Open Access Article
Open Access ArticleA palladium-catalyzed approach to allenic aromatic ethers and first total synthesis of terricollene A†
Chaofan
Huang
a,
Fuchun
Shi
b,
Yifan
Cui‡
b,
Can
Li‡
b,
Jie
Lin‡
a,
Qi
Liu‡
a,
Anni
Qin‡
a,
Huanan
Wang‡
a,
Guolin
Wu‡
a,
Penglin
Wu‡
a,
Junzhe
Xiao‡
 b,
Haibo
Xu‡
b,
Yuan
Yuan‡
a,
Yizhan
Zhai‡
b,
Wei-Feng
Zheng‡
a,
Yangguangyan
Zheng‡
b,
Haibo
Xu‡
b,
Yuan
Yuan‡
a,
Yizhan
Zhai‡
b,
Wei-Feng
Zheng‡
a,
Yangguangyan
Zheng‡
 a,
Biao
Yu
*b and
Shengming
Ma
a,
Biao
Yu
*b and
Shengming
Ma
 *ab
*ab
aResearch Center for Molecular Recognition and Synthesis, Department of Chemistry, Fudan University, 220 Handan Lu, Shanghai 200433, P. R. China
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, P. R. China. E-mail: masm@sioc.ac.cn
First published on 9th June 2021
Abstract
A palladium-catalyzed C–O bond formation reaction between phenols and allenylic carbonates to give 2,3-allenic aromatic ethers with decent to excellent yields under mild reaction conditions has been described. A variety of synthetically useful functional groups are tolerated and the synthetic utility of this method has been demonstrated through a series of transformations of the allene moiety. By applying this reaction as the key step, the total syntheses of naturally occurring allenic aromatic ethers, eucalyptene and terricollene A (first synthesis; 4.5 g gram scale), have been accomplished.
Introduction
Aromatic ethers are prevalent and prominent in a variety of natural products, pharmaceuticals, and agrochemicals.1 Traditional methods for the preparation of aromatic ethers include the Williamson ether synthesis,2 direct nucleophilic substitution reactions,3 Mitsunobu reaction,4 Ullman-type couplings of alkoxides with aryl halides,5etc. On the other hand, due to their unique chemical properties as well as the substituent-loading capability derived from the distinctive structure, allenes have been demonstrated as powerful platform molecules for the efficient syntheses of other functional organic compounds.6,7 Interestingly, many aromatic 2,3-allenylic ethers are also found in the nature (Scheme 1a).8 Reports on the syntheses of such aromatic ethers are very limited. Thus, development of new methods for the efficient synthesis of aromatic 2,3-allenylic ethers is of high interest currently. Herein, we disclose the development of palladium-catalyzed C–O bond formation between aryl phenols and allenylic carbonates to give allenic aromatic ethers in 70–99% yields under mild reaction conditions (Scheme 1c).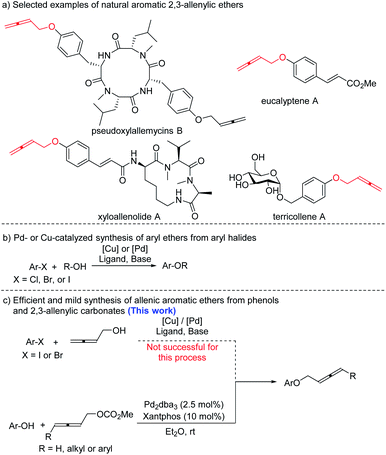 | ||
| Scheme 1 Naturally occurring aromatic 2,3-butadienyl ethers and the metal-catalyzed C–O bond formation for the synthesis of aromatic ethers. | ||
Results and discussion
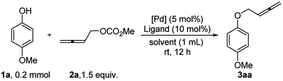
In contrast to the well-established metal-catalyzed coupling reactions between aryl halides and phenols or aliphatic alcohols (Scheme 1b),9 metal-catalyzed coupling reactions of aryl halides and allenols have not been developed (Scheme 1c). In a preliminary survey, the palladium-10 and copper-catalyzed11 coupling reactions between 4-iodoanisole and allenols were studied; however, only a trace amount of the desired allenyl aryl ether product was obtained (Scheme 1c, for details, see the ESI‡). Then 4-methoxyphenol 1a was treated with 1.5 equiv. of 2,3-butadienyl carbonate 2a in the presence of 2.5 mol% [PdCl(π-allyl)]2 and 10 mol% BINAP with toluene as the solvent at room temperature for 12 h. Interestingly, 39% yield of the expected allenol ether product 3aa was formed with 48% recovery of 4-methoxyphenol 1a. Various palladium catalysts and ligands were then screened (Table 1, entries 1–7). The combination of Pd2dba3 and Xantphos as the catalyst delivered the best result, affording 80% yield of 3aa with 10% recovery of 1a (Table 1, entry 4). Subsequent solvent screening (Table 1, entries 8–11) showed that Et2O or MTBE was the best solvent, affording the product 3aa in 99% or 96% yield, respectively (entries 8 and 9).| Entry | [Pd] | Ligand | Solvent | NMR yield of 3aa (%) | Recovery of 1a (%) |
|---|---|---|---|---|---|
| a Yield and recovery were determined by 1H NMR analysis using CH3NO2 as the internal standard. b The reaction was carried out with 1 mmol of 1a and the isolated yield is shown in parentheses. | |||||
| 1 | [PdCl(π-allyl)]2 | BINAP | Toluene | 39 | 48 |
| 2 | Pd2dba3 | BINAP | Toluene | 68 | 26 |
| 3 | Pd(PPh3)4 | BINAP | Toluene | 43 | 49 |
| 4 | Pd2dba3 | Xantphos | Toluene | 80 | 10 |
| 5 | Pd2dba3 | DPEPhos | Toluene | 28 | 70 |
| 6 | Pd2dba3 | BIPHEP | Toluene | 10 | 89 |
| 7 | Pd2dba3 | SPhos | Toluene | 68 | 13 |
| 8 | Pd2dba3 | Xantphos | MTBE | 96 | — |
| 9 | Pd2dba3 | Xantphos | Et2O | 99 (95b) | — |
| 10 | Pd2dba3 | Xantphos | Dioxane | 67 | 28 |
| 11 | Pd2dba3 | Xantphos | THF | 49 | 47 |
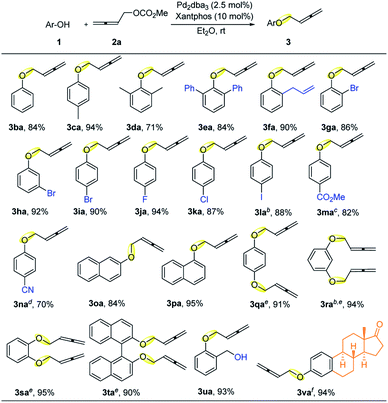
With the optimized conditions in hand, the scope of phenols was evaluated (Table 2). To our delight, parent phenol 1b and electron-rich p-cresol 1c gave high yields of 3ba and 3ca; even the reaction of highly sterically hindered 2,6-dimethyl or 2,6-diphenyl substituted phenols proceeded smoothly to afford 3da or 3ea in 71% or 84% yield, respectively. The ortho-allyl substituent also survived, allowing further modification based on the reactivity of the C–C double bond. Bromo-substituents at the ortho- (3ga), meta- (3ha), and para-positions (3ia) were all well tolerated without much difference, indicating that the steric effect is negligible and the relatively reactive C–Br bonds were tolerated. In addition, other synthetically useful halogen substituents such as F, Cl, and even I were all compatible, delivering the corresponding products 3ja, 3ka, and 3la in 94%, 87%, and 88% yields, respectively. It is worth noting that the highly reactive C–I bond is generally less compatible in palladium-catalyzed reactions.12 We reasoned that the relatively strong coordination effect of the allene unit brought the palladium catalyst to the carbonate, so that the oxidative addition of the palladium catalyst occurred exclusively to the carbonate unit, leaving the C–Br/I bond intact.13 Furthermore, phenols with electron-withdrawing and synthetically versatile substituents such as CO2Me and CN could also deliver the corresponding products 3ma and 3na in good to excellent yields upon changing the catalyst to Pd(PPh3)4. Moreover, 1- or 2-naphthols could also afford the corresponding products 3oa or 3pa in 84% and 95% yield, respectively. With 2.5 equiv. of allene 2a, the coupling reactions of hydroquinone, resorcinol, catechol, and 1,1′-binaphthol gave the double-coupling products 3qa–3ta in excellent yields. Interestingly, only the phenol hydroxy group was exclusively coupled leaving the benzylic hydroxyl group untouched to afford 3ua. Such a coupling reaction of the hydroxyl group in estrone was also realized by changing the solvent to MTBE for a reaction at 60 °C to afford 3va in an excellent yield.
| a Reaction conditions: 1 (1.0 mmol), 2a (1.5 mmol), Pd2dba3 (2.5 mol%), and Xantphos (10 mol%) in Et2O (5 mL) at rt; isolated yields are shown. b In MTBE at 35 °C. c With 5 mol% Pd(PPh3)4 in dioxane at 35 °C. d With 5 mol% Pd(PPh3)4 in MTBE at 40 °C. e With 2.5 mmol of 2a. f In MTBE at 60 °C in a sealed tube. |
|---|
|
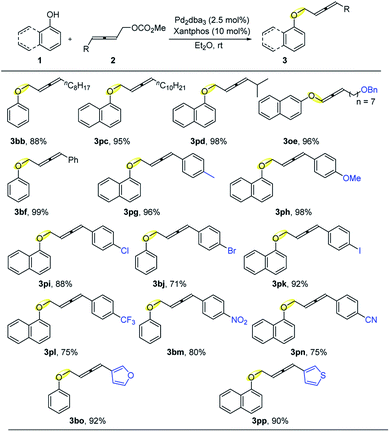
We then evaluated the scope of 2,3-allenylic carbonates (Table 3): carbonates with 4-alkyl and isopropyl substituents delivered the coupling products 3bb, 3pc, and 3pd in decent to excellent yields. Benzyl ether, which is a common and practical protecting group for the hydroxyl group, was also viable in this transformation affording 3oe in 96% yield. 4-Phenyl-2,3-butadienyl carbonate 1f reacted with phenol to afford 3bf in an almost quantitative yield. Moreover, electron-donating groups such as p-methyl and p-methoxy and electron withdrawing yet potentially useful p-Cl, p-Br,13p-I,13p-CF3, p-NO2, and p-CN were all accommodated yielding the corresponding products 3pg–3pn in decent yields. Last but not the least, 4-heteroaryl substituents such as a furyl and a thienyl group in allenylic carbonates 2 were also successfully tolerated affording corresponding products 3bo and 3pp in excellent yields.
| a Reaction conditions: 1 (1.0 mmol), 2 (1.5 mmol), Pd2dba3 (2.5 mol%), and Xantphos (10 mol%) in Et2O (5 mL) at rt; isolated yield. |
|---|
|
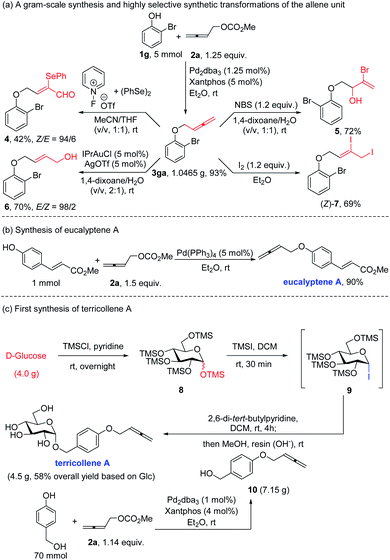
A gram-scale reaction between 1g and 2a delivered 1.0465 g of 3ga in 93% yield with a reduced loading of both the catalyst and ligand (Scheme 2a). As a class of synthetically useful chemicals, the versatile allenyl unit could be transferred into different structure units: selenohydroxylation and oxidation of 3ga with 1-fluoropyridinium/1,2-diphenyldiselane could deliver 2-selenoenal 4 with a Z/E selectivity of 94/6;14 bromohydroxylation of this allenyl group in aqueous dioxane afforded terminal 2-bromoallylic alcohol 5 as the sole product in 72% yield;15 the allenyl group may also be selectively transformed into the corresponding primary allylic alcohol 6 with an E/Z selectivity of 98/2 by a gold-catalyzed hydration reaction;16 iodination of the allenyl group exclusively gave diiodide product (Z)-7 in 69% yield.17 It should be noted that these products are difficult to synthesize through traditional transition-metal catalyzed coupling reactions.10,18
Finally this method has been applied to the efficient syntheses of two naturally occurring allenes: eucalyptene A has been successfully synthesized under the catalysis of 5 mol% Pd(PPh3)4 in 90% yield from methyl 4-hydroxycinnamate and 2,3-butadienyl methyl carbonate (Scheme 2b); 70 mmol scale reaction of p-hydroxymethylphenol with 1.14 equiv. of 2,3-butadienyl methyl carbonate 2a afforded the corresponding p-hydroxymethylphenyl 2,3-butadienyl ether 10. D-Glucose (4.0 g) was etherified with chlorotrimethylsilane in pyridine to give penta-O-trimethylsilyl-D-glucopyranose 8, which was then reacted with iodotrimethylsilane to generate the glucosyl iodide 9 within 30 min at room temperature. The reaction of allene 10 (7.15 g) with 9 in the presence of 2,6-di-tert-butylpyridine in dry dichloromethane at room temperature for 4 h followed by treatment with methanol and resin(OH−) to remove the silyl protecting groups afforded 4.5 g of terricollene A in 58% overall yield as the sole anomer, which is the first total synthesis of this natural product (Scheme 2c).19
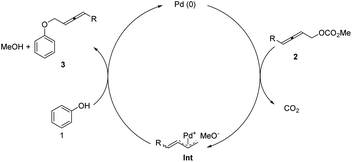
A plausible mechanism is proposed (Scheme 3): the oxidative addition of Pd(0) with 2,3-allenylic carbonate would yield, after releasing CO2, an α-methylene-π-allylic Pd intermediate Int,20,13c,d which would subsequently undergo a nucleophilic allenylation reaction with phenol to deliver the allenic aromatic ether 3 with regeneration of the catalytically active Pd(0) to finish the catalytic cycle.
Conclusions
In conclusion, we have described a general method of palladium-catalyzed C–O bond formation reaction between phenols and allenylic carbonates. This first example of such a C–O bond formation reaction exhibits a broad scope of both phenols and carbonates under very mild conditions, providing an attractive complement to traditional C–O bond formation reactions. The attractive reactivities of the allene unit in the products have been demonstrated. Moreover, the present catalytic system offers a convenient route for the efficient synthesis of naturally occurring aromatic 2,3-allenylic ethers, eucalyptene A and terricollene A, which exhibited cytotoxic activity against KB/KBv200 cells and HeLa/MCF-7 cells, respectively.8b,d Further studies are currently under way in our laboratories.Data availability
The electronic supplementary information include experimental detail, NMR data, MS data, IR data, elemental analysis data, and all the spectra.Author contributions
C. H. and S. M. conceived the idea. C. H. conducted the most of experiments. B. Y. supervised the total synthesis of terricollene A. C. H. and F. S. co-synthesized the terricollene A. Y. C., C. L., J. L., Q. L., A. Q., H. W., G. W., P. W., J. X., H. X., Y. Y., Y. Z., W. Z., and Y. Z. co-synthesized part of substrates and they contribute equally to this paper. C. H. and S. M. co-wrote the paper. All the authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Natural Science Foundation of China (21690063 & 21988101) is greatly appreciated.Notes and references
- (a) D. A. Evans and K. M. DeVries, in Glycopeptide Antibiotics, Drugs and the Pharmaceutical Sciences, ed. R. Nagarajan, Marcel Decker, Inc., New York, 1994, vol. 63, pp. 63–104 Search PubMed; (b) A. V. R. Rao, M. K. Gurjar, K. L. Reddy and A. S. Rao, Chem. Rev., 1995, 95, 2135 Search PubMed; (c) J. Zhu, Synlett, 1997, 133 Search PubMed; (d) K. C. Nicolaou, C. N. C. Boddy, S. Bräse and N. Winssinger, Angew. Chem., Int. Ed., 1999, 38, 2096 Search PubMed; (e) J. Blankenstein and J. Zhu, Eur. J. Org. Chem., 2005, 1949 Search PubMed; (f) S. D. Roughley and A. M. Jordan, J. Med. Chem., 2011, 54, 3451 Search PubMed; (g) X. Wang, A. Liu, L. Cao, M. Lei, Y. Ren, B. Zhou, L. Huang, D. Gao, H. Chen and L. Cheng, CN105985246A, 2016.
- For selected examples, see: (a) B. Juršić, Tetrahedron, 1988, 44, 6677 Search PubMed; (b) E. Fuhrmann and J. Talbiersky, Org. Process Res. Dev., 2005, 9, 206 Search PubMed; (c) A. R. Massah, M. Mosharafian, A. R. Momeni, H. Aliyan and H. J. Naghash, Synth. Commun., 2007, 37, 1807 Search PubMed.
- For selected examples, see: (a) J. F. Bunnett and R. E. Zahler, Chem. Rev., 1951, 49, 273 Search PubMed; (b) J. Lindley, Tetrahedron, 1984, 40, 1433 Search PubMed; (c) A. J. Pearson and A. M. Gelormini, J. Org. Chem., 1994, 59, 4561 Search PubMed; (d) T. F. Woiwode, C. Rose and T. J. Wandless, J. Org. Chem., 1998, 63, 9594 Search PubMed; (e) J. Zilberman, Org. Process Res. Dev., 2003, 7, 303 Search PubMed.
- For selected examples, see: (a) R. G. Gentles, D. Wodka, D. C. Park and A. Vasudevan, J. Comb. Chem., 2002, 4, 442 Search PubMed; (b) K. C. K. Swamy, N. N. B. Kumar, E. Balaraman and K. V. P. P. Kumar, Chem. Rev., 2009, 109, 2551 Search PubMed.
- For selected examples, see: (a) F. Ullmann, Chem. Ber., 1904, 37, 853 Search PubMed; (b) I. Beletskaya and A. Cheprakov, Coord. Chem. Rev., 2004, 248, 2337 Search PubMed.
- N. Krause and A. S. K. Hashimi, Modern Allene Chemistry, Wiley-VCH, Weinheim, Germany, 2004 Search PubMed.
- For selected reviews, see: (a) A. S. K. Hashmi, Angew. Chem., Int. Ed., 2000, 39, 3590 Search PubMed; (b) S. Ma, Acc. Chem. Res., 2003, 36, 701 Search PubMed; (c) S. Ma, Chem. Rev., 2005, 105, 2829 Search PubMed; (d) M. Jeganmohan and C. Cheng, Chem. Commun., 2008, 3101 Search PubMed; (e) S. Ma, Acc. Chem. Res., 2009, 42, 1679 Search PubMed; (f) N. Krause and C. Winter, Chem. Rev., 2011, 111, 1994 Search PubMed; (g) S. Yu and S. Ma, Angew. Chem., Int. Ed., 2012, 51, 3074 Search PubMed; (h) M. P. Muñoz, Org. Biomol. Chem., 2012, 10, 3584 Search PubMed; (i) R. Zimmer and H.-U. Reissig, Chem. Soc. Rev., 2014, 43, 2888 Search PubMed; (j) F. López and J. L. Mascareñas, Chem. Soc. Rev., 2014, 43, 2904 Search PubMed; (k) T. Cañeque, F. M. Truscott, R. Rodriguez, G. Maestri and M. Malacria, Chem. Soc. Rev., 2014, 43, 2916 Search PubMed; (l) Z. Wang, X. Xu and O. Kwon, Chem. Soc. Rev., 2014, 43, 2927 Search PubMed; (m) W. Yang and A. S. K. Hashmi, Chem. Soc. Rev., 2014, 43, 2941 Search PubMed; (n) S. Kitagaki, F. Inagaki and C. Mukai, Chem. Soc. Rev., 2014, 43, 2956 Search PubMed; (o) M. A. Tius, Chem. Soc. Rev., 2014, 43, 2979 Search PubMed; (p) J. Le Bras and J. Muzart, Chem. Soc. Rev., 2014, 43, 3003 Search PubMed; (q) E. Soriano and I. Fernández, Chem. Soc. Rev., 2014, 43, 3041 Search PubMed; (r) B. Alcaide, P. Almendros and C. Aragoncillo, Chem. Soc. Rev., 2014, 43, 3106 Search PubMed; (s) C. S. Adams, C. D. Weatherly, E. G. Burke and J. M. Schomaker, Chem. Soc. Rev., 2014, 43, 3136 Search PubMed; (t) M. P. Muñoz, Chem. Soc. Rev., 2014, 43, 3164 Search PubMed; (u) P. Koschker and B. Breit, Acc. Chem. Res., 2016, 49, 1524 Search PubMed; (v) A. P. Pulis, K. Yeung and D. J. Procter, Chem. Sci., 2017, 8, 5240 Search PubMed; (w) Y. Wei and M. Shi, Org. Chem. Front., 2017, 4, 1876 Search PubMed; (x) B. Yang, Y. Qiu and J. Bäckvall, Acc. Chem. Res., 2018, 51, 1520 Search PubMed; (y) J. Mascareñas, I. Varela and F. López, Acc. Chem. Res., 2019, 52, 465 Search PubMed.
- (a) A. Hoffmann-Röder and N. Krause, Angew. Chem., Int. Ed., 2004, 43, 1196 Search PubMed; (b) S.-Y. Wang, W.-W. Mao, Z.-G. She, C.-R. Li, D.-Q. Yang, Y.-C. Lin and L.-W. Fu, Bioorg. Med. Chem. Lett., 2007, 17, 2785 Search PubMed; (c) V. M. Dembitsky and T. Maoka, Prog. Lipid Res., 2007, 46, 328 Search PubMed; (d) F. Zhang, S. Liu, X. Lu, L. Guo, H. Zhang and Y. Che, J. Nat. Prod., 2009, 72, 1782 Search PubMed; (e) X.-L. Lu, Z.-L. Xu, X.-L. Yao, F.-J. Su, C.-H. Ye, J. Li, Y.-C. Lin, G.-L. Wang, J.-S. Zeng, R.-X. Huang, J.-S. Ou, H.-S. Sun, L.-P. Wang, J.-Y. Pang and Z. Pei, Mar. Drugs, 2012, 10, 1307 Search PubMed; (f) H. Guo, N.-B. Kreuzenbeck, S. Otani, M. Garciaaltares, H.-M. Dahse, C. Weigel, D.-K. Aanen, C. Hertweck, M. Poulsen and C. Beemelmanns, Org. Lett., 2016, 18, 3338 Search PubMed; (g) A. J. Cameron, C. J. Squire, A. Gérenton, L. A. Stubbing, P. W. R. Harris and M. A. Brimble, Org. Biomol. Chem., 2019, 17, 3902 Search PubMed.
- For reviews of palladium-mediated ether synthesis, see: (a) J. F. Hartwig, in Handbook of Organopalladium Chemistry for Organic Synthesis, ed. E.-i. Negishi, J. Wiley& Sons Inc., New York, 2002, pp. 1097–1106 Search PubMed; (b) A. R. Muci and S. L. Buchwald, Top. Curr. Chem., 2002, 219, 131–209 Search PubMed; (c) S. Enthaler and A. Company, Chem. Soc. Rev., 2011, 40, 4912 Search PubMed. For reviews of copper-mediated ether synthesis, see: (d) S. Bhunia, G. G. Pawar, S. V. Kumar, Y. Jiang and D. Ma, Angew. Chem., Int. Ed., 2017, 56, 16136 Search PubMed. For reviews of other metal-mediated ether synthesis, see: (e) R. I. Khusnutdinov and A. R. Bayguzina, Russ. J. Org. Chem., 2019, 55, 903 Search PubMed; (f) J. Zhang, S. Wang, Y. Zhang and Z. Feng, Asian J. Org. Chem., 2020, 9, 1519 Search PubMed.
- A. V. Vorogushin, X. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 8146 Search PubMed.
- (a) M. Wolter, G. Nordmann, G. E. Job and S. L. Buchwald, Org. Lett., 2002, 4, 973 Search PubMed; (b) D. Ma and Q. Cai, Org. Lett., 2003, 5, 3799 Search PubMed.
- (a) K. Okuro and H. Alper, J. Org. Chem., 1997, 62, 1566 Search PubMed; (b) S. Husinec, M. Jadranin, R. Markovic, M. Petkovic, V. Savic and N. Todorovic, Tetrahedron Lett., 2010, 51, 4066 Search PubMed; (c) A. Kumbhar, J. Organomet. Chem., 2017, 848, 22 Search PubMed.
- For the coordination effect of allenes with transition metal catalysts, see: (a) X. Pu, X. Qi and J. M. Ready, J. Am. Chem. Soc., 2009, 131, 10364 Search PubMed; (b) F. Cai, X. Pu, X. Qi, V. Lynch, A. Radha and J. M. Ready, J. Am. Chem. Soc., 2011, 133, 18066 Search PubMed; (c) S. Song, J. Zhou, C. Fu and S. Ma, Nat. Commun., 2019, 10, 507 Search PubMed; (d) S. Song and S. Ma, Chin. J. Chem., 2020, 38, 1233 Search PubMed; (e) H. K. Maliszewska, D. L. Hughes and M. P. Muñoz, Dalton Trans., 2020, 49, 4034 Search PubMed.
- B. Alcaide, P. Almendros, T. Martínez del Campo, L. Martín, G. Palop and M. Toledano-Pinedo, Org. Chem. Front., 2019, 6, 2447 Search PubMed.
- W. Kong, B. Guo, C. Fu and S. Ma, Eur. J. Org. Chem., 2011, 2278 Search PubMed.
- Z. Zhang, S. Du Lee, A. S. Fisher and R. A. Widenhoefer, Tetrahedron, 2009, 65, 1794 Search PubMed.
- M. J. Campbell, P. D. Pohlhaus, G. Min, K. Ohmatsu and J. S. Johnson, J. Am. Chem. Soc., 2008, 130, 9180 Search PubMed.
- (a) P. A. Evans and D. K. Leahy, J. Am. Chem. Soc., 2000, 122, 5012 Search PubMed; (b) D. F. Taber, M. I. Sikkander, J. F. Berry and K. J. Frankowski, J. Org. Chem., 2008, 73, 1605 Search PubMed; (c) A. Arfaoui, F. Saâdi, A. Nefzi and H. Amri, Synth. Commun., 2015, 45, 2627 Search PubMed.
- (a) W. Fan, Y. Wu, X.-K. Li, N. Yao, X. Li, Y.-G. Yu and L. Hai, Eur. J. Med. Chem., 2011, 46, 3651 Search PubMed; (b) M. Furukawa, S. Kamo, M. Makino, M. Kurita, K. Tabata, K. Matsuzaki, T. Suzuki and T. Uchiyama, Phytochemistry, 2017, 136, 147 Search PubMed.
- For selected examples of Pd-catalyzed reactions involving the α-methylene-π-allylic Pd intermediate with malonates and amides, see: (a) D. Djahanbini, B. Cazes and J. Gore, Tetrahedron Lett., 1984, 25, 203 Search PubMed; (b) B. M. Trost and J. M. Tour, J. Org. Chem., 1989, 54, 484 Search PubMed; (c) J. Nokami, A. Maihara and J. Tsuji, Tetrahedron Lett., 1990, 31, 5629 Search PubMed; (d) M. E. Piotti and H. Alper, J. Org. Chem., 1994, 59, 1956 Search PubMed; (e) Y. Imada, K. Ueno, K. Kutsuwa and S. Murahashi, Chem. Lett., 2002, 140 Search PubMed; (f) M. Rubin, J. Markov, S. Chuprakov, D. J. Wink and V. Gevorgyan, J. Org. Chem., 2003, 68, 6251 Search PubMed; (g) M. Ogasawara, A. Okada, S. Watanabe, L. Fan, K. Uetake, K. Nakajima and T. Takahashi, Organometallics, 2007, 26, 5025 Search PubMed; (h) S. Song, J. Zhou, C. Fu and S. Ma, Nat. Commun., 2019, 10, 507 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc01896e |
| ‡ These authors contributed equally to this work and are sorted in alphabetical order of last name. |
| This journal is © The Royal Society of Chemistry 2021 |