Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Zirconium-catalyzed asymmetric Kabachnik–Fields reactions of aromatic and aliphatic aldehydes†
Yijing
Dai
,
Li
Zheng
,
Debarshi
Chakraborty
,
Babak
Borhan
 and
William D.
Wulff
and
William D.
Wulff
 *
*
Department of Chemistry, Michigan State University, East Lansing, MI 48824, USA. E-mail: wulff@chemistry.msu.edu
First published on 3rd August 2021
Abstract
An effective catalyst has been developed for the three-component reaction of aldehydes, anilines and phosphites in an asymmetric catalytic Kabachnik–Fields reaction to give α-aminophosphonates. A catalyst was sought that would give high asymmetric inductions for aromatic and, and more particularly, for aliphatic aldehydes since there has not previously been an effective catalyst developed for this class of aldehydes. The optimal catalyst is prepared from three equivalents of the 7,7′-di-t-butylVANOL ligand, one equivalent of N-methylimidazole and one equivalent of zirconium tetraisopropoxide. This catalyst was most efficient in the presence of 10 mol% benzoic acid. Optimal conditions for aryl aldehydes required the use of 3,5-diisopropyl-2-hydroxyaniline and gave the aryl α-aminophosphonates in up to 96% yield and 98% ee over 11 different aryl aldehydes. The best aniline for aliphatic aldehydes was found to be 3-t-butyl-2-hydroxyaniline and gave the corresponding phosphonates in up to 83% yield and 97% ee over 18 examples. The asymmetric inductions for aliphatic aldehydes were comparable with those for aromatic aldehydes with a mean induction of 90% ee for the former and 91% ee for the latter. The best method for the liberation of the free amine from the aniline substituted α-aminophosphonates involved oxidation with N-iodosuccinimide.
1. Introduction
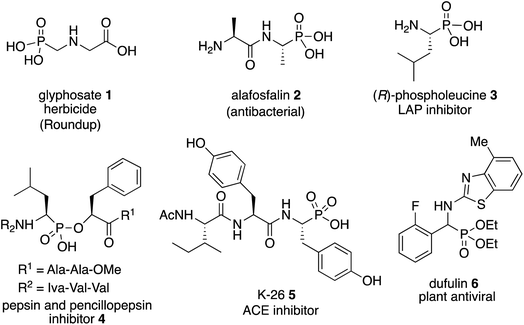
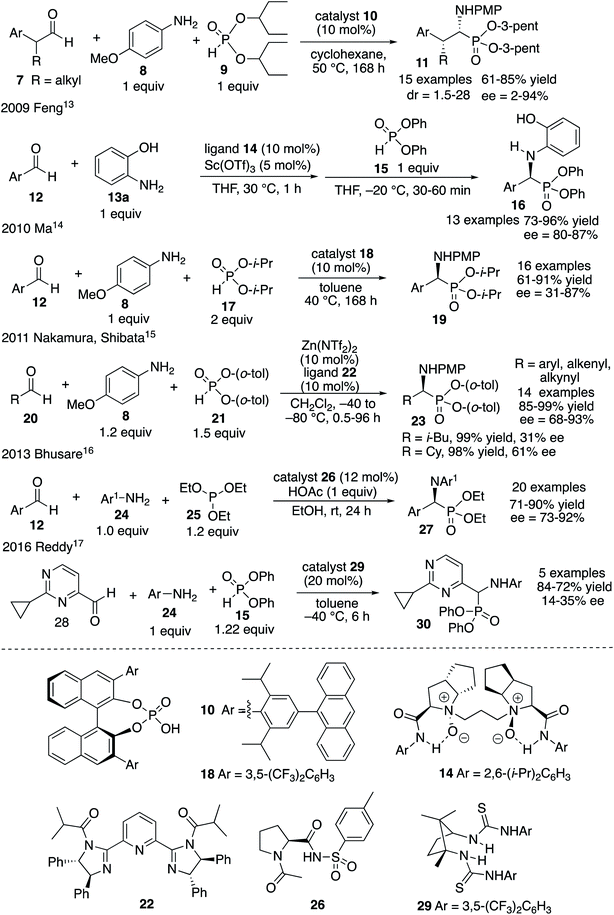
The most important analogs of α-amino acids are α-aminophosphonic acids and examples include both natural and synthetic derivatives.1 Among their biological activities many are related to their ability to inhibit enzymes that are involved in cleavage of peptide bonds since they can serve as transition-state analogs of a tetrahedral intermediate formed from an amide carbonyl during hydrolysis.1,2 Examples of biologically active α-aminophosphonic acids and esters are shown in Scheme 1 and include glyphosate 1 (Roundup),3 the antibacterial alafosfalin 2 (the other three isomers are less active)4 and phospholeucine 3 which is a leucine aminopeptidase inhibitor (the (S)-enantiomer is 103 times less active than the (R)-enantiomer).5 The phospholeucine 3 is a key component of, and plays an important role in, the activity of the pepsin and penicillopepsin inhibitor 4.6 The naturally occurring phosphotyrosine tripeptide K-26 5 is an ACE inhibitor with comparable activity to captopril,1b,7a although analogs of 5 were found to be more active.7b,c Dufulin 6 has been widely used to treat viral diseases in agricultural crops in China.8The most common methods for the synthesis of α-aminophosphonic acids involve the two component reaction of an imine and a phosphite (Pudovik reaction9) and the three component reaction of an aldehyde, an amine and a phosphite (Kabachnik–Fields reaction10,11). Given the level of difficulty it is not surprising that the three component Kabachnik–Fields reaction has been the more difficult of the two to develop asymmetric catalytic versions.11 The first catalytic asymmetric Kabachnik–Fields reaction was reported by List and coworkers in 2008 (Fig. 1).12 They developed the BINOL hydrogen phosphate catalyst 10 for the three component reaction to give α-aminophosphonic esters 11 with high enantioselectivity. Interestingly, this reaction also involved a dynamic kinetic resolution of the aldehyde, yielding product 11 with high diastereoselectivity. The transformation was limited to aryl acetaldehydes and the reaction times were quite long. Feng and coworkers reported that a scandium catalyst with the bis-amine oxide ligand 14 gives good asymmetric inductions with aryl aldehydes and aniline 13a with the diphenyl phosphite 15.13 However, this was not actually a Kabachnik–Fields reaction since the imine was generated first at 30 °C and then the phosphite was added at −20 °C. A second example involving a BINOL hydrogen phosphate catalyst was published by Ma and coworkers in 2010 and gave the α-aminophosphonate 19 in low to high asymmetric induction with 16 different aromatic aldehydes.14 In 2011 Nakamura and Shibata and coworkers reported the success of a zinc-bis-imidazolidine catalyst in the reaction of 16 different aldehydes with the aniline 8 and the bis-o-tolylphosphite 21.15 The α-aminophosphonate diester 23 was obtained in 68–93% ee with aryl aldehydes but the two alkyl aldehydes that were examined gave low to moderate induction (31–61% ee). The organocatalyst 26 has been reported by Bhusare and coworkers to give good to excellent asymmetric inductions in the Kabachnik–Fields reaction for a variety of aryl aldehydes.16 This reaction is unusual in that, instead of a phosphite diester, a phosphite triester was employed in this Kabachnik–Fields reaction where one of the ethyl groups was cleaved during the reaction to give the phophonate diester 27. Finally, Reddy and coworkers reported the use of the bis-thiourea catalyst 29 to give the α-aminophosphonate 30 from the aldehyde 28 in low enantioselectivity.17
To summarize, the existing methods for the asymmetric catalytic Kabachnik–Fields reaction work well only for aromatic aldehydes. The only two examples with aliphatic aldehydes come from the work of Nakamura and Shibata who were able to prepare the α-aminophosphonate 23 from cyclohexanecarboxaldehyde and isovaleraldehyde in 61% and 31% ee, respectively.15 Given that α-aminophosphonates are largely of interest as analogs of α-amino acids, and that most α-amino acids do not contain aromatic groups, it is clear that improved methods for the catalytic asymmetric Kabachnik–Fields reaction are needed.
2. Results and discussion
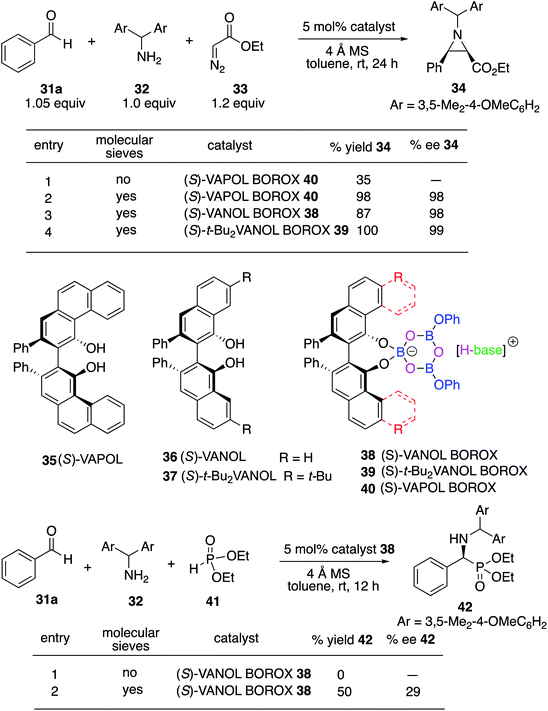
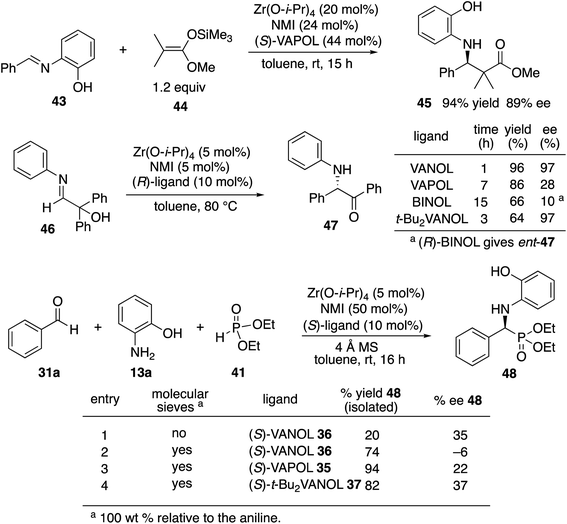
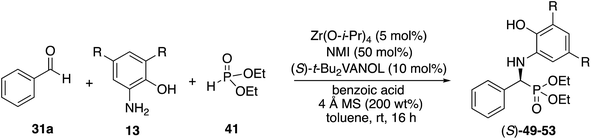
We decided to begin the search for new catalysts for the Kabachnik–Field reaction by examining aromatic aldehydes since it was the expectation that reaction optimization would not be as challenging. We had previously reported a three-component reaction for the catalytic asymmetric synthesis of aziridines from an aldehyde, amine and an α-diazoacetate (Scheme 2).18 The catalyst was a boroxinate (BOROX) derived from either the VAPOL or VANOL ligands and both gave the aziridines 34 in 98% ee. The reaction gives low conversion in the absence of molecular sieves. This was not surprising given that an imine is generated in situ. Later it was found that the BOROX catalyst 39 generated from the t-Bu2VANOL ligand 37 gives the highest yields and asymmetric inductions.19 Unfortunately, the success with the BOROX catalysts did not transfer from the three component aziridination reaction to the three component Kabachnik–Fields reaction since the VANOL-BOROX catalyst 38 only gave the α-aminophosphonate 42 in 29% ee.Although not three-component reactions, we have had success with zirconium catalysts in the asymmetric catalytic transformation of imines in Mannich reactions20 and α-imino rearrangements21 (Scheme 3). In each case the catalyst is generated by combining zirconium tetraisopropoxide, a vaulted biaryl ligand and N-methylimidazole. This catalyst type was initially screened in the reaction of benzaldehyde, 2-hydroxyaniline 13a and diethyl phosphite 41 with three different vaulted biaryl ligands (Scheme 3). In the absence of molecular sieves, only a low 20% yield of the α-aminophosphonate 48 was obtained with the VANOL ligand 36 in 35% ee (entry 1). The yield was greatly increased in the presence of molecular sieves, but the product was nearly racemic (entry 2). The yield was excellent with a catalyst generated from the VAPOL ligand 35 but the asymmetric induction was low (22% ee) (entry 3). The best result was achieved with the 7,7′-di-t-butylVANOL ligand 37 giving 48 in 82% yield and 37% ee (entry 4).
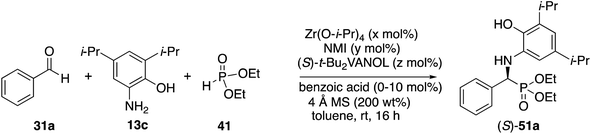
In the Mannich reaction with the zirconium catalyst indicated in Scheme 3, we had observed that higher asymmetric inductions and yields were obtained with the 3,5-dimethyl-2-hydroxyaniline 13b.20 This was also the case in the present study where the asymmetric induction of the α-aminophosphonate increased from 37 to 67% ee (Table 1, entries 1 and 2). Further studies revealed that if the amount of molecular sieves was increased from 100 to 200 wt% relative to the aniline, the asymmetric induction with aniline 13b could be increased from 67 to 82% ee (entries 2 vs. 3). Additional amounts of molecular sieves were not beneficial (see the ESI†). Further optimization was possible by screening other 3,5-disubstituted-2-hydroxyanilines in this reaction. The α-aminophosphonate 51 could be obtained in 90% ee with the 3,5-diisopropyl-2-hydroxyaniline 13c (entry 5). We had also observed that if benzaldehyde 31a was not freshly distilled, the yield improved. Assuming that the difference here was due to benzoic acid, this prompted a study into the effect of added benzoic acid. Here the yield increased by 10% upon the addition of 5 mol% benzoic acid (entries 5 vs. 6), and the induction was slightly higher with 10 mol% benzoic acid but dropped with 100 mol% benzoic acid (entries 5 to 8). This effect of benzoic acid was not noted for the 3,5-dimethyl-2-hydroxyaniline 13b (entries 3 and 4). The di-n-butylaniline 13d did not give as high an asymmetric induction as the di-i-propylaniline 13c (82% vs. 90%, entries 5 vs. 9) and the highest induction was observed with the di-t-butylaniline 13e (95%, entry 10). Unfortunately in the latter case, the reaction was very slow and in the same time period, only a 12% yield of 53 was isolated. The yield could only be slightly recovered upon the addition of benzoic acid (entries 10 vs. 13). The yield could be increased to 52% with a reaction temperature of 60 °C, but the induction fell to 89%. The absolute configuration of the phosphonate 51 from the (S)-catalyst was determined to be (S)-51 after deprotection to the free amine as indicated in Scheme 6.
| Entry | R | Aniline | Temp (°C) | Benzoic acid (mol%) | Product | % Yieldb | % ee |
|---|---|---|---|---|---|---|---|
| a Unless otherwise specified, the reactions were carried out on 0.1 mmol of aldehyde with 1.0 equiv. of aniline and 1.0 equiv. of phosphite and with 200 wt% of 4 Å MS relative to the aniline. b Isolated yield. c 100 wt% MS relative to the aniline. d (R)-t-Bu2 VANOL ligand was used and ent-52/53 was obtained. | |||||||
| 1 | Hc | 13a | rt | 0 | 49 | 82 | 37 |
| 2 | Mec | 13b | rt | 0 | 50 | 55 | 67 |
| 3 | Me | 13b | rt | 0 | 50 | 87 | 82 |
| 4 | Me | 13b | rt | 10 | 50 | 87 | 80 |
| 5 | i-Pr | 13c | rt | 0 | 51 | 76 | 90 |
| 6 | i-Pr | 13c | rt | 5 | 51 | 86 | 92 |
| 7 | i-Pr | 13c | rt | 10 | 51 | 80 | 94 |
| 8 | i-Pr | 13c | rt | 100 | 51 | 59 | 77 |
| 9 | n-Bu | 13d | rt | 0 | 52 | 73 | −82d |
| 10 | t-Bu | 13e | rt | 0 | 53 | 12 | 95 |
| 11 | t-Bu | 13e | 40 | 0 | 53 | 41 | 92 |
| 12 | t-Bu | 13e | 60 | 0 | 53 | 52 | −89d |
| 13 | t-Bu | 13e | rt | 10 | 53 | 17 | 95 |
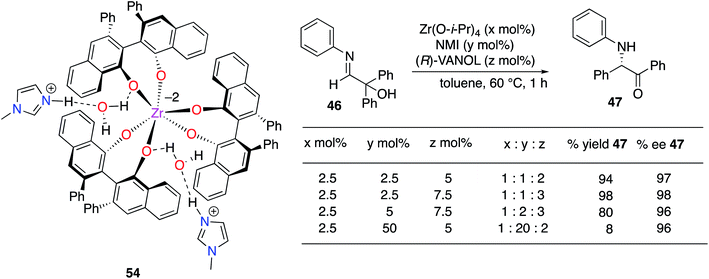
The structure(s) of the zirconium catalysts shown in Scheme 3 are not known with great certainty. We had assumed that the structure of the catalyst for the Mannich reaction in Scheme 3 (ref. 20) had two molecules of ligand per zirconium as this had been reported for the BINOL analog.22 However, in studies on the α-imino rearrangements21 we were able to grow crystals of the zirconium complex 54 (Scheme 4) which revealed that the zirconium has three VANOL ligands around the zirconium with two protonated N-methylimidazoles to balance the charge. A homoleptic zirconium complex with three bis-phenol ligands has not been reported before, but Shibasaki has reported that rare earth catalysts with three BINOL ligands are effective for a number of reactions.23 However, later, Schelter and Walsh have shown that Shibasaki's catalysts are in equilibrium with species that only have two BINOL ligands and may be the actual active catalyst species.24 Although we have examined the structure of the zirconium catalyst in the present work by NMR the results were not conclusive. Thus, presently it is not known whether the complex 54 is the actual catalyst or if it loses a VANOL ligand in solution to give the active catalyst with only two molecules of VANOL per zirconium. It was found that if the catalyst was prepared from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mixture of Zr(O-i-Pr)4, N-methylimidazole and VANOL the rearrangement of 46 to 47 occurred with essentially the same result as from a 1
3 mixture of Zr(O-i-Pr)4, N-methylimidazole and VANOL the rearrangement of 46 to 47 occurred with essentially the same result as from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture (Scheme 4). If the catalyst was prepared from a 1
2 mixture (Scheme 4). If the catalyst was prepared from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mixture the reaction was a little slower and with a 1
3 mixture the reaction was a little slower and with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio the yield drops to 8% in the same time (Scheme 4). This may suggest that one of the imidazoles needs to dissociate to initiate the reaction. Additionally, crystals were grown from both the 1
2 ratio the yield drops to 8% in the same time (Scheme 4). This may suggest that one of the imidazoles needs to dissociate to initiate the reaction. Additionally, crystals were grown from both the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and the 1
2 and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 : 3 mixtures, their structures were solved, and both were found to be zirconium complex 54.
1 : 3 mixtures, their structures were solved, and both were found to be zirconium complex 54.
Based on the above discussion, the final optimization of the Kabachnik–Field reaction of aryl aldehydes involved examining the effect of the ratio of the components in catalyst formation (Table 2). Unlike the case with α-imino rearrangements (Scheme 4) the use of a large amount of N-methylimidazole (10 equiv.) relative to zirconium was not noticeably detrimental to the reaction (entry 1). The yield was slightly higher with three equivalents of VANOL per zirconium than with two but the asymmetric induction was the same (entries 3 vs. 5). The yield dropped a bit with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio of zirconium to NMI to ligand (entry 7 vs. entries 3 and 5). The outcomes of the reactions with all variations of the ratio of catalyst components were greatly dependent on the presence of benzoic acid with a decrease in yields of 30 to 69% in the absence of benzoic acid although the asymmetric inductions only decreased by 5 to 10% (entries 1 vs. 2, 3 vs. 4 and 5 vs. 6). The decrease in yields was smaller for a larger ratio of NMI to zirconium (entries 1 vs. 2).
3 ratio of zirconium to NMI to ligand (entry 7 vs. entries 3 and 5). The outcomes of the reactions with all variations of the ratio of catalyst components were greatly dependent on the presence of benzoic acid with a decrease in yields of 30 to 69% in the absence of benzoic acid although the asymmetric inductions only decreased by 5 to 10% (entries 1 vs. 2, 3 vs. 4 and 5 vs. 6). The decrease in yields was smaller for a larger ratio of NMI to zirconium (entries 1 vs. 2).
| Entry | x mol% | y mol% | z mol% |
x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) z z |
Benzoic acid (mol%) | % Yield 51a | % ee 51a |
|---|---|---|---|---|---|---|---|
| a Unless otherwise specified, the reactions were carried out on 0.1 mmol of aldehyde with 1.0 equiv. of aniline and 1.0 equiv. of phosphite and with 200 wt% of 4 Å MS relative to the aniline. | |||||||
| 1 | 5 | 50 | 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
10 | 80 | 94 |
| 2 | 5 | 50 | 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0 | 50 | 89 |
| 3 | 5 | 5 | 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
10 | 83 | 93 |
| 4 | 5 | 5 | 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0 | 30 | 85 |
| 5 | 5 | 5 | 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
10 | 90 | 93 |
| 6 | 5 | 5 | 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0 | 21 | 83 |
| 7 | 5 | 10 | 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
10 | 70 | 94 |
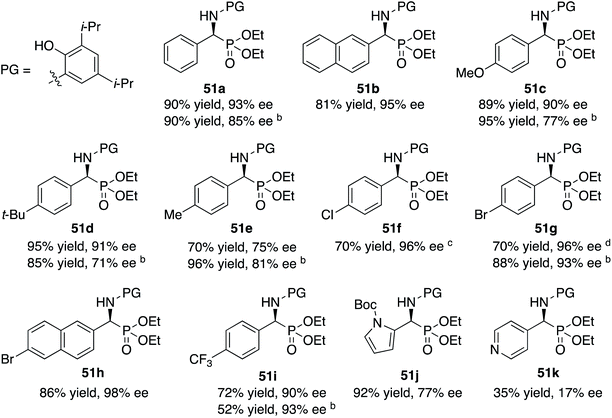
The scope of the Kabachnik–Fields reaction with aromatic aldehydes is summarized in Table 3 and the optimal conditions identified in entry 5 of Table 2 are employed. Both electron rich and electron poor substituted benzaldehydes are tolerated by this catalyst and all but one give α-amino phosphonates 51 with 90 to 98% ee and in 70 to 96% yield. The exception is 4-methylbenzaldehyde 31e. This aldehyde gives the α-aminophosphonate 51e in 70% yield and 75% ee under the standard conditions indicated in Table 3. However, if the amount of benzoic acid is lowered from 10 mol% to 5 mol% the % ee increases to 81% with 96% yield. This stands out from most of the other benzaldehydes that were investigated with both 5 and 10 mol% benzoic acid that are indicated in Table 3 where the higher asymmetric induction is observed with 10 mol% benzoic acid. The reaction with pyrrole-2-carboxaldehyde immediately turns very dark and led to the consumption of the starting material with no detectable product formation. However, the reaction with Boc protected pyrrole-2-carboxaldehyde proceeds to give a 92% yield of the α-aminophosphonate 51j in 77% ee. Pyridine-4-carboxaldehyde 31k was not a suitable substrate giving the α-aminophosphonate 51k in 35% yield with 17% ee. The absolute configuration of the α-aminophosphonate 51a from the (S)-catalyst was determined to be (S)-51a after deprotection to the free amine as shown in Scheme 6. The products from the other aromatic aldehydes were assumed to be homo-chiral.
| a Zirconium catalysts were prepared by stirring a mixture of 5 mol% Zr(O-i-Pr)4(HO-i-Pr), 5 mol% N-methylimidazole (NMI) and 15 mol% (S)-t-Bu2VANOL ligand in dry toluene for 30 min at rt under air. Unless otherwise specified, all reactions were carrired out under nitrogen on 0.1 mmol of aldehyde 31 with 1 equiv. of aniline 13c and 1 equiv. of diethylphosphite 41 in toluene at rt for 16 h in the presence of 4 Å molecular sieves (200 wt% relative to 13c) and 10 mol% of benzoic acid. All yields are isolated yields. The % ee was determined by HPLC. b Reaction with 5 mol% benzoic acid. c With a reaction time of 40 h the yield was 80% with 96% ee. d With a reaction time of 40 h the yield was 92% with 96% ee. |
|---|
|
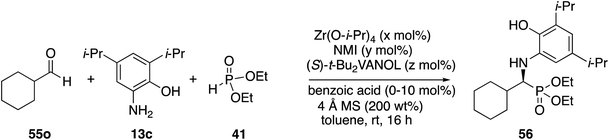
Next, attention was turned to aliphatic aldehydes. The cyclohexanecarboxaldehyde 55o served as a prototypical aliphatic aldehyde and the initial results from its reaction with aniline 13c and phosphite 41 are presented in Table 4. It was quite interesting to observe that there is a vast difference between the reaction carried out with the catalyst prepared from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of zirconium/NMI/ligand and that with a 1
2 mixture of zirconium/NMI/ligand and that with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mixture (Table 4, entry 1 vs. 3). The α-aminophosphonate 56 was isolated in 45% yield with 11% ee with the former and 80% yield and 82% ee with the latter. This is in sharp contrast to the observation with benzaldehyde, where no real significant difference was observed between a 1
3 mixture (Table 4, entry 1 vs. 3). The α-aminophosphonate 56 was isolated in 45% yield with 11% ee with the former and 80% yield and 82% ee with the latter. This is in sharp contrast to the observation with benzaldehyde, where no real significant difference was observed between a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 catalyst ratio (Table 2, entries 3 vs. entry 5). However, as with benzaldehyde (Table 2), there is a significant difference between the effect of benzoic acid on the reaction of cyclohexanecarboxaldehyde 55o. With the 1
3 catalyst ratio (Table 2, entries 3 vs. entry 5). However, as with benzaldehyde (Table 2), there is a significant difference between the effect of benzoic acid on the reaction of cyclohexanecarboxaldehyde 55o. With the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 catalyst, the yield dropped from 45% to 9% without benzoic acid although the % ee was slightly enhanced (Table 4, entries 1 and 2). This strong dependence was also observed with the 1
2 catalyst, the yield dropped from 45% to 9% without benzoic acid although the % ee was slightly enhanced (Table 4, entries 1 and 2). This strong dependence was also observed with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 catalyst where the yield dropped from 80% to 14% without benzoic acid but in this case the % ee also dropped significantly (entries 3 vs. 4). Such a strong dependence on benzoic acid was not seen for a 1
3 catalyst where the yield dropped from 80% to 14% without benzoic acid but in this case the % ee also dropped significantly (entries 3 vs. 4). Such a strong dependence on benzoic acid was not seen for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 catalyst preparation (entries 5 vs. 6). The optimal conditions for the reaction of cyclohexanecarboxaldehyde gave phosphonate 56 in 80% yield with 82% ee (Table 4, entry 3). This is to be compared to the same reaction of benzaldehyde under the same conditions which gave phosphonate 51a in 90% yield and 93% ee (Table 2, entry 5). This is a less than desirable outcome for the reaction of this aliphatic aldehyde, and thus further optimization was needed.
3 catalyst preparation (entries 5 vs. 6). The optimal conditions for the reaction of cyclohexanecarboxaldehyde gave phosphonate 56 in 80% yield with 82% ee (Table 4, entry 3). This is to be compared to the same reaction of benzaldehyde under the same conditions which gave phosphonate 51a in 90% yield and 93% ee (Table 2, entry 5). This is a less than desirable outcome for the reaction of this aliphatic aldehyde, and thus further optimization was needed.
| Entry | x mol% | y mol% | z mol% |
x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) z z |
mol% benzoic acid | % Yield 56 | % ee 56 |
|---|---|---|---|---|---|---|---|
| a Unless otherwise specified, the reactions were carried out on 0.1 mmol of aldehyde with 1.0 equiv. of aniline and 1.0 equiv. of phosphite and with 200 wt% of 4 Å MS relative to the aniline. | |||||||
| 1 | 5 | 5 | 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
10 | 45 | 11 |
| 2 | 5 | 5 | 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0 | 9 | 39 |
| 3 | 5 | 5 | 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
10 | 80 | 82 |
| 4 | 5 | 5 | 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0 | 14 | 31 |
| 5 | 5 | 50 | 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
10 | 78 | 80 |
| 6 | 5 | 50 | 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0 | 82 | 68 |
| 7 | 5 | 100 | 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
10 | 60 | 82 |
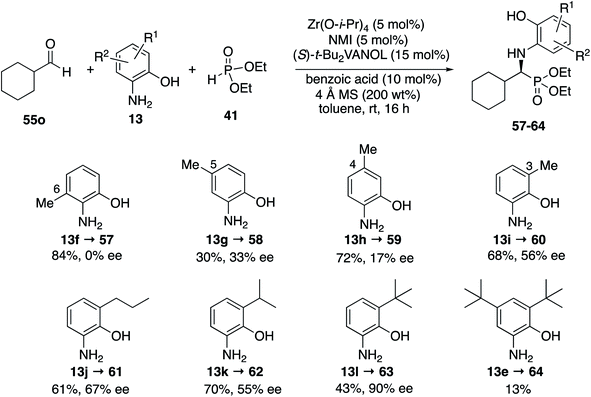
It was decided to probe the effect of substituents on all four of the aryl positions of the aniline 13 and determine the consequence of their resulting interactions with the catalyst in a systematic way. All four methyl derivatives 13f to 13i were prepared and their reactions with cyclohexanecarboxaldehyde 55o were examined under the optimal conditions given in Table 4 (entry 3), with the results outlined in Scheme 5. The greatest asymmetric induction (56%) was observed with a methyl group in the 3-position. A further increase to 67% ee was observed when the larger n-propyl group was introduced into the 3-position (13j) but this was found to decrease to 55% ee when replaced with an iso-propyl (13k). Surprisingly, the induction was found to increase again to 90% when the even larger t-butyl group was introduced into the 3-position (13l), leading to the isolation of the phosphonate 63 in 43% yield. The introduction of t-butyl groups into both the 3- and 5-positions leads to a slow reaction and the isolation of phosphonate 64 in only 13% yield. As a result of the screening of the various anilines shown in Scheme 5, the highest asymmetric induction (90% ee) was realized with the 3-t-butyl-2-hydroxyaniline 13l and this was identified as the aniline of choice for screening additional aliphatic aldehydes. The set of anilines shown in Scheme 5 was also employed in the screening of the reaction of benzaldehyde but none gave higher asymmetric inductions than 3,5-diisopropyl-2-hydroxyaniline 13c used in Table 3 for aromatic aldehydes, although the 3-t-butyl-2-hydroxyaniline 13l gave an identical induction of 93% ee (see the ESI†).
Before committing to a broad investigation of the scope of the reactions of aliphatic aldehydes, we probed the use of 3-t-butyl substituted aniline 13l instead of 3,5-diisopropyl substituted aniline 13c. The data for the reactions of butanal 55a are presented in Table 5. The t-Bu2VANOL ligand 37 was superior to the VANOL ligand 36, giving the α-aminophosphonate 65 in 81% ee vs. 39% ee with aniline 13c (Table 5, entries 1 vs. 2). A slight increase in induction to 85% was observed with the catalyst prepared from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mixture of zirconium/NMI/ligand and the yield was increased slightly when the catalyst loading was increased to 10 mol% (entries 3 and 4). Finally, the reaction of butanal was compared under the same conditions with both anilines 13c and 13l with the result that the t-butyl substituted aniline 13l gave both higher yield and higher asymmetric induction than aniline 13c (entries 4 vs. 5).
3 mixture of zirconium/NMI/ligand and the yield was increased slightly when the catalyst loading was increased to 10 mol% (entries 3 and 4). Finally, the reaction of butanal was compared under the same conditions with both anilines 13c and 13l with the result that the t-butyl substituted aniline 13l gave both higher yield and higher asymmetric induction than aniline 13c (entries 4 vs. 5).
| Entry | Ligand | x mol% | y mol% | z mol% |
x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) z z |
Aniline | % Yield 65/66a | % ee 65/66a |
|---|---|---|---|---|---|---|---|---|
| a Unless otherwise specified, the reactions were carried out on 0.1 mmol of aldehyde with 1.0 equiv. of aniline and 1.0 equiv. of phosphite and with 200 wt% of 4 Å MS relative to the aniline and 10 mol% benzoic acid. | ||||||||
| 1 | VANOL 36 | 5 | 10 | 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
13c | 52 | 39 |
| 2 | t-Bu2VANOL 37 | 5 | 10 | 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
13c | 51 | 81 |
| 3 | t-Bu2VANOL 37 | 5 | 5 | 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
13c | 48 | 85 |
| 4 | t-Bu2VANOL 37 | 10 | 10 | 30 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 : 3 1 : 3 |
13c | 56 | 85 |
| 5 | t-Bu2VANOL 37 | 10 | 10 | 30 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
13l | 80 | 89 |
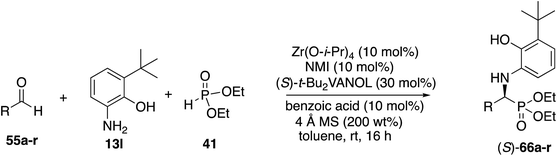
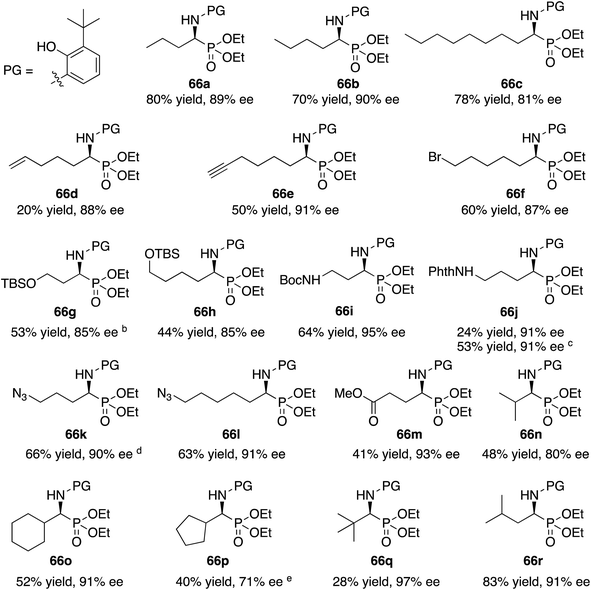
With the identification of the aniline 13l as the optimal third component for the Kabachnik–Fields reaction of aliphatic aldehydes, a general study of the scope was undertaken with a set of 18 aliphatic aldehydes (Table 6). The yields for these reactions varied from 20 to 83% and the asymmetric inductions ranged from 71–97%. The asymmetric inductions for the aliphatic aldehydes compared favorably with those for the aromatic aldehydes with the mean asymmetric induction of 90% ee for the aliphatic aldehydes and 91% ee for the aromatic aldehydes shown in Table 3. The best yields were observed in general for α-unbranched aldehydes while α,α-dibranched aldehydes generally gave less product. 2,2-Dimethylpropanal 55q gave the highest asymmetric induction of 97% but the yield of α-aminophosphonate 66q was only 28%. Extension of the reaction time from 16 to 48 h did not improve the yield. The reaction is tolerant of a number of functional groups including alkyne (66e), a primary bromide (66f), silyl ethers (66g and 66h), protected amines (66i and 66j), azides (66k and 66l) and an ester (66m). Interestingly, for reasons that we do not understand at this point, the terminal olefin in 5-hexenal 55d only reacted to give the α-aminophosphonate 66d in 20% yield. Increasing the reaction time with aldehyde 55d from 16 to 48 h did not improve the yield of 66d. This was true of many of the aldehydes in Table 6, however, an increase in the reaction time with the phthalimide protected γ-amino butanal 55j from 16 to 72 h increased the yield of 66j from 24% to 53%. The α-branched aldehydes 55n, 55o and 55p gave the corresponding α-aminophosphonates in moderate yields and good to excellent asymmetric inductions. The β-branched aldehyde isovaleraldehyde 55r gave the phosphonoleucine diethyl ester 66r in 83% yield and 91% ee. The absolute configuration of the α-aminophosphonate 66r from the (S)-catalyst was determined to be (S)-66r after deprotection to the free amine as shown in Scheme 6. The products from the other aliphatic aldehydes in Table 6 were assumed to be homo-chiral.
| a Zirconium catalysts were prepared by stirring a mixture of 10 mol% Zr(O-i-Pr)4(HO-i-Pr), 10 mol% N-methylimidazole (NMI) and 30 mol% (S)-t-Bu2VANOL ligand in dry toluene for 30 min at rt under air. Unless otherwise specified, all reactions were carried out under nitrogen on 0.1 mmol of aldehyde 55 with 1 equiv. of aniline 13l and 1 equiv. of diethylphosphite 41 in toluene at rt for 16 h in the presence of 4 Å molecular sieves (200 wt% relative to 13l) and 10 mol% of benzoic acid. All yields are isolated yields. The % ee was determined by HPLC. b Reaction time was 24 h. c Reaction time was 72 h. d Reaction time was 48 h. e This reaction was repeated and both times 71% ee was obtained. |
|---|
|
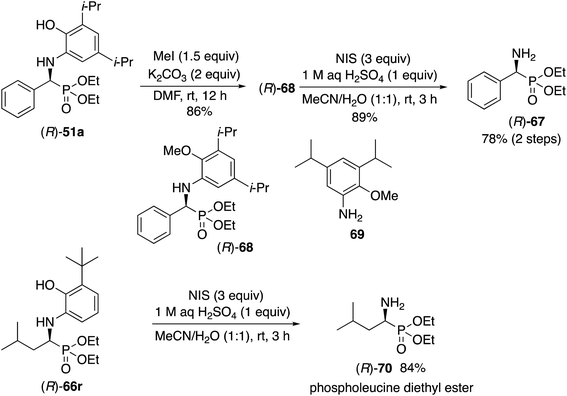
The liberation of the α-aminophosphonates requires a slightly different protocol for the aryl and aliphatic aldehydes. For the phosphonate (R)-51a from benzaldehyde it is first necessary to methylate the phenol function to give the methyl ether (R)-68. The free amine can then be obtained by oxidative deprotection of (R)-68 with N-iodosuccinimide to give the α-aminophosphonate (R)-67 in 78% yield in two steps. The absolute configuration of 67 was determined by comparison of its optical rotation with that previously reported for this compound25 and by the ECCD method26 (see the ESI†). Direct oxidation of the unprotected phenol unit in (R)-51a with N-iodosuccinimide gave the desired deprotected product (R)-67 but in only 10% yield along with several other products. Direct oxidation of (R)-51a with ceric ammonium nitrate gave a benzoxazole (see the ESI†). As a consequence of this failure, the Kabachnick–Fields reaction of benzaldehyde 31a was performed with the O-methylated aniline derivative 69 according to the optimized procedure in Table 3 but only gave a 9% yield of 68 as a racemic compound. Thus, the phenol function in the aniline 13c must play an important role in the reaction by either H-bonding to the catalyst center or by forming a covalent bond with the zirconium. The deprotection of the α-amino phosphonates derived from 3-t-butyl-2-hydroxyaniline 13l and aliphatic aldehydes is much more straightforward. Treatment of the α-amino phosphonate (R)-66r directly with three equivalents of N-iodosuccinimide and 1 equivalent of sulfuric acid in a mixture of water and acetonitrile gave phospholeucine diethyl ester (R)-70 in 84% yield. The absolute configuration of 70 was determined by comparison of its optical rotation with that previously reported for this compound27 and by the ECCD method developed previously26 (see the ESI†).
3. Conclusion
An effective asymmetric catalyst has been developed for the Kabachnik–Fields three component reaction of aldehydes, amines and phosphites to give α-aminophosphonates. This catalyst was first optimized for aromatic aldehydes and later was extensively re-optimized to find a suitable catalyst for aliphatic aldehydes. The catalyst is generated in situ from zirconium tetraisopropoxide, N-methylimidazole (NMI) and a vaulted biaryl ligand with the optimal ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Several different vaulted biaryl ligands were examined including VAPOL, VANOL and several 7,7′-disubstituted VANOL ligands with 7,7′-di-t-butylVANOL found to be the most effective. It was found that the yields and to some extent the asymmetric inductions could be increased in the presence of 10 mol% benzoic acid. For aromatic aldehydes the optimal amine was found to be a substituted 2-hydroxyaniline and of the several anilines screened 3,5-diisopropyl-2-hydroxyaniline was identified as superior and the optimal phosphite was diethyl phosphite. To achieve the desired level of asymmetric induction with aliphatic aldehydes an additional set of eight substituted 2-hydroxyanilines was prepared and screened and the most effective was found to be 3-t-butyl-2-hydroxyaniline. The asymmetric inductions for aliphatic aldehydes were comparable with those for aromatic aldehydes with a mean induction of 90% ee for the former and 91% ee for the latter. The best method for the liberation of the free amine from the aniline substituted α-aminophosphonates involved oxidation with N-iodosuccinimide and in the case of aromatic substrates, this first required the O-methylated phosphonate.
3. Several different vaulted biaryl ligands were examined including VAPOL, VANOL and several 7,7′-disubstituted VANOL ligands with 7,7′-di-t-butylVANOL found to be the most effective. It was found that the yields and to some extent the asymmetric inductions could be increased in the presence of 10 mol% benzoic acid. For aromatic aldehydes the optimal amine was found to be a substituted 2-hydroxyaniline and of the several anilines screened 3,5-diisopropyl-2-hydroxyaniline was identified as superior and the optimal phosphite was diethyl phosphite. To achieve the desired level of asymmetric induction with aliphatic aldehydes an additional set of eight substituted 2-hydroxyanilines was prepared and screened and the most effective was found to be 3-t-butyl-2-hydroxyaniline. The asymmetric inductions for aliphatic aldehydes were comparable with those for aromatic aldehydes with a mean induction of 90% ee for the former and 91% ee for the latter. The best method for the liberation of the free amine from the aniline substituted α-aminophosphonates involved oxidation with N-iodosuccinimide and in the case of aromatic substrates, this first required the O-methylated phosphonate.
Data availability
The data is in the ESI.†Author contributions
YD conceived the project and carried out the large majority of the reactions. WW wrote the manuscript and the other authors were minor contributors in the laboratory. All authors contributed to discussions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a grant from the National Institute of General Medical Sciences (GM094478) and the NSF (CHE-1213759).References
- (a) B. Lejczak and P. Kafarski, Biological Activity of Aminophosphonic acids and Their Short Peptides, Topics in Heterocyclic Chemistry; Phosphorus Heterocyclies I, 2009, vol. 20, pp. 31–63 Search PubMed; (b) A. Mucha, P. Kafarski and L. Berlicki, Remarkable Potential of the a-Aminophosphonate/Phosphinate Stuctural Motif in Medicinal Chemistry, J. Med. Chem., 2011, 54, 5955 CrossRef CAS.
- J. Hiratake and J. Oda, Aminophosphonic and Aminoboronic Acids as Key Elements of a Transition State Analogue Inhibitor of Enzymes, Biosci. Biotechnol. Biochem., 1997, 61, 211–218 CrossRef CAS.
- D. Cattani, P. A. Cesconetto, M. K. Tavares, E. B. Parisotto, P. A. De Oliveira, C. A. Heinz Rieg, M. C. Leite, R. D. Schroder Prediger, N. C. Wendt, G. Fazzera, D. W. Filho and A. Zamoner, Developmental exposure to glyphosate-based herbicide and depressive-like behavior in adult offspring: implication of glutamet excitotoxicity and oxidative stress, Toxicology, 2018, 387, 67–80 CrossRef PubMed.
- (a) F. R. Atherton, C. H. Hassall and R. W. Lambert, Synthesis and Structure-Activity Relationships of Antibacterial phosphonopeptides Incorporating (1-Aminoethyl)phosphonic Acid and (Aminomethyl)phosphonic Acid, J. Med. Chem., 1986, 29, 29–41 CrossRef CAS PubMed; (b) V. A. Solodenko and V. P. Kukhar, Stereoselective Papain-Catalyzed Synthesis of alafosfalin, Tetrahedron Lett., 1989, 30, 6917–6918 CrossRef CAS.
- (a) P. P. Giannousis and P. A. Bartlett, Phosphorus Amino Acid Analogues as Inhibitors of Leucine Aminopeptidase, J. Med. Chem., 1987, 30, 1603 CrossRef CAS PubMed; (b) C. Stamper, B. Bennett, T. Edwards, R. C. Holz, D. Ringe and G. Petsko, Isolation of the Aminopeptidase from Aeromonas proteolytica by L-Leucinephosphonic Acid. Spectroscopic and Crystallographic Characterization of the Transition State of Peptide Hydrolysis, Biochemistry, 2001, 40, 7035–7046 CrossRef CAS PubMed.
- P. A. Bartlett, J. E. Hanson and Giannousia, Potent Inhibition of pepsin and penicillipepsin by phosphorus-Containing Peptide Analogues, J. Org. Chem., 1990, 55, 6268–6274 CrossRef CAS.
- (a) H. Seto and T. Kuzuyama, Bioactive natural products with carbon-phosphorus bonds and their biosynthesis, Nat. Prod. Rep., 1999, 16, 589–596 RSC; (b) M. Akif, I. Ntai, E. D. Sturrock, R. E. Isaac, B. O. Bachmann and K. R. Acharya, Crystal structure of phosphonotripeptide K-26 in complex with angiotensin converting enzyme homologue (AnCE) from Drosophila melanogaster, Biochem. Biophys. Res. Commun., 2010, 398, 532–536 CrossRef CAS PubMed; (c) I. Ntai and B. O. Bachmann, Identification of ACE pharmacophore in the phosphonopeptide metabolite K-26, Bioorg. Med. Chem. Lett., 2008, 18, 3068–3071 CrossRef CAS PubMed.
- G. Zhang, G. Hao, J. Pan, J. Zhang, D. Hu and B. Song, Asymmetric Synthesis of Bioselective Activities of a-Aminophosphonates Based on the Dufulin Motif, J. Agric. Food Chem., 2016, 64, 4207 CrossRef CAS PubMed.
- (a) A. N. Pudovik, Dokl. Akad. Nauk SSSR, 1952, 83, 865 CAS; (b) A. N. Pudovik and I. V. Konovalova, Addition Reactions of Esters of Phosphorus(III) Acids with Unsaturated Systems, Synthesis, 1979, 81–96 CrossRef CAS; (c) R. Engel, Phosphorus addition at sp2 carbon, Org. React., 1988, 36, 175–248 CAS. For selective examples of the asymmetric catalytic Pudovik reaction, see: (d) H. Sasai, S. Arai, Y. Tahara and M. Shibasaki, Catalytic Asymmetric Synthesis of α-Amino Phosphonates using Lanthanoid-Potassium-BINOL Complexes, J. Org. Chem., 1995, 60, 6656–6657 CrossRef CAS; (e) G. D. Joly and E. N. Jacobsen, Thiourea-Catalyzed Enantioselective Hydrophosphonylation of Imines: Practical Access to Enantiomerically Enriched α-Amino Phosphonic Acids, J. Am. Chem. Soc., 2004, 126, 4102–4103 CrossRef CAS; (f) B. Saito, H. Egami and T. Katsuki, Synthesis of an Optically Active Al(salalen) Complex and its Application to Catalytic Hydrophosphonylation of Aldehydes and Aldimines, J. Am. Chem. Soc., 2007, 129, 1978–1986 CrossRef CAS PubMed; (g) J. P. Abell and H. Yamanoto, Catalytic Enantioselective Pudovik Reaction of Aldehydes and Aldimines with Tethered Bis(8-quinolinato) (TBOx) Aluminum Complex, J. Am. Chem. Soc., 2008, 130, 10521–10523 CrossRef CAS.
- (a) M. I. Kabachnik and T. Y. Medved, Dokl. Akad. Nauk SSSR, 1952, 83, 689–691 CAS; (b) E. Fields, The Synthesis of Esters of Substituted Amino Phosphonic Acids, J. Am. Chem. Soc., 1952, 74, 1528–1531 CrossRef CAS.
- For reviews, see: (a) N. S. Zefirov and E. D. Matveeva, Catalytic Kabachnik-Fields reaction: new horizons for old reaction, ARKIVOC, 2008,(i), 1–17 Search PubMed; (b) M. Ordonez, J. L. Viveros-Ceballos, C. Cativiela and F. J. Sayago, An update on the stereoselective synthesis of α-aminophosphonic acids and derivatives, Tetrahedron, 2015, 71, 1745–1784 CrossRef CAS; (c) M. Ordonez and J. L. Viveros-Ceballos, Stereoselective Synthesis of α-Aminophosphonic Acids through Pudovik and Kabachnik-Fields Reaction, Romero-Estudillo, I. INTECH Open Science, Amino Acid, New Insights and Roles in Plant and Animal, ed. T. Asao, 2017, ch. 6 Search PubMed; (d) T. Shilpa, N. A. Harry, S. M. Ujwaldev and G. Anilkumar, An Overview of Microwave-Assisted Kabachnik-Field Reactions, ChemistrySelect, 2020, 5, 4422–4436 CrossRef CAS.
- X. Cheng, R. Goddard, G. Buth and B. List, Direct Catalytic Asymmetric Three-Component Kabachnik-Fields Reaction, Angew. Chem., Int. Ed., 2008, 47, 5079–5081 CrossRef CAS PubMed.
- X. Zhou, D. Shang, Q. Zhang, L. Lin, X. Liu and X. Feng, Enantioselective Three-Component Kabachnik-Fields Reaction Catalyzed by Chiral Scandium(III)–N,N’-Dioxide Complexes, Org. Lett., 2009, 11, 1401–1404 CrossRef CAS PubMed.
- L. Wang, S.-M. Cui, W. Meng, G.-W. Zhang, J. Nie and J.-A. Ma, Asymmetric synthesis of α-aminophosphonates by means of direct organocatalytic three-component hydrophosphonylation, Chin. Sci. Bull., 2010, 55, 1729–1731 CrossRef CAS.
- M. Ohara, S. Nakamura and N. Shibata, Direct Enantioselective Three-Component Kabachnik-Fields Reaction Catalyzed by Chiral Bis(imidazoline)-Zinc(II) Catalysts, Adv. Synth. Catal., 2011, 353, 3285–3289 CrossRef CAS.
- P. B. Thorat, S. V. Goswami, R. L. Magar, B. R. Patil and S. R. Bhusare, An Efficient Organocatalysis: A One-Pot Highly Enantioselective Synthesis of α-Aminophosphonates, Eur. J. Org. Chem., 2013, 5509–5516 CrossRef CAS.
- P. S. Reddy, M. V. K. Reddy and P. V. G. Reddy, Camphor-derived thioureas: Synthesis and application in asymmetric Kabachnik-Fields reaction, Chin. Chem. Lett., 2016, 27, 943–947 CrossRef CAS.
- A. K. Gupta, M. Mukherjee and W. D. Wulff, Multicomponent Catalytic Asymmetric Aziridination of Aldehydes, Org. Lett., 2011, 13, 5866–5869 CrossRef CAS PubMed.
- M. l. Mukherjee, Y. Zhou, A. K. Gupta, Y. Guan and W. D. Wulff, A General Synthesis of Sphinganines through Multicomponent Catalytic Asymmetric Aziridination, Eur. J. Org. Chem., 2014, 1386–1390 CrossRef CAS.
- S. Xue, S. Yu, Y. Deng and W. D. Wulff, Active Site Design in a Chemzyme: Development of a Highly Asymmetric and Remarkably Temperature-Independent Catalyst for the Imino Aldol Reaction, Angew. Chem., Int. Ed., 2001, 40, 2271–2274 CrossRef CAS.
- X. Zhang, R. J. Staples, A. L. Rheingold and W. D. Wulff, Catalytic Asymmetric α-Iminol Rearrangement: New Chiral Platforms, J. Am. Chem. Soc., 2014, 136, 13971–13974 CrossRef CAS.
- H. Ishitani, M. Ueno and S. Kobayashi, Catalytic Enantioselective Mannich-Type Reactions Using a Novel Chiral Zirconium Catalyst, J. Am. Chem. Soc., 1997, 119, 7153–7154 CrossRef.
- M. Shibasaki, H. Sasai and T. Arai, Asymmetric Catalysis with Heterobimetallic Compounds, Angew. Chem., Int. Ed., 1997, 36, 1236–1256 CrossRef.
- J. R. Robinson, J. Gu, P. J. Carroll, E. J. Schelter and P. Walsh, Exchange Processes in Shibasaki's Rare Earth Alkali Metal BINOLate Frameworks and Their Relevance in Multifunctaional Asymmetric Catalysis, J. Am. Chem. Soc., 2015, 137, 7135–7144 CrossRef CAS PubMed.
- F. A. Davies, S. Lee, H. X. Yang and D. D. Titus, Asymmetric synthesis of quaternary α-amino phosphonates using sulfinimines, Org. Lett., 2001, 2, 1757–1759 CrossRef PubMed.
- X. Y. Li, M. Tanasova, C. Vasileiou and B. Borhan, Fluorinated porphyrin tweezer: a powerful reporter of absolute configuration for erythro and threo diols, amino alcohols and diamines, J. Am. Chem. Soc., 2008, 130, 1885–1893 CrossRef CAS PubMed.
- A. B. Smith, K. M. Yager, B. W. Phillips and C. M. Taylor, Asymmetric synthesis of diethyl (R)-(–)-(1-amino-3-methylbutyl)phosphonate, Org. Synth., 1998, 75, 19–30 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Procedures for the preparation of new compounds and characterization data for all new compounds. See DOI: 10.1039/d1sc03222d |
| This journal is © The Royal Society of Chemistry 2021 |