Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
DOI: 10.1039/D1SC90165F
(Correction)
Chem. Sci., 2021, 12, 10956-10957
Correction: Hydrogen-activation mechanism of [Fe] hydrogenase revealed by multi-scale modeling
Arndt Robert Finkelmanna,
Hans Martin Senn*b and
Markus Reiher*a
aLaboratory of Physical Chemistry, ETH Zürich, Vladimir-Prelog-Weg 2, Zürich, Switzerland. E-mail: markus.reiher@phys.chem.ethz.ch
bWestCHEM and School of Chemistry, University of Glasgow, Glasgow G12 8QQ, UK. E-mail: hans.senn@glasgow.ac.uk
Received
22nd July 2021
, Accepted 22nd July 2021
First published on 9th August 2021
Abstract
Correction for ‘Hydrogen-activation mechanism of [Fe] hydrogenase revealed by multi-scale modeling’ by Arndt Robert Finkelmann et al., Chem. Sci., 2014, 5, 4474–4482, DOI: 10.1039/C4SC01605J.
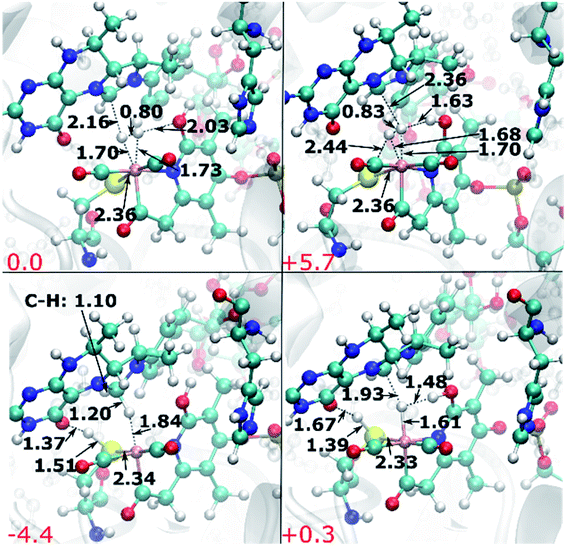
The authors regret that there were minor typographical errors in two figures. In Fig. 9 and 11, the internuclear distances were swapped. The Fe-bound hydrogen atoms are affected, where Hp is the hydrogen atom proximal to the oxypyridine ligand and Hd is the hydrogen atom distal to the oxypyridine ligand. In Fig. 9, left panel, the distance between Hp and the oxypyridine O atom was given as 1.82 Å and the distance between Hp and the Fe atom was given as 1.7 Å. However, it should read 1.82 Å between Hp and Fe and 1.70 Å between Hp and the oxypyridine O atom. In Fig. 11, top left panel, the distance between Hp and Fe was shown to be 1.70 Å and the distance between Hd and Fe was given as 1.73 Å. However, it should read 1.73 Å between Hp and Fe and 1.70 Å between Hd and Fe. The correct versions of these figures are given below. The results and conclusions are not affected by these typographical errors.
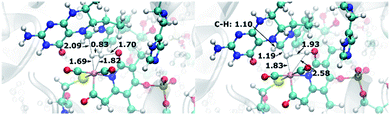 | ||
| Fig. 9 QM/MM-optimized reactant (left) and product (right) structures of the H2 cleavage reaction for the scenario with oxypyridine ligand. Distances are given in Å. | ||
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2021 |