCs is beneficial to the production routes without and with CCS, it is still not enough to comfortably compete with the conventional diesel, only the LCOF of FTS without CCS is close enough; which entails that tax exemptions may also be required for the feasibility of the biorefineries.
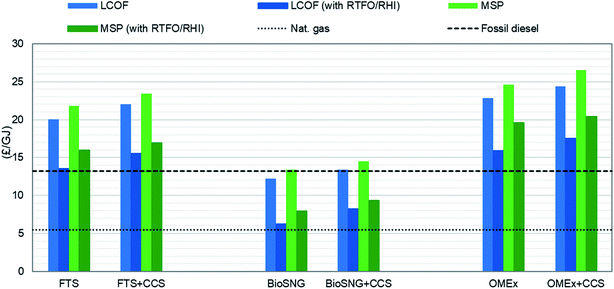 | ||
| Fig. 6 Effect of RTFO and RHI on the production cost and minimum fuel selling price of three production routes without and with CCS. | ||
The implementaion of the RTFO results in a decrease of the MSP of up to 28% for FT-fuels and up to 24% decrease for OMEx. While the implementation of the RHI to bioSNG production decreases the minimum selling price by 41% without CCS and 36% with CCS, it is still not profitable and competitive with natural gas production. Further, decreasing the MSP of all production routes will require switching to waste as feedstock so that the bioSNG process can qualify for 3.8 RTFCs kg−1 under the RTFO and the FT-fuels and OMEx can qualify for 2 RTFCs/L; also, this switch could result in a decrease in the feedstock price but at the same time the technical feasibility of waste to fuels is more challenging and less mature compared to virgin wood.
Carbon price is an effective tool in reducing GHG emissions when applied effectively. In the current EU emissions trading scheme applied by the UK, there is no credit for negative emissions. Applying a sufficient carbon price to close the carbon pricing gap will assist renewable technologies to fairly compete with fossil-fuel technologies and eventually decrease the dependence on fossil fuels by lowering the levelised cost of fuel of biofuels whilst increasing that of fossil-based fuels. Introducing a carbon price for negative emissions could boost the feasibility of producing biofuels because while revenue will be generated through this means, the price of fossil-derived fuels will increase as a result of paying the environmental price for positive emissions. The emission factors used for fossil derived diesel (0.26880 kg CO2e kW h−1) and natural gas (0.20428 kg CO2e kW h−1) in this section were the scope 1 factors reported by the BEIS.78
Fig. 7 illustrates the dependency of the minimum selling price for the CCS cases on the carbon price (RTFO and RHI payments are not included). In this study, a break-even carbon price of £48 per tCO2 is required for FT-fuels to compete with fossil-derived diesel and a price of £86 per tCO2 is required for OMEx to compete. Higher carbon prices will discourage the production of diesel and favour the production of FT-fuels with CCS and OMEx with CCS as well as drive the development and deployment of this technology. With bioSNG, even with the increase in natural gas prices where a carbon price is introduced, a carbon price up to £63 per tCO2 is required to break even. The major barrier to the deployment of the bioSNG route is the very low price of natural gas. These break-even carbon prices are dependent on the emission factor of the production route. To further decrease the minimum carbon price required, the routes, especially the OMEx route will need to generate more negative emissions; doubling the emission factor halves the break-even carbon price.
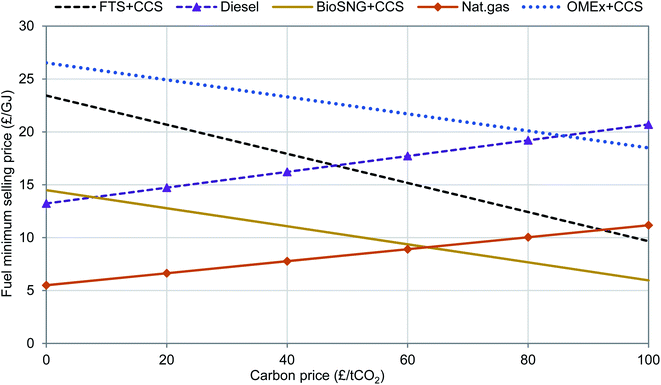 | ||
| Fig. 7 Effect of negative emission credit on minimum selling price of biofuels. The fossil-derived fuel prices are presented for comparisons. | ||
Natural gas production currently enjoys a lot of benefits as it produces 50% less CO2 emissions than coal85 and is currently being used as a transition fuel. However, now is the time to prepare and cut the costs associated with bioSNG production to boost its feasibility as economies move towards decarbonisation.
3.10 Sensitivity analysis
The production cost of biofuel is dependent on different parameters. In this section, a sensitivity analysis is performed to pinpoint the parameters that have a significant effect on the production cost. This analysis highlights the parameters to be focused on making each route feasible and competitive. Generally, based on previous studies86,87 the parameters (CAPEX, plant life, biomass cost, CCS) are changed by ±30% of the base case scenario with the exceptions of the fuel output at ±10%, the interest rate at ±20% and the operating hours where the capacity factor is between 70% and 90% as not to exceed the maximum hours in a year. Also, this analysis is only carried out for the CCS cases as the focus is on BECCS technologies and it is only the CCS cases that provide negative emissions. The results of the analysis are displayed in Fig. 8.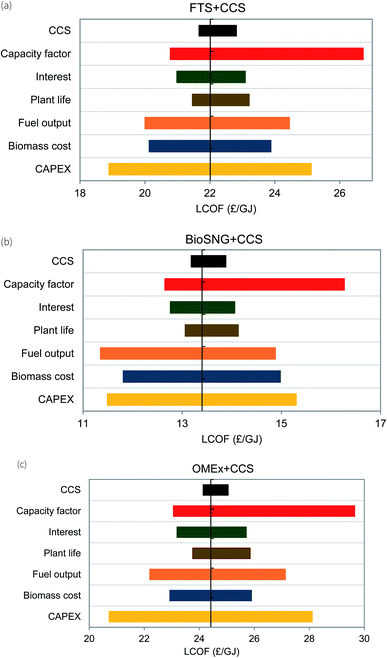 | ||
| Fig. 8 Sensitivity analysis on the production cost for three production routes with CCS (a) Fischer–Tropsch synthesis, (b) methanation and (c) oxymethylene ether synthesis. | ||
The bars in the Fig. 8 represent the sensitivity of each parameter to the production cost. Longer bars depict higher sensitivity while shorter bars depict lower sensitivity. From this, the most significant factor that will affect the production cost is the operating hours. The plant operating hours in a year is dependent on the capacity factor. Increasing the capacity factor to 90% while other factors are kept constant, decreases the production cost by 6% while decreasing the capacity factor to 70% results in a 21% increase in the production cost. Apart from maintenance, the capacity factor is affected by the feedstock availability. Where there is a shortage of supply, the capacity factor is reduced, thus resulting in decreased output. It is important that the supply of the biomass is sustainable and secure for each production route; this should also be applied for the biomass price to avoid fluctuations in price (at 30% uncertainty of feedstock price, fluctuations in production cost are −6% to +10%). Alternatively, considering a mix of biomass sources could be beneficial.
The next factors that significantly affect the production cost are the capital investment and the fuel output. Fluctuations of −15% to +15% in production cost are seen for an uncertainty of 30% in the capital investment while fluctuations of −9% to +11% are seen for an uncertainty of 10% in the fuel output. The possible decrease in production cost highlights the importance of improving the plant efficiency to increase the fuel output. As the data for the capital investment is acquired from factorial estimation and open literature, it is difficult to accurately predict the capital expenditure. Also, the capital investment is subject to location; importation of equipment attracts import duties and could result in increased CAPEX while producing within the country will not have such duties. Accurate data will be available where there are existing commercial plants. Until then, the initial estimates provided in this chapter are suitable for preliminary feasibility studies. Other parameters – CO2 compression, transport & storage costs, and plant life – have minimal effect on the production cost.
4 Conclusion
BECCS is an integral concept to stabilizing and eventually reducing CO2 levels in the atmosphere. Fuel generation already has an advantage due to the intrinsic capture unit and pure stream of CO2 available providing an opportunity for easy CCS retrofitting. This work investigated the technical, economic, and environmental performance of three biofuel production routes that have the potential to be BECCS technologies. The fuel synthesis routes – Fischer–Tropsch synthesis, methanation and oxymethylene ethers synthesis – were modelled using Aspen Plus. A sensitivity analysis was performed to highlight the parameters for process improvements.The energy conversion was in the range of 44.9% to 59.7% without CCS and 44.0% to 58.2% with CCS. BioSNG production had the highest efficiency due to high CO conversion to CH4 as well as optimised heat integration with no external heat input. FT synthesis had the least efficiency due to the relatively low CO conversion, i.e.72.7%. In all three cases, the energy conversion decreases by less than 1.5 percentage points when CCS is added to the plant due to intrinsic nature of CO2 capture in biofuel generation.
Regarding the effect on the environment, adding CCS results in substantial negative emissions for the three routes considered. Overall, 301![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 519
000 to 519![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tCO2 can be removed from the atmosphere per year using the investigated routes. The OMEx route has the least emission factor at −0.2892 kg CO2 kW h−1 due to purchased heat, steam, and electricity to meet the plant demand. The bioSNG route has a lower emission factor than the FT-fuel route due to the amount of CO2 that is vented to the atmosphere during the process especially in the gasification section that uses dual fluidised bed gasifier to maximise the product yield. However, more negative emissions can be achieved by capturing CO2 in the flue gas and limiting electricity imports which increase indirect emissions, thus maximising carbon removal.
000 tCO2 can be removed from the atmosphere per year using the investigated routes. The OMEx route has the least emission factor at −0.2892 kg CO2 kW h−1 due to purchased heat, steam, and electricity to meet the plant demand. The bioSNG route has a lower emission factor than the FT-fuel route due to the amount of CO2 that is vented to the atmosphere during the process especially in the gasification section that uses dual fluidised bed gasifier to maximise the product yield. However, more negative emissions can be achieved by capturing CO2 in the flue gas and limiting electricity imports which increase indirect emissions, thus maximising carbon removal.
The production costs are in the range of £12.2 to £22.9 per GJ without CCS and £13.4 to £24.4 per GJ with CCS. BioSNG is relatively the cheapest fuel to produce while OMEx is relatively the most expensive to produce as a result of the high capital investment and the high operating expenditure. The addition of CCS does not have a drastic effect on the production costs while providing huge environmental benefits. However, in comparison to fossil-derived fuels on the market, the biofuel counterparts cannot feasibly compete without financial incentives due to minimum selling prices of the biofuels being at least two times more than fossil-derived fuels. With the application of the current RTFO and the RHI prices, competition is still not feasible with the current feedstock – woody biomass. Applying carbon pricing as an economic tool, a price in the range of £48 to £86 per tCO2 is required to break-even with the current market price of the fossil-derive fuels. These figures are a function of the emission factor hence more negative emission credits need to be generated by the plants to achieve a lower carbon price. Combining the current financial incentives (RTFO and RHI) with carbon pricing could result in a much lower breakeven price. The sensitivity analysis highlights the capacity factor as the critical process parameter affecting the production cost. Other important parameters include the fuel output, the capital expenditure and feedstock costs.
Overall, the three routes with CCS are promising BECCS technologies but cannot currently compete with fossil-fuels without carbon pricing. Process improvements to boost the product yield and capture more CO2 are required. Also, more aggressive policies and incentives focused on decarbonisation are needed to drive development and deployment and eventually favour competition to phase out fossil-derived fuels.
Appendix
| Process unit | Design variable | Base capacity | Base cost (£ million) | Base year | Scaling factor | References |
|---|---|---|---|---|---|---|
| Pretreatment | Biomass input | 2200 dt d−1 | 11.34 | 2007 | 0.77 | 8 and 26 |
| Gasification (EFG) | Biomass input | 2200 dt d−1 | 33.87 | 2007 | 0.66 | 8 and 26 |
| Gasification (DFB) | LHV (MWSNG) | 20 MW | 2.61 | 2014 | 0.66 | 69 |
| Syngas cleaning | Syngas output | 3823 t d−1 | 16.74 | 2007 | 0.7 | 8 and 26 |
| Primary cleaning | SNG output | 20 MW | 2.11 | 2014 | 0.67 | 69 |
| WGS & cracking | SNG output | 20 MW | 2.09 | 2014 | 0.7 | 69 |
| FT synthesis | Biomass input | 2200 dt d−1 | 24.68 | 2007 | 0.7 | 8 and 26 |
| Methanation | SNG output | 20 MW | 2.18 | 2014 | 0.7 | 69 |
| Methanol synthesis | Methanol output | 44.3 t d−1 | 2.18 | 2011 | 0.8 | 88 |
| OMEx synthesis | OMEx output | 1 million t y−1 | 166.29 | 2014 | 0.65 | 35 |
| Pressure swing adsorption | PSA input | 797.6 t d−1 | 8.37 | 2015 | 0.7 | 26 and 89 |
| Hydrocracking | Flowrate | 378 t d−1 | 16.49 | 2007 | 0.67 | 8 and 26 |
| Upgrading unit | SNG output | 20 MW | 0.44 | 2014 | 0.7 | 69 |
| Power generation | Power | 35.9 MW | 29.83 | 2015 | 0.75 | 8 |
| Air separation | Air input | 2903 t d−1 | 12.14 | 2007 | 0.8 | 8 and 26 |
| Centrifugal compression | Power | 500 kW | 0.91 | 2010 | 0.6 | 68 |
| Reciprocal compression | Power | 500 kW | 0.36 | 2010 | 0.75 | 68 |
| Cooling system | Flowrate | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L s−1 000 L s−1 |
3.45 | 2010 | 0.9 | 68 |
| Position | Salary | Number | Total |
|---|---|---|---|
| Plant manager | £60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1 | £60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Plant engineer | £40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2 | £80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Maintenance supervisor | £30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1 | £30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Lab manager | £38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1 | £38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Shift supervisor | £26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5 | £130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Lab technician | £22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4 | £88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Maintenance technician | £29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
8/12 | £232![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000/£348 000/£348![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Shift operators | £29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
20/30 | £580![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000/£870 000/£870![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Yard employees | £20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
12 | £240![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Clerks & secretaries | £20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3 | £60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Total | £1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 538 538![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000/£1 000/£1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 944 944![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
||
| Parameter | Value |
|---|---|
| Fixed costs | |
| Insurance, taxes & rent | 2.5% fixed capital investment |
| Maintenance and repairs | 2% fixed capital investment |
| Operating supplies | 15% maintenance & repairs |
| Laboratory charges | 10% operating labour |
| Overhead costs | 50% operating labour |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Variable costs | |
| Feedstock | £50 per dry tonne |
| Ash disposal | £19 per tonne |
| Wastewater treatment | £4.52 per m3 |
| MEA | £1.92 per tCO2 |
| WGS catalyst | £16 per kg |
| FTS catalyst | £30 per kg |
| Methanation catalyst | £0.02 per GJ of SNG |
| Methanol catalyst | £18.8 per kg |
| OMEx catalyst | £33.2 per kg |
| Formaldehyde catalyst | £437 per kg |
| Activated carbon | £10.4 per kW h |
| Boiler feed water | £0.50 per tonne |
| Cooling water | £0.023 per tonne |
| Fired heat | £8.06 per MW h |
| Electricity to grid | £58 per MW h |
| Electricity from grid | £104 per MW h |
| LO-CAT chemicals | £160 per tonne S |
| Hydroprocessing | £3.63 per barrel produced |
| PSA | £3.31 per kg |
| CO2 transportation and storage | £19 per tCO2 |
Conflicts of interest
There are no conflicts to declare.References
- EASAC, Negative Emission Technologies: What Role in Meeting Paris Agreement Targets?, 2018 Search PubMed.
- M. Fajardy and N. Mac Dowell, Can BECCS deliver sustainable and resource efficient negative emissions?, Energy Environ. Sci., 2017, 10, 1389–1426, 10.1039/c7ee00465f.
- P. Ibarra-Gonzalez and B. G. Rong, A review of the current state of biofuels production from lignocellulosic biomass using thermochemical conversion routes, Chin. J. Chem. Eng., 2019, 27, 1523–1535, DOI:10.1016/j.cjche.2018.09.018.
- IEA, From 1 st- to 2nd-Generation Biofuel technologies, Paris, 2008, https://www.iea.org/reports/from-1st-to-2nd-generation-biofuel-technologies, accessed 30 June 2020 Search PubMed.
- S. N. Naik, V. V. Goud, P. K. Rout and A. K. Dalai, Production of first and second generation biofuels: A comprehensive review, Renew. Sustain. Energy Rev., 2010, 14, 578–597, DOI:10.1016/j.rser.2009.10.003.
- A. Demirbas, Competitive liquid biofuels from biomass, Appl. Energy, 2011, 88, 17–28, DOI:10.1016/j.apenergy.2010.07.016.
- M. Stöcker, Biofuels and Biomass-To-Liquid Fuels in the Biorefinery: Catalytic Conversion of Lignocellulosic Biomass using Porous Materials, Angew. Chem. Int. Ed., 2008, 47, 9200–9211, DOI:10.1002/anie.200801476.
- R. M. Swanson, A. Platon, J. A. Satrio and R. C. Brown, Techno-economic analysis of biomass-to-liquids production based on gasification, Fuel, 2010, 89, S11–S19, DOI:10.1016/j.fuel.2010.07.027.
- F. Trippe, M. Fröhling, F. Schultmann, R. Stahl and E. Henrich, Techno-economic assessment of gasification as a process step within biomass-to-liquid (BtL) fuel and chemicals production, Fuel Process. Technol., 2011, 92, 2169–2184, DOI:10.1016/j.fuproc.2011.06.026.
- F. Trippe, M. Fröhling, F. Schultmann, R. Stahl, E. Henrich and A. Dalai, Comprehensive techno-economic assessment of dimethyl ether (DME) synthesis and Fischer-Tropsch synthesis as alternative process steps within biomass-to-liquid production, Fuel Process. Technol., 2013, 106, 577–586, DOI:10.1016/j.fuproc.2012.09.029.
- M. Fajardy, S. Chiquier and N. Mac Dowell, Investigating the BECCS resource nexus: Delivering sustainable negative emissions, Energy Environ. Sci., 2018, 11, 3408–3430, 10.1039/c8ee01676c.
- S. Deutz, D. Bongartz, B. Heuser, A. Kätelhön, L. Schulze Langenhorst, A. Omari, M. Walters, J. Klankermayer, W. Leitner, A. Mitsos, S. Pischinger and A. Bardow, Cleaner production of cleaner fuels: Wind-to-wheel-environmental assessment of CO2-based oxymethylene ether as a drop-in fuel, Energy Environ. Sci., 2018, 11, 331–343, 10.1039/c7ee01657c.
- H. Mahmoudi, M. Mahmoudi, O. Doustdar, H. Jahangiri, A. Tsolakis, S. Gu and M. LechWyszynski, A review of Fischer Tropsch synthesis process, mechanism, surface chemistry and catalyst formulation, Biofuels Eng., 2018, 2, 11–31, DOI:10.1515/bfuel-2017-0002.
- 10.2. Fischer-Tropsch Synthesis, https://www.netl.doe.gov/research/coal/energy-systems/gasification/gasifipedia/ftsynthesis, accessed 29 June 2020 Search PubMed.
- S. S. Ail and S. Dasappa, Biomass to liquid transportation fuel via Fischer Tropsch synthesis - Technology review and current scenario, Renew. Sustain. Energy Rev., 2016, 58, 267–286, DOI:10.1016/j.rser.2015.12.143.
- Approval granted for £150m renewable gas plant, in Cheshire, Energy Live News, assessed in 25/3/2021, https://www.energylivenews.com/2019/11/06/approval-granted-for-150m-renewable-gas-plant-in-cheshire/ Search PubMed.
- Bioenergy International, Göteborg Energi winds down GoBIGas 1 project in advance, assessed in 25/3/2021, https://bioenergyinternational.com/research-development/goteborg-energi-winds-gobigas-1-project-advance Search PubMed.
- Green Car Congress, Germany launches new study of oxymethylene ethers for optimizing clean diesel combustion, assessed in 25/3/2021, https://www.greencarcongress.com/2016/02/20160215-ome.html.
- M. Härtl, K. Gaukel, D. Pélerin and G. Wachtmeister, Oxymethylene Ether as Potentially CO2-neutral Fuel for Clean Diesel Engines Part 1: Engine Testing, MTZ Worldw, 2017, 78, 52–59, DOI:10.1007/s38313-016-0163-6.
- D. Zhang, M. Bui, M. Fajardy, P. Patrizio, F. Kraxner and N. Mac Dowell, Unlocking the potential of BECCS with indigenous sources of biomass at a national scale, Sustainable Energy Fuels, 2019, 4, 226–253, 10.1039/c9se00609e.
- C. Cumicheo, N. Mac Dowell and N. Shah, Natural gas and BECCS: A comparative analysis of alternative configurations for negative emissions power generation, Int. J. Greenhouse Gas Control, 2019, 90, 102798, DOI:10.1016/j.ijggc.2019.102798.
- R. P. Cabral, M. Bui and N. Mac Dowell, A synergistic approach for the simultaneous decarbonisation of power and industry via bioenergy with carbon capture and storage (BECCS), Int. J. Greenhouse Gas Control, 2019, 87, 221–237, DOI:10.1016/j.ijggc.2019.05.020.
- M. Bui, M. Fajardy and N. Mac Dowell, Bio-energy with carbon capture and storage (BECCS): Opportunities for performance improvement, Fuel, 2018, 213, 164–175, DOI:10.1016/j.fuel.2017.10.100.
- O. Emenike, S. Michailos, K. N. Finney, K. J. Hughes, D. Ingham and M. Pourkashanian, Initial techno-economic screening of BECCS technologies in power generation for a range of biomass feedstock, Sustain. Energy Technol. Assessments., 2020, 40, 100743, DOI:10.1016/j.seta.2020.100743.
- M. Fajardy and N. Mac Dowell, The energy return on investment of BECCS: Is BECCS a threat to energy security?, Energy Environ. Sci., 2018, 11, 1581–1594, 10.1039/c7ee03610h.
- I. S. Tagomori, P. R. R. Rochedo and A. Szklo, Techno-economic and georeferenced analysis of forestry residues-based Fischer-Tropsch diesel with carbon capture in Brazil, Biomass Bioenergy, 2019, 123, 134–148, DOI:10.1016/j.biombioe.2019.02.018.
- G. Song, J. Xiao, Y. Yu and L. Shen, A techno-economic assessment of SNG production from agriculture residuals in China, Energy Sources, Part B, 2016, 11, 465–471, DOI:10.1080/15567249.2012.654595.
- S. Michailos, O. Emenike, D. Ingham, K. J. Hughes and M. Pourkashanian, Methane production via syngas fermentation within the bio-CCS concept: A techno-economic assessment, Biochem. Eng. J., 2019, 150, 107290, DOI:10.1016/j.bej.2019.107290.
- G. de Álamo, J. Sandquist, B. J. Vreugdenhill, G. A. Almansa and M. Carbo, Implementation of bio-CCS in biofuels production - IEA Bionergy Task 33 special report, 2018 Search PubMed.
- I Aspen Technology, Aspen Physical Property System 11.1 Physical Property Methods and Models, 2001, https://esupport.aspentech.com/S_Article?id=000062950, accessed 6 July 2020 Search PubMed.
- I. Aspen Technology, Aspen Plus 11.1 Getting Started Modeling Processes with Solids, 2001, https://esupport.aspentech.com/S_Article?id=000064733, accessed 6 July 2020 Search PubMed.
- N. Jand, V. Brandani and P. U. Foscolo, Thermodynamic limits and actual product yields and compositions in biomass gasification processes, Ind. Eng. Chem. Res., 2006, 45, 834–843, DOI:10.1021/ie050824v.
- X. T. Li, J. R. Grace, C. J. Lim, A. P. Watkinson, H. P. Chen and J. R. Kim, Biomass Bioenergy, 2004, 26(2), 171–193 CrossRef CAS.
- J. R. Thome, Engineering Data Book III.pdf, 2004 Search PubMed.
- N. Schmitz, J. Burger, E. Ströfer and H. Hasse, From methanol to the oxygenated diesel fuel poly(oxymethylene) dimethyl ether: An assessment of the production costs, Fuel, 2016, 185, 67–72, DOI:10.1016/j.fuel.2016.07.085.
- E. E. Baruth, Water Treatment Plant Design, 1988, DOI:10.1061/(ASCE)0733-9372(1988)114:6(1487).
- L. Creech, Waste wood processing rises by six per cent in 2019, Resour. Mag., 2020, https://resource.co/article/waste-wood-processing-rises-six-cent-2019, accessed 24 March 2021 Search PubMed.
- John Clegg Consulting Ltd, WOOD FIBRE AVAILABILITY & DEMAND IN BRITAIN 2013-2035, Edinburgh, 2016 Search PubMed.
- P. Harrison, C. Malins, S. Searle, A. Baral, D. Turley and L. Hopwood, National case studies on potential waste and residue availability for cellulosic biofuel production in the EU, 2014, http://www.theicct.org/wasted-europes-untapped-resource-report Search PubMed.
- E4Tech, Review of Technologies for Gasification of Biomass and Wastes Final report, NNFCC Bioeconomy Consult, 2009, pp. 1–130, http://www.e4tech.com/wp-content/uploads/2016/01/gasification2009.pdf%0Ahttp://www.nnfcc.co.uk/tools/review-of-technologies-for-gasification-of-biomass-and-wastes-nnfcc-09-008 Search PubMed.
- I. Dimitriou, H. Goldingay and A. V. Bridgwater, Techno-economic and uncertainty analysis of Biomass to Liquid (BTL) systems for transport fuel production, Renew. Sustain. Energy Rev., 2018, 88, 160–175, DOI:10.1016/j.rser.2018.02.023.
- J. Szuhanszki, O. F. Moguel, K. Finney, M. Akram and M. Pourkashanian, Biomass, combustion under oxy-fuel and post combustion capture conditions at the PACT 250 kW air/oxy-fuel CTF, 2017 Search PubMed.
- S. Mani, L. G. Tabil and S. Sokhansanj, Grinding performance and physical properties of wheat and barley straws, corn stover and switchgrass, Biomass Bioenergy, 2004, 27, 339–352, DOI:10.1016/j.biombioe.2004.03.007.
- P. Basu, Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory, 2013, DOI:10.1016/C2011-0-07564-6.
- H. Ghassemi and R. Shahsavan-Markadeh, Effects of various operational parameters on biomass gasification process; A modified equilibrium model, Energy Convers. Manage., 2014, 79, 18–24, DOI:10.1016/j.enconman.2013.12.007.
- R. F. Probstein and R. E. Hicks, Synthetic fuels, Dover Publications, 2006 Search PubMed.
- C. Higman and M. van der Burgt, Gasification Processes, Gasification, ed. C. Higman and M. van der Burgt, Gulf Professional Publishing, Burlington, 2003, ch. 5, pp. 85–170 Search PubMed.
- H. Thunmann, GoBiGas demonstration – a vital step for a large-scale transition from fossil fuels to advanced biofuels and electrofuels, 2018 Search PubMed.
- A. Alamia, S. Òsk Gardarsdòttir, A. Larsson, F. Normann and H. Thunman, Efficiency Comparison of Large-Scale Standalone, Centralized, and Distributed Thermochemical Biorefineries, Energy Technol., 2017, 5, 1435–1448, DOI:10.1002/ente.201600719.
- L. P. R. Pala, Q. Wang, G. Kolb and V. Hessel, Steam gasification of biomass with subsequent syngas adjustment using shift reaction for syngas production: An Aspen Plus model, Renewable Energy, 2017, 101, 484–492, DOI:10.1016/j.renene.2016.08.069.
- G. Gautam, S. Adhikari and S. Bhavnani, Estimation of biomass synthesis gas composition using equilibrium modeling, Energy Fuels, 2010 DOI:10.1021/ef901477c.
- K. D. Panopoulos, L. E. Fryda, J. Karl, S. Poulou and E. Kakaras, High temperature solid oxide fuel cell integrated with novel allothermal biomass gasification. Part I: Modelling and feasibility study, J. Power Sources, 2006, 159, 570–585, DOI:10.1016/j.jpowsour.2005.12.024.
- G. Schuster, G. Löffler, K. Weigl and H. Hofbauer, Biomass steam gasification - An extensive parametric modeling study, Bioresour. Technol., 2001, 77, 71–79, DOI:10.1016/S0960-8524(00)00115-2.
- W. Doherty, A. Reynolds and D. Kennedy, Aspen plus simulation of biomass gasification in a steam blown dual fluidised bed, Mater. Process Energy., 2013, 212–220 Search PubMed.
- A. Alamia, H. Thunman and M. Seemann, Process Simulation of Dual Fluidized Bed Gasifiers Using Experimental Data, Energy Fuels, 2016, 30, 4017–4033, DOI:10.1021/acs.energyfuels.6b00122.
- M. Asadullah, Biomass gasification gas cleaning for downstream applications: A comparative critical review, Renew. Sustain. Energy Rev., 2014, 40, 118–132, DOI:10.1016/j.rser.2014.07.132.
- P. J. Woolcock and R. C. Brown, A review of cleaning technologies for biomass-derived syngas, Biomass Bioenergy, 2013, 52, 54–84, DOI:10.1016/j.biombioe.2013.02.036.
- S. D. Phillips, J. K. Tarud, M. J. Biddy and A. Dutta, Gasoline from Woody Biomass via Thermochemical Gasification, Methanol Synthesis, and Methanol-to-Gasoline Technologies, A Technoeconomic Analysis, Ind. Eng. Chem. Res., 2011, 50(20), 11734–11745 CrossRef CAS.
- H.-S. Song, D. Ramkrishna, S. Trinh and H. Wright, Operating Strategies for Fischer-Tropsch Reactors: A Model-Directed Study, 2004 Search PubMed.
- M. N. Rosli and N. Aziz, Simulation of ethane steam cracking with severity evaluation, IOP Conf. Ser. Mater. Sci. Eng., 2016, 162(1), 012017 CrossRef.
- H. Topsøe, From solid fuels to substitute natural gas (SNG) using TREMP, Tech. Report, Halder Topsøe, 2009, vol. 8, http://scholar.google.com/scholar?hl=en%26btnG=Search%26q=intitle:From+solid+fuels+to+substitute+natural+gas+(SNG)+using+TREMP?#4 Search PubMed.
- N. Schmitz, F. Homberg, J. Berje, J. Burger and H. Hasse, Chemical Equilibrium of the Synthesis of Poly(oxymethylene) Dimethyl Ethers from Formaldehyde and Methanol in Aqueous Solutions, 2015, DOI:10.1021/acs.iecr.5b01148.
- Z. J. Ai, C. Y. Chung and I. L. Chien, Design and Control of Poly(Oxymethylene) Dimethyl Ethers Production Process Directly From Formaldehyde and Methanol in Aqueous Solutions, IFAC-PapersOnLine, 2018, 51, 578–583, DOI:10.1016/j.ifacol.2018.09.362.
- V. E. Onyebuchi, A. Kolios, D. P. Hanak, C. Biliyok and V. Manovic, A systematic review of key challenges of CO2 transport via pipelines, Renew. Sustain. Energy Rev., 2018, 81, 2563–2583, DOI:10.1016/j.rser.2017.06.064.
- I. S. Cole, P. Corrigan, S. Sim and N. Birbilis, Corrosion of pipelines used for CO 2 transport in CCS: Is it a real problem?, Int. J. Greenhouse Gas Control, 2011, 5, 749–756, DOI:10.1016/j.ijggc.2011.05.010.
- A. O. Oyedun and A. Kumar, The development of the production cost of oxymethylene ethers as diesel additives from biomass, 2018, DOI:10.1002/bbb.1887.
- M. S. Peters, K. D. Timmerhaus and R. E. West, Plant Design and Economics for Chemical Engineers, 5th edn, 2003 Search PubMed.
- R. K. Sinnott and G. Towler, Chemical Engineering Design, 5th edn, 2009, DOI:10.1016/C2009-0-61216-2.
- H. Thunman, C. Gustavsson, A. Larsson, I. Gunnarsson and F. Tengberg, Economic assessment of advanced biofuel production via gasification using real cost data from GoBiGas, a first-of-its-kind industrial installation, Submitt. Energy Sci. Eng., 2018, 105–118, DOI:10.1002/ese3.271.
- Company Salaries, Glassdoor, 2019, https://www.glassdoor.com/Salaries/index.htm.
- Payscale, PayScale - Salary Comparison, Salary Survey, Search Wages, Payscale, 2013, http://www.payscale.com/.
- S. Phillips, A. Aden, J. Jechura, D. Dayton and T. Eggeman, Thermochemical Ethanol via Direct Gasification and Mixed Alcohol Synthesis of Lignocellulosic Biomass. Technical Report NREL/TP-510-41168, NREL, Natl. Renew. Energy Lab., 2007, p. 125, http://www.nrel.gov/docs/fy07osti/41168.pdf Search PubMed.
- UK Parliament, The Renewable Transport Fuel Obligations Order 2007, No. 3072, 2007, https://www.legislation.gov.uk/uksi/2007/3072/contents/made, accessed 25 February 2021 Search PubMed.
- UK Parliament, The Renewable Heat Incentive Scheme Regulations 2011 (revoked), No. 2860, 2011 Search PubMed.
- X. Zhang, A. O. Oyedun, A. Kumar, D. Oestreich, U. Arnold and J. Sauer, An optimized process design for oxymethylene ether production from woody-biomass-derived syngas, Biomass Bioenergy, 2016, 90, 7–14, DOI:10.1016/j.biombioe.2016.03.032.
- C. R. Vitasari, M. Jurascik and K. J. Ptasinski, Exergy analysis of biomass-to-synthetic natural gas (SNG) process via indirect gasification of various biomass feedstock, Energy, 2011, 36, 3825–3837, DOI:10.1016/j.energy.2010.09.026.
- A. Brown, L. Waldheim, I. Landälv, J. Saddler, M. Ebadian, J. D. McMillan, A. Bonomi and B. Klein, Advanced Biofuels, 2020, 88 Search PubMed.
- Department of Business Energy & Industrial Strategy, Greenhouse gas reporting: conversion factors 2019, GOV.UK, Dep. Business, Energy Ind. Strateg., 2019, https://www.gov.uk/government/publications/greenhouse-gas-reporting-conversion-factors-2019, accessed 24 January 2021 Search PubMed.
- C. Charles, R. Bridle and T. Moerenhout, Biofuels—At What Cost? A review of costs and benefits of U.K. biofuel policies, 2013 Search PubMed.
- Wholesale gas charts and indicators, Ofgem|Ofgem, https://www.ofgem.gov.uk/data-portal/all-charts/policy-area/gas-wholesale-markets Search PubMed.
- Petrol and diesel prices since 2014 - wholesale and average pump, The RAC Media Centre Search PubMed.
- Bioenergy Insight, Generators of biomethane can claim RHI and RTFO payments, assessed in 25/03/2021, https://www.bioenergy-news.com/news/generators-of-biomethane-can-claim-rhi-and-rtfo-payments/ Search PubMed.
- Local authorities|ADBA|Anaerobic Digestion & Bioresources Association, http://adbioresources.org/about-ad/how-ad-benefits-everyone/local-authority-and-government/local-authorities Search PubMed.
- Ofgem, Non-Domestic RHI tariff table, Ofgem, 2020 Search PubMed.
- T. Fout, A. Zoelle, D. Keairns, M. Turner, M. Woods, N. Kuehn, V. Shah, V. Chou and L. Pinkerton, Cost and Performance Baseline for Fossil Energy Plants Volume 1a: Bituminous Coal (PC) and Natural Gas to Electricity Revision 3, 2015, DOE/NETL-2010/1397 Search PubMed.
- D. H. König, M. Freiberg, R. U. Dietrich and A. Wörner, Techno-economic study of the storage of fluctuating renewable energy in liquid hydrocarbons, Fuel, 2015, 159(1), 289–297 CrossRef.
- F. G. Albrecht, D. H. König, N. Baucks and R. U. Dietrich, A standardized methodology for the techno-economic evaluation of alternative fuels – A case study, Fuel, 2017, 194, 511–526, DOI:10.1016/j.fuel.2016.12.003.
- Er. C. Tan, M. Talmadge, A. Dutta, J. Hensley, J. Schaidle, M. Biddy, D. Humbird, L. J. Snowden-Swan, J. Ross, D. Sexton, R. Yap and J. Lukas, Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons via Indirect Liquefaction, Natl. Renew. Energy Lab. Pacific Northwest Natl. Lab., 2015, 2 Search PubMed.
- J. C. Meerman, M. M. J. Knoope, A. Ramírez, W. C. Turkenburg and A. P. C. Faaij, Technical and economic prospects of coal- and biomass-fired integrated gasification facilities equipped with CCS over time, Int. J. Greenhouse Gas Control, 2013, 16, 311–323, DOI:10.1016/j.ijggc.2013.01.051.
- Amec Foster Wheeler Group Limited, Assessing the Cost Reduction Potential and Competitiveness of Novel (Next Generation) UK Carbon Capture Technology, Benchmarking State-of-the-art and Next Generation Technologies, 13333-8820-RP-001Rev4A, 2018, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/864688/BEIS_Final_Benchmarks_Report_Rev_4A.pdf Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1se00123j |
| This journal is © The Royal Society of Chemistry 2021 |