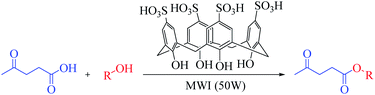
Microwave-assisted green synthesis of levulinate esters as biofuel precursors using calix[4]arene as an organocatalyst under solvent-free conditions†
Gabriel Abranches Dias
Castro
 and
Sergio Antonio
Fernandes
and
Sergio Antonio
Fernandes
 *
*
Grupo de Química Supramolecular e Biomimética (GQSB), Departamento de Química, CCE, Universidade Federal de Viçosa, Viçosa, MG 36570-900, Brazil. E-mail: santonio@ufv.br; sefernandes@gmail.com; Tel: +55-31-3612-6647
First published on 28th October 2020
Abstract
Levulinic acid, one of the top 12 value-added chemicals, can be obtained by the transformation of biomass by acid catalysis. Alkyl levulinate has been widely explored as a precursor for obtaining renewable fuel additives. In this work, the organocatalyst CX4SO3H was employed for the first time as an organocatalyst for levulinic acid esterification reactions. Various parameters, such as the temperature, reaction time, and catalyst load, were investigated. The optimized reaction conditions were a reaction temperature of 80 °C (MWI), a reaction time of 2.5 min, a CX4SO3H catalyst load of 1 mol% and solvent-free conditions. Ten different alcohols were evaluated for the synthesis of alkyl levulinate with high yields (ca. 99%), with the exception of tert-butyl alcohol (13% yield). The levulinic acid esterification reaction, a type of green chemistry reaction, has many advantages such as (i) creation of a new C–O bonds, (ii) water being the sole waste, (iii) 100% carbon economy, (iv) metal- and solvent-free processes, (v) short time and (vi) nontoxic and reusable organocatalysts. These advantages, along with the simple workup procedure, make this efficient protocol a greener alternative to the traditional methods used for the synthesis of levulinate esters to generate biofuels.
Introduction
Growing concern about environmental issues and the depletion of fossil fuels, which are the principal source of essential chemicals and fuels, have attracted significant attention in recent years from the industry and academia.1–3 The demand for renewable resources, clean energy alternatives and green chemicals derived from biomass is a field experiencing intense transformation. Among the various renewable resources, biomass, with an estimated annual production of 1 × 1011 tons per year, is considered sustainable, abundant, and biodegradable and is a renewable source of clean energy and high value-added chemicals.4Levulinic acid (LA), one of the top 12 value-added chemicals, is one of the platform chemicals from biomass screened by the United States Department of Energy (U.S. DOE) and National Renewable Energy Laboratory (NREL).5,6 As one of the most promising and sustainable platform chemicals, LA can be a versatile building block for fuel additives, resins, eco-friendly herbicides, flavour and fragrance ingredients, skin creams and degreasers, pharmaceuticals and chemical intermediates, with wide potential industrial applications.7–12
Levulinic acid-derived esters and ethers such as ethyl levulinate and 2-methyltetrahydrofuran (MTHF) are promising fuel additives. Ethyl levulinate has been studied for its use as a biobased replacement and renewable fuel additive similar to methyl tert-butyl ether (MTBE) and tertiary amyl methyl ether (TAME).13
In this context, high-efficiency catalysis is an inevitable topic of discussion in biomass transformation for sustainable synthetic chemistry. This esterification of the LA reaction is reported to be catalysed by different mineral acid catalysts, such as H2SO4, HCl, and H3PO4,14–16 various solid acids, such as sulfonic acid functionalized materials,17,18 metal oxides,19,20 MOFs,21 zeolites,22,23 supported heteropolyacids24,25 and ionic liquids.26 Conversely, the use of organocatalysts has still received little attention for esterification reactions of LA.27,28
Among the available organocatalysts, calix[n]arenes have been gaining prominence in several organic transformations in recent years.29–31 Calix[n]arenes are macrocyclic compounds composed of phenolic units that are linked by methylene or sulfur groups at the 2- and 6-positions, with defined upper and lower rims and a central annulus. The simplicity of modifying these structures makes calix[n]arenes versatile organocatalysts with Brønsted or Lewis acidity and hydrophobic cavities.29–31
Herein, we report a novel application of esterification reactions with a p-sulfonic acid calix[4]arene (CX4SO3H) organocatalyst to synthesize alkyl esters of levulinic acid under solvent-free and microwave-assisted conditions.
Experimental section
Synthesis of p-sulfonic acid calix[4]arene (CX4SO3H)
CX4SO3H is synthesized in our laboratory by following procedures published elsewhere (ESI†).32–34General procedure for the preparation of alkyl levulinates
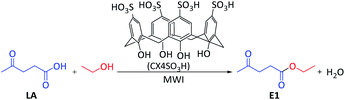
Microwave (MW) approach: a mixture of levulinic acid (1 mmol), alcohol (1 mmol) and CX4SO3H (1 mol%) was added to a vial, which was sealed and placed in a CEM Discover microwave oven. The temperature of the reaction was monitored using an internal probe. The reaction mixture was subjected to solvent-free microwave irradiation for 2.5 minutes under stirring at a temperature of 80 °C and power of 50 W (Scheme 1). Subsequently, ethyl acetate was added to the reaction mixture for organocatalyst (CX4SO3H) precipitation. The mixture was filtered, and the filtrate was evaporated under reduced pressure. Ten alkyl levulinates were obtained, and GC-MS was used for characterization.Ethyl levulinate, which formed during the reaction, was quantified based on the external standard technique. Standard solutions were prepared in methanol at concentrations of 0.25–1.5 mg mL−1 and injected into the GC-MS system (injected volume: 1 μL).
The chromatograms and mass spectra were obtained by gas chromatography-mass spectrometry using a SHIMADZU GCMS-QP2010C Ultra mass spectrometer and a method with the following specifications. Column: RTx-5 MS, 30 m, ID 0.25 mm; carrier gas: helium; injector temperature: 220 °C; oven temperature: 40 °C (2 min), increased at 5 °C min−1 up to 100 °C (held for 5 min) and increased at 30 °C min−1 up to 200 °C (held for 5 min). The calibration curve (R2 = 0.990) was obtained with respect to the mass of the standard injected into the GC-MS system (0.25–1.5 μg of E1), as presented in the ESI (Fig. S10).† The percentage yield of E1 (%) was calculated based on the calibration curve.
Catalyst recycling
The catalyst recycling experiment was conducted using a model reaction of levulinic acid, ethanol, and 1 mol% CX4SO3H used as the organocatalyst at a temperature at 80 °C for 2.5 min. After the reaction was completed, CX4SO3H was precipitated by the addition of ethyl acetate (5 mL). The mixture was then centrifuged and filtered, and CX4SO3H was recovered as a solid. CX4SO3H was oven dried at 85 °C and then added for a new reaction cycle.Results and discussion
We started our investigation by optimizing the reaction conditions: the time, temperature and organocatalyst amount under solvent-free conditions (Table 1) were tested to determine the best reaction conditions for the synthesis of ethyl levulinate (E1). For this purpose, a model reaction using LA and ethanol was chosen, and the reactions were conducted in the presence of a CX4SO3H organocatalyst under microwave irradiation (MWI). The yield was determined by standard (ethyl levulinate) calibration curve GC-MS analysis (Fig. S10†). The yield of E1 was good (92%) when the reaction time was 1 min (Table 1, entry 1), and excellent yields (>99%) were achieved for a reaction time of 2.5 min or higher (Table 1, entries 2–5). The second parameter evaluated was the temperature (Table 1, entries 2, 6 and 7).| Entry | Time (min) | T (°C) | CX4SO3H (mol%) | Yieldd (%) |
|---|---|---|---|---|
| a Reagents and conditions: levulinic acid (1.0 mmol), ethanol (1.0 mmol), MWI (50 W) and solvent-free conditions. b Conventional heating. c Gram-scale experiment: levulinic acid (1 g, 8.6 mmol), ethanol (500 μL, 8.6 mmol). d Yield was determined by standard (ethyl levulinate) calibration curve GC-MS analysis. | ||||
| 1 | 1 | 80 | 1.0 | 92 |
| 2 | 2.5 | 80 | 1.0 | >99 |
| 3 | 5 | 80 | 1.0 | >99 |
| 4 | 10 | 80 | 1.0 | >99 |
| 5 | 15 | 80 | 1.0 | >99 |
| 6 | 2.5 | 70 | 1.0 | 63 |
| 7 | 2.5 | 60 | 1.0 | 50 |
| 8 | 2.5 | 80 | 0.75 | 90 |
| 9 | 2.5 | 80 | 0.5 | 78 |
| 10 | 2.5 | 80 | — | 30 |
| 11 | 2.5 | 80 | 1.0 | 49b |
| 12 | 2.5 | 80 | 1.0 | >99c |
The E1 yield decreased to 63% and 50% when the temperature was decreased from 80 °C to 70 °C and 60 °C, respectively (Table 1, entries 2, 6 and 7). The effect of the load of the organocatalyst on the reaction efficiency was also evaluated (Table 1). Excellent yields were obtained for the 1 mol% organocatalyst load (Table 1, entry 2). The yield of E1 decreased to 90% and 78% when the amount of CX4SO3H was decreased to 0.75 mol% and 0.5 mol%, respectively (Table 1, entries 8 and 9).
A catalyst-free experiment was carried out, and only a 30% yield of E1 was observed, which suggests that the LA esterification reaction cannot achieve a high yield without any catalyst (Table 1, entry 10). To verify the importance of the heating source, an experiment was carried out using conventional heating and only a 49% yield of E1 was observed (Table 1, entry 11).
An experiment with 1 gram of levulinic acid was carried out and there was no change in E1 yield, which makes it possible to use the synthetic methodology to obtain esters in the gram-scale (Table 1, entry 12).
The E1 yield measured in the presence of the organocatalyst CX4SO3H is comparable with the values reported in the literature in the presence of different catalysts, for instance, Brønsted acid employing MWI, as shown in Table 2.
| Entry | Catalyst | Experimental conditions | Molar ratio (LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol) ethanol) |
Conversion | Reference |
|---|---|---|---|---|---|
| 1 | CX4SO3H | T = 80 °C, cat = 1 mol%, time = 2.5 min | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
>99% | This study |
| 2 | H2SO4 | T = 120 °C, cat = 2.5 mol%, time = 5 min | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
100% | 27 |
| 3 | PTSA | T = 120 °C, cat = 2.5 mol%, time = 5 min | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
100% | 27 |
| 4 | Silicotungstic acid | T = 110 °C, cat = 0.5 mol%, time = 90 min | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42 |
97% | 35 |
Leadbeater et al. (2015)27, who studied the H2SO4- or PTSA-catalysed esterification reaction of levulinic acid with ethanol, obtained results similar to those obtained in this work (Table 2, entries 1–3). However, the experimental conditions were drastic, with excess ethanol (10 times), a temperature of 120 °C, a time of 5 min and a catalyst load 2.5 times higher than that used in this work (Table 2, entries 1–3).
Haider et al. 2019,35 proposed the use of silicotungstic acid for the synthesis of ethyl levulinate with a conversion similar to that obtained in this work (Table 2, entries 1 and 4). The authors used drastic conditions, namely, a temperature of 110 °C, a 90 min reaction time and excess ethanol (42 times), compared to the present work (80 °C, 2.5 min and solvent-free).
Despite the small difference between the yields obtained with CX4SO3H and the other Brønsted acids (H2SO4, PTSA and silicotungstic acid), the use of CX4SO3H has the advantages of good thermal and chemical stability, not being corrosive, recyclability, low volatility, and low toxicity, which are important for green and sustainable chemistry.
The microwave-assisted reactions proceeded efficiently, had short reaction times, were metal- and solvent-free, had low-costs, and used an inexpensive, readily available and low-toxic catalyst compared to the catalysts reported in the literature (Table 2).
Next, using the optimal conditions (solvent-free, 2.5 min, 80 °C (MWI) and 1 mol% CX4SO3H), we examined the substrate scope of the esterification reactions by using different alcohols. The reactions using seven primary alcohols proceeded smoothly and afforded the corresponding esters in excellent yields ranging to greater than 99% (Scheme 2, E1–E7). The two secondary alcohols (Scheme 2, E8 and E9) were converted into esters in good yields, 89% each. The tert-butyl alcohol product was evaluated, and the yields were only 13% (E10).
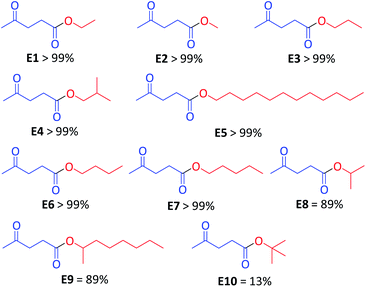 | ||
| Scheme 2 Scope of the reaction for different alcohols, employing CX4SO3H as the organocatalyst. Yield determined by GC-MS. | ||
The reaction reactivity decreased with the increase in the number of carbons linked to hydroxyl carbon (Scheme 2), and the following order of substrate reactivity was observed: primary alcohol > secondary alcohol > tertiary alcohol. Silva et al. (2020)36 and Tang et al. (2019)37 described the same order of reactivity for the alcohols observed in this work. The authors attributed the decreased reactivity to factors including steric hindrance on the hydroxyl group of the alcohol, making nucleophilic attack on the carbonyl group of the carboxylic acid difficult.
According to the principles of green chemistry and considering sustainable processes that seek to minimize chemical waste, the recovery and reuse of the catalyst is quite important.38 Therefore, after the reaction was completed, the organocatalyst was separated from the reaction mixture by the addition of ethyl acetate.
The reaction mixture was centrifuged, the organocatalyst was recovered after filtration (greater than 97% yield), and the organocatalyst was again used for five reaction cycles (Fig. 1). The organocatalytic activity of CX4SO3H was approximately constant until the fourth reaction (>95% yield) and a good yield was still obtained in the fifth reaction (87% yield).
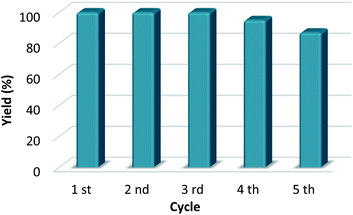 | ||
| Fig. 1 Reuse of CX4SO3H as the catalyst for the synthesis of levulinic acid-derived esters. Yield was determined by standard (ethyl levulinate) calibration curve GC-MS analysis. | ||
Conclusions
Furthermore, the potential of this reaction, in terms of coherence with green chemistry principles, was addressed, and several sustainable features of this transformation have been achieved. In particular, it was demonstrated that the conversion of LA to alkyl levulinate has many advantages, such as (i) creation of a new C–O bonds, (ii) water being the sole waste, (iii) 100% carbon economy, (iv) metal- and solvent-free processes, (v) short time and (vi) nontoxic and reusable organocatalysts. A yield of >99% could be obtained by the reaction using only 1 mol% CX4SO3H at 80 °C for 2.5 min.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful for the financial support provided by Fundação de Amparo à Pesquisa do Estado de Minas Gerais – Brazil (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico – Brazil (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES – Finance Code 001). S. A. F. is supported by Research Fellowships from CNPq.Notes and references
- G. F. David, A. M. Ríos-Ríos, Â. de Fátima, V. H. Perez and S. A. Fernandes, Ind. Crops Prod., 2019, 134, 382–387 CrossRef CAS.
- S. P. S. Pereira, J. O. S. Varejão, Â. de Fátima and S. A. Fernandes, Ind. Crops Prod., 2019, 138, 111492 CrossRef.
- M. V. Aristizábal, P. Á. Gómez and C. A. A. Cardona, Bioresour. Technol., 2015, 196, 480–489 CrossRef.
- A. Khan, Z. Man, M. Bustam, C. Kait, A. Nasrullah, Z. Ullah, A. Sarwono, P. Ahamd and N. Muhammad, J. Cleaner Prod., 2018, 170, 591–600 CrossRef CAS.
- K. Huang, S. Ma, S. Wang, Q. Li, Z. Wu, J. Liu, R. Liu and J. Zhu, Green Chem., 2019, 21, 4964–4970 RSC.
- T. Werpy and G. Petersen, Top Value Added Chemicals from Biomass: Volume I - Results of Screening for Potential Candidates from Sugars and Synthesis Gas, NREL, 2004 Search PubMed.
- E. I. Gürbüz, D. M. Alonso, J. Q. Bond and J. A. Dumesic, ChemSusChem, 2011, 4, 357–361 CrossRef.
- J. C. Serrano-Ruiz, A. Pineda, A. M. Balu, R. Luque, J. M. Campelo, A. A. Romero and J. M. Ramos-Fernández, Catal. Today, 2012, 195, 162–168 CrossRef CAS.
- K. Yan, C. Jarvis, J. Gu and Y. Yan, Renewable Sustainable Energy Rev., 2015, 51, 986–997 CrossRef CAS.
- C. Antonetti, D. Licursi, S. Fulignati, G. Valentini and A. M. R. Galletti, Catalysts, 2016, 6, 1–29 CrossRef.
- F. D. Pileidis and M. Titirici, ChemSusChem, 2016, 9, 562–582 CrossRef CAS.
- L. Yan, Q. Yao and Y. Fu, Green Chem., 2017, 19, 5527–5547 RSC.
- G. Shrivastav, T. S. Khan, M. Agarwal and M. A. Haider, ACS Sustainable Chem. Eng., 2017, 5, 7118–7127 CrossRef CAS.
- H. J. Bart, J. Reidetschläger, K. Schatka and A. Lehmann, Ind. Eng. Chem. Res., 1994, 33, 21–25 CrossRef CAS.
- Y. Liu, E. Lotero and J. G. Goodwin, J. Catal., 2006, 242, 278–286 CrossRef CAS.
- F. D. Pileidis, M. Tabassum, S. Coutts and M. Ttitirici, Chin. J. Catal., 2014, 35, 1571–1577 CrossRef.
- D. Song, S. An, B. Lu, Y. Guo and J. Leng, Appl. Catal., B, 2015, 179, 445–457 CrossRef CAS.
- B. L. Oliveira and V. Teixeira Da Silva, Catal. Today, 2014, 234, 257–263 CrossRef CAS.
- L. Peng, L. Lin, J. Zhang, J. Shi and S. Liu, Appl. Catal., A, 2011, 397, 259–265 CrossRef CAS.
- J. P. Lange, W. D. van de Graaf and R. J. Haan, ChemSusChem, 2009, 2, 437–441 CrossRef CAS.
- F. G. Cirujano, A. Corma and F. X. Llabrés i Xamena, Chem. Eng. Sci., 2015, 124, 52–60 CrossRef CAS.
- K. Y. Nandiwale and V. V. Bokade, Chem. Eng. Technol., 2015, 38, 246–252 CrossRef CAS.
- C. R. Patil, P. S. Niphadkar, V. V. Bokade and P. N. Joshi, Catal. Commun., 2014, 43, 188–191 CrossRef CAS.
- G. Pasquale, P. Vázquez, G. Romanelli and G. Baronetti, Catal. Commun., 2012, 18, 115–120 CrossRef CAS.
- K. Yan, G. Wu, J. Wen and A. Chen, Catal. Commun., 2013, 34, 58–63 CrossRef CAS.
- A. G. Khiratkar, K. R. Balinge, M. Krishnamurthy, K. K. Cheralathan, D. S. Patle, V. Singh, S. Arora and P. R. Bhagat, Catal. Lett., 2018, 148, 680–690 CrossRef CAS.
- M. P. Negus, A. C. Mansfield and N. E. Leadbeater, J. Flow Chem., 2015, 5, 148–150 CrossRef CAS.
- T. Funatomi, K. Wakasugi, T. Misaki and Y. Tanabe, Green Chem., 2006, 8, 1022–1027 RSC.
- P. A. S. Abranches, W. F. de Paiva, Â. De Fátima, F. T. Martins and S. A. Fernandes, J. Org. Chem., 2018, 83, 1761–1771 CrossRef CAS.
- R. Natalino, E. V. V. Varejão, M. J. da Silva, A. L. Cardoso and S. A. Fernandes, Catal. Sci. Technol., 2014, 4, 1369–1375 RSC.
- N. A. Liberto, J. B. Simões, S. de Paiva Silva, C. J. da Silva, L. V. Modolo, Â. de Fátima, L. M. Silva, M. Derita, S. Zacchino, O. M. P. Zuñiga, G. P. Romanelli and S. A. Fernandes, Bioorg. Med. Chem., 2017, 25, 1153–1162 CrossRef CAS.
- C. Gutsche and M. Iqbal, Org. Synth., 1990, 68, 234 CrossRef CAS.
- A. Casnati, N. Della Ca', F. Sansone, F. Ugozzoli and R. Ungaro, Tetrahedron, 2004, 60, 7869–7876 CrossRef CAS.
- S. Shinkai, T. Tsubaki, T. Sone and O. Manabe, Tetrahedron Lett., 1985, 26, 3343–3344 CrossRef CAS.
- E. Ahmad, M. I. Alam, K. K. Pant and M. A. Haider, Ind. Eng. Chem. Res., 2019, 58, 16055–16064 CrossRef CAS.
- C. B. Vilanculo, L. C. de Andrade Leles and M. J. da Silva, Waste Biomass Valorization, 2020, 11, 1895–1904 CrossRef CAS.
- F. Yang and J. Tang, ChemistrySelect, 2019, 4, 1403–1409 CrossRef CAS.
- R. A. Sheldon, Green Chem., 2017, 19, 18–43 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0se01257b |
| This journal is © The Royal Society of Chemistry 2021 |