Open Access Article
Open Access ArticleStereoselective tandem iridium-catalyzed alkene isomerization-cope rearrangement of ω-diene epoxides: efficient access to acyclic 1,6-dicarbonyl compounds†
Rahul
Suresh
 ,
Itai
Massad
,
Itai
Massad
 and
Ilan
Marek
and
Ilan
Marek
 *
*
Schulich Faculty of Chemistry, Technion – Israel Institute of Technology Technion City, 3200009 Haifa, Israel. E-mail: chilanm@technion.ac.il
First published on 9th June 2021
Abstract
The Cope rearrangement of 2,3-divinyloxiranes, a rare example of epoxide C–C bond cleavage, results in 4,5-dihydrooxepines which are amenable to hydrolysis, furnishing 1,6-dicarbonyl compounds containing two contiguous stereocenters at the 3- and 4-positions. We employ an Ir-based alkene isomerization catalyst to form the reactive 2,3-divinyloxirane in situ with complete regio- and stereocontrol, which translates into excellent control over the stereochemistry of the resulting oxepines and ultimately to an attractive strategy towards 1,6-dicarbonyl compounds.
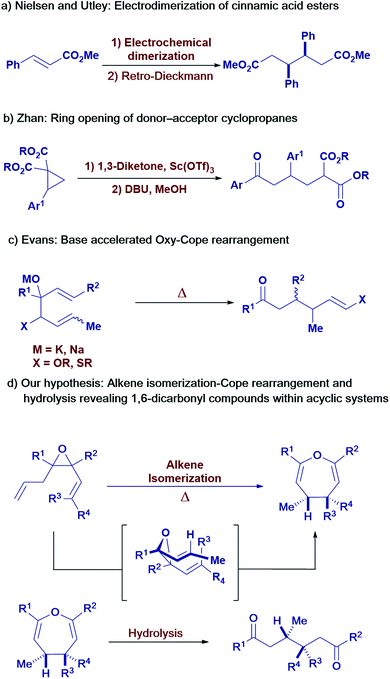
1,6-Dicarbonyl compounds are widespread as targets and intermediates in organic synthesis.1 Due to the “dissonant” polarizing effect induced by the two carbonyl groups,2 these motifs are challenging to retrosynthetically disconnect into classical synthons. Unsurprisingly, many approaches toward 1,6-dicarbonyls rely on dimerization of α,β-unsaturated carbonyl compounds (Scheme 1a)3 or oxidative cleavage of substituted cyclohexene derivatives4 which significantly limits the range of possible products. Alternative strategies, such as the ring-opening of donor–acceptor cyclopropanes with enolate nucleophiles, efficiently form the 1,6-dicarbonyl skeleton, albeit with limited substrate scope (Scheme 1b).5 The Cope rearrangement of 1,5-dienes, featuring oxygen functionality in the 3- and 4-positions,6 represents a promising strategy towards 1,6-dicarbonyl compounds but suffers from lack of stereocontrol over the diene substrates, resulting in diastereomeric mixtures of products (Scheme 1c).
 | ||
| Scheme 1 Selected approaches towards the formation of 1,6-dicarbonyl compounds and our proposed approach. | ||
A conceptually related approach towards the preparation of 1,6-dicarbonyl compounds is through the hydrolysis of 3,4-dihydrooxepines (Scheme 1d), which are in turn generated through the Cope rearrangement of 2,3-divinyloxiranes.7 Such a sigmatropic rearrangement is also noteworthy as a rare example where an epoxide C–C bond is selectively cleaved over the usually more reactive C–O bond. This intriguing rearrangement has been studied but its use in synthesis is scarce, presumably due to difficulties in the stereoselective synthesis and handling of the key divinyl epoxides.
In line with our interest in the strategic application of alkene isomerization to generate reactive synthetic intermediates in stereodefined form,8 we posited to form the reactive 2,3-divinyloxiranes in situ, through alkene isomerization9,10 of the simpler allyl epoxides, which are accessible in enantiomerically enriched form.11 Such a strategy might greatly facilitate access to these intermediates and therefore uncover a synthetically attractive route toward 1,6-dicarbonyl compounds featuring two contiguous stereocenters.
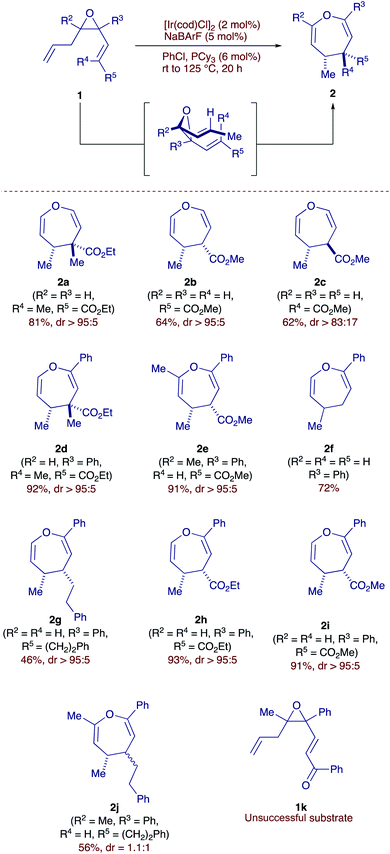
With this idea in mind, we first explored the isomerization and subsequent Cope rearrangement of allyl-vinyl epoxides 1 (Scheme 2). To induce isomerization, we employed a cationic iridium-based catalytic system,12 which is known to reliably isomerize alkenes with high degrees of regio- and stereocontrol.13
 | ||
| Scheme 2 Substrate scope for the tandem iridium-catalyzed alkene isomerization-Cope rearrangement of allyl-vinyl epoxides. | ||
In line with our expectations, our model substrate 1a (R2 = R3 = H, R4 = Me, R5 = CO2Et) was smoothly isomerized at 65 °C in the presence of 1.5 mol% of Ir dimer to obtain the corresponding divinyl epoxide with a complete E-selectivity. With suitable conditions for alkene isomerization in hand, we exposed substrate 1a to the Ir-based catalytic system at 120 °C and were equally pleased to observe the 4,5-dihydrooxepine product 2a, resulting from the tandem isomerization-Cope rearrangement as a single diastereoisomer in 81% yield. We proceeded to test the generality of our protocol with respect to different alkene and epoxide substitution patterns. Pleasingly, product 2b was generated with complete stereoselectivity, showcasing the compatibility of the reaction conditions with potentially labile tertiary stereocenters α to the ester group. We then wondered whether the anti-diastereomer could be accessed starting from the corresponding cis allyl-vinyl epoxide. Indeed, in line with the known stereospecific behavior of the Cope rearrangement, we obtained the complementary diastereomer 2c. Turning our attention to more highly substituted epoxides, we were pleased to observe the formation of dihydrooxepines 2d and 2e, which correspond to 1,6-keto-aldehyde and diketone products, respectively. Substrate 1f (R2 = R4 = R5 = H, R3 = Ph), which features an unactivated vinyl group, also underwent the rearrangement, demonstrating that an activated alkenyl group is not required for a successful outcome. Similarly, product 2g featuring two alkyl groups is also generated, with high diastereoselectivity albeit in moderate yield. Products featuring ethyl and methyl ester 2h, 2i could also be obtained in good yields and diastereoselectivity. We next tested substrate 1j (R2 = Me, R3 = Ph, R4 = CH2CH2Ph, R5 = H), as a geometric-mixture of the double bond (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 1.1
Z = 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and in accordance with the stereospecificity of the process, the oxepine 2j was obtained as a mixture of two diastereomers with the same ratio. Disappointingly, substrate 1k did not undergo isomerization, presumably due to the Lewis basic nature of the ketone, likely poisoning the Ir-catalyst.
1) and in accordance with the stereospecificity of the process, the oxepine 2j was obtained as a mixture of two diastereomers with the same ratio. Disappointingly, substrate 1k did not undergo isomerization, presumably due to the Lewis basic nature of the ketone, likely poisoning the Ir-catalyst.
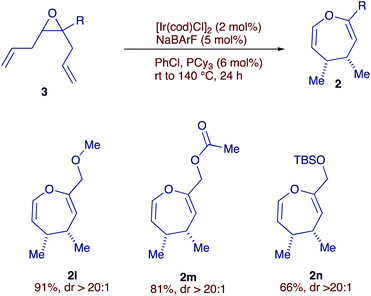
During our study, we noticed that allyl-vinyl epoxides bearing electron donating groups on the vinyl moiety tend to decompose during purification by column chromatography on silica gel. This obstacle further motivated us to explore diallyl epoxides 3 as substrates, where the reactive divinyl epoxide would be generated by isomerization of both allyl fragments. Notably, these diallyl epoxides are much more stable compared to their vinyl counterparts and can be readily prepared in two steps from simple alkynes.14 To our delight, diallyl epoxide 3a (R = CH2OMe) smoothly underwent the double isomerization-Cope rearrangement cascade at 140 °C, furnishing oxepine 2l with impressive yield and diastereoselectivity (Scheme 3). The use of alkene isomerization to form the reactive divinyl epoxide in situ avoids the isolation of the unstable divinyl epoxide, while controlling the stereochemistry of both double bonds, particularly not trivial to achieve using classical olefination reactions. Products 2m and 2n feature ester and silyl groups, highlighting the functional group tolerance of the catalytic system.
 | ||
| Scheme 3 Substrate scope for tandem iridium-catalyzed double alkene isomerization-Cope rearrangement of diallyl epoxides. | ||
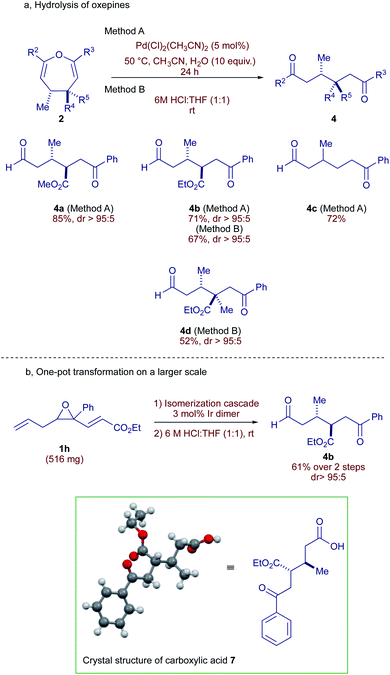
Our next objective was to hydrolyze the diastereomerically pure oxepines obtained through the rearrangement in a stereoretentive fashion, revealing the acyclic 1,6-dicarbonyl motif. Pleasingly, diversely substituted oxepines 2 underwent smooth hydrolysis either using 5 mol% of Pd(MeCN)2Cl215 at 50 °C or an acidic aqueous solution to form 1,6-dicarbonyls 4 in diastereomerically pure form (Scheme 4).16 Dicarbonyl products featuring labile tertiary centers 4a and 4b are formed under these conditions with excellent diastereoselectivities and yields. Without surprise, oxepine 2f (R2 = R4 = R5 = H, R3 = Ph) furnished the keto-substituted product 4c in good yield. The relative stereochemistry of 4b was unambiguously confirmed by single crystal X-ray diffraction analysis of the corresponding carboxylic acid 7 (Scheme 4b).17 The reaction is scalable to ½ gram of substrate and could be performed in a single-pot operation without isolation of the intermediate oxepine (Scheme 4b). By using this approach, 1h provides 4b in 61% yield as a single diastereomer, underlining the synthetic potential and efficiency of this method.
Conclusions
In summary, we report a highly diastereoselective alkene isomerization-Cope rearrangement cascade, affording 3,4-dihydrooxepines bearing two contiguous stereocenters. These oxepines were hydrolyzed to obtain stereodefined acyclic 1,6-dicarbonyl compounds bearing two contiguous stereocenters, which are challenging to access through other means. Forming the key divinyl epoxides in situ through alkene isomerization allows excellent control over alkene stereochemistry, while sidestepping stability issues associated with divinyl epoxides.Author contributions
RS and IM planned, conducted and analyzed the experiments. IM conceived and directed the project and wrote the manuscript with contribution sof RS and IM. All authors contributed to discussions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation program under Grant Agreement No. 786976 and from the Israel Science Foundation administrated by the Israel Academy of Sciences and Humanities (Grant No. 330/17). IM is holder of the Sir Michael and Lady Sobell Academic Chair.Notes and references
- Organic Synthesis: The Disconnection Approach, ed. S. Warren and P. Wyatt, Wiley 2008, p. 199 Search PubMed.
- D. Seebach, Angew. Chem., Int. Ed., 1979, 18, 239 Search PubMed.
- (a) H. Maekawa, K. Nakano, T. Hirashima and I. Nishiguchi, Chem. Lett., 1991, 20, 1661 Search PubMed; (b) I. Fussing, O. Hammerich, A. Hussain, M. F. Nielsen and J. H. P. Utley, Acta Chem. Scand., 1998, 52, 328 Search PubMed.
- (a) F. Plavac and C. H. Heathcock, Tetrahedron Lett., 1979, 2115 Search PubMed; (b) A. Eschenmoser and C. E. Winter, Science, 1977, 196, 1410 Search PubMed; (c) J. E. McMurry, J. Org. Chem., 1987, 52, 4885 Search PubMed; (d) C. Iwata, Y. Takemoto, M. Doi and T. Imanishi, J. Org. Chem., 1988, 53, 1623 Search PubMed.
- (a) J. P. Qu, C. Deng, J. Zhou, X. L. Sun and Y. Tang, J. Org. Chem., 2009, 74, 7684 Search PubMed; (b) D. Zhang, H. Cai, Y. Chen, L. Yin, J. Zhong, Y. Zhang and Q. F. Zhang, J. Org. Chem., 2020, 85, 14262 Search PubMed; (c) J. Fang, J. Ren and Z. Wang, Tetrahedron Lett., 2008, 49, 6659 Search PubMed; (d) For a review, see T. F. Schneider, J. Kaschel and D. B. Werz, Angew. Chem., Int. Ed., 2014, 53, 5504 Search PubMed e:.
- D. A. Evans, D. J. Baillargeon and J. V. Nelson, J. Am. Chem. Soc., 1978, 100, 2242 Search PubMed.
- (a) R. A. Braun, J. Org. Chem., 1963, 28, 1383 Search PubMed; (b) E. L. Stogryn, M. H. Gianni and A. J. Passannante, J. Org. Chem., 1964, 29, 1275 Search PubMed; (c) D. L. Clark, W. N. Chou and J. B. White, J. Org. Chem., 1990, 55, 3975 Search PubMed; (d) W. N. Chou and J. B. White, Tetrahedron Lett., 1991, 32, 157 Search PubMed; (e) W. N. Chou, J. B. White and W. B. Smith, J. Am. Chem. Soc., 1992, 114, 4658 Search PubMed; (f) A. Mayasundari, U. Peters and D. G. J. Young, Tetrahedron Lett., 2003, 44, 2633 Search PubMed; (g) M. Shimizu, T. Fujimoto, X. Liu and T. Hiyama, Chem. Lett., 2004, 33, 438 Search PubMed; (h) X. Xu, W. H. Hu, P. Y. Zavalij and M. P. Doyle, Angew. Chem., Int. Ed., 2011, 50, 11152 Search PubMed; (i) K. C. Nicolaou, R. Yu, L. Shi, Q. Cai, M. Lu and P. Heretsch, Org. Lett., 2013, 15, 1994 Search PubMed; (j) M. Zora, J. Org. Chem., 2005, 70, 6018 Search PubMed.
- (a) N. Chinkov, S. Majumdar and I. Marek, J. Am. Chem. Soc., 2003, 125, 13258 Search PubMed; (b) N. Chinkov, A. Levin and I. Marek, Angew. Chem., Int. Ed., 2006, 45, 465 Search PubMed; (c) A. Masarwa, D. Didier, T. Zabrodsky, M. Schinkel, L. Ackermann and I. Marek, Nature, 2014, 505, 199 Search PubMed; (d) A. Vasseur, L. Perrin, O. Eisenstein and I. Marek, Chem. Sci., 2015, 6, 2770 Search PubMed; (e) A. Vasseur and I. Marek, Nat. Protoc., 2017, 12, 74 Search PubMed; (f) S. Singh, J. Bruffaerts, A. Vasseur and I. Marek, Nat. Commun., 2017, 8, 14200 Search PubMed; (g) J. Bruffaerts, A. Vasseur and I. Marek, Adv. Synth. Catal., 2018, 360, 1389 Search PubMed; (h) S. Singh, M. Simaan and I. Marek, Eur. J. Org. Chem., 2018, 24, 8553 Search PubMed; (i) G.-M. Ho, L. Judkele, J. Bruffaerts and I. Marek, Angew. Chem., Int. Ed., 2018, 57, 8012 Search PubMed; (j) J. Bruffaerts, D. Pierrot and I. Marek, Nat. Chem., 2018, 10, 1164 Search PubMed; (k) H. Sommer, T. Weissbrod and I. Marek, ACS Catal., 2019, 9, 2400 Search PubMed; (l) I. Massad, H. Sommer and I. Marek, Angew. Chem., Int. Ed., 2020, 59, 15549 Search PubMed; (m) G.-M. Ho, L. Segura and I. Marek, Chem. Sci., 2020, 11, 5944 Search PubMed; (n) A. Cohen, J. Chagneau and I. Marek, ACS Catal., 2020, 10, 7154 Search PubMed.
- For reviews, see: (a) E. Larionov, H. Li and C. Mazet, Chem. Commun., 2014, 50, 9816 Search PubMed; (b) A. Vasseur, J. Bruffaerts and I. Marek, Nat. Chem., 2016, 8, 209 Search PubMed; (c) J. Bruffaerts, D. Pierrot and I. Marek, Org. Biomol. Chem., 2016, 14, 10325 Search PubMed; (d) H. Sommer, F. Juliá-Hernández, R. Martin and I. Marek, ACS Cent. Sci., 2018, 4, 153 Search PubMed; (e) T. Kochi, S. Kanno and F. Kakiuchi, Tetrahedron Lett., 2019, 60, 150938 Search PubMed; (f) J. J. Molloy, T. Morack and R. Gilmour, Angew. Chem., Int. Ed., 2019, 58, 13654 Search PubMed; (g) I. Massad and I. Marek, ACS Catal., 2020, 10, 5793 Search PubMed; (h) D. Janssen-Müller, B. Sahoo, S. Z. Sun and R. Martin, Isr. J. Chem., 2020, 60, 195 Search PubMed; (i) D. Fiorito, S. Scaringi and C. Mazet, Chem. Soc. Rev., 2021, 50, 1391 Search PubMed.
- For last year reports on alkene isomerization, see: (a) J. J. Molloy, M. Scafer, M. Wienhold, T. Morack, C. G. Daniliuc and R. Gilmour, Science, 2020, 369, 302 Search PubMed; (b) M. R. Becker, T. Morack, J. Robertson, J. B. Metternich, C. Muck-Lichtenfeld, C. Daniliuc, G. A. Burley and R. Gilmour, Tetrahedron, 2020, 76, 131198 Search PubMed; (c) G. Kundu, T. Sperger, K. Rissanen and F. Schoenebeck, Angew. Chem., Int. Ed., 2020, 59, 21930 Search PubMed; (d) S. Hanna, T. Wills, T. W. Butcher and J. F. Hartwig, ACS Catal., 2020, 10, 8736 Search PubMed; (e) J. Li, S. Qu and Z. Zhao, Angew. Chem., Int. Ed., 2020, 59, 2360 Search PubMed; (f) E. Matsuura, M. K. Karunananda, M. Liu, N. Nguyen, D. G. Blackmond and K. M. Engle, ACS Catal., 2021, 11, 4239 Search PubMed; (g) A. M. Camp, M. R. Kita, T. Blackburn, H. M. Dodge, C.-H. Chen and A. J. M. Miller, J. Am. Chem. Soc., 2021, 143, 2792 Search PubMed; (h) X. Yu, H. Zhao, P. Li and M. J. Koh, J. Am. Chem. Soc., 2020, 142, 18223 Search PubMed; (i) H. E. Bonfield, D. Valette, D. M. Linsay and M. Reid, Chem.–Eur. J., 2021, 27, 158–174 Search PubMed; (j) S. P. Ross, A. A. Rahman and M. S. Sigman, J. Am. Chem. Soc., 2020, 142, 10516 Search PubMed; (k) M. Hu and S. Ge, Nat. Commun., 2020, 11, 765 Search PubMed; (l) Y. Gao, C. Yang, S. Bai, X. Liu, Q. Wu, J. Wang, C. Jiang and Z. Qi, Chem, 2020, 6, 675 Search PubMed; (m) Y. Baumgartner and O. Baudoin, ACS Catal., 2020, 10, 10508 Search PubMed; (n) H. Liu, C. Cai, Y. Ding, J. Chen, B. Liu and Y. Xia, ACS Omega, 2020, 5, 1165 Search PubMed.
- (a) T. Katsuki and K. B. Sharpless, J. Am. Chem. Soc., 1980, 102, 5974 Search PubMed; (b) Y. Gao, R. M. Hanson, J. M. Klunder, S. Y. Ko, H. Masamune and K. B. Sharpless, J. Am. Chem. Soc., 1987, 109, 5765 Search PubMed; (c) K. Purushotham Reddy, D. Vasudeva Reddy and G. Sabitha, Eur. J. Org. Chem., 2018, 2018, 4389 Search PubMed.
- (a) D. Baudry, M. Ephritikhine and H. Felkin, J. Chem. Soc., Chem. Commun., 1978, 694 Search PubMed; (b) J. H. van Boom, J. J. Oltvoort, C. A. A. Van Boeckel and J. H. de Koning, Synthesis, 1981, 305 Search PubMed; (c) I. Matsuda, T. Kato, S. Sato and Y. Izumi, Tetrahedron Lett., 1986, 27, 5747 Search PubMed; (d) T. Moriya, A. Suzuki and N. Miyaura, Tetrahedron Lett., 1995, 36, 1887 Search PubMed; (e) T. Ohmura, Y. Shirai, Y. Yamamoto and N. Miyaura, Chem. Commun., 1998, 6, 1337 Search PubMed; (f) T. Ohmura, Y. Yamamoto and N. Miyaura, Tetrahedron Lett., 1999, 18, 413 Search PubMed; (g) Y. Yamamoto, T. Miyairi, T. Ohmura and N. Miyaura, J. Org. Chem., 1999, 64, 296 Search PubMed; (h) S. G. Nelson, C. J. Bungard and K. Wang, J. Am. Chem. Soc., 2003, 125, 13000 Search PubMed; (i) M. Krel, J. Y. Lallemand and C. Guillou, Synlett, 2005, 2043 Search PubMed; (j) S. G. Nelson and K. Wang, J. Am. Chem. Soc., 2006, 128, 4232 Search PubMed; (k) B. D. Stevens, C. J. Bungard and S. G. Nelson, J. Org. Chem., 2006, 71, 6397 Search PubMed; (l) K. Wang, C. J. Bungard and S. G. Nelson, Org. Lett., 2007, 9, 2325 Search PubMed; (m) Y. Kavanagh, C. M. Chaney, J. Muldoon and P. Evans, J. Org. Chem., 2008, 73, 8601 Search PubMed.
- (a) L. Mantilli, D. Gérard, S. Torche, C. Besnard and C. Mazet, Angew. Chem., Int. Ed., 2009, 48, 5143 Search PubMed; (b) L. Mantilli and C. Mazet, Chem. Commun., 2010, 46, 445 Search PubMed; (c) L. Mantilli, D. Gérard, S. Torche, C. Besnard and C. Mazet, Pure Appl. Chem., 2010, 82, 1461 Search PubMed; (d) L. Mantilli, D. Gérard, S. Torche, C. Besnard and C. Mazet, Eur. J. Org. Chem., 2010, 16, 12736 Search PubMed; (e) A. Quintard, A. Alexakis and C. Mazet, Angew. Chem., Int. Ed., 2011, 50, 2354 Search PubMed; (f) M. G. McLaughlin and M. J. Cook, J. Org. Chem., 2012, 77, 2058 Search PubMed; (g) S. Biswas, Z. Huang, Y. Choliy, D. Y. Wang, M. Brookhart, K. Krogh-Jespersen and A. S. Goldman, J. Am. Chem. Soc., 2012, 134, 13276 Search PubMed; (h) L. Mantilli, D. Gérard, C. Besnard and C. Mazet, Eur. J. Inorg. Chem., 2012, 3320 Search PubMed; (i) T. Miura, Y. Nishida, M. Morimoto and M. Murakami, J. Am. Chem. Soc., 2013, 135, 11497 Search PubMed; (j) H. Li and C. Mazet, Org. Lett., 2013, 15, 6170 Search PubMed; (k) H. Li and C. Mazet, J. Am. Chem. Soc., 2015, 137, 10720 Search PubMed; (l) L. Lin, K. Yamamoto, H. Mitsunuma, Y. Kanzaki, S. Matsunaga and M. Kanai, J. Am. Chem. Soc., 2015, 137, 15418 Search PubMed; (m) H. Li and C. Mazet, Acc. Chem. Res., 2016, 49, 1232 Search PubMed; (n) C. Romano and C. Mazet, J. Am. Chem. Soc., 2018, 140, 4743 Search PubMed.
- K. Komeyama, Y. Yamahata and I. Osaka, Org. Lett., 2018, 20, 1457 Search PubMed.
- H. Aoyama, M. Tokunaga, S. I. Hiraiwa, Y. Shirogane, Y. Obora and Y. Tsuji, Org. Lett., 2004, 6, 509 Search PubMed.
- In some cases, during the Pd-catalyzed hydrolysis of oxepines, the intramolecular aldol products of 4 were observed, particularly when diketones were concerned. All data and complete analysis can be found in the ESI†.
- The relative stereochemistry of 7 was determined by X-ray structure analysis. CCDC 2074187 contains the supplementary crystallographic data for this paper†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2074187. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc02575a |
| This journal is © The Royal Society of Chemistry 2021 |