Open Access Article
Open Access ArticleConversion of bile salts from inferior emulsifier to efficient smart emulsifier assisted by negatively charged nanoparticles at low concentrations†
Haojie
Zhang
a,
Miao
Lv
a,
Jianzhong
Jiang
 *a,
Zhenggang
Cui
*a,
Zhenggang
Cui
 a,
Wenshui
Xia
b and
Bernard P.
Binks
a,
Wenshui
Xia
b and
Bernard P.
Binks
 *c
*c
aThe Key Laboratory of Synthetic and Biological Colloids, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, 1800 Lihu Road, Wuxi, Jiangsu, P. R. China. E-mail: jzjiang@jiangnan.edu.cn
bState Key Laboratory of Food Science and Technology, School of Food Science and Technology, Jiangnan University, 1800 Lihu Road, Wuxi, Jiangsu, P. R. China
cDepartment of Chemistry, University of Hull, Hull HU6 7RX, UK. E-mail: b.p.binks@hull.ac.uk
First published on 4th August 2021
Abstract
Bile salts (BS), one of the biological amphiphiles, are usually used as solubilizing/emulsifying agents of lipids or drugs. However, BS such as sodium deoxycholate (NaDC) can't stabilize an oil-in-water (O/W) emulsion alone due to its unusual molecular structure. In this paper we report that these emulsifiers with poor emulsifying ability can be transformed to highly efficient emulsifiers by combining with negatively charged particles (silica or montmorillonite). Both together can synergistically co-stabilize oil-in-water emulsions at extremely low concentrations (minimum 0.01 mM NaDC plus 0.003 wt% particles). Moreover, the emulsions can be reversibly switched between stable and unstable triggered by CO2/N2 at room temperature. This strategy is universal for emulsions containing different oils (alkanes, aromatic hydrocarbons and triglycerides) and for different BS and offers a generic model for a variety of BS of different molecular structure, which will extend their applications in more technical fields such as emulsion polymerization, biphasic catalysis and emulsion extraction.
Introduction
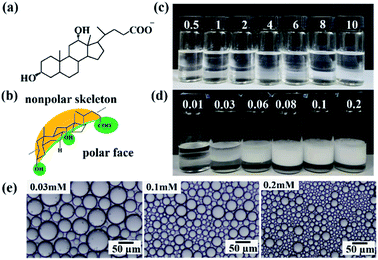
Emulsions are multiphase dispersions composed of immiscible liquid phases which have a variety of applications in many fields such as pharmaceuticals, cosmetics, textiles and agrochemicals.1–5 Emulsions are commonly stabilized by surfactants or surface-active polymers to prevent droplets from flocculation and coalescence,1,2 and the stability is an important consideration with respect to long-term storage.Bile salts (BS) are important biosurfactants responsible for digestion and adsorption of nutrients.6–11 Unlike conventional surfactants with linear hydrocarbon tails, BS have a facial amphiphilic structure composed of a hydrophobic face with a rigid steroid backbone and a hydrophilic face with hydroxyl/carboxylate groups (e.g. NaDC, Fig. 1a and b), which facilitates BS molecules to form self-assembled structures like micelles and gels in water.12–17 The interfacial properties of BS are different to those of conventional surfactants. Since the rigid sterol ring has higher hydrophobicity than the hydroxyl group, BS have affinity to the oil-water interface.18–22 On the one hand, BS tend to adsorb at the interface and replace other adsorbed surfactant or emulsifier molecules already present.11,19 Although some recent studies focus on the interaction of BS with other emulsifiers or proteins at the interface,23–27 studies dealing with BS as a unique emulsifier are very limited. On the other hand, some BS were observed to desorb from the interface at low concentrations.20,28,29 The peculiar adsorption/desorption profile of BS at the interface always depends on their molecular structure.20,30 For example, sodium glycodeoxycholate (NaGDC) was reported to fully desorb from an interface to form clusters or micelles in water.20 Unfortunately, such desorption from interfaces of an emulsion always leads to coalescence of droplets or demulsification,24,25 which hinders the use of BS as an emulsifier or emulsion stabilizer for long-term storage. Fig. 1c shows that NaDC can't stabilize an octane-water emulsion alone even at high concentrations (critical micelle concentration (cmc) = 2.4 mM). To date therefore, it is still a challenge to manipulate the adsorption/desorption behaviour of BS at an oil-water interface.20
Herein we report on the conversion of bile salts from inferior emulsifier to efficient smart emulsifier assisted by a trace amount of negatively charged nanoparticles. Although neither NaDC nor silica particles can stabilize emulsions alone, both together can synergistically inhibit desorption of NaDC from the oil–water interface through electrical repulsion, which significantly improves its emulsifying ability. In addition, the concentrations of NaDC and silica particles required for stabilization of the emulsions are extremely low, and the synergistic effects between NaDC and particles can be triggered by CO2/N2, yielding switchable oil-in-dispersion emulsions.31
Results and discussion
Fig. 1d shows that n-octane-in-water emulsions stable to coalescence can be obtained at a NaDC concentration as low as 0.08 mM after addition of 0.1 wt% hydrophilic silica nanoparticles (diameter = 20 nm,32ζ = −29 mV in pure water, Fig. S1†). The average droplet size in emulsions decreases from 40–50 μm to 8–10 μm with increasing NaDC concentration from 0.01 mM to 0.2 mM (Fig. 1e and S2†), while it was less affected by changing the silica particle concentration (Fig. S3†). Interestingly, the minimum concentrations of silica nanoparticles and NaDC required for stabilization of the emulsion are only 0.003 wt% and 0.08 mM, respectively (Fig. S4 and S5†). Moreover, the emulsions are stable to coalescence for at least two weeks over a wide range of oil:water volume ratios (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 to 7
6 to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) with no oil being released at NaDC concentrations above 0.05 mM (Fig. S6†).
3) with no oil being released at NaDC concentrations above 0.05 mM (Fig. S6†).
The type of emulsion is oil-in-water, which is identified by dilution and fluorescence staining methods. Red spherical droplets were observed as shown in Fig. 2a when the emulsion is stained with oil-soluble Nile Red, and the dried emulsion shows spherical holes free of any particles via SEM indicating no adsorption of silica particles at the oil–water interface (Fig. 2b and c). The fluorescence image (particles labelled green in Fig. S7†) also proves the position of the particles is mainly in the aqueous continuous phase.31–35 This microstructure is different from that of Pickering emulsions stabilized by NaDC and positively charged alumina nanoparticles (Fig. 2d), in which particles attach to the oil–water borders forming winkled films on the surfaces of dried oil droplets. We note that some Pickering emulsions can also be stabilized when the interfacial coverage by particles is quite low.36,37 Here, we believe that only NaDC molecules adsorb at the oil–water interface with silica particles dispersed in the continuous phase between droplets31–35 producing an oil-in-dispersion emulsion35 as shown by the SEM and fluorescence microscopy images (Fig. 2 and S7†).
It was further observed that this strategy of improving the emulsifying ability of BS by addition of silica particles is universal for other BS. As shown in Fig. 3a, stable n-octane-in-water emulsions were prepared using silica particles and a variety of BS containing different numbers and orientations of hydroxyl groups (Fig. S8†). Even the BS without hydroxyl groups (sodium dehydrocholate) yields a stable emulsion in combination with silica particles. On the other hand, if octane is replaced by other oils such as olive oil, soybean oil, diesel oil, kerosene, toluene, liquid paraffin, dimethicone and cyclohexene, oil-in-water emulsions with droplet diameters of 15–65 μm can also be obtained (Fig. 3b and S9†).
We can then reveal the stabilization mechanism involved in the above oil-in-water emulsions by considering the interaction of NaDC and negatively charged silica particles. First the zeta potential of the silica nanoparticles was measured (−29 mV in pure water). When they are dispersed (0.1 wt%) in NaDC solutions the zeta potential changes from −20 mV to −11 mV with increasing NaDC concentration from 0.001 mM to 1 mM, as shown in Fig. 4. Since both the particles and NaDC are negatively charged, NaDC is not expected to adsorb on particle surfaces but may act as an electrolyte to screen the electrical double layer repulsion around particles, resulting in a decrease of the zeta potential.31 In contrast, if silica nanoparticles are replaced by positively charged particles such as alumina nanoparticles (Fig. S10†),31 the zeta potential was observed to decrease from +34 mV to −10 mV, indicating strong adsorption of NaDC on alumina particle surfaces.
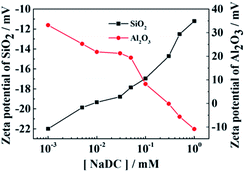 | ||
| Fig. 4 Zeta potential of 0.1 wt% silica nanoparticles or alumina nanoparticles dispersed in NaDC solutions of different concentration. | ||
The electrostatic repulsion between droplets induced by the negatively charged carboxylate group in NaDC and that between particles and droplets is responsible for the stabilization of the emulsion,31–35 whereas the hydrogen bonding between NaDC and silica particles if present is not crucial to emulsion stabilization, since BS without hydroxyl groups (sodium dehydrocholate) also yield a stable emulsion in combination with silica particles. However, in contrast to the oil-in-dispersion emulsions stabilized by CTAB in combination with alumina nanoparticles where CTAB adsorbs at the oil–water interface as a good emulsifier,31 the current system seems to be unusual since NaDC is an inferior emulsifier (Fig. 1c) and may desorb from the interface similar to other BS.11,20
In order to confirm whether there is an adsorption behaviour of NaDC on the surface of the particles through its hydroxyl groups, the interfacial behavior of NaDC was then investigated which is molecular structure dependent.20,30 Normally for a nonionic surfactant solution the surface tension will increase upon addition of silica nanoparticles due to adsorption of the surfactant on particle surfaces via hydrogen bonding.38 But here we observed that the surface tension of NaDC solutions decreases slightly upon addition of silica particles (Fig. 5a), implying no adsorption of NaDC on the particles. It has been reported that the surface tension of NaDC solutions is relatively higher than that of conventional surfactants due to the loose arrangement at the air–water interface.20 Interestingly, the reduction in surface tension induced by addition of silica particles becomes larger upon increasing the silica particle concentration, as shown by Fig. 5b where the pc20 value (the negative log of the surfactant concentration C20), which is used to evaluate the adsorption efficiency of a surfactant at the interface,39 increased from 3.32 to 3.66 upon raising the particle concentration from 0 to 1.5 wt%. This evidence indicates that the adsorption efficiency of NaDC at a fluid interface is enhanced by addition of silica nanoparticles, probably via the electrostatic repulsion between the negative charge on particles and the anionic carboxylate of NaDC.
The interfacial tensions of NaDC solutions against n-octane measured by the pendant drop method also show an interesting change upon addition of silica nanoparticles, as shown in Fig. 5c and d. The interfacial tension decreases after a fresh interface is created due to adsorption of NaDC from bulk to the interface. With a large hydrophobic group, the NaDC molecule has a slow diffusion speed so that it takes over 1000 s for the tension to reach equilibrium. However, the interfacial tension was observed to increase slightly with time, probably caused by desorption of NaDC molecules from the interface. Such a non-monotonic change in surface tension has also been reported in the literature40 but the underlying cause is unknown. It may be due to the formation of non-equilibrium aggregates.20,28 In contrast, this phenomenon was not observed for sodium dodecyl sulphate (SDS) which has a simple amphiphilic structure as shown in Fig. S11.† However, such desorption can be weakened by addition of a small amount of silica particles (0.05–0.25 wt%) and be inhibited completely at a particle concentration of 1.5 wt%, resulting in a decrease in equilibrium interfacial tension (Fig. 5d). Therefore, the electrostatic repulsion between NaDC and silica particles may improve adsorption of NaDC at the oil–water interface. At such a low concentration it is believed that the adsorbed amount of NaDC at the oil–water interface is not sufficient to form a close packed monolayer to prevent flocculation and coalescence of the droplets. The stabilization of the emulsion therefore relies on the presence of particles dispersed in the aqueous phase. As shown by the SEM images (Fig. 2b and c), the particles constitute thick lamellae between droplets resulting in an increase of the inter-droplet spacing and thus a reduction of the van der Waals attraction between droplets.31–35 However, no stable oil-in-dispersion emulsion can be obtained above the cmc of NaDC (>2 mM, Fig. S12†). On the one hand, NaDC alone is a poor emulsifier and cannot stabilize an O/W emulsion. On the other hand, it behaves as an electrolyte reducing the interfacial potential of both oil droplets and particles so that the electrical double layer repulsion between particles and between particles and droplets is screened resulting in instability of the oil-in-dispersion emulsion. In fact, this effect appears when the NaDC concentration is increased beyond 0.1 mM where the particle concentration should be increased to compensate this effect giving a V-shape relationship between the particle and surfactant concentrations required for emulsion stabilization (Fig. S5†). Such a trend was also observed for the like charged alumina + CTAB system.31
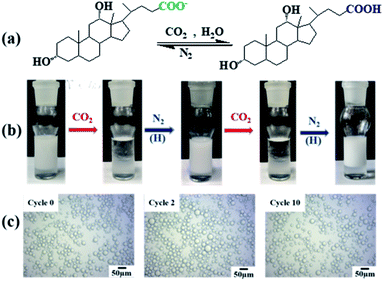
In order to reveal the stabilization mechanism further, the responsiveness of the emulsion to a CO2/N2 trigger was examined. Since NaDC has a pKa of 6.58,41 it can be protonated into acid form (deoxycholic acid, HDC) by bubbling CO2 removing its negative charge and be recovered to its original carboxylate form by bubbling N2 (Fig. 6a). After bubbling CO2 (10 mL min−1, 10 min) into a neutral and transparent aqueous NaDC solution (20 mL, 0.05 mM), the solution is turned to acidic (pH = 4.2 ± 0.2) and turbid (15 NTU) due to the insolubility of HDC in water. However, the neutral and transparent solution can be recovered following bubbling N2 (10 mL min−1, 10 min, Fig. S13†). Fig. 6b shows that for a n-octane-in-water emulsion stabilized by 0.1 wt% silica nanoparticles plus 0.05 mM NaDC, efficient demulsification is easily achieved by bubbling CO2 (10 mL min−1, 10 min) into the emulsion at room temperature. In contrast, no demulsification was observed by bubbling N2. Obviously, the negative charge of NaDC is neutralized by protons produced by bubbling CO2, which destroys the electrical double layer repulsion between droplets and between droplets and particles resulting in demulsification. A stable emulsion was formed again by bubbling N2 (10 mL min−1, 10 min) at room temperature into the separated oil–water mixture followed by homogenization, which recovered the negative charge of NaDC. The demulsification/re-stabilization processes can be reversibly switched for at least 10 times by bubbling CO2/N2 alternately without degradation, and the average droplet diameter of the emulsion after 10 cycles is similar to that of the original emulsion (Fig. 6c, 15–25 μm). A similar switchable emulsification protocol can also be achieved by adding HCl/NaOH (Fig. S14†), whereas no stable emulsions can be stabilized by silica particles alone at pH values between 3 and 11 (Fig. S15†). Furthermore, demulsification can also be initiated after adding various inorganic salts,31e.g. 0.1 mM FeCl3 (Fig. S16†). The results confirm that the electrostatic repulsion between the droplets and between the droplets and particles is crucial for stabilization of the oil-in-dispersion emulsions of NaDC, which can be weakened or destroyed by bubbling CO2 or adding salt.
Switchable emulsions in response to various environmental triggers have recently attracted increasing attention due to smart control of the stable/unstable states of emulsions.42,43 Compared with other triggers, the CO2/N2 trigger has been recognized as low cost and environmentally benign.42 Normally the CO2-responsive emulsions are prepared by using amine-containing compounds as smart emulsifiers and the trigger has to work at high temperature (∼65 °C).42,44 In our method here, the same trigger works well at ambient temperature which is convenient, energy saving and therefore beneficial in practical applications.45,46
The above findings are also applicable to other negatively charged particles such as montmorillonite (pharmaceutical grade) with larger average diameter (680 nm) and smaller zeta potential (−12 mV) in pure water (Fig. S17 and S18†). It is found that the larger size and lower zeta potential of the montmorillonite particles makes it less efficient for stabilization of the oil-in-dispersion emulsion (Fig. S19†), but can be compensated by increasing the concentration of both particles and NaDC (Fig. S20†).
Conclusions
In summary, we have demonstrated that bile salts can be converted to efficient emulsifiers in combination with a trace amount of similarly charged nanoparticles, which enhance adsorption of them at the oil–water interface and inhibit their desorption from it. The concentrations of bile salts and particles required for stabilization of oil-in-water emulsions are very low (e.g. minimum 0.003 wt% silica particles plus 0.01 mM NaDC) and the emulsions formed are CO2/N2 switchable at room temperature. The low adsorption of negatively charged bile salt molecules at the oil–water interface, although not sufficient to stabilize oil droplets alone, endows droplets with negative charge which prevents flocculation and coalescence via electrical double layer repulsion when negatively charged nanoparticles are dispersed in the aqueous phase between them. Such synergistic stabilization can be manipulated by a CO2/N2 trigger through protonation (removing charge)/deprotonation (recovering charge) of the bile salt. This strategy is universal for a variety of bile salts of different molecular structure and offers a generic model enabling bile salts and negatively charged particles to act as an efficient and switchable emulsifier for a variety of practical applications.Author contributions
H. Z. and M. L. performed the experiment. All authors discussed the results. The manuscript was written through contributions of all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21872064, 21573096, 21473080) and the National First-Class Discipline Program of Food Science and Technology (JUFSTR20180201).Notes and references
- P. Becher, Encyclopedia of Emulsion Technology, Marcel Dekker, New York, 1983, vol. 1 Search PubMed.
- M. J. Rosen and J. T. Kunjappu, Surfactants and Interfacial Phenomena, Wiley, Hoboken, 4th edn, 2012 Search PubMed.
- R. Aveyard, B. P. Binks and J. H. Clint, Adv. Colloid Interface Sci., 2003, 100–102, 503–546 CrossRef CAS.
- T. Tadros, P. Izquierdo, J. Esquena and C. Solans, Adv. Colloid Interface Sci., 2004, 108, 303–318 CrossRef.
- M. Golding and T. J. Wooster, Curr. Opin. Colloid Interface Sci., 2010, 15, 90–101 CrossRef CAS.
- S. Mukhopadhyay and U. Maitra, Curr. Sci., 2004, 87, 1666–1683 CAS.
- M. Makishima, A. Y. Okamoto, J. J. Repa, H. Tu, R. M. Learned, A. Luk, M. V. Hull, K. D. Lustig, D. J. Mangelsdorf and B. Shan, Science, 1999, 284, 1362–1365 CrossRef CAS PubMed.
- M. Watanabe, S. M. Houten, C. Mataki, M. A. Christoffolete, B. W. Kim, H. Sato, N. Messaddeq, J. W. Harney, O. Ezaki, T. Kodama, K. Schoonjans, A. C. Bianco and J. Auwerx, Nature, 2006, 439, 484–489 CrossRef CAS PubMed.
- J. Maldonado-Valderrama, P. J. Wilde, A. Macierzanka and A. R. Mackie, Adv. Colloid Interface Sci., 2011, 165, 36–46 CrossRef CAS.
- R. Holm, A. Mullertz and H. L. Mu, Int. J. Pharm., 2013, 453, 44–55 CrossRef CAS.
- A. Macierzanka, A. Torcello-Gómez, C. Jungnickel and J. Maldonado-Valderrama, Adv. Colloid Interface Sci., 2019, 274, 102045 CrossRef CAS.
- D. Madenci and S. U. Egelhaaf, Curr. Opin. Colloid Interface Sci., 2010, 15, 109–115 CrossRef CAS.
- L. Galantini, C. Leggio, A. Jover, F. Meijide, N. V. Pavel, V. H. S. Tellini, J. V. Tato, R. D. Leonardo and G. Ruocco, Soft Matter, 2009, 5, 3018–3025 RSC.
- M. Gubitosi, L. Travaglini, M. C. Di Gregorio, N. V. Pavel, J. Vázquez Tato, S. Sennato, U. Olsson, K. Schillén and L. Galantini, Angew. Chem., Int. Ed., 2015, 54, 7018–7021 CrossRef CAS PubMed.
- S. H. Tung, Y. E. Huang and S. R. Raghavan, J. Am. Chem. Soc., 2006, 128, 5751–5756 CrossRef CAS.
- S. Salentinig, S. Phan, A. Hawley and B. J. Boyd, Angew. Chem., Int. Ed., 2015, 54, 1600–1603 CrossRef CAS PubMed.
- A. Sadeghpour, M. Rappolt, S. Misra and C. V. Kulkarni, Langmuir, 2018, 34, 13626–13637 CrossRef CAS.
- L. Galantini, M. C. Gregorio, M. Gubitosi, L. Travaglini, J. V. Tato, A. Jover, F. Meijide, V. H. S. Tellini and N. V. Pavel, Curr. Opin. Colloid Interface Sci., 2015, 20, 170–182 CrossRef CAS.
- L. O. Pabois, C. D. Lorenz, R. D. Harvey, I. Grillo, M. M. L. Grundy, P. J. Wilde, Y. Gerelli and C. A. Dreiss, J. Colloid Interface Sci., 2019, 556, 266–277 CrossRef PubMed.
- J. Maldonado-Valderrama, J. L. Muros-Cobos, J. A. Holgado-Terriza and M. A. Cabrerizo-Vílchez, Colloids Surf., B, 2014, 120, 176–183 CrossRef CAS PubMed.
- A. Torcello-Gómez, J. Maldonado-Valderrama, A. B. Jódar-Reyes and T. J. Foster, Langmuir, 2013, 29, 2520–2529 CrossRef.
- S. R. Euston, W. G. Baird, L. Campbell and M. Kuhns, Biomacromolecules, 2013, 14, 1850–1858 CrossRef CAS.
- J. N. Naso, F. A. Bellesi, V. M. Pizones Ruiz-Henestrosa and A. M. R. Pilosof, Colloids Surf., B, 2019, 174, 493–500 CrossRef CAS PubMed.
- A. Torcello-Gómez, A. B. Jódar-Reyes, J. Maldonado-Valderrama and A. Martín-Rodríguez, Food Res. Int., 2012, 48, 140–147 CrossRef.
- J. Zornjak, J. Liu, A. Esker, T. Lin and C. Fernandez-Fraguas, Food Hydrocolloids, 2020, 106, 105867 CrossRef CAS.
- F. A. Bellesi, V. M. Pizones Ruiz-Henestrosa and A. M. R. Pilosof, Food Hydrocolloids, 2014, 36, 115–122 CrossRef CAS.
- C. M. Cremers, D. Knoefler, V. Vitvitsky, R. Banerjee and U. Jakob, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, E1610–E1619 CrossRef CAS.
- S. R. Euston, U. Bellstedt, K. Schillbach and P. S. Hughes, Soft Matter, 2011, 7, 8942–8951 RSC.
- S. Gallier, E. Shaw, A. Laubscher, D. Gragson, H. Singh and R. Jimenez-Flores, J. Agric. Food Chem., 2014, 62, 1363–1372 CrossRef CAS PubMed.
- R. Parker, N. M. Rigby, M. J. Ridout, A. P. Gunning and P. J. Wilde, Soft Matter, 2014, 10, 6457–6466 RSC.
- M. Xu, J. Jiang, X. Pei, B. Song, Z. Cui and B. P. Binks, Angew. Chem., Int. Ed., 2018, 130, 7864–7868 CrossRef.
- J. Jiang, S. Yu, W. Zhang, H. Zhang, Z. Cui, W. Xia and B. P. Binks, Angew. Chem., Int. Ed., 2021, 60, 11793–11798 CrossRef CAS.
- M. Xu, L. Xu, Q. Lin, X. Pei, J. Jiang, H. Zhu, Z. Cui and B. P. Binks, Langmuir, 2019, 35, 4058–4067 CrossRef CAS PubMed.
- H. Zhang, J. Wu, J. Jiang, Z. Cui and W. Xia, Langmuir, 2020, 36, 14589–14596 CrossRef CAS.
- M. Xu, W. Zhang, J. Jiang, X. Pei, H. Zhu, Z. Cui and B. P. Binks, Langmuir, 2020, 36, 15543–15551 CrossRef CAS PubMed.
- M. E. Leunissen, A. van Blaaderen, A. D. Hollingsworth, M. T. Sullivan and P. M. Chaikin, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 2585–2590 CrossRef CAS.
- D. J. French, A. T. Brown, A. B. Schofield, J. Fowler, P. Taylor and P. S. Clegg, Sci. Rep., 2016, 6, 31401 CrossRef CAS PubMed.
- Y. Zhu, T. Fu, K. Liu, Q. Ling, X. Pei, J. Jiang, Z. Cui and B. P. Binks, Langmuir, 2017, 33, 5724–5733 CrossRef CAS.
- F. He, G. Xu, J. Pang, M. Ao, T. Han and H. Gong, Langmuir, 2011, 27, 538–545 CrossRef CAS.
- Z. Zhang, Y. Jiang, C. Huang, Y. Chai, E. Goldfine, F. Liu, W. Feng, J. Forth, T. E. Williams, P. D. Ashby, T. P. Russell and B. A. Helms, Sci. Adv., 2018, 4, eaap8045 CrossRef CAS.
- A. Iyire, M. Alaayedi and A. R. Mohammed, Sci. Rep., 2016, 6, 32498 CrossRef CAS.
- Y. X. Liu, P. G. Jessop, M. Cunningham, C. A. Eckert and C. L. Liotta, Science, 2006, 313, 958–960 CrossRef CAS.
- J. T. Tang, P. J. Quinlan and K. C. Tam, Soft Matter, 2015, 11, 3512–3529 RSC.
- J. Jiang, Y. Zhu, Z. Cui and B. P. Binks, Angew. Chem., Int. Ed., 2013, 52, 12373–12376 CrossRef CAS.
- P. Liu, W. Lu, W. Wang, B. Li and S. Zhu, Langmuir, 2014, 30, 10248–10255 CrossRef CAS.
- S. Yu, D. Zhang, J. Jiang, Z. Cui, W. Xia, B. P. Binks and H. Yang, Green Chem., 2019, 21, 4062–4068 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc02596a |
| This journal is © The Royal Society of Chemistry 2021 |