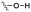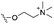Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The phospholipid membrane compositions of bacterial cells, cancer cell lines and biological samples from cancer patients†
Kira L. F.
Hilton‡
a,
Chandni
Manwani‡
ab,
Jessica E.
Boles
a,
Lisa J.
White
a,
Sena
Ozturk
a,
Michelle D.
Garrett
 *b and
Jennifer R.
Hiscock
*b and
Jennifer R.
Hiscock
 *a
*a
aSchool of Physical Sciences, University of Kent, Canterbury, Kent CT2 7NH, UK. E-mail: J.R.Hiscock@Kent.ac.uk
bSchool of Biosciences, University of Kent, Canterbury, Kent CT2 7NJ, UK. E-mail: M.D.Garrett@Kent.ac.uk
First published on 28th September 2021
Abstract
While cancer now impacts the health and well-being of more of the human population than ever before, the exponential rise in antimicrobial resistant (AMR) bacterial infections means AMR is predicted to become one of the greatest future threats to human health. It is therefore vital that novel therapeutic strategies are developed that can be used in the treatment of both cancer and AMR infections. Whether the target of a therapeutic agent be inside the cell or in the cell membrane, it must either interact with or cross this phospholipid barrier to elicit the desired cellular effect. Here we summarise findings from published research into the phospholipid membrane composition of bacterial and cancer cell lines and biological samples from cancer patients. These data not only highlight key differences in the membrane composition of these biological samples, but also the methods used to elucidate and report the results of this analogous research between the microbial and cancer fields.
Introduction
Cancer remains one of the most significant threats to human health, resulting in around 10 million deaths worldwide in 2020.1 However, it has been predicted that by the year 2050, an alarming rise in the rates of antimicrobial resistant (AMR) bacterial infections will result in approximately 10 million deaths globally per year, matching the number of deaths currently caused by cancer.2Cancer is caused by gene mutations that can progressively drive the transformation of normal human cells into a malignant form.3 Being able to therapeutically target the differences between cancer and normal cells is therefore key for the effective treatment of this disease.4 However, many currently marketed cancer drugs exhibit high levels of toxicity towards normal cells, due to the biological similarity that remains between them and their cancerous counterparts.5 This means that the therapeutic efficacy of these agents can be limited by deleterious side effects, including neuro- and cardio-toxicity.6,7 Intrinsic and acquired resistance to these therapeutic agents is also an issue.8
While bacteria possess a far greater degree of structural-diversity compared to healthy eukaryotic cells, making them easier to selectively target, AMR has given rise to bacteria that are resistant to all currently marketed antimicrobial agents.9 Moreover, a 34 year hiatus in the identification of novel antibiotics,10 primarily caused because of the costs associated with the discovery of new drugs, combined with poor market returns,9,11,12 has hampered the development of novel antimicrobial therapeutic agents to meet the growing challenge from AMR infections.
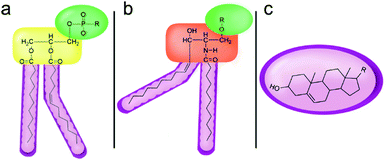
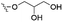
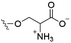
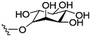
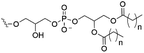
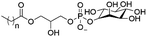
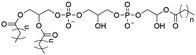
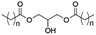
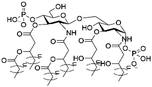
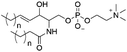
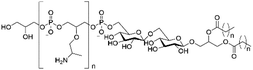
However, despite the many differences between normal human cells, their cancerous counterparts and bacteria, the cytosol of each is packaged within a cell membrane, that plays essential roles in diverse processes including cellular communication,13 energy storage14 and structural rigidity.15 A major, but more commonly overlooked constituent of this membrane are the lipids. These amphiphilic small molecules, which incorporate a hydrophilic head group and hydrophobic tail group,16 can be split into three main classes: glycerophospholipids (commonly referred to as phospholipids), sphingolipids, and sterols (mainly cholesterol in mammals).17 The general structures of these lipids are shown in Fig. 1.
Lipid molecules self-assemble, resulting in the formation of a semi-permeable bilayer, in which the polar lipid head groups interact with the aqueous environment, while the hydrophobic tail groups are concealed within the structures core. These bilayers allow passive diffusion of small neutral molecules (O2, CO2) and solvents (H2O), but prevent the diffusion of larger molecules (e.g. glucose) and ionic species (Na+, Cl−).18 The incorporation of proteins, carbohydrates and other molecular species within these phospholipid bilayers results in the production of the fully functioning cell membrane, that is able to control the composition of the intracellular environment through closely-regulated molecular transport events.18,19
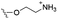
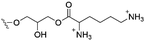
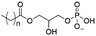
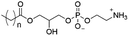
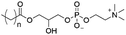
Of the three main classes of lipid found in the cell membrane, phospholipids represent the most prevalent and structurally significant. Alterations to the general chemical structure of the phospholipid head and tail groups, gives rise to an array of amphiphilic molecules, each exhibiting different physicochemical properties. A summary of these structural modifications accompanied by common phospholipid nomenclature has been provided for reference in Tables 1 and 2.20 It is important to note that fatty acid tails not only vary in length (typically between 14 and 24 carbon atoms),21 but also saturation.21,22 Furthermore, R group modifications also occur within the phospholipid head group.20 This leads to high levels of chemical and conformational diversity. Further compositional diversity within the cell membrane is brought about by variation in the relative proportion of the different lipids present within it.17 It is because of the complexity associated with these amphiphilic molecules, combined with the limitations associated with the construction of synthetic systems, such as membrane transition temperatures, availability of phospholipid samples and associated purchase costs, that we have chosen to limit the focus of our discussion within this review to the phospholipid headgroups only. However, further information relating to the alkyl component of the phospholipids discussed can be found within the ESI,† where available.
As important as the phospholipid component of a cell membrane is for cell viability, it can act as a barrier for effective drug delivery,23–25 or become the cause of cellular resistance.26,27 Alternatively, it can act as a drug target, where cell death is the desired therapeutic outcome.28–30 Therefore, understanding interactions between existing or proposed new drugs and phospholipid membranes is of great importance. Examples are emerging of novel membrane-targeting technologies, designed to alter membrane fluidity31 or permeability.32 Craik and co-workers have designed a novel class of cyclotides identified as potential anticancer therapeutic agents.33 Furthermore, O'Shea and co-workers have shown that the anticancer agent, Ophiobolin A (OPA) functions through interaction with specific phospholipids leading to the formation of cytotoxic adducts that cause irreversible lipid bilayer destabilisation.34 Gale and co-workers have developed low molecular weight synthetic anion transporter technologies;35–37 whilst Jolliffe and co-workers, have recently developed a fluorogenic probe for cell surface PS.38
Additionally, there are multiple examples of antimicrobial peptides (AMP) where the mode of action is known to rely upon interaction with, and destabilisation of, bacterial phospholipid membranes.39 Finally, within the scope of our own work, we have shown that a novel class of supramolecular self-associating amphiphiles (SSAs) can coordinate to and permeate both bacterial40 and cancer cell membranes,41 causing SSAs to be identified as anticancer agents,41 antimicrobials40,42,43 and efficacy enhancers for known therapeutic agents.41,44
The analysis herein seeks to aid the effort to understand and characterise interactions between therapeutic candidates and phospholipid membranes by creating a resource detailing the phospholipid membrane composition of cancer and bacterial cells.
Phospholipid composition of bacterial membranes
Within the scope of this review, we have focused our efforts detailing the phospholipid composition of the ESKAPE pathogens, along with the commonly used models of Gram-negative (Escherichia coli – E. coli) and Gram-positive (Bacillus subtilis – B. subtilis) organisms. The ESKAPE pathogens are a group of six nosocomial bacteria (Enterococcus faecium – E. faecium, Staphylococcus aureus – S. aureus, Klebsiella pneumoniae – K. pneumoniae, Acinetobacter baumannii – A. baumannii, Pseudomonas aeruginosa – P. aeruginosa, and Enterobacter spp.) that the CDC has listed as the ‘biggest’ threats to human health.45–50 This is due to the ability of these bacteria to evolve new resistance mechanisms in addition to those currently identified for this group of pathogens.51–53In general, the outer phospholipid membrane of bacterial cells contains mixtures of polar phospholipids such as PE and PG.54–56 Therefore, traditionally these bacterial phospholipid membranes have been modelled in synthetic systems using a mixture of PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PG in a 3
PG in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio.55,57 However, as detailed in Table 3, this is not representative of many naturally derived bacterial membranes. For example, although Gram-negative E. coli has a bacterial phospholipid composition that is very similar to these conventional model systems (PE
1 ratio.55,57 However, as detailed in Table 3, this is not representative of many naturally derived bacterial membranes. For example, although Gram-negative E. coli has a bacterial phospholipid composition that is very similar to these conventional model systems (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PG
PG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CL 75
CL 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5),57 this is not the case for Gram-positive methicillin-sensitive S. aureus U-71 (PG
5),57 this is not the case for Gram-positive methicillin-sensitive S. aureus U-71 (PG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-PG
L-PG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CL 80
CL 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5).58 Furthermore, the composition of the phospholipid membrane has also been found to be dependent on growth phase59,60 and life-cycle,61,62 as highlighted with methicillin sensitive S. aureus 209P. In log phase growth the phospholipid composition of this bacteria was found to be: PG
5).58 Furthermore, the composition of the phospholipid membrane has also been found to be dependent on growth phase59,60 and life-cycle,61,62 as highlighted with methicillin sensitive S. aureus 209P. In log phase growth the phospholipid composition of this bacteria was found to be: PG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-PG
L-PG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CL 79
CL 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 however, in stationary phase the phospholipid membrane composition alters to PG
4 however, in stationary phase the phospholipid membrane composition alters to PG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-PG
L-PG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CL 66
CL 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.63 Additionally, it is well known that phospholipid membrane composition is dependent on bacterial culture conditions. For example, P. aeruginosa grown in synthetic cystic fibrosis media (SCFM) showed lower levels of PC compared to P. aeruginosa grown in Muller–Hilton broth (MHB).64 To more accurately simulate the natural biological environment, the phospholipid composition of the bacterial were also studied in SCFM with added DOPC (SCFM-PC).65 As with SCFM only, there was a general increase in comparative quantities of those lipids present within P. aeruginosa grown in SCFM-PC, compared to that grown in MHB. This was most notable for PC and PG phospholipids. Interestingly, the minimum inhibitory concentration (MIC) values obtained for a variety of well-known antibiotics (carbenicillin, gentamicin, ciprofloxacin, colistin) against P. aeruginosa grown in these different media was also found to alter. With the bacteria grown in MHB showing higher MIC values compared to SCFM.64
14.63 Additionally, it is well known that phospholipid membrane composition is dependent on bacterial culture conditions. For example, P. aeruginosa grown in synthetic cystic fibrosis media (SCFM) showed lower levels of PC compared to P. aeruginosa grown in Muller–Hilton broth (MHB).64 To more accurately simulate the natural biological environment, the phospholipid composition of the bacterial were also studied in SCFM with added DOPC (SCFM-PC).65 As with SCFM only, there was a general increase in comparative quantities of those lipids present within P. aeruginosa grown in SCFM-PC, compared to that grown in MHB. This was most notable for PC and PG phospholipids. Interestingly, the minimum inhibitory concentration (MIC) values obtained for a variety of well-known antibiotics (carbenicillin, gentamicin, ciprofloxacin, colistin) against P. aeruginosa grown in these different media was also found to alter. With the bacteria grown in MHB showing higher MIC values compared to SCFM.64
| Bacterial strain | PG | L-PG | DAG | CL | PE | Growth phase | Analysis method |
|---|---|---|---|---|---|---|---|
| a MS = mass spectrometry. TLC = thin layer chromatography, GC = gas chromatography. | |||||||
| E. faecium (Gram +ve) | |||||||
| R446 (ref. 66) | 15 | 16 | 23 | 47 | — | Stationary | MS |
| S447 (ref. 66) | 34 | 14 | 13 | 29 | — | Stationary | MS |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| S. aureus (Gram +ve) | |||||||
| USA300 (ref. 67) | 60 | 30 | 4 | 2 | — | Log | MS |
| USA300 (ref. 67) | 62 | 20 | 3 | 4 | — | Stationary | MS |
| DSM 20233 (ref. 68) | 50 | 20 | — | 1 | — | Log | TLC |
| fakA mutant67 | 70 | 25 | 3 | 1 | — | Log | MS |
| fakA mutant67 | 60 | 22 | 5 | 6 | — | Stationary | MS |
| CB1118 (ref. 69) | 84 | 12 | — | 7 | — | Unknown | TLC |
| CB2205 (ref. 69) | 84 | 25 | — | 7 | — | Unknown | TLC |
| 209P70 | 79 | 14 | — | 4 | — | Log | TLC |
| 209P70 | 66 | 10 | — | 5 | — | Stationary | TLC |
| U-71 (ref. 58) | 80 | 12 | — | 5 | — | Unknown | TLC |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| K. pneumonia (Gram −ve) | |||||||
| Smooth mutant71 | 5 | — | — | 6.5 | 82 | Unknown | TLC |
| 005 (ref. 72) | 35 | — | — | — | 59 | Unknown | MS |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| A. baumannii (Gram −ve) | |||||||
| HO1–N73 | 10 | — | — | 7 | 73 | Unknown | GC |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| P. aeruginosa (Gram −ve) | |||||||
| PAO1 (ref. 74) | 27 | — | — | — | 69 | Stationary | MS |
| B-219 (ref. 75) | 41 | — | — | — | 65 | Unknown | MS |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Enterobacter cloacae (E. cloacae) (Gram −ve) | |||||||
| Sw1 (ref. 76) | 23 | — | — | 3.5 | 73 | Stationary | TLC |
| Sw1 (ref. 76) | 6 | — | — | 8 | 77 | Log | TLC |
| 012 (ref. 72) | 35 | — | — | — | 65 | Unknown | MS |
| 008 (ref. 72) | 42 | — | — | — | 50 | Unknown | MS |
| AZT-R76 | 17 | — | — | 2 | 80 | Log | TLC |
| AZT-R76 | 5 | — | — | 9 | 76 | Stationary | TLC |
| AMA-R76 | 17 | — | — | 3 | 79 | Log | TLC |
| AMA-R76 | 5 | — | — | 6 | 82 | Stationary | TLC |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| B. subtilis (Gram +ve) | |||||||
| I′1a (ref. 77) | 70 | 3 | — | 4 | 22 | Log | MS |
| I′1a (ref. 77) | 75 | 2 | — | 7 | 17 | Stationary | MS |
| DSM 3257 (ref. 77) | 65 | 2 | — | 4 | 35 | Log | MS |
| DSM 3257 (ref. 77) | 30 | 2 | — | 10 | 70 | Stationary | MS |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| E. coli (Gram −ve) | |||||||
| B78 | 20 | — | — | 5 | 75 | Log | TLC |
For these reasons, the culture conditions used have been detailed in the ESI (Table S1†).
As exemplified in Table 3, the phospholipid composition of AMR bacteria differs significantly from those membranes studied from analogous non-resistant bacterial strains. For example, the clinical pair of daptomycin susceptible (S447) and daptomycin resistant (R446) strains of Enterococcus faecium66 have a phospholipid composition PG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-PG
L-PG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CL
CL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DAG 34
DAG 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 and PG
13 and PG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-PG
L-PG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CL
CL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DAG 15
DAG 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47
47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 respectively.66 This was interesting, as there are a number of unique bacterial lipids that are already associated with antimicrobial resistance including lipid A and teichoic acids.79 Therefore, when considering the design and delivery of novel therapeutics to treat AMR infections where interaction with, or permeation through the cell membrane is critical, the differences in phospholipid membrane composition should also be taken into consideration.
23 respectively.66 This was interesting, as there are a number of unique bacterial lipids that are already associated with antimicrobial resistance including lipid A and teichoic acids.79 Therefore, when considering the design and delivery of novel therapeutics to treat AMR infections where interaction with, or permeation through the cell membrane is critical, the differences in phospholipid membrane composition should also be taken into consideration.
Phospholipid composition of cancer cell membranes
Although cancer initiation normally has a genetic trigger, it has been shown that changes in membrane biophysical properties occur when non-malignant cells undergo malignant transformation.80 Quantitative changes in all major classes of phospholipids caused by alterations in lipid regulation pathways, have been identified in many types of cancer cell.81 For example, upregulation of PA leads to changes in tumour metabolism, such as the activation of kinases involved in intracellular stress signalling pathways,82 while elevated levels of PC result in modulation of enzymes involved in metabolic pathways causing higher proliferation rates.81 PI is important for a number of signalling pathways involved in cellular processes such as DNA damage, cell differentiation, proliferation, survival and trafficking, with PI5P known to modulate acetylation of p53 and activity of histone deacetylases and acetylases.83PS in the outer leaflet of the cell membrane is an early marker of apoptosis and has also been shown to play a pivotal role in non-apoptotic cell death as changes in its regulation affect necrosis.84 Moreover, increased levels of PS are responsible for the weakening of the immune system in response to cancer.81 Furthermore, elevated levels of lyso-PA (Table 2) are considered a potential clinical biomarker in ovarian, colorectal85 and pancreatic cancer86,87 and, are a potential therapeutic target for novel anti-cancer treatments.88 PE is present on both the inner and outer leaflet of the plasma membrane and under normal physiological conditions it is more concentrated in the cytosolic region (inner leaflet). However, in cancer cells, this distribution is reversed with PE being more concentrated in the outer leaflet of the plasma membrane.81
In addition to those phospholipids detailed within the scope of this review, the levels of plasmalogens (a subclass of cell membrane glycerophospholipids)89 have also been linked to cancer, as effectively reviewed by Messias and co-workers.90 This review summarises the regulation of plasmalogens in patients suffering from liver, colon, oesophageal, colorectal and pancreatic cancer. Therefore these data have not be reproduced herein, although we do urge the readers of this review to consider this class of lipids within the study of phospholipid cancer cell membranes, the importance of which has been further highlighted by the work of Fernandes and co-workers.91
Table 4 summarises the changes in phospholipid composition in cancer versus control samples as defined within the table. We note that there are a number of distinct sample types, exemplified by cancer cell lines and biological samples from cancer patients including tissue, plasma and urine. For this review we have focussed on human cancer cell lines and tissues from patients and have omitted other biological sample types such as urine and plasma, which are not representative of cells. We have also only included those data sets that provide a comparison between the cancer sample and a control sample. Two key examples of this are the sampling of adjacent normal tissue when tumour tissue is analysed from a cancer patient, and the use of a non-cancerous cell line (e.g. MCF10A for breast cancer) when a cancer cell line is being studied. This is important as it relates to the question of the differences between the normal versus cancer cell, which is then translated into the identification of candidate cancer specific biomarkers. Whilst quantitative numerical data on phospholipid content is provided, there is no standardised format in which these data have been reported. It is often summarised in tables that show whether a specific phospholipid is upregulated or downregulated in the cancer versus control sample, therefore this is the approach we have taken when summarising these data in Tables 4 and S2.† Within Table 4, an upwards arrow represents upregulation of a specific phospholipid type and a downwards arrow represents downregulation of this phospholipid in the cancer versus control sample. Where there are both upwards and downwards arrows present, this reflects the fact there will be multiple forms of a particular phospholipid present in a cell, due to different alkyl chain lengths and their degree of saturation. Some of these maybe upregulated in a cancer cell, whereas others maybe downregulated.
| Sample type | PC | PE | PI | PG | PS | SM | PA | Control |
|---|---|---|---|---|---|---|---|---|
| Lung | ||||||||
| Tissue92 |
|
|
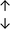
|
— |
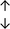
|
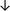
|
— | Matched distant lung tissue |
| Tissue93 |

|

|
— | — | — | — | — | Distal non-cancerous tissue |
| Tissue94 |

|
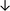
|
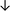
|
— | — |
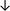
|

|
Adjacent normal tissue |
| Tissue95 |

|
— | — | — | — |

|
— | Adjacent normal lung tissue |
| Tissue96 |

|
— | — | — | — |
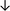
|
— | Normal lung tissue |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Breast | ||||||||
| Cell lines97 |

|
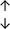
|
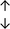
|
— | — |
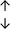
|
— | MCF10A cell line |
| Cell lines97 |

|

|

|
— | — |
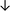
|
— | MCF10A cell line |
| Cell lines97 |

|
— |

|
— | — |

|
— | MCF10A cell line |
| Cell lines98 |

|
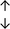
|
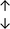
|
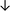
|

|
— | — | MCF10A cell line |
| Cell lines98 |

|

|

|

|

|
— | — | MCF10A cell line |
| Tissue94 |
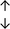
|
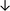
|
|
— | — |
|
|
Adjacent normal tissue |
| Tissue99 |

|

|

|
— | — |

|
— | Adjacent normal tissue |
| Tissue100 |

|
— | — | — | — |

|
— | Adjacent normal breast tissue |
| Tissue101 |

|

|

|
— | — |
|
— | Normal breast tissue |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Prostate | ||||||||
| Cell lines102 |

|

|
— | — | — |

|
— | PNT1a cell line |
| Tissue103 |

|

|

|
— | — |

|
— | Tissue from healthy males |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Colorectal | ||||||||
| Tissue104 | — | — | — |

|

|
— |

|
Adjacent normal mucosa |
| Tissue94 |
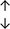
|
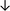
|
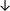
|
— | — |
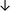
|
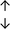
|
Adjacent normal tissue |
| Tissue105 |

|

|

|
— |
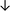
|
— | — | Adjacent normal mucosa |
| Tissue106 |

|

|
— | — | — | — | — | Adjacent normal mucosa |
| Tissue107 |

|

|
— |

|

|

|
— | Tumour adjacent tissue |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Colorectal liver metastasis | ||||||||
| Tissue108 |
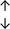
|
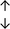
|
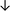
|
— |
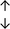
|

|
— | Normal liver parenchyma |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Ovarian | ||||||||
| Tissue109 |

|
— |

|
— | — | — | — | Adjacent normal tissue |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Urothelial cancer of the bladder | ||||||||
| Tissue110 |

|

|

|
— |

|
— | — | Benign adjacent tissue |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Gastric | ||||||||
| Tissue94 |

|
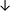
|
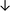
|
— | — |
|

|
Adjacent normal tissue |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Oesophageal | ||||||||
| Tissue94 |
|
|
|
— | — |
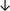
|
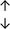
|
Adjacent normal tissue |
| Tissue111 | — |

|

|

|

|
— |

|
Matched healthy oesophageal epithelium |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Thyroid | ||||||||
| Tissue94 |

|
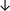
|
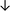
|
— | — |
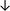
|

|
Adjacent normal tissue |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Hepatocellular | ||||||||
| Tissue112 |
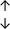
|
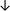
|
— | — | — |
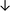
|
— | Benign tissue |
From the data summarised in Table 4, we observe a general trend in which more phospholipids are upregulated in the cancer versus the control sample, than downregulated. PC is generally upregulated in both breast cancer cell lines and tissue while the regulation of the remaining phospholipids present appears to be sample specific. A key limitation is the number of reports available for a particular cancer type, which for some such as bladder cancer, is limited to just one. Finally, a key factor in cancer treatment failure is drug resistance. Due to data limitations, it is currently not possible to comment on the differences between the phospholipid content of drug sensitive versus resistant cancer cells/samples.
Conclusions
An increased understanding of cell membrane phospholipid composition in cancer and bacterial cells can provide opportunities to develop novel treatment strategies for cancer and AMR, and thereby begin to countermeasure shortfalls of current therapies. It is the intention that this review of current knowledge into the membrane composition of cancer and AMR cells and, cancerous tissue from cancer patients will aid those wishing to develop novel membrane transport technologies, drug delivery technologies, drug adjuvant technologies, therapeutics, or repurpose approved therapeutics against these diseases. While it is clear that a far greater library of such data would aid current research in these areas, it is also apparent that phospholipid composition studies in the microbiology and cancer fields are undertaken on a different basis. Specifically, we believe that the inclusion of percentage phospholipid composition data, as well as studies into the differences between the phospholipid content of drug sensitive versus resistant cancer cells/samples will greatly enhance the applicability of these data.Data availability
The datasets supporting this article have been uploaded as part of the ESI† and article references.Author contributions
Kira L. F. Hilton: investigation; validation; writing – original draft. Chandni Manwani: investigation; validation; writing – original draft. Jessica E. Boles: investigation; writing – original draft. Lisa J. White: supervision; writing – original draft. Sena Ozturk: writing – original draft. Michelle D. Garrett: funding acquisition; project administration; supervision; writing – review & editing. Jennifer R. Hiscock: conceptualization; funding acquisition; project administration; supervision; writing – original draft, review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
KH would like to thank the University of Kent for funding. CM would like to thank the Jane Irons Scholarship fund for the funding of her PhD studentship. JH and MG would like to thank Prof. Dan Mulvihill and Dr John Stolz for their advice during the drafting of this review. JB would like to thank Public Health England and the University of Kent for PhD studentship funding. MDG would like to thank the University of Kent, the Jane Irons Scholarship fund and EB Hutchinson Charitable Trust for funding. JH and LW would like to thank the UKRI for JH's Future Leaders Fellowship (MR/T020415/1).Notes and references
- H. Sung, J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal, F. Bray and C. A. Cancer, J. Clin., 2021, 71, 209–249 Search PubMed.
- S. Davies, J. Farrar, J. Rex, L. White, R. Murry and J. O'Neill, Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations, 2014 Search PubMed.
- R. A. Weinberg and D. Hanahan, Cell, 2000, 100, 57–70 CrossRef.
- P. L. Bedard, D. M. Hyman, M. S. Davids and L. L. Siu, Lancet, 2020, 395, 1078–1088 CrossRef CAS.
- C. S. Cleeland, J. D. Allen, S. A. Roberts, J. M. Brell, S. A. Giralt, A. Y. Khakoo, R. A. Kirch, V. E. Kwitkowski, Z. Liao and J. Skillings, Nat. Rev. Clin. Oncol., 2012, 9, 471 CrossRef CAS PubMed.
- J. B. Stone and L. M. DeAngelis, Nat. Rev. Clin. Oncol., 2016, 13, 92 CrossRef CAS PubMed.
- J. Herrmann, Nat. Rev. Cardiol., 2020, 17, 503–522 CrossRef CAS PubMed.
- C. Holohan, S. Van Schaeybroeck, D. B. Longley and P. G. Johnston, Nat. Rev. Cancer, 2013, 13, 714–726 CrossRef CAS PubMed.
- E. D. Brown and G. D. Wright, Nature, 2016, 529, 336–343 CrossRef CAS PubMed.
- L. L. Silver, Clin. Microbiol. Rev., 2011, 24, 71–109 CrossRef CAS PubMed.
- J. A. DiMasi, H. G. Grabowski and R. W. Hansen, J. Health Econ., 2016, 47, 20–33 CrossRef PubMed.
- M. J. Renwick, D. M. Brogan and E. Mossialos, J. Antibiot., 2016, 69, 73–88 CrossRef CAS PubMed.
- H. Sunshine and M. L. Iruela-Arispe, Curr. Opin. Lipidol., 2017, 28, 408–413 CrossRef CAS PubMed.
- M. T. Nakamura, B. E. Yudell and J. J. Loor, Prog. Lipid Res., 2014, 53, 124–144 CrossRef CAS PubMed.
- D. Casares, P. V. Escribá and C. A. Rosselló, Int. J. Mol. Sci., 2019, 9, 2167 CrossRef PubMed.
- M. Alagumuthu, D. Dahiya and P. Singh Nigam, AIMS Mol. Sci., 2019, 6, 1–19 CAS.
- T. Harayama and H. Riezman, Nat. Rev. Mol. Cell, Biol., 2018, 19, 281–296 CrossRef CAS PubMed.
- G. M. Cooper, The Cell: A molecular Approach, Sinauer Associates, Sunderland (MA), 2nd edn, 2000 Search PubMed.
- J. Gruenberg and M. J. Clague, Curr. Opin. Cell Biol., 1992, 4, 593–599 CrossRef CAS PubMed.
- C. Sohlenkamp and O. Geiger, FEMS Microbiol. Rev., 2016, 40, 133–159 CrossRef CAS PubMed.
- D. A. Los and N. Murata, Biochim. Biophys. Acta, Biomembr., 2004, 1666, 142–157 CrossRef CAS PubMed.
- B. Alberts, A. Johnson, J. Lewis, M. Raff, K. Roberts and P. Walter, Mol. Biol. Cell. Garl. Sci., 2008, 617–1115 Search PubMed.
- B. Chertok, M. J. Webber, M. D. Succi and R. Langer, Mol. Pharm., 2013, 10, 3531–3543 CrossRef CAS PubMed.
- G. Tiwari, R. Tiwari, B. Sriwastawa, L. Bhati, S. Pandey, P. Pandey and S. K. Bannerjee, Int. J. Pharm. Investig., 2012, 2, 2–11 CrossRef PubMed.
- T. M. Allen and P. R. Cullis, Science, 2004, 303, 1818–1822 CrossRef CAS PubMed.
- A. York, Nat. Rev. Microbiol., 2020, 18, 63 Search PubMed.
- C. P. J. Vendrik, J. J. Bergers, W. H. De Jong and P. A. Steerenberg, Cancer Chemother. Pharmacol., 1992, 29, 413–429 CrossRef CAS PubMed.
- Y. Ma, K. Gao, H. Yu, W. Liu, Y. Qin, R. Xing, S. Liu and P. Li, Carbohydr. Polym., 2021, 261, 117821 CrossRef CAS PubMed.
- H. Cheng, R.-R. Zheng, G.-L. Fan, J.-H. Fan, L.-P. Zhao, X.-Y. Jiang, B. Yang, X.-Y. Yu, S.-Y. Li and X.-Z. Zhang, Biomaterials, 2019, 188, 1–11 CrossRef CAS PubMed.
- M. Rucevic, D. Hixson and D. Josic, Electrophoresis, 2011, 32, 1549–1564 CrossRef CAS PubMed.
- D. B. Goldstein, Annu. Rev. Pharmacol. Toxicol., 1984, 24, 43–64 CrossRef CAS PubMed.
- K. Hu, Y. Jiang, Y. Xie, H. Liu, R. Liu, Z. Zhao, R. Lai and L. Yang, J. Phys. Chem. B, 2015, 119, 8553–8560 CrossRef CAS PubMed.
- S. Troeira Henriques, Y.-H. Huang, S. Chaousis, C. K. Wang and D. J. Craik, ChemBioChem, 2014, 15, 1956–1965 CrossRef CAS PubMed.
- C. Chidley, S. A. Trauger, K. Birsoy and E. K. O'Shea, Elife, 2016, 5, e14601 CrossRef PubMed.
- P. A. Gale, Chem, 2020, 6, 2873–2875 CAS.
- E. N. W. Howe and P. A. Gale, J. Am. Chem. Soc., 2019, 141, 10654–10660 CrossRef CAS PubMed.
- M. Fares, X. Wu, D. Ramesh, W. Lewis, P. A. Keller, E. N. W. Howe, R. Pérez-Tomás and P. A. Gale, Angew. Chemie, 2020, 132, 17767–17774 CrossRef.
- V. E. Zwicker, B. L. Oliveira, J. H. Yeo, S. T. Fraser, G. J. L. Bernardes, E. J. New and K. A. Jolliffe, Angew. Chemie, 2019, 131, 3119–3123 CrossRef.
- M. Magana, M. Pushpanathan, A. L. Santos, L. Leanse, M. Fernandez, A. Ioannidis, M. A. Giulianotti, Y. Apidianakis, S. Bradfute, A. L. Ferguson, A. Cherkasov, M. N. Seleem, C. Pinilla, C. de la Fuente-Nunez, T. Lazaridis, T. Dai, R. A. Houghten, R. E. W. Hancock and G. P. Tegos, Lancet Infect. Dis., 2020, 20, e216–e230 CrossRef CAS PubMed.
- L. J. White, J. E. Boles, N. Allen, L. S. Alesbrook, M. J. Sutton, C. K. Hind, K. L. F. Hilton, L. R. Blackholly, R. J. Ellaby, G. T. Williams, D. P. Mulvihill and J. R. Hiscock, J. Mater. Chem. B, 2020, 8, 4694–4700 RSC.
- N. O. Dora, E. Blackburn, J. E. Boles, G. T. Williams, L. J. White, S. E. G. Turner, J. D. Hothersall, T. Askwith, J. A. Doolan, D. P. Mulvihill, M. D. Garrett and J. R. Hiscock, RSC Adv., 2021, 11, 14213–14217 RSC.
- S. N. Tyuleva, N. Allen, L. J. White, A. Pépés, H. J. Shepherd, P. J. Saines, R. J. Ellaby, D. P. Mulvihill and J. R. Hiscock, Chem. Commun., 2018, 55, 95–98 RSC.
- G. Townshend, G. S. Thompson, L. J. White, J. R. Hiscock and J. L. Ortega-Roldan, Chem. Commun., 2020, 56, 4015–4018 RSC.
- J. E. Boles, R. J. Ellaby, H. J. Shepherd and J. R. Hiscock, RSC Adv., 2021, 11, 9550–9556 RSC.
- R. E. Nelson, K. M. Hatfield, H. Wolford, M. H. Samore, R. D. Scott II, S. C. Reddy, B. Olubajo, P. Paul, J. A. Jernigan and J. Baggs, Clin. Infect. Dis., 2021, 72, S17–S26 CrossRef PubMed.
- S. L. Solomon and K. B. Oliver, Am. Fam. Physician, 2014, 89, 938 Search PubMed.
- J. A. Jernigan, K. M. Hatfield, H. Wolford, R. E. Nelson, B. Olubajo, S. C. Reddy, N. McCarthy, P. Paul, L. C. McDonald, A. Kallen, A. Fiore, M. Craig and J. Baggs, N. Engl. J. Med., 2020, 382, 1309–1319 CrossRef CAS PubMed.
- M. Mendelson and M. P. Matsoso, SAMJ South African Med. J., 2015, 105, 325 CrossRef PubMed.
- E. Tacconelli, N. Magrini, G. Kahlmeter and N. Singh, World Heal. Organ., 2017, 27, 318–327 Search PubMed.
- M. S. Mulani, E. E. Kamble, S. N. Kumkar, M. S. Tawre and K. R. Pardesi, Front. Microbiol., 2019, 10, 539 CrossRef PubMed.
- S. Santajit and N. Indrawattana, Biomed Res. Int., 2016, 2016, 2475067 Search PubMed.
- L. B. Rice, Infect. Control Hosp. Epidemiol., 2010, 31, S7–S10 CrossRef PubMed.
- S. A. Beatson and M. J. Walker, Science, 2014, 345, 1454–1455 CrossRef CAS PubMed.
- W. Dowhan, Annu. Rev. Biochem., 1997, 66, 199–232 CrossRef CAS PubMed.
- H. Goldfine, J. Lipid Res., 1984, 25, 1501–1507 CrossRef CAS.
- R. M. Epand and R. F. Epand, Biochim. Biophys. Acta, Biomembr., 2009, 1788, 289–294 CrossRef CAS PubMed.
- K. Murzyn, T. Róg and M. Pasenkiewicz-Gierula, Biophys. J., 2005, 88, 1091–1103 CrossRef CAS PubMed.
- P. H. Ray and D. C. White, J. Bacteriol., 1972, 109, 668–677 CrossRef CAS PubMed.
- C. Le Sénéchal, M. Crouzet, P. Costaglioli, C. Barthe, C. Buré and S. Vilain, Lipids, 2019, 54, 519–529 CrossRef PubMed.
- J. Gidden, J. Denson, R. Liyanage, D. M. Ivey and J. O. Lay, Int. J. Mass Spectrom., 2009, 283, 178–184 CrossRef CAS PubMed.
- S. Furse, H. Wienk, R. Boelens, A. I. P. M. de Kroon and J. A. Killian, FEBS Lett., 2015, 589, 2726–2730 CrossRef CAS PubMed.
- M. E. Pedrido, P. de Oña, W. Ramirez, C. Leñini, A. Goñi and R. Grau, Mol. Microbiol., 2013, 87, 348–367 CrossRef CAS PubMed.
- A. Okabe, Y. Hirai, H. Hayashi and Y. Kanemasa, Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab., 1980, 617, 28–35 CrossRef CAS.
- E. Deschamps, A. Schaumann, I. Schmitz-Afonso, C. Afonso, E. Dé, C. Loutelier-Bourhis and S. Alexandre, Biochim. Biophys. Acta, Biomembr., 2021, 1863, 183482 CrossRef CAS PubMed.
- M. S. Son, W. J. Matthews, Y. Kang, D. T. Nguyen and T. T. Hoang, Infect. Immun., 2007, 75, 5313–5324 CrossRef CAS PubMed.
- N. N. Mishra, A. S. Bayer, T. T. Tran, Y. Shamoo, E. Mileykovskaya, W. Dowhan, Z. Guan and C. A. Arias, PLoS One, 2012, 7, e43958 CrossRef CAS PubMed.
- Z. DeMars, V. K. Singh and J. L. Bose, J. Bacteriol., 2020, 202, e128-20 CrossRef PubMed.
- H. U. Koch, R. Haas and W. Fischer, Eur. J. Biochem., 1984, 138, 357–363 CrossRef CAS PubMed.
- N. N. Mishra, S.-J. Yang, A. Sawa, A. Rubio, C. C. Nast, M. R. Yeaman and A. S. Bayer, Antimicrob. Agents Chemother., 2009, 53, 2312–2318 CrossRef CAS PubMed.
- K. Yasuhiro, Y. Tieko and H. Hideo, Biochim. Biophys. Acta, Lipids Lipid Metab., 1972, 280, 444–450 CrossRef.
- M. K. Wassef, Lipids, 1976, 11, 364–369 CrossRef CAS PubMed.
- H. S. Aluyi, V. Boote, D. B. Drucker, J. M. Wilson and Y. H. Ling, J. Appl. Bacteriol., 1992, 73, 426–432 CrossRef CAS PubMed.
- C. C. Scott, S. R. Makula and W. R. Finnerty, J. Bacteriol., 1976, 127, 469–480 CrossRef CAS PubMed.
- T. Yosuke, I. Aya, S. Motoyuki, I. Sosaku, T. Naoki, N.-K. Toshiaki, U. Hiroo and N. Nobuhiko, Biotechnol. Biochem., 2011, 75, 605–607 CrossRef PubMed.
- P. Bernat, P. Siewiera, A. Soboń and J. Długoński, World J. Microbiol. Biotechnol., 2014, 30, 2343–2350 CrossRef CAS PubMed.
- R. Aggeler, R. L. Then and R. Ghosh, J. Gen. Microbiol., 1987, 133, 3383–3392 CAS.
- P. Bernat, K. Paraszkiewicz, P. Siewiera, M. Moryl, G. Płaza and J. Chojniak, World J. Microbiol. Biotechnol., 2016, 32, 1–13 CrossRef CAS PubMed.
- S. Hiraoka, H. Matsuzaki and I. Shibuya, FEBS Lett., 1993, 336, 221–224 CrossRef CAS PubMed.
- M. Abdi, S. Mirkalantari and N. Amirmozafari, J. Pept. Sci., 2019, 25, e3210 CrossRef CAS PubMed.
- P. V Escribá, J. M. González-Ros, F. M. Goñi, P. K. Kinnunen, L. Vigh, L. Sánchez-Magraner, A. M. Fernández, X. Busquets, I. Horváth and G. Barceló-Coblijn, J Cell Mol Med, 2008, 12, 829–875 CrossRef PubMed.
- W. Szlasa, I. Zendran, A. Zalesińska, M. Tarek and J. Kulbacka, J. Bioenerg. Biomembr., 2020, 52, 321–342 CrossRef CAS PubMed.
- P. Putta, J. Rankenberg, R. A. Korver, R. van Wijk, T. Munnik, C. Testerink and E. E. Kooijman, Biochim. Biophys. Acta, 2016, 1858, 2709–2716 CrossRef CAS PubMed.
- A. Poli, A. E. Zaurito, S. Abdul-Hamid, R. Fiume, I. Faenza and N. Divecha, Int. J. Mol. Sci., 2019, 20, 2080 CrossRef CAS PubMed.
- I. Shlomovitz, M. Speir and M. Gerlic, Cell Commun Signal, 2019, 17, 139 CrossRef PubMed.
- M. Meleh, B. Pozlep, A. Mlakar, H. Meden-Vrtovec and L. Zupancic-Kralj, J Chromatogr B Anal. Technol Biomed Life Sci, 2007, 858, 287–291 CrossRef CAS PubMed.
- T. Yamada, K. Sato, M. Komachi, E. Malchinkhuu, M. Tobo, T. Kimura, A. Kuwabara, Y. Yanagita, T. Ikeya, Y. Tanahashi, T. Ogawa, S. Ohwada, Y. Morishita, H. Ohta, D. S. Im, K. Tamoto, H. Tomura and F. Okajima, J. Biol. Chem., 2004, 279, 6595–6605 CrossRef CAS PubMed.
- Y. Hou, J. Li, H. Xie, F. Sun, K. Yang, J. Wang, C. Ke, G. Lou and K. Li, Metabolomics, 2016, 12, 1–12 CrossRef CAS.
- Y. Xu, Cancers, 2018, 10, 227 CrossRef PubMed.
- Z. A. Almsherqi, Front. Cell Dev. Biol., 2021, 9, 67317 Search PubMed.
- M. Cristina, F. Messias, G. C. Mecatti, D. G. Priolli, P. De and O. Carvalho, Lipids Health Dis, 2018, 17, 1–12 CrossRef PubMed.
- A. M. A. P. Fernandes, M. C. F. Messias, G. H. B. Duarte, G. K. D. de Santis, G. C. Mecatti, A. M. Porcari, M. Murgu, A. V. C. Simionato, T. Rocha, C. A. R. Martinez and P. O. Carvalho, Metabolites, 2020, 10, 262 CrossRef CAS PubMed.
- E. Marien, M. Meister, T. Muley, S. Fieuws, S. Bordel, R. Derua, J. Spraggins, R. de Plas, J. Dehairs, J. Wouters, M. Bagadi, H. Dienemann, M. Thomas, P. A. Schnabel, R. M. Caprioli, E. Waelkens and J. V Swinnen, Int. J. Cancer, 2015, 137, 1539–1548 CrossRef CAS PubMed.
- L. You, Y. Fan, X. Liu, S. Shao, L. Guo, H. A. A. Noreldeen, Z. Li, Y. Ouyang, E. Li, X. Pan, T. Liu, X. Tian, F. Ye, X. Li and G. Xu, J. Proteome Res., 2020, 19, 3750–3760 CrossRef CAS PubMed.
- S. Guo, Y. Wang, D. Zhou and Z. Li, Sci. Rep., 2014, 4, 5959 CrossRef CAS PubMed.
- G. K. Lee, H. S. Lee, Y. S. Park, J. H. Lee, S. C. Lee, J. H. Lee, S. J. Lee, S. R. Shanta, H. M. Park, H. R. Kim, I. H. Kim, Y. H. Kim, J. I. Zo, K. P. Kim and H. K. Kim, Lung Cancer, 2012, 76, 197–203 CrossRef PubMed.
- Y. Muranishi, T. Sato, S. Ito, J. Satoh, A. Yoshizawa, S. Tamari, Y. Ueda, Y. Yutaka, T. Menju, T. Nakamura and H. Date, Sci. Reports, 2019, 9, 1–10 CAS.
- M. L. Dória, C. Z. Cotrim, C. Simões, B. Macedo, P. Domingues, M. R. Domingues and L. A. Helguero, J. Cell. Physiol., 2013, 228, 457–468 CrossRef PubMed.
- H. Y. Kim, K. M. Lee, S. H. Kim, Y. J. Kwon, Y. J. Chun and H. K. Choi, Oncotarget, 2016, 7, 67111–67128 CrossRef PubMed.
- M. Hilvo, C. Denkert, L. Lehtinen, B. Müller, S. Brockmöller, T. Seppänen-Laakso, J. Budczies, E. Bucher, L. Yetukuri, S. Castillo, E. Berg, H. Nygren, M. Sysi-Aho, J. L. Griffin, O. Fiehn, S. Loibl, C. Richter-Ehrenstein, C. Radke, T. Hyötyläinen, O. Kallioniemi, K. Iljin and M. Oresic, Cancer Res., 2011, 71, 3236–3245 CrossRef CAS PubMed.
- S. Kang, A. Lee, Y. S. Park, S. C. Lee, S. Y. Park, S. Y. Han, K. P. Kim, Y. H. Kim, C. W. Yoo and H. K. Kim, Int J Gynecol Cancer, 2011, 21, 1566–1572 CrossRef PubMed.
- E. Cífková, M. Holčapek, M. Lísa, D. Vrána, J. Gatěk and B. Melichar, Anal. Bioanal. Chem., 2015, 407, 991–1002 CrossRef PubMed.
- A. Sorvina, C. A. Bader, C. Caporale, E. A. Carter, I. R. D. Johnson, E. J. Parkinson-Lawrence, P. V. Simpson, P. J. Wright, S. Stagni, P. A. Lay, M. Massi, D. A. Brooks and S. E. Plush, Oncotarget, 2018, 9, 35541 CrossRef PubMed.
- N. Morse, T. Jamaspishvili, D. Simon, P. G. Patel, K. Y. M. Ren, J. Wang, R. Oleschuk, M. Kaufmann, R. J. Gooding and D. M. Berman, Lab. Investig., 2019, 99, 1561–1571 CrossRef CAS PubMed.
- J. Alexander, L. Gildea, J. Balog, A. Speller, J. McKenzie, L. Muirhead, A. Scott, C. Kontovounisios, S. Rasheed, J. Teare, J. Hoare, K. Veselkov, R. Goldin, P. Tekkis, A. Darzi, J. Nicholson, J. Kinross and Z. Takats, Surg Endosc, 2017, 31, 1361–1370 CrossRef PubMed.
- T. E. Merchant, J. N. Kasimos, P. W. de Graaf, B. D. Minsky, L. W. Gierke and T. Glonek, Int J Color. Dis, 1991, 6, 121–126 CrossRef CAS PubMed.
- D. A. Dueck, M. Chan, K. Tran, J. T. Wong, F. T. Jay, C. Littman, R. Stimpson and P. C. Choy, Mol. Cell. Biochem., 1996, 162, 97–103 CrossRef CAS PubMed.
- A. Pakiet, K. Sikora, J. Kobiela, O. Rostkowska, A. Mika and T. Sledzinski, Lipids Health Dis, 2021, 20, 85 CrossRef CAS PubMed.
- S. Kiritani, K. Yoshimura, J. Arita, T. Kokudo, H. Hakoda, M. Tanimoto, T. Ishizawa, N. Akamatsu, J. Kaneko, S. Takeda and K. Hasegawa, BMC Cancer, 2021, 21, 1–9 CrossRef PubMed.
- S. Kang, A. Lee, Y. S. Park, S. C. Lee, S. Y. Park, S. Y. Han, K. P. Kim, Y. H. Kim, C. W. Yoo and H. K. Kim, Int. J. Gynecol. Cancer, 2011, 21, 1566–1572 CrossRef PubMed.
- D. W. B. Piyarathna, T. M. Rajendiran, V. Putluri, V. Vantaku, T. Soni, F. C. von Rundstedt, S. R. Donepudi, F. Jin, S. Maity, C. R. Ambati, J. Dong, D. Gödde, S. Roth, S. Störkel, S. Degener, G. Michailidis, S. P. Lerner, S. Pennathur, Y. Lotan, C. Coarfa, A. Sreekumar and N. Putluri, Eur. Urol. Focus, 2018, 4, 907–915 CrossRef PubMed.
- N. Abbassi-Ghadi, S. S. Antonowicz, J. S. McKenzie, S. Kumar, J. Huang, E. A. Jones, N. Strittmatter, G. Petts, H. Kudo, S. Court, J. M. Hoare, K. Veselkov, R. Goldin, Z. Takáts and G. B. Hanna, Cancer Res., 2020, 80, 2764–2774 CrossRef CAS PubMed.
- Z. Liu, Z. Zhang, H. Mei, J. Mao and X. Zhou, Hepatol. Int., 2020, 14, 544–555 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: This includes full data tables, listing the phospholipid compositions of the bacterial, cancer cell lines and biological samples from cancer patients. See DOI: 10.1039/d1sc03597e |
| ‡ Denotes equal scientific input and joint authorship status. |
| This journal is © The Royal Society of Chemistry 2021 |