Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Suppressing carboxylate nucleophilicity with inorganic salts enables selective electrocarboxylation without sacrificial anodes
Nathan
Corbin
,
Deng-Tao
Yang
,
Nikifar
Lazouski
,
Katherine
Steinberg
and
Karthish
Manthiram
*
Department of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, Massachusetts 02139, USA. E-mail: karthish@mit.edu
First published on 23rd September 2021
Abstract
Correction for ‘Suppressing carboxylate nucleophilicity with inorganic salts enables selective electrocarboxylation without sacrificial anodes’ by Nathan Corbin et al., Chem. Sci., 2021, DOI: 10.1039/D1SC02413B.
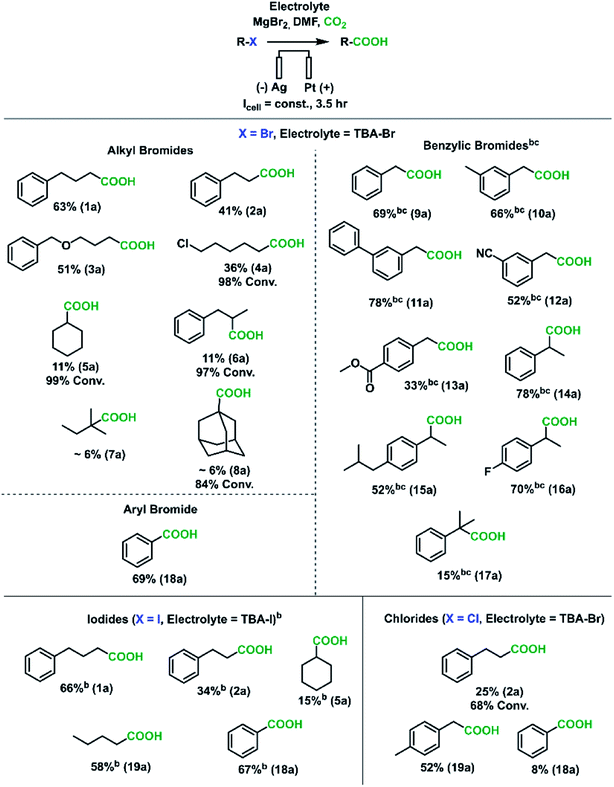
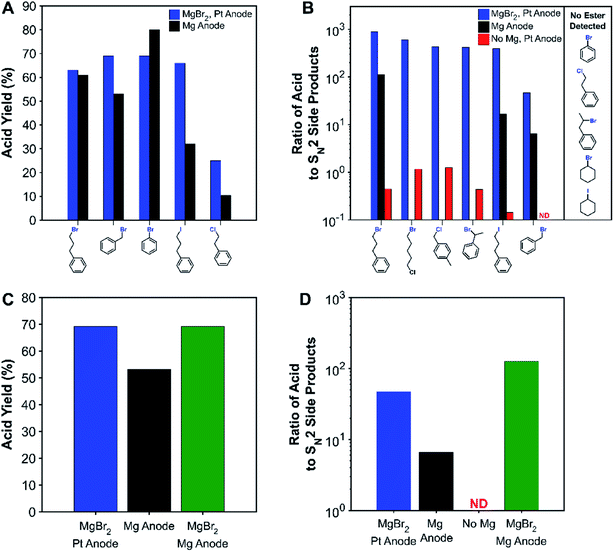
We regret that there was a minor error in the structure of the benzyl chloride in Scheme 2, Fig. 2 and the ESI. The structure of the benzyl chloride should be 4-methyl benzyl chloride but was instead given as 3-methyl benzyl. The correct figure and scheme are shown below, and the ESI has been updated.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2021 |