Facile and low-cost synthesis of a novel dopant-free hole transporting material that rivals Spiro-OMeTAD for high efficiency perovskite solar cells†
Received
5th September 2020
, Accepted 2nd November 2020
First published on 17th November 2020
Abstract
A Spiro fluorene-based dopant-free hole-transporting material denoted as Spiro-IA has been designed and developed from inexpensive starting materials with high yield via a simple synthetic approach for application in perovskite solar cells (PSCs). The unit cost of Spiro-IA can be as low as 1/9th that of the conventional Spiro-OMeTAD. Moreover, Spiro-IA shows good solubility in different organic solvents, e.g. CHCl3, acetone, EtOH, and DMF, and showed favorable charge-transport ability and greater photocurrent density compared to Spiro-OMeTAD. The UV absorption/emission spectra of Spiro-IA (λmax = 430 nm, Emax = 601 nm) are red shifted compared to those of Spiro-OMeTAD (λmax = 388 nm, Emax = 414 nm) with larger stokes shift values (171 nm) which helps suppress the loss of incident photons absorbed by the HTM and is more beneficial for improving the performance of PSCs. Optical and electrochemical studies show that Spiro-IA fulfilled the basic requirements of the hole transfer and electron regeneration process in the fabricated devices. PSCs fabricated (surface area = 1.02 cm2) with dopant-free Spiro-IA achieved a maximum power conversion efficiency (PCE) of 15.66% (JSC = 22.14 mA cm−2, VOC = 1.042 V, FF = 0.679%), which was comparable to that of the most commonly used Li-doped Spiro-OMeTAD (PCE = 15.93%, JSC = 20.37 mA cm−2, VOC = 1.057 V, FF = 0.74%) and surpassed that of the dopant-free Spiro-OMeTAD (PCE = 9.34%). Additionally, the PSCs based on dopant-free Spiro-IA achieved outstanding long-term stability and favorable conductivity (σ = 2.104 × 10−4 S cm−1) compared to those based on Spiro-OMeTAD (σ = 9.00 × 10−8 S cm−1). DFT studies were performed using Gaussian 09 at the B3LYP/6-31G (d/p) level to investigate their electron cloud delocalization in HOMO/LUMO levels. These results showed that Spiro-IA could be a promising candidate for low-cost PSC technology and has a great chance to supersede the expensive Spiro-OMeTAD.
1. Introduction
Perovskite solar cells (PSCs) have attracted considerable attention due to their low manufacturing costs, light weight, and superior photovoltaic efficiency.1–8 The PCSs' power conversion efficiency (PCE) has improved dramatically from 3.8% to 23.7% in the last few years.9–14 High-efficiency PSCs are achieved by sandwiching the perovskite absorber layer between the electron selective layers (ESLs, n-type) and the hole transport materials (HTMs, p-type), to achieve better extraction of the charge into the corresponding electrodes. Optimal HTMs will have a lower molecular orbital energy level than the total valence band of the perovskite absorber. However, high-hole mobility and excellent thermal and photochemical stability are also key criteria for preferred PSC HTMs.15–17 To date, Spiro-OMeTAD has been the most widely used HTM for high-performance PSCs because it is characterized by a rigid Spiro structure that enables high thermal stability, ensures the formation of a high-quality film, minimizes the tendency to form aggregates and provides sufficient energy levels to block electrons and pass holes at the same time.18–23 The commercial Spiro-OMeTAD has been reported to involve five different reaction steps with a total yield of less than 40%, including the Grignard, cyclization, bromination and the Hartwig–Buchwald coupling reactions.24,25 As a result, the time-consuming synthesis and high synthetic cost of Spiro-OMeTAD hinder its large-scale commercial application, as it requires low temperature (−78 °C) and harsh reagent (Br) conditions. In addition, expensive sublimation steps are required to obtain high-purity Spiro-OMeTAD materials. Therefore, there is an urgent need to develop new HTMs with low cost and easy tuning while maintaining performance. A great deal of effort has been made to explore the replacement of Spiro-OMeTAD with doped or dopant-free alternative HTMs that could improve both the PCE and PSC stability.26–38 Several promising dopant-free HTMs such as 6,13-bis(triisopropylsilylethynyl) pentacene and spiro-based 4,4′,4′′,4′′′-(2H,2′H,4H,4′H-3,3′-spiro-bi[thieno[3,4-b][1,4]dioxepine]-6,6′,8,8′-tetrayl)tetrakis(N,N-bis(4-methoxyphenyl)aniline) have been reported with PCEs of 11.8%39 and 13.44%,40 respectively. Recently, HTMs developed with a spiro[fluorene-9,9-xanthene] core were characterized by a PCE of up to 14.4%41 and, more recently, an HTM based on a quinacridone dye, named ACE-QA-ACE, achieved 18.2% efficiency.42 Additionally, polymer HTMs based on poly(triarylamine) (PTAA) with a chemical dopant have achieved a PCE of up to 21% in perovskite solar cells but polymeric HTMs suffer from relatively poor device reproducibility and fuzzy synthetic and purification routes.43 Despite the increased PCE of PSCs, the presence of dopants led to a decrease in the stability of the devices and an increase in the cost of production.42 Therefore, much effort has been made to use cheap dopant-free HTMs for PSCs achieving a PCE above 16% and higher stability than that of the classic Spiro-OMeTAD, which has attracted attention as it opens the way for cost-effective solar cell growth.41–47 Spiro[fluorene-9,9′-xanthene] is one of the various spiro cores that has attracted great interest due to its simple one-pot synthesis which includes the cost-effective use of 9-fluorenone and phenols as the starting molecules.48 Besides the low cost, the HTMs based on Spiro[fluorene-9,9′-xanthene] exhibit remarkable properties such as great charge transporting ability, good energy matching with perovskite, transparency to solar radiation, large stokes shift, good solubility in different organic solvents, and morphologically stable film formation.49–54
Herein, the low-cost fluorene-based hole transport material Spiro-IA as an alternative to the expensive Spiro-OMeTAD has been designed and synthesized for application in PSCs. The Spiro-IA was prepared with a yield higher than 78% through a facile reaction and cheap starting material. The synthesized Spiro-IA is characterized by a small dimensional structure, low molecular weight, strong electron-donating properties, suitable energy levels, and good environmental stability as well as having great potential for structural modification by integrating different alkyl or functional groups into the outer benzene ring which allows easy tuning of the optoelectronic properties, solubility, and molecular packing.55–57 Our PSCs fabricated with a surface area of 1.02 cm2 based on dopant-free Spiro-IA achieved a PCE of 15.66% with good reproducibility and stability compared to the reported hydroxyl free Spiro[fluorene-9,9-xanthene]-based HTMs which were characterized by a PCE up to 14.4%;41 the new design in this work is the introduction of –OH groups into Spiro-IA molecules. The hydroxyl groups in the fluorene core of Spiro-IA help to increase the electron donation within the HTM molecule which plays an important role to destabilize the ground state oxidation potential (GSOP) and thus reducing the energy gap (E0-0) of the molecule, increasing the charge mobility and achieving excellent optoelectronic properties and a photovoltaic performance (PCE) of 15.66% (Fig. 1).
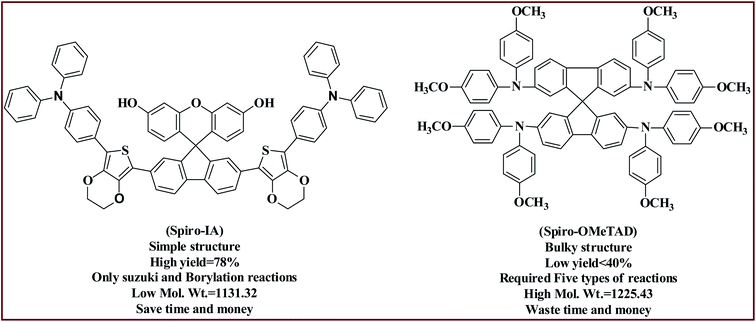 |
| | Fig. 1 Chemical structures of Spiro-OMeTAD and Spiro-IA. | |
2. Experimental
2.1. Materials and methods
The starting materials such as 4-bromotriphenylamine, bis(pinacolato)diboron, AcOK, Pd(dppf)2Cl2, K2CO3, Pd(PPh3)4, 5-dibromo-3,4-ethylenedioxythiophene, resorcinol and 9-fluorenone were purchased from Sigma-Aldrich, Alfa Aesar and Ark Pharm Companies. Further, benzenamine, N,N-diphenyl-4(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) (1),58 2-triphenyl amine 5-bromo-3,4-ethylenedioxythiophene (2),59,60 and 2,7-dibromo-spiro[9H-fluorene-9,9′-[9H]xanthene]-3′,6′-diol (3)61 were synthesized as shown in the ESI.† A Bruker Avance instrument was used to record the spectra of 1H-NMR at 500 MHZ using CDCl3 as the solvent and TMS as the internal standard. A Bruker Alpha spectrophotometer was used to record the FTIR spectra to determine the functional groups. A Thermo-Scientific EXACTIVE (ESI-MS) instrument was employed to record the mass spectra. A SPECORD S600 spectrophotometer and a Horiba Fluoromax-4 spectrofluorometer were used to record the UV-Vis and fluorescence spectra, respectively, using a 10−5 M chloroform solution. Conductivity measurements were obtained from the current–voltage (J–V) curves recorded using an ALS Electrochemical Analyzer 624D using hole only devices (ITO/PEDOT:PSS/(Spiro-IA/Spiro-OMeTAD)/Au). Spectroscopic ellipsometry measurements were conducted with a J. A. Woollam M-2000U to determine the thickness of the Spiro-IA at 1765 nm. The thermogravimetric analysis was completed using a Rigaku Thermo plus differential thermal analyzer with a heating rate of 20 °C min−1 from the room temperature to 1000 °C in air. Molecular engineering calculations (DFT) were performed using the Gaussian 09 software package with the B3LYP exchange–correlation functional and a 6-31G(d,p) basis set in the gas phase, and all operations were submitted remotely to the NC State University High Performance Computer.
2.2. Synthesis and characterization
2.2.1. 2,7-Bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2-yl)-spiro[9H-fluorene-9,9′[9H]xanthene]-3′,6′-diol (4).
Under an argon atmosphere, a mixture consisting of 2.0 g (3.84 mmol) of 2,7-dibromo-spiro[9H-fluorene-9,9′-[9H]xanthene]-3′,6′-diol (3), 2.3 g (8.5 mmol) of bis(pinacoloto)diboron, 2.20 g (22.42 mmol) of potassium acetate, and 80 mL of DMF was deoxygenated at room temperature. Subsequently, 0.32 mg (0.392 mmol) of PdCl2(dppf) was added, and the mixture was stirred at 80–90 °C for 64 hours. After the reaction was completed, the DMF was evaporated, using rotovap, from the reaction mixture, and water was added to the residues followed by extraction using methylene chloride (50 mL × 2). The resulting organic residues were collected, dried, and purified by column chromatography utilizing a silica gel adsorbent (particle size is 63–200 μm), and eluting with hexane/ethyl acetate (2/1) afforded pale-yellow crystals. Yield (90.3%), and MP (230 °C). FT-IR (cm−1): 3301 (OH), 2976 (CH of CH3), 1658 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic), 1346 (C–N aromatic amine). 1H-NMR (500 MHz, chloroform-d, δ): 7.92 (d, J = 7.5 Hz, 2H), 7.65 (d, J = 1.6 Hz, 2H), 7.53 (dd, J = 7.5, 1.5 Hz, 2H), 7.00 (s, 2H), 6.94 (d, J = 7.5 Hz, 2H), 6.42 (d, J = 2.0 Hz, 2H), 6.15 (dd, J = 7.5, 2.0 Hz, 2H), 1.38 (s, 24H). MS: m/z (C37H39B2O7) found = 617.28799 (calcd 617.28764 for [M + H]+) with an error of ΔM = 0.5716 ppm.
C aromatic), 1346 (C–N aromatic amine). 1H-NMR (500 MHz, chloroform-d, δ): 7.92 (d, J = 7.5 Hz, 2H), 7.65 (d, J = 1.6 Hz, 2H), 7.53 (dd, J = 7.5, 1.5 Hz, 2H), 7.00 (s, 2H), 6.94 (d, J = 7.5 Hz, 2H), 6.42 (d, J = 2.0 Hz, 2H), 6.15 (dd, J = 7.5, 2.0 Hz, 2H), 1.38 (s, 24H). MS: m/z (C37H39B2O7) found = 617.28799 (calcd 617.28764 for [M + H]+) with an error of ΔM = 0.5716 ppm.
2.2.2. 2,7-Bis(7-(4-(diphenylamino)phenyl)-2,3-dihydrothieno[3,4-b][1,4]dioxin-5-yl)spiro[fluorene-9,9′-xanthene]-3′,6′-diol (Spiro-IA).
In a three necked flask, 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2-yl)-spiro[9H-fluorene-9,9′[9H]xanthene]-3′,6′-diol (4) (0.677 g, 1.1 mmol) and 2-triphenyl amine 5-bromo-3,4-ethylenedioxythiophene (2) (0.928 g, 1 mmol) were dissolved in a mixture of toluene and ethanol (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 mL) and a solution of K2CO3 (2 M, 10 mL) was added and degassed under argon for 30 minutes. Then tetrakis(triphenyl-phosphine)palladium(0) (0.068 g, 0.06 mmol) was added to this solution. The reaction mixture was refluxed and stirred at 90 °C for 6 hours. The solvent in the reaction mixture was evaporated via rotovap followed by adding water and extraction with methylene chloride (40 mL × 2). The resulting yellow colored organic layer was collected, dried over Na2SO4, and purified by column chromatography utilizing a silica gel adsorbent (particle size is 63–200 μm) and eluting with chloroform to give a yellow solid. Yield (78%), and MP (278 °C). FT-IR (cm−1): 3401 (OH), 3060 & 2926 (CH of CH3), 1588 (C
20 mL) and a solution of K2CO3 (2 M, 10 mL) was added and degassed under argon for 30 minutes. Then tetrakis(triphenyl-phosphine)palladium(0) (0.068 g, 0.06 mmol) was added to this solution. The reaction mixture was refluxed and stirred at 90 °C for 6 hours. The solvent in the reaction mixture was evaporated via rotovap followed by adding water and extraction with methylene chloride (40 mL × 2). The resulting yellow colored organic layer was collected, dried over Na2SO4, and purified by column chromatography utilizing a silica gel adsorbent (particle size is 63–200 μm) and eluting with chloroform to give a yellow solid. Yield (78%), and MP (278 °C). FT-IR (cm−1): 3401 (OH), 3060 & 2926 (CH of CH3), 1588 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic), 1085 (C–N aromatic amine). 1H NMR (500 MHz, chloroform-d, δ): 7.61–7.58 (m, 12H), 7.45–7.49 (m, 8H), 7.41–7.38 (m, 14H), 7.31 (d, 4.2 Hz, 2H), 6.64 (s, 2H), 6.29–6.27 (m, 2H), 6.24–6.22 (m, 2H), 2.10 (s, 8H). MS: m/z (C73H50N2O7S2) found = 1130.30 (calcd 1131.32 for [M+]) with an error of ΔM = 1.08 ppm.
C aromatic), 1085 (C–N aromatic amine). 1H NMR (500 MHz, chloroform-d, δ): 7.61–7.58 (m, 12H), 7.45–7.49 (m, 8H), 7.41–7.38 (m, 14H), 7.31 (d, 4.2 Hz, 2H), 6.64 (s, 2H), 6.29–6.27 (m, 2H), 6.24–6.22 (m, 2H), 2.10 (s, 8H). MS: m/z (C73H50N2O7S2) found = 1130.30 (calcd 1131.32 for [M+]) with an error of ΔM = 1.08 ppm.
2.3. Perovskite solar cell fabrication
Fluorine doped tin oxide (FTO) coated glass was cleaned using a detergent solution for 30 min with sonication. After the glass was rinsed and washed with water, the FTO coated glass was washed with ethanol and acetone. A dense compact layer of TiO2 was deposited on top of FTO by spray pyrolysis from a 20 mM titanium diisopropoxide bis(acetylacetonate) solution at 500 °C. To prepare the mp-TiO2 layer, the TiO2 nanoparticle (30 NRD, Dyesol) paste from an ethanol solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, weight ratio) was spin coated on top of the compact TiO2 layer and sintered at 500 °C (30 min). The precursor solution of the mixed perovskite was prepared by dissolving a mixture of FAI (1.1 M), PbI2 (1.15 M), MABr (0.2 M) and PbBr2 (0.2 M) in DMF
6, weight ratio) was spin coated on top of the compact TiO2 layer and sintered at 500 °C (30 min). The precursor solution of the mixed perovskite was prepared by dissolving a mixture of FAI (1.1 M), PbI2 (1.15 M), MABr (0.2 M) and PbBr2 (0.2 M) in DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (anhydrous) with a volume ratio of 4
DMSO (anhydrous) with a volume ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. A layer of (FAPbI3)0.85(MAPbBr3)0.15 (ref. 62) was coated on top of the TiO2 nanoparticle layer through spin-coating of (FAPbI3)0.85(MAPbBr3)0.15 solution in a two step program at 1000 rpm and 5000 rpm for 10 s and 30 s, respectively. During the 2nd step of spin coating, 100 μL of chlorobenzene was dropped at 15 s before the end of the spin coating. The (FAPbI3)0.85(MAPbBr3)0.15 coated substrate was annealed at 100 °C for 60 min. Spiro-IA and Spiro-OMeTAD HTMs were spin-coated at 4000 rpm for 30 s. The Spiro-OMeTAD solution was prepared by dissolving 70 mg Spiro-MeOTAD in 1 mL chlorobenzene, with additives of 49 μL Li-bis(trifluoromethanesulfonyl) imide (LiTFSI)/acetonitrile (200 mg mL−1) and 28.5 μL 4-tert-butylpyridine. For the Spiro-IA based HTM no additives were added during the solution preparation. The device fabrication was finally completed by thermal evaporation under high vacuum (<1 × 10−6 Pa) of 50 nm thick gold and 100 nm thick silver. The active area of all the PSCs was 1.02 cm2.
1. A layer of (FAPbI3)0.85(MAPbBr3)0.15 (ref. 62) was coated on top of the TiO2 nanoparticle layer through spin-coating of (FAPbI3)0.85(MAPbBr3)0.15 solution in a two step program at 1000 rpm and 5000 rpm for 10 s and 30 s, respectively. During the 2nd step of spin coating, 100 μL of chlorobenzene was dropped at 15 s before the end of the spin coating. The (FAPbI3)0.85(MAPbBr3)0.15 coated substrate was annealed at 100 °C for 60 min. Spiro-IA and Spiro-OMeTAD HTMs were spin-coated at 4000 rpm for 30 s. The Spiro-OMeTAD solution was prepared by dissolving 70 mg Spiro-MeOTAD in 1 mL chlorobenzene, with additives of 49 μL Li-bis(trifluoromethanesulfonyl) imide (LiTFSI)/acetonitrile (200 mg mL−1) and 28.5 μL 4-tert-butylpyridine. For the Spiro-IA based HTM no additives were added during the solution preparation. The device fabrication was finally completed by thermal evaporation under high vacuum (<1 × 10−6 Pa) of 50 nm thick gold and 100 nm thick silver. The active area of all the PSCs was 1.02 cm2.
2.3.1 Photovoltaic measurements of the fabricated PSCs.
A Hitachi S-4800 is a field emission scanning electron microscope was used to obtain a high resolution images of the fabricated PSCs. The current–voltage (J–V) curves were measured using a solar simulator with standard air mass 1.5 G sunlight (100 mW cm−2, WXS-155S-10: Wacom Denso Co., Japan) under ambient conditions. Moreover, the IPCE spectra were measured by applying monochromatic incident light (1 × 1016 photons cm−2) in direct current mode (CEP-2000BX, Bunkoukeiki Co., Ltd). The light intensity was calibrated to simulate solar energy with a standard silicon solar cell (PV measurements). The operational stability was tested on a solar cell resistance test system (BIR-50, Bunko-Keiki), and a Keithley instrument was used to record the J–V curves automatically.
2.4. Cyclic voltammetry measurements
The CV plots of Spiro-IA in dichloromethane solvent were obtained in the presence of a 0.1 M [N-Bu4PF6] electrolyte using a Bio-Logic SP-150 electrochemical workstation and a three-electrode system with a glass carbon electrode acting as the working electrode, a Pt electrode as the counter and Ag/AgCl as the reference electrode at a 100 mV s−1 scan rate.
3. Results and discussion
3.1. Synthesis
The synthetic routes to Spiro-IA are shown in Scheme 1. Spiro-IA was prepared from the starting materials (1) and (3). The first step of the synthesis included the C–C Suzuki coupling reaction of benzenamine and N,N-diphenyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) (1)58 with 2,5-dibromo-3,4-ethylenedioxythiophene to form 2-triphenyl amine 5-bromo-3,4-ethylenedioxy thiophene (2).59,60 The second step involved bi-borylation of 2,7-dibromo-spiro[9H-fluorene-9,9′-[9H]xanthene]-3′,6′-diol (3)61 to afford 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2-yl)-spiro[9H-fluorene-9,9′[9H]xanthene]-3′,6′-diol (4). The third and final step was a one pot C–C Suzuki coupling of compounds (2) and (4) to give the target hole transport material (Spiro-IA). The structures of the newly synthesized HTMs and their intermediates have been verified using various spectral techniques as shown in Fig. S4–S15 (see the ESI†).
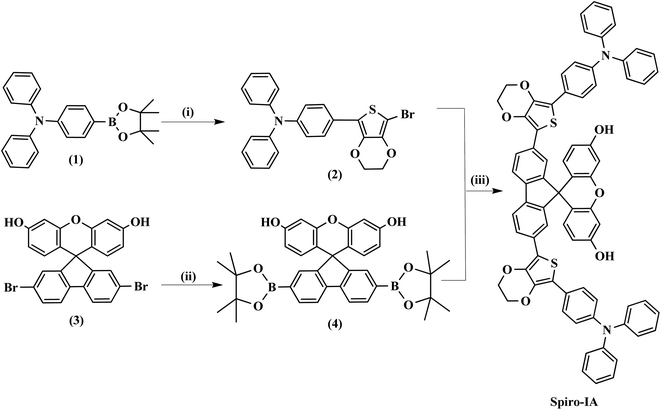 |
| | Scheme 1 The synthetic routes to Spiro-IA, where (i) 2,5-dibromo-3,4-ethylenedioxythiophene, K2CO3, Aliquat-336, Pd(PPh3)4, toluene–EtOH–H2O/90 °C, (ii) ACOK, Pd(dppf)2Cl2, DMF, 80–90 °C, bis(pinacolato)diboron, and (iii) Aliquat-336, K2CO3, Pd(PPh3)4, toluene–EtOH–H2O/90 °C. | |
3.2. Photophysical and electrochemical properties
The UV-vis absorption and emission spectra of the Spiro-IA were recorded from a CH2Cl2 solution (10−5 M) and are displayed in Fig. 2a and the detailed spectral data are collected in Table 1. It was apparent that Spiro-IA and Spiro-OMeTAD showed absorption spectra which are quite similar in the 385–430 nm wavelength range; this behavior explains the very relevant chemical composition. The UV absorption spectrum of Spiro-IA (λmax = 430 nm) is red shifted by Δλ = 42 nm compared to that of Spiro-OMeTAD (λmax = 388 nm).63 Absorption in the visible region for Spiro-IA is useful because it helps to suppress the loss of incident photons absorbed by the HTM compared to Spiro-OMeTAD which absorbs at a lower wavelength.57,63 The emission spectrum of Spiro-IA (Emax = 601 nm) is significantly red-shifted compared to that of Spiro-OMeTAD (Emax = 414 nm)53 by ΔEmax = 187 nm. For this reason, we can expect the newly designed HTM (Spiro-IA) to have a better effect on improving the power conversion efficiency of fabricated PSCs compared to that of Spiro-OMeTAD based PSCs. Stokes shifts were calculated from the difference between the maxima of the absorption and emission spectra. The Spiro-IA obviously has a larger Stokes shift (171 nm) compared to Spiro-OMeTAD (28 nm). The large Stokes shift of Spiro-IA could be an attractive sign that there is a significant structural change between the ground and the excited states, resulting in good molecular flexibility of the excited state. In addition, the large Stokes shift coupled with the small molecular size of Spiro-IA would be synergistic for infiltration and pore-filling of an HTM by simple annealing or light soaking post-treatment.36,64 In general, larger Stokes shifts help provide better stability, good solubility, small exciton binding energy, and higher hole mobility and are more beneficial for improving the performance of PSCs.65 Additionally, when the UV-Vis spectrum of Spiro-IA was measured on compact TiO2/mp-TiO2, showed red-shifted electronic absorption in the 450–550 nm region compared to Spiro-OMeTAD, which confirms the ability of Spiro-IA to suppress the loss of incident photons absorbed, as shown in Fig. 2b. Further, the HTMs fabricated on top of the perovskite absorber showed a similar level of absorption with an onset up to 800 nm; Fig. 2c.
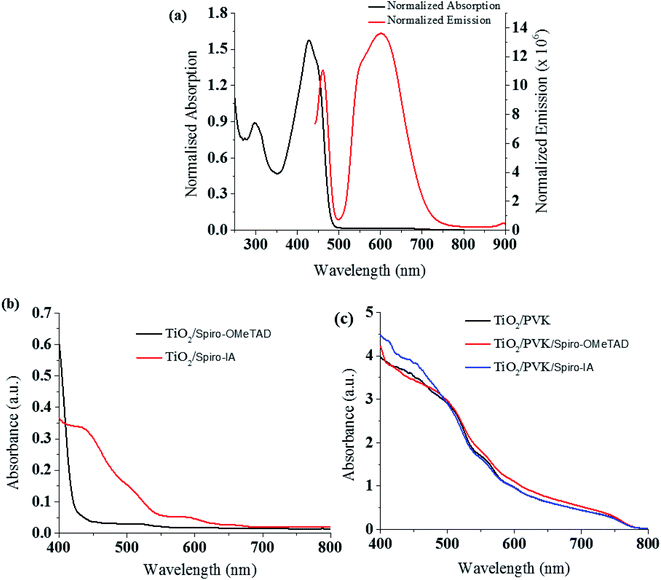 |
| | Fig. 2 (a) UV-visible absorption and emission spectra of Spiro-IA in CH2Cl2 (10−5 M). (b) UV-vis absorption spectra of Spiro-IA and Spiro-OMeTAD on TiO2 films. (c) The absorption spectra of perovskite films with different HTMs. | |
Table 1 Optical and electrochemical properties of Spiro-IA and Spiro-OMeTADa
| HTM |
λ
max (nm) |
E
max (nm) |
Stokes shift (nm) |
I (nm) |
E
0-0 (eV) |
E
oxdonset (NHE) |
GSOP (eV) |
ESOP (eV) |
σ (S cm−1) |
|
Where I : intersection of absorption and emission spectra, Emax: wavelength of maximum emission, E0-0: experimental energy gap, and σ = conductivity of the HTM without doping.
|
|
Spiro-IA
|
430 |
601 |
171 |
489 |
2.54 |
−0.537 |
−5.24 |
−2.70 |
2.10 × 10−4 |
|
Spiro-OMeTAD
|
388 |
414 |
28 |
406 |
3.05 |
0.63 |
−5.33 |
−2.28 |
9.00 × 10−8 |
The electronic properties of Spiro-IA were determined by cyclic voltammetry as shown in Fig. 3. The CV curves were registered at a 100 mV s−1 scan rate and the corresponding data are collected in Table 1. The initial oxidation potential Eoxdonset was obtained from the CV oxidation peak using eqn (1) to calculate the ground state oxidation potential (GSOP). In addition, the optical band gap (E0-0) and GSOP values were used to quantify their excited state oxidation potential (ESOP) according to eqn (2).60,66
| | | GSOP = −[Eoxdonset + 4.7]eV | (1) |
| | | ESOP = [GSOP − E0-0]eV | (2) |
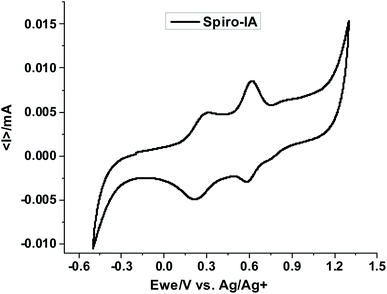 |
| | Fig. 3 The cyclic voltammetry (CV) curve for Spiro-IA. | |
As shown in Fig. 4, the estimated ground state oxidation potentials of Spiro-OMeTAD (−5.33 eV)53 and Spiro-IA (−5.24 eV) were less negative than that of the perovskite (−5.65 eV) allowing effective hole transfer mobility generated from the perovskite layer to the HTM layer. Meanwhile, the excited state oxidation potential of Spiro-OMeTAD (−2.28 eV)53 and Spiro-IA (−2.70 eV) was higher than that of perovskite, which prevents electron transport from perovskite to the counter electrode, decreasing the recombination rate. From the results, it is clear that Spiro-IA fulfilled the prerequisites for hole transfer from the perovskite to the HTM and the electron regeneration process in the fabricated devices.
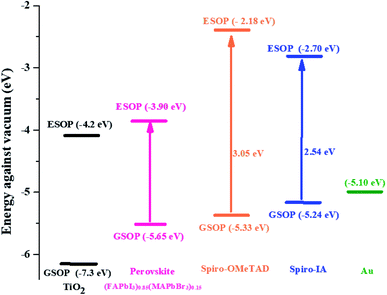 |
| | Fig. 4 Energy level diagram for perovskite solar cells. | |
Furthermore, an appropriate HTM should have high hole mobility and enough conductivity to minimize losses during hole transport to the contact.67 The conductivity (σ) is calculated using the relationships in eqn (3)–(5).68
The voltage (V) and current (J) are computed from the J–V curve output for the PSC devices. The resistance (R), electrical resistivity (ρ), conductor surface area (A), and pathlength (L) of the conductor were measured via the ellipsometry optical technique. To measure the vertical conductivity of the Spiro-IA HTM against the substrate, hole-only devices were fabricated in ITO/PEDOT:PSS/Spiro-IA/Au configuration. The conductivity of the Spiro-IA HTM is depicted in Fig. S1 and Table S1 (see the ESI†). Spiro-IA showed a conductivity of 2.104 × 10−4 S cm−1 which was slightly higher than that of Spiro-OMeTAD (σ = 9.00 × 10−8 S cm−1).69 This might be relevant to the larger conjugated system in the Spiro-IA molecule material, leading to more efficient π–π stacking in the resulting film. This indicates that the Spiro-IA HTM could contribute to better photovoltaic performance as compared to that of undoped Spiro-OMeTAD based PSCs.
3.3. Molecular modeling
In order to gain deep insights into the geometric and electronic properties of the new Spiro-IA, density functional theory (DFT) calculations were performed using Gaussian 09 (ref. 70) at the B3LYP/6-31G (d/p) level of theory71,72 to identify the optimized structural conformations as shown in Fig. 5. It was obvious that Spiro-IA exhibited highly twisted nonplanar dimensional molecular structures, most likely highlighting the reason for their high solubility in most commonly used organic solvents. Good solubility allows Spiro-IA to be easily spin-coated forming a very smooth thin film. Moreover, the electron density distribution of the HOMOs for the Spiro-IA is delocalized throughout the central fluorine core and the terminal EDOT–TPA groups, while the electron density distribution of the LUMOs is mainly located on the central fluorine core and EDOT segments. Hence, sufficient orbital overlap between the HOMOs and LUMOs suggest that fast formation of neutral excitons and hole transfer transition may take place.30
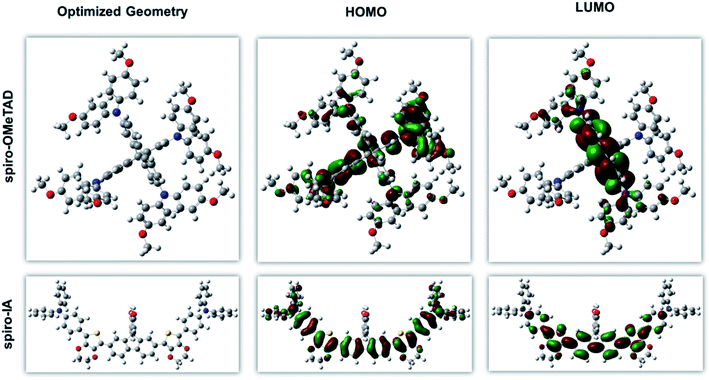 |
| | Fig. 5 Optimized geometry and simulated MO energy levels of Spiro-IA and Spiro-OMeTAD. | |
3.4. Photovoltaic measurements
We fabricated PSCs with Spiro-IA and Spiro-OMeTAD HTMs with the solar cell structure of cl-TiO2/mp-TiO2/(FAPbI3)0.85(MAPbBr3)0.15/HTMs/Au/Ag. The surface morphology of the fabricated (FAPbI3)0.85(MAPbBr3)0.15 on top of the TiO2 underlayers is shown in Fig. S2(a)† and the complete PSC is shown in Fig. S2(b).† The overall performance of the perovskite solar cell depends to a large extent on the thickness of both the absorber layer and the hole transport material. The thickness of the perovskite layer used in solar cell fabrication was 320 nm whereas the thickness of Spiro-IA was 226 nm as observed from the cross sectional scanning electron microscope (SEM) image (see the ESI, Fig. S2(c)†). For the fabrication of PSCs with Spiro-OMeTAD, a standard amount of the dopant was used as mentioned in the device fabrication section. In contrast, for the fabrication of Spiro-IA HTM based PSCs no dopant was added. It has been well described in the literature that the addition of dopants such as LiTFSI has a mixed effect on the performance of the PSC. As LiTFSI itself requires the ingress of oxygen for increasing the conductivity of Spiro-OMeTAD resulting in a higher PCE of PSCs controlling the amount of the oxidized HTM has remained critical.73Fig. 6a shows the current density–voltage (J–V) curves of PSCs based on the Spiro-IA and Spiro-OMeTAD measured under forward and reverse bias and AM1.5 G solar illumination (100 mW cm2), and the relevant PV parameters are outlined in Table 2. From the results, the doped Spiro-OMeTAD based PSCs showed a short-circuit current density (JSC) of 20.37 mA cm−2, an open-circuit voltage (VOC) of 1.057 V and a fill factor (FF) of 0.74, affording an overall PCE of 15.93% immediately after fabrication. Under the same fabrication and measurement conditions, the dopant-free Spiro-IA-based PSC provided comparable photovoltaic parameters to those of the cell based on doped Spiro-OMeTAD, showing a short-circuit current density (JSC) of 22.14 mA cm−2, an open-circuit voltage (VOC) of 1.042 V and a fill factor (FF) of 0.679, affording an overall PCE of 15.66%. Interestingly, the dopant-free Spiro-IA-based PSC outperformed the dopant-free Spiro-OMeTAD based cell which showed an inferior PCE of 9.34% with a VOC of 0.91 V, JSC of 20.64 mA cm−2, and FF of 0.54, which is mainly ascribed to the low conductivity and high charge-transport resistance of the non-doped Spiro-OMeTAD film.74 The dopant-free Spiro-IA based device showed a 1.77 mA cm−2 higher JSC value but a lower fill factor compared to the Li-doped Spiro-OMeTAD based device. The increased JSC of dopant-free Spiro-IA may be attributed to the lower band gap in the case of Spiro-IA (E0-0 = 2.54 eV) compared to Spiro-OMeTAD (E0-0 = 3.05 eV) which enhances the hole transfer mobility and decreases recombination in the PSC device. Interestingly, the forward and reverse scan J–V curves of the PSC with dopant-free Spiro-IA show negligible J–V hysteretic behavior (HI = 0.02) compared to Spiro-OMeTAD PSCs, where the HI values for dopant-free and doped Spiro-OMeTAD based PSCs are 0.39 and 0.05%, respectively, as shown in Fig. S3 (see the ESI†). The notable hysteresis values ensure better charge transfer and fewer charge trapping sites in the PSC with dopant-free Spiro-IA than in Spiro-OMeTAD. The IPCE curves of dopant-free Spiro-IA and doped Spiro-OMeTAD-based PSCs are depicted in Fig. 6b. From the results, it is clear that the IPCE spectrum of Spiro-IA exhibits a broad absorption peak in the region of 300–800 nm which shows comparable behavior to that of the cell based on Spiro-OMeTAD. In addition, from the IPCE plots of the fabricated PSCs (Fig. 6b) and the absorption plots of HTMs on the surface of TiO2 (Fig. 2b), it was obvious that the wavelength response range of PSCs in the case of Spiro-IA had slightly shifted to a longer wavelength compared to that for the Spiro-OMeTAD which improves light absorption, suppresses charge recombination, and improves charge collection helping to generate a higher photocurrent in the device and reflecting the better performance of the PSC based on the Spiro-IA HTM.75,76 Further, the PCE and IPCE values of the Spiro-IA-based PSC agree well with those of Spiro-OMeTAD based PSCs. Clearly, our present results demonstrate that the low-cost dopant-free Spiro-IA exhibited good photovoltaic performance in PSCs.
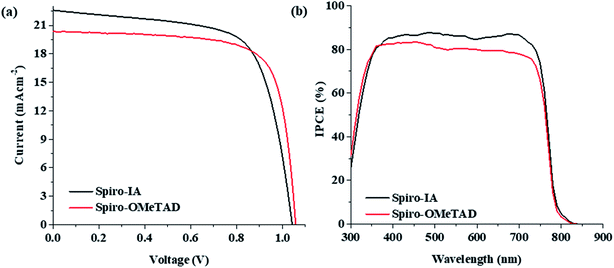 |
| | Fig. 6 (a) J–V curves; (b) IPCE curves of the fabricated PSCs with dopant-free Spiro-IA and doped Spiro-OMeTAD HTMs. | |
Table 2 Photovoltaic measurement parameters of fabricated PSCs with Spiro-IA and Spiro-OMeTAD under forward and reverse bias conditions
| HTM |
Bias condition |
J
SC (mA cm−2) |
V
OC (V) |
FF |
PCE (%) |
HIa |
|
The hysteresis index (HI) was calculated using HI = (PCEreverse − PCEforward)/PCEreverse.
|
| Dopant-free Spiro-IA |
Reverse |
21.11 |
1.003 |
0.724 |
15.33 |
0.02 |
| Forward |
22.14 |
1.042 |
0.679 |
15.66 |
|
| Dopant-free Spiro-OMeTAD |
Reverse |
19.28 |
0.829 |
0.42 |
6.71 |
0.39 |
| Forward |
20.64 |
0.91 |
0.54 |
9.34 |
|
| Doped Spiro-OMeTAD |
Reverse |
20.44 |
1.028 |
0.718 |
15.09 |
0.05 |
| Forward |
20.37 |
1.057 |
0.740 |
15.93 |
|
The laboratory cost (synthetic cost) of both Spiro-IA and Spiro-OMeTAD HTMs is shown in Table 3. The synthesis cost of Spiro-IA is calculated by using the price as provided from the chemical suppliers as mentioned in Tables S2–S5 (ESI†) and following previous attempts.77–79 The cost of our newly developed high-performance Spiro-IA HTM is almost 1/2 that of the synthetic Spiro-OMeTAD and nearly 1/9th the commercial price of Spiro-OMeTAD.79,80 This cost can further drop if large-scale synthesis is performed.
Table 3 Laboratory synthesis costs of Spiro-IA and Spiro-OMeTAD
| HTM |
Synthesis cost ($ g−1) |
Commercial price ($ g−1) |
|
Spiro-IA
|
55.28 |
— |
|
Spiro-OMeTAD
|
91.67 |
170–475 |
3.5. Durability and reproducibility of PSCs fabricated with the Spiro-IA based HTM
The long-term stability (durability) represents a great challenge for PSCs. We investigated the stability of the PSC devices based on the Spiro-IA HTM, which were kept in the dark with a humidity of 50%. The solar cell parameters of PSCs were measured over a period of 500 h to observe their performances in the long run. Fig. 7a shows the results of the shelf-stability tests with PCEs measured under forward and reverse bias scans during the first 500 h of measurement. The photovoltaic performance of Spiro-IA based PSCs did not degrade at all and showed no hysteresis behavior. During the 200th hour measurement the performance of PSCs showed a small decline to 15.07% from its initial efficiency of 15.66%. During the 336th hour measurement, a slight difference in efficiency between the forward and reverse bias scan was observed with 13.68% and 13.39%, respectively. At the 500th hour the PCE of the Spiro-IA based PSC was recorded as 11.19% and 10.8%, respectively. From the results, it was clear that Spiro-IA is a promising candidate for a stable PSC. It was clear that the hysteretic response between the forward and reverse scans observed after more than 200 hours completes the actual determination of the cell efficiency. It has recently been documented that the localization of positively charged ions at the interface between the perovskite layer and the electron transport layer (ETL) causes PCE hysteresis in PSCs. This can be attributed to the acceleration of non-radiative recombination due to the effects of localized positive ions, which often degrade PSCs.81 On the other hand, reproducibility of PSCs fabricated with the Spiro-IA HTM was studied in order to meet the commercial demand for perovskite solar cell modules. A great challenge is to realize highly reproducible PSCs with perfect thin film morphology and interfaces, which can be of great benefit to the large-area fabrication process of low-cost PSCs. Fig. 7b displays the histograms of the PCE of 10 devices. It is shown that the PCEs were distributed within 11–16%.
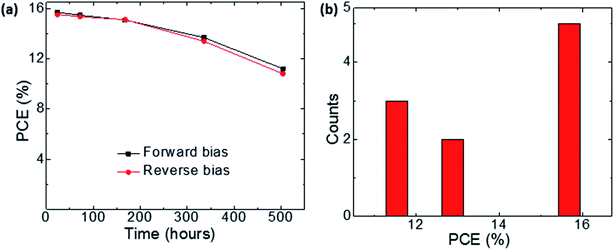 |
| | Fig. 7 (a) Stability (dark conditions); (b) reproducibility results of PSCs fabricated with the Spiro-IA based HTM. | |
4. Conclusions
In summary, we have designed a new fluorene-based HTM with D–D–π–D–D architecture as alternatives to the expensive Spiro-OMeTAD for application in PSCs. This HTM features low-cost, facile synthesis, good solubility in most organic solvents and good charge-transport ability. The photovoltaic studies on Spiro-IA in perovskite solar cells without any dopants showed excellent optoelectronic properties and a photovoltaic performance comparable to that of the Li-doped Spiro-OMeTAD. The PSC based on dopant-free Spiro-IA as the HTM afforded an overall conversion efficiency of 15.66%, thus showing a competitive photovoltaic performance compared to the cell based on Li-doped Spiro-OMeTAD (PCE = 15.93%) and outperforming the dopant-free Spiro-OMeTAD (PCE = 9.34%). Meanwhile Spiro-IA showed a higher current density of JSC = 22.14 mA cm−2 compared to the JSC = 20.37 mA cm−2 for the Spiro-OMeTAD HTM. In addition, Spiro-IA showed a conductivity of 2.104 × 10−4 S cm−1 which was slightly higher than that of Spiro-OMeTAD. These findings encourage the use of Spiro-IA as a competitive HTM in future PSC applications.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
The authors would like to thank the Department of Textile Engineering, Chemistry, and Science at NC State University, USA. Thanks to the National Institute for Materials Science, Tsukuba, Japan and the support from his work group (JSPS KAKENHI Grant No. 18H02079. J.-J. Lee and T. H. C. NRF-2016M1A2A2940912 and 2015M1A2A2054996) for helping us with device fabrication and characterization.
References
- C. Chen, S. Wu, J. Wang, S. Chen, T. Peng and R. Li, Improved photovoltaic performance of perovskite solar cells based on three-dimensional rutile TiO2 nanodendrite array film, Nanoscale, 2018, 10, 20836–20843, 10.1039/c8nr06899b.
- W. J. Yin, T. Shi and Y. Yan, Unique properties of halide perovskites as possible origins of the superior solar cell performance, Adv. Mater., 2014, 26, 4653–4658, DOI:10.1002/adma.201306281.
- D. P. McMeekin, G. Sadoughi, W. Rehman, G. E. Eperon, M. Saliba, M. T. Hörantner, A. Haghighirad, N. Sakai, L. Korte, B. Rech, M. B. Johnston, L. M. Herz and H. J. Snaith, A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells, Science, 2016, 351, 151–155, DOI:10.1126/science.aad5845.
- M. Saliba, T. Matsui, K. Domanski, J. Y. Seo, A. Ummadisingu, S. M. Zakeeruddin, J. P. Correa-Baena, W. R. Tress, A. Abate, A. Hagfeldt and M. Grätzel, Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance, Science, 2016, 354, 206–209, DOI:10.1126/science.aah5557.
- J. P. Correa-Baena, A. Abate, M. Saliba, W. Tress, T. Jesper Jacobsson, M. Grätzel and A. Hagfeldt, The rapid evolution of highly efficient perovskite solar cells, Energy Environ. Sci., 2017, 10, 710–727, 10.1039/c6ee03397k.
- L. Meng, J. You, T. F. Guo and Y. Yang, Recent Advances in the Inverted Planar Structure of Perovskite Solar Cells, Acc. Chem. Res., 2016, 49, 155–165, DOI:10.1021/acs.accounts.5b00404.
- Y. M. You, W. Q. Liao, D. Zhao, H. Y. Ye, Y. Zhang, Q. Zhou, X. Niu, J. Wang, P. F. Li, D. W. Fu, Z. Wang, S. Gao, K. Yang, J. M. Liu, J. Li, Y. Yan and R. G. Xiong, An organic-inorganic perovskite ferroelectric with large piezoelectric response, Science, 2017, 357, 306–309, DOI:10.1126/science.aai8535.
- W. Q. Liao, Y. Zhang, C. L. Hu, J. G. Mao, H. Y. Ye, P. F. Li, S. D. Huang and R. G. Xiong, A lead-halide perovskite molecular ferroelectric semiconductor, Nat. Commun., 2015, 6, 1–7, DOI:10.1038/ncomms8338.
- P. Agarwala and D. Kabra, A review on triphenylamine (TPA) based organic hole transport materials (HTMs) for dye sensitized solar cells (DSSCs) and perovskite solar cells (PSCs): Evolution and molecular engineering, J. Mater. Chem. A, 2017, 5, 1348–1373, 10.1039/c6ta08449d.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, Organometal halide perovskites as visible-light sensitizers for photovoltaic cells, J. Am. Chem. Soc., 2009, 131, 6050–6051, DOI:10.1021/ja809598r.
- M. Saliba, S. Orlandi, T. Matsui, S. Aghazada, M. Cavazzini, J. P. Correa-Baena, P. Gao, R. Scopelliti, E. Mosconi, K. H. Dahmen, F. De Angelis, A. Abate, A. Hagfeldt, G. Pozzi, M. Graetzel and M. K. Nazeeruddin, A molecularly engineered hole-transporting material for efficient perovskite solar cells, Nat. Energy, 2016, 1, 1–7, DOI:10.1038/nenergy.2015.17.
- S. S. Shin, E. J. Yeom, W. S. Yang, S. Hur, M. G. Kim, J. Im, J. Seo, J. H. Noh and S. Il Seok, Colloidally prepared La-doped BaSnO3 electrodes for efficient,
photostable perovskite solar cells, Science, 2017, 356, 167–171, DOI:10.1126/science.aam6620.
- Y. Liu, B. He, J. Duan, Y. Zhao, Y. Ding, M. Tang, H. Chen and Q. Tang, Poly(3-hexylthiophene)/zinc phthalocyanine composites for advanced interface engineering of 10.03%-efficiency CsPbBr3 perovskite solar cells, J. Mater. Chem. A, 2019, 7, 12635–12644, 10.1039/c9ta01151j.
- W. Liao, D. Zhao, Y. Yu, C. R. Grice, C. Wang, A. J. Cimaroli, P. Schulz, W. Meng, K. Zhu, R. G. Xiong and Y. Yan, Lead-free inverted planar Formamidinium Tin Triiodide perovskite solar cells achieving power conversion efficiencies up to 6.22%, Adv. Mater., 2016, 28, 9333–9340, DOI:10.1002/adma.201602992.
- S. Ameen, M. A. Rub, S. A. Kosa, K. A. Alamry, M. S. Akhtar, H. S. Shin, H. K. Seo, A. M. Asiri and M. K. Nazeeruddin, Perovskite solar cells: Influence of hole transporting materials on power conversion efficiency, ChemSusChem, 2016, 9, 10–27, DOI:10.1002/cssc.201501228.
- R. Fan, Y. Huang, L. Wang, L. Li, G. Zheng and H. Zhou, The progress of interface design in perovskite-based solar cells, Adv. Energy Mater., 2016, 6, 1600460, DOI:10.1002/aenm.201600460.
- L. Calió, S. Kazim, M. Grätzel and S. Ahmad, Hole-transport materials for perovskite solar cells, Angew. Chem., Int. Ed., 2016, 55, 14522–14545, DOI:10.1002/anie.201601757.
- D. Bi, C. Yi, J. Luo, J. D. Décoppet, F. Zhang, S. M. Zakeeruddin, X. Li, A. Hagfeldt and M. Grätzel, Polymer-templated nucleation and crystal growth of perovskite films for solar cells with efficiency greater than 21%, Nat. Energy, 2016, 1, 1–5, DOI:10.1038/nenergy.2016.142.
- H. Yoon, S. M. Kang, J. K. Lee and M. Choi, Hysteresis-free low-temperature-processed planar perovskite solar cells with 19.1% efficiency, Energy Environ. Sci., 2016, 9, 2262–2266, 10.1039/c6ee01037g.
- D. Bi, W. Tress, M. I. Dar, P. Gao, J. Luo, C. Renevier, K. Schenk, A. Abate, F. Giordano, J. P. Correa Baena, J. D. Decoppet, S. M. Zakeeruddin, M. K. Nazeeruddin, M. Grätzel and A. Hagfeldt, Efficient luminescent solar cells based on tailored mixed-cation perovskites, Sci. Adv., 2016, 2, e1501170, DOI:10.1126/sciadv.1501170.
- J. Melas-Kyriazi, I. K. Ding, A. Marchioro, A. Punzi, B. E. Hardin, G. F. Burkhard, N. Tétreault, M. Grätzel, J. E. Moser and M. D. McGehee, The effect of hole transport material pore filling on photovoltaic performance in solid-state dye-sensitized solar cells, Adv. Energy Mater., 2011, 1, 407–414, DOI:10.1002/aenm.201100046.
- A. Dualeh, T. Moehl, M. K. Nazeeruddin and M. Grätzel, Temperature dependence of transport properties of spiro-MeOTAD as a hole transport material in solid-state dye-sensitized solar cells, ACS Nano, 2013, 7, 2292–2301, DOI:10.1021/nn4005473.
- M. Wang, S. J. Moon, D. Zhou, F. Le Formal, N. L. Cevey-Ha, R. Humphry-Baker, C. Grätzel, P. Wang, S. M. Zakeeruddin and M. Grätzel, Enhanced-light-harvesting amphiphilic ruthenium dye for efficient solid-state dye-sensitized solar cells, Adv. Funct. Mater., 2010, 20, 1821–1826, DOI:10.1002/adfm.200902396.
- T. P. I. Saragi, T. Spehr, A. Siebert, T. Fuhrmann-Lieker and J. Salbeck, Spiro compounds for
organic optoelectronics, Chem. Rev., 2007, 107, 1011–1065, DOI:10.1021/cr0501341.
- J. M. Tour, R. Wu and J. S. Schumm, Approaches to orthogonally fused conducting polymers for molecular electronics, J. Am. Chem. Soc., 1990, 112, 5662–5663, DOI:10.1021/ja00170a053.
- B. Xu, E. Sheibani, P. Liu, J. Zhang, H. Tian, N. Vlachopoulos, G. Boschloo, L. Kloo, A. Hagfeldt and L. Sun, Carbazole-based hole-transport materials for efficient solid-state dye-sensitized solar cells and perovskite solar cells, Adv. Mater., 2014, 26, 6629–6634, DOI:10.1002/adma.201402415.
- J. Wang, S. Wang, X. Li, L. Zhu, Q. Meng, Y. Xiao and D. Li, Novel hole transporting materials with a linear π-conjugated structure for highly efficient perovskite solar cells, Chem. Commun., 2014, 50, 5829–5832, 10.1039/c4cc01637h.
- L. Zheng, Y. H. Chung, Y. Ma, L. Zhang, L. Xiao, Z. Chen, S. Wang, B. Qu and Q. Gong, A hydrophobic hole transporting oligothiophene for planar perovskite solar cells with improved stability, Chem. Commun., 2014, 50, 11196–11199, 10.1039/c4cc04680c.
- J. A. Christians, R. C. M. Fung and P. V. Kamat, An inorganic hole conductor for organo-lead halide perovskite solar cells: improved hole conductivity with copper iodide, J. Am. Chem. Soc., 2014, 136, 758–764, DOI:10.1021/ja411014k.
- A. Krishna, D. Sabba, H. Li, J. Yin, P. P. Boix, C. Soci, S. G. Mhaisalkar and A. C. Grimsdale, Novel hole transporting materials based on triptycene core for high efficiency mesoscopic perovskite solar cells, Chem. Sci., 2014, 5, 2702–2709, 10.1039/c4sc00814f.
- H. Choi, S. Park, M. S. Kang and J. Ko, Efficient, symmetric oligomer hole transporting materials with different cores for high performance perovskite solar cells, Chem. Commun., 2015, 51, 15506–15509, 10.1039/c5cc05814g.
- P. Qin, N. Tetreault, M. I. Dar, P. Gao, K. L. McCall, S. R. Rutter, S. D. Ogier, N. D. Forrest, J. S. Bissett, M. J. Simms, A. J. Page, R. Fisher, M. Grätzel and M. K. Nazeeruddin, A novel oligomer as a hole transporting material for efficient perovskite solar cells, Adv. Energy Mater., 2015, 5, 1400980, DOI:10.1002/aenm.201400980.
- Y. Song, S. Lv, X. Liu, X. Li, S. Wang, H. Wei, D. Li, Y. Xiao and Q. Meng, Energy level tuning of TPB-based hole-transporting materials for highly efficient perovskite solar cells, Chem. Commun., 2014, 50, 15239–15242, 10.1039/c4cc06493c.
- J. W. Jung, C. C. Chueh and A. K. Y. Jen, High-performance semitransparent perovskite solar cells with 10% power conversion efficiency and 25% average visible transmittance based on transparent CuSCN as the hole-transporting material, Adv. Energy Mater., 2015, 5, 1500486, DOI:10.1002/aenm.201500486.
- G. A. Sepalage, S. Meyer, A. Pascoe, A. D. Scully, F. Huang, U. Bach, Y. B. Cheng and L. Spiccia, Copper(I) iodide as hole-conductor in planar perovskite solar cells: Probing the origin of J-V hysteresis, Adv. Funct. Mater., 2015, 25, 5650–5661, DOI:10.1002/adfm.201502541.
- H. Li, K. Fu, A. Hagfeldt, M. Grätzel, S. G. Mhaisalkar and A. C. Grimsdale, A simple 3,4-ethylenedioxythiophene based hole-transporting material for perovskite solar cells, Angew. Chem., Int. Ed., 2014, 53, 4085–4088, DOI:10.1002/anie.201310877.
- H. Kim, K. G. Lim and T. W. Lee, Planar heterojunction organometal halide perovskite solar cells: Roles of interfacial layers, Energy Environ. Sci., 2016, 9, 12–30, 10.1039/c5ee02194d.
- K. G. Lim, H. B. Kim, J. Jeong, H. Kim, J. Y. Kim and T. W. Lee, Boosting the power conversion efficiency of perovskite solar cells using self-organized polymeric hole extraction layers with high work function, Adv. Mater., 2014, 26, 6461–6466, DOI:10.1002/adma.201401775.
- S. Kazim, F. J. Ramos, P. Gao, M. K. Nazeeruddin, M. Grätzel and S. Ahmad, A dopant-free linear acene derivative as a hole transport material for perovskite pigmented solar cells, Energy Environ. Sci., 2015, 8, 1816–1823, 10.1039/c5ee00599j.
- P. Ganesan, K. Fu, P. Gao, I. Raabe, K. Schenk, R. Scopelliti, J. Luo, L. H. Wong, M. Grätzel and M. K. Nazeeruddin, A simple spiro-type hole transporting material for efficient perovskite solar cells, Energy Environ. Sci., 2015, 8, 1986–1991, 10.1039/c4ee03773a.
- G. Wu, Y. Zhang, R. Kaneko, Y. Kojima, K. Sugawa, T. H. Chowdhury, A. Islam, Q. Shen, M. Akhtaruzzaman, T. Noda and J. Otsuki, Hole-transport materials containing triphenylamine donors with a spiro[fluorene-9,9′-xanthene] core for efficient and stable large area perovskite solar cells, Sol. RRL, 2017, 1, 1700096, DOI:10.1002/solr.201700096.
- H. D. Pham, S. M. Jain, M. Li, S. Manzhos, K. Feron, S. Pitchaimuthu, Z. Liu, N. Motta, H. Wang, J. R. Durrant and P. Sonar, Dopant-free novel hole-transporting materials based on quinacridone dye for high-performance and humidity-stable mesoporous perovskite solar cells, J. Mater. Chem. A, 2019, 7, 5315–5323, 10.1039/c8ta11361k.
- Y. Kim, E. H. Jung, G. Kim, D. Kim, B. J. Kim and J. Seo, Sequentially fluorinated PTAA polymers for enhancing VOC of high-performance perovskite solar cells, Adv. Energy Mater., 2018, 8, 1801668, DOI:10.1002/aenm.201801668.
- N. J. Jeon, H. G. Lee, Y. C. Kim, J. Seo, J. H. Noh, J. Lee and S. Il Seok,
O-Methoxy substituents in spiro-OMeTAD for efficient inorganic-organic hybrid perovskite solar cells, J. Am. Chem. Soc., 2014, 136, 7837–7840, DOI:10.1021/ja502824c.
- P. Gratia, A. Magomedov, T. Malinauskas, M. Daskeviciene, A. Abate, S. Ahmad, M. Grätzel, V. Getautis and M. K. Nazeeruddin, A methoxydiphenylamine-substituted carbazole twin derivative: An efficient hole-transporting material for perovskite solar cells, Angew. Chem., Int. Ed., 2015, 54, 11409–11413, DOI:10.1002/anie.201504666.
- M. H. Li, C. W. Hsu, P. S. Shen, H. M. Cheng, Y. Chi, P. Chen and T. F. Guo, Novel spiro-based hole transporting materials for efficient perovskite solar cells, Chem. Commun., 2015, 51, 15518–15521, 10.1039/c5cc04405g.
- H. Choi, J. W. Cho, M. S. Kang and J. Ko, Stable and efficient hole transporting materials with a dimethylfluorenylamino moiety for perovskite solar cells, Chem. Commun., 2015, 51, 9305–9308, 10.1039/c5cc01471a.
- D. Y. Lee, G. Sivakumar, M. Manju, R. Misra and S. Il Seok, Carbazole-based Spiro[fluorene-9,9′-xanthene] as an efficient hole-transporting material for perovskite solar cells, ACS Appl. Mater. Interfaces, 2020, 12, 28246–28252, DOI:10.1021/acsami.0c06318.
- Š. Daškevičiū
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tė, N. Sakai, M. Franckevičius, M. Daškevičienė, A. Magomedov, V. Jankauskas, H. J. Snaith and V. Getautis, Nonspiro, fluorene-based, amorphous hole transporting materials for efficient and stable perovskite solar cells, Adv. Sci., 2018, 5, 1700811, DOI:10.1002/advs.201700811.
tė, N. Sakai, M. Franckevičius, M. Daškevičienė, A. Magomedov, V. Jankauskas, H. J. Snaith and V. Getautis, Nonspiro, fluorene-based, amorphous hole transporting materials for efficient and stable perovskite solar cells, Adv. Sci., 2018, 5, 1700811, DOI:10.1002/advs.201700811.
- M. Maciejczyk, A. Ivaturi and N. Robertson, SFX as a low-cost “Spiro” hole-transport material for efficient perovskite solar cells, J. Mater. Chem. A, 2016, 4, 4855–4863, 10.1039/c6ta00110f.
- S. Gangala and R. Misra, Spiro-linked organic small molecules as hole-transport materials for perovskite solar cells, J. Mater. Chem. A, 2018, 6, 18750, 10.1039/c8ta08503j.
- D. Bi, B. Xu, P. Gao, L. Sun, M. Grätzel and A. Hagfeldt, Facile synthesized organic hole transporting material for perovskite solar cell with efficiency of 19.8%, Nano Energy, 2016, 23, 138–144, DOI:10.1016/j.nanoen.2016.03.020.
- B. Xu, J. Zhang, Y. Hua, P. Liu, L. Wang, C. Ruan, Y. Li, G. Boschloo, E. M. J. Johansson, L. Kloo, A. Hagfeldt, A. K.-Y. Jen and L. Sun, Tailor-making low-cost Spiro[fluorene-9,90-xanthene]-based 3D oligomers for perovskite solar cells, Chem, 2017, 2, 676–687, DOI:10.1016/J.CHEMPR.2017.03.011.
- R. Nakar, F. J. Ramos, C. Dalinot, P. S. Marques, C. Cabanetos, P. Leriche, L. Sanguinet, M. Kobeissi, P. Blanchard, J. Faure-Vincent, F. Tran-Van, N. Berton, J. Rousset and B. Schmaltz, Cyclopentadithiophene and fluorene Spiro-core-based hole-transporting materials for perovskite solar cells, J. Phys. Chem. C, 2019, 123, 22767–22774, DOI:10.1021/acs.jpcc.9b05931.
- M. Daskeviciene, S. Paek, Z. Wang, T. Malinauskas, G. Jokubauskaite, K. Rakstys, K. T. Cho, A. Magomedov, V. Jankauskas, S. Ahmad, H. J. Snaith, V. Getautis and M. K. Nazeeruddin, Carbazole-based enamine: Low-cost and efficient hole transporting material for perovskite solar cells, Nano Energy, 2017, 32, 551–557, DOI:10.1016/j.nanoen.2017.01.015.
- X. Li, M. Cai, Z. Zhou, K. Yun, F. Xie, Z. Lan, J. Hua and L. Han, A comparative study of o,p-dimethoxyphenyl-based hole transport materials by altering π-linker units for highly efficient and stable perovskite solar cells, J. Mater. Chem. A, 2017, 5, 10480–10485, 10.1039/c7ta02556d.
- S. Do Sung, M. S. Kang, I. T. Choi, H. M. Kim, H. Kim, M. Hong, H. K. Kim and W. I. Lee, 14.8% perovskite solar cells employing carbazole derivatives as hole transporting materials, Chem. Commun., 2014, 50, 14161–14163, 10.1039/c4cc06716a.
- I. M. Abdellah and A. El-Shafei, Influence of carbonyl group on photocurrent density of novel fluorene-based D–π–A photosensitizers: Synthesis, photophysical and photovoltaic studies, J. Photochem. Photobiol., A, 2020, 387, 112133, DOI:10.1016/j.jphotochem.2019.112133.
- I. M. Abdellah, A. I. Koraiem and A. El-Shafei, Structure-property relationship of novel monosubstituted Ru(II) complexes for high photocurrent and high efficiency DSSCs: Influence of donor versus acceptor ancillary ligand on DSSCs performance, Sol. Energy, 2019, 177, 642–651, DOI:10.1016/j.solener.2018.11.047.
- I. M. Abdellah, T. H. Chowdhury, J. J. Lee, A. Islam and A. El-Shafei, Novel dopant-free hole-transporting materials for efficient perovskite solar cells, Sol. Energy, 2020, 206, 279–286, DOI:10.1016/j.solener.2020.06.016.
- Y. Zhou, Y. Song, Y. Liu and J. Qu, A convenient one-pot preparation of spiro[fluorene-9,9′-xanthene]-3′,6′-diol derivatives, ARKIVOC, 2015, 2015, 99–109, DOI:10.3998/ark.5550190.p008.980.
- Y. Reyna, M. Salado, S. Kazim, A. Pérez-Tomas, S. Ahmad and M. Lira-Cantu, Performance and stability of mixed FAPbI3(0.85)MAPbBr3(0.15) halide perovskite solar cells under outdoor conditions and the
effect of low light irradiation, Nano Energy, 2016, 30, 570–579, DOI:10.1016/j.nanoen.2016.10.053.
- Y.-K. Wang, Z.-C. Yuan, G.-Z. Shi, Y.-X. Li, Q. Li, F. Hui, B.-Q. Sun, Z.-Q. Jiang and L.-S. Liao, Dopant-free spiro-triphenylamine/fluorene as hole-transporting material for perovskite solar cells with enhanced efficiency and stability, Adv. Funct. Mater., 2016, 26, 1375–1381, DOI:10.1002/adfm.201504245.
- W. J. Chi, P. P. Sun and Z. S. Li, A strategy to improve the efficiency of hole transporting materials: Introduction of a highly symmetrical core, Nanoscale, 2016, 8, 17752–17756, 10.1039/c6nr06116h.
- H. Liu and X. Liu, Strategy to modulate the π-bridged units in bis(4-methoxyphenyl)amine-based hole-transporting materials for improvement of perovskite solar cell performance, J. Mater. Chem. C, 2018, 6, 6816–6822, 10.1039/c8tc01891j.
- H.-Y. Wang, J.-H. Wan, H.-J. Jiang, G.-A. Wen, J.-C. Feng, Z.-J. Zhang, B. Peng, W. Huang and W. Wei, Synthesis and characterization of cross-shaped p–n diblock oligomers, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 1066–1073, DOI:10.1002/pola.21864.
- K. Rakstys, C. Igci and M. K. Nazeeruddin, Efficiency: vs. stability: Dopant-free hole transporting materials towards stabilized perovskite solar cells, Chem. Sci., 2019, 10, 6748–6769, 10.1039/c9sc01184f.
-
M. B. Heaney, Electrical conductivity and resistivity, in Electrical Measurement, Signal Processing, and Displays, ed. J. G. Webster, CRC Press, 2003, ch. 7, pp. 1–7 Search PubMed.
- M. L. Petrus, A. Music, A. C. Closs, J. C. Bijleveld, M. T. Sirtl, Y. Hu, T. J. Dingemans, T. Bein and P. Docampo, Design rules for the preparation of low-cost hole transporting materials for perovskite solar cells with moisture barrier properties, J. Mater. Chem. A, 2017, 5, 25200–25210, 10.1039/c7ta06452g.
-
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, and D. J. Fox, Gaussian 09, Revision A.02, Gaussian Inc., Wallingford CT, 2016 Search PubMed.
- J. Tirado-Rives and W. L. Jorgensen, Performance of B3LYP density functional methods for a large set of organic molecules, J. Chem. Theory Comput., 2008, 4, 297–306, DOI:10.1021/ct700248k.
- W. J. Chi, D. Y. Zheng, X. F. Chen and Z. S. Li, Optimizing thienothiophene chain lengths of D–π–D hole transport materials in perovskite solar cells for improving energy levels and hole mobility, J. Mater. Chem. C, 2017, 5, 10055–10060, 10.1039/c7tc03232c.
- W. H. Nguyen, C. D. Bailie, E. L. Unger and M. D. McGehee, Enhancing the hole-conductivity of Spiro-OMeTAD without oxygen or lithium salts by using Spiro(TFSI)2 in perovskite and dye-sensitized solar cells, J. Am. Chem. Soc., 2014, 136, 10996–11001, DOI:10.1021/ja504539w.
- D. Zhang, P. Xu, T. Wu, Y. Ou, X. Yang, A. Sun, B. Cui, H. Sun and Y. Hua, Cyclopenta[hi] aceanthrylene-based dopant-free hole-transport material for organic-inorganic hybrid and all-inorganic perovskite solar cells, J. Mater. Chem. A, 2019, 7, 5221–5226, 10.1039/c8ta12139g.
- S. Pitchaiya, M. Natarajan, A. Santhanam, V. Asokan, A. Yuvapragasam, V. Madurai Ramakrishnan, S. E. Palanisamy, S. Sundaram and D. Velauthapillai, A review on the classification of organic/inorganic/carbonaceous hole transporting materials for perovskite solar cell application, Arab. J. Chem., 2020, 13, 2526–2557, DOI:10.1016/j.arabjc.2018.06.006.
- Y. Liu, H. Zhang, Y. Zhang, B. Xu, L. Liu, G. Chen, C. Im and W. Tian, Influence of hole transport layers on internal absorption, charge recombination and collection in HC(NH2)2PbI3 perovskite solar cells, J. Mater. Chem. A, 2018, 6, 7922–7932, 10.1039/c8ta01617h.
- M. L. Petrus, T. Bein, T. J. Dingemans and P. Docampo, A low cost azomethine-based hole transporting material for perovskite photovoltaics, J. Mater. Chem. A, 2015, 3, 12159–12162, 10.1039/c5ta03046c.
- T. P. Osedach, T. L. Andrew and V. Bulović, Effect of synthetic accessibility on the commercial viability of organic photovoltaics, Energy Environ. Sci., 2013, 6, 711–718, 10.1039/c3ee24138f.
- H. D. Pham, T. T. Do, J. Kim, C. Charbonneau, S. Manzhos, K. Feron, W. C. Tsoi, J. R. Durrant, S. M. Jain and P. Sonar, Molecular engineering using an anthanthrone dye for low-cost hole transport materials: A strategy for dopant-free, high-efficiency, and stable perovskite solar cells, Adv. Energy Mater., 2018, 8, 1703007, DOI:10.1002/aenm.201703007.
- X. Liu, F. Kong, T. Cheng, W. Chen, Z. Tan, T. Yu, F. Guo, J. Chen, J. Yao and S. Dai, Tetraphenylmethane-arylamine hole-transporting materials for perovskite solar cells, ChemSusChem, 2017, 10, 968–975, DOI:10.1002/cssc.201601683.
- G. Tumen-Ulzii, T. Matsushima, D. Klotz, M. R. Leyden, P. Wang, C. Qin, J.-W. Lee, S.-J. Lee, Y. Yang and C. Adachi, Hysteresis-less and stable perovskite solar cells with a self-assembled monolayer, Commun. Mater., 2020, 1, 1–7, DOI:10.1038/s43246-020-0028-z.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0se01323d |
|
| This journal is © The Royal Society of Chemistry 2021 |
Click here to see how this site uses Cookies. View our privacy policy here.  a,
Towhid H.
Chowdhury
bc,
Jae-Joon
Lee
a,
Towhid H.
Chowdhury
bc,
Jae-Joon
Lee
 c,
Ashraful
Islam
c,
Ashraful
Islam
 *b,
Mohamad K.
Nazeeruddin
*b,
Mohamad K.
Nazeeruddin
 d,
Michael
Gräetzel
d and
Ahmed
El-Shafei
d,
Michael
Gräetzel
d and
Ahmed
El-Shafei
 *e
*e
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic), 1346 (C–N aromatic amine). 1H-NMR (500 MHz, chloroform-d, δ): 7.92 (d, J = 7.5 Hz, 2H), 7.65 (d, J = 1.6 Hz, 2H), 7.53 (dd, J = 7.5, 1.5 Hz, 2H), 7.00 (s, 2H), 6.94 (d, J = 7.5 Hz, 2H), 6.42 (d, J = 2.0 Hz, 2H), 6.15 (dd, J = 7.5, 2.0 Hz, 2H), 1.38 (s, 24H). MS: m/z (C37H39B2O7) found = 617.28799 (calcd 617.28764 for [M + H]+) with an error of ΔM = 0.5716 ppm.
C aromatic), 1346 (C–N aromatic amine). 1H-NMR (500 MHz, chloroform-d, δ): 7.92 (d, J = 7.5 Hz, 2H), 7.65 (d, J = 1.6 Hz, 2H), 7.53 (dd, J = 7.5, 1.5 Hz, 2H), 7.00 (s, 2H), 6.94 (d, J = 7.5 Hz, 2H), 6.42 (d, J = 2.0 Hz, 2H), 6.15 (dd, J = 7.5, 2.0 Hz, 2H), 1.38 (s, 24H). MS: m/z (C37H39B2O7) found = 617.28799 (calcd 617.28764 for [M + H]+) with an error of ΔM = 0.5716 ppm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 mL) and a solution of K2CO3 (2 M, 10 mL) was added and degassed under argon for 30 minutes. Then tetrakis(triphenyl-phosphine)palladium(0) (0.068 g, 0.06 mmol) was added to this solution. The reaction mixture was refluxed and stirred at 90 °C for 6 hours. The solvent in the reaction mixture was evaporated via rotovap followed by adding water and extraction with methylene chloride (40 mL × 2). The resulting yellow colored organic layer was collected, dried over Na2SO4, and purified by column chromatography utilizing a silica gel adsorbent (particle size is 63–200 μm) and eluting with chloroform to give a yellow solid. Yield (78%), and MP (278 °C). FT-IR (cm−1): 3401 (OH), 3060 & 2926 (CH of CH3), 1588 (C
20 mL) and a solution of K2CO3 (2 M, 10 mL) was added and degassed under argon for 30 minutes. Then tetrakis(triphenyl-phosphine)palladium(0) (0.068 g, 0.06 mmol) was added to this solution. The reaction mixture was refluxed and stirred at 90 °C for 6 hours. The solvent in the reaction mixture was evaporated via rotovap followed by adding water and extraction with methylene chloride (40 mL × 2). The resulting yellow colored organic layer was collected, dried over Na2SO4, and purified by column chromatography utilizing a silica gel adsorbent (particle size is 63–200 μm) and eluting with chloroform to give a yellow solid. Yield (78%), and MP (278 °C). FT-IR (cm−1): 3401 (OH), 3060 & 2926 (CH of CH3), 1588 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic), 1085 (C–N aromatic amine). 1H NMR (500 MHz, chloroform-d, δ): 7.61–7.58 (m, 12H), 7.45–7.49 (m, 8H), 7.41–7.38 (m, 14H), 7.31 (d, 4.2 Hz, 2H), 6.64 (s, 2H), 6.29–6.27 (m, 2H), 6.24–6.22 (m, 2H), 2.10 (s, 8H). MS: m/z (C73H50N2O7S2) found = 1130.30 (calcd 1131.32 for [M+]) with an error of ΔM = 1.08 ppm.
C aromatic), 1085 (C–N aromatic amine). 1H NMR (500 MHz, chloroform-d, δ): 7.61–7.58 (m, 12H), 7.45–7.49 (m, 8H), 7.41–7.38 (m, 14H), 7.31 (d, 4.2 Hz, 2H), 6.64 (s, 2H), 6.29–6.27 (m, 2H), 6.24–6.22 (m, 2H), 2.10 (s, 8H). MS: m/z (C73H50N2O7S2) found = 1130.30 (calcd 1131.32 for [M+]) with an error of ΔM = 1.08 ppm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, weight ratio) was spin coated on top of the compact TiO2 layer and sintered at 500 °C (30 min). The precursor solution of the mixed perovskite was prepared by dissolving a mixture of FAI (1.1 M), PbI2 (1.15 M), MABr (0.2 M) and PbBr2 (0.2 M) in DMF
6, weight ratio) was spin coated on top of the compact TiO2 layer and sintered at 500 °C (30 min). The precursor solution of the mixed perovskite was prepared by dissolving a mixture of FAI (1.1 M), PbI2 (1.15 M), MABr (0.2 M) and PbBr2 (0.2 M) in DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (anhydrous) with a volume ratio of 4
DMSO (anhydrous) with a volume ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. A layer of (FAPbI3)0.85(MAPbBr3)0.15 (ref. 62) was coated on top of the TiO2 nanoparticle layer through spin-coating of (FAPbI3)0.85(MAPbBr3)0.15 solution in a two step program at 1000 rpm and 5000 rpm for 10 s and 30 s, respectively. During the 2nd step of spin coating, 100 μL of chlorobenzene was dropped at 15 s before the end of the spin coating. The (FAPbI3)0.85(MAPbBr3)0.15 coated substrate was annealed at 100 °C for 60 min. Spiro-IA and Spiro-OMeTAD HTMs were spin-coated at 4000 rpm for 30 s. The Spiro-OMeTAD solution was prepared by dissolving 70 mg Spiro-MeOTAD in 1 mL chlorobenzene, with additives of 49 μL Li-bis(trifluoromethanesulfonyl) imide (LiTFSI)/acetonitrile (200 mg mL−1) and 28.5 μL 4-tert-butylpyridine. For the Spiro-IA based HTM no additives were added during the solution preparation. The device fabrication was finally completed by thermal evaporation under high vacuum (<1 × 10−6 Pa) of 50 nm thick gold and 100 nm thick silver. The active area of all the PSCs was 1.02 cm2.
1. A layer of (FAPbI3)0.85(MAPbBr3)0.15 (ref. 62) was coated on top of the TiO2 nanoparticle layer through spin-coating of (FAPbI3)0.85(MAPbBr3)0.15 solution in a two step program at 1000 rpm and 5000 rpm for 10 s and 30 s, respectively. During the 2nd step of spin coating, 100 μL of chlorobenzene was dropped at 15 s before the end of the spin coating. The (FAPbI3)0.85(MAPbBr3)0.15 coated substrate was annealed at 100 °C for 60 min. Spiro-IA and Spiro-OMeTAD HTMs were spin-coated at 4000 rpm for 30 s. The Spiro-OMeTAD solution was prepared by dissolving 70 mg Spiro-MeOTAD in 1 mL chlorobenzene, with additives of 49 μL Li-bis(trifluoromethanesulfonyl) imide (LiTFSI)/acetonitrile (200 mg mL−1) and 28.5 μL 4-tert-butylpyridine. For the Spiro-IA based HTM no additives were added during the solution preparation. The device fabrication was finally completed by thermal evaporation under high vacuum (<1 × 10−6 Pa) of 50 nm thick gold and 100 nm thick silver. The active area of all the PSCs was 1.02 cm2.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tė, N. Sakai, M. Franckevičius, M. Daškevičienė, A. Magomedov, V. Jankauskas, H. J. Snaith and V. Getautis, Nonspiro, fluorene-based, amorphous hole transporting materials for efficient and stable perovskite solar cells, Adv. Sci., 2018, 5, 1700811, DOI:10.1002/advs.201700811.
tė, N. Sakai, M. Franckevičius, M. Daškevičienė, A. Magomedov, V. Jankauskas, H. J. Snaith and V. Getautis, Nonspiro, fluorene-based, amorphous hole transporting materials for efficient and stable perovskite solar cells, Adv. Sci., 2018, 5, 1700811, DOI:10.1002/advs.201700811.





