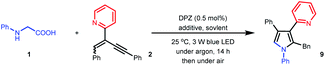Open Access Article
Open Access ArticleRadical-based functionalization-oriented construction: rapid assembly of azaarene-substituted highly functionalized pyrroles†
Weigao
Hu‡
a,
Qiangqiang
Zhan‡
ab,
Hongwei
Zhou
 b,
Shanshan
Cao
*c and
Zhiyong
Jiang
b,
Shanshan
Cao
*c and
Zhiyong
Jiang
 *ac
*ac
aInternational Scientific and Technological Cooperation Base of Chiral Chemistry, Henan University, Kaifeng, Henan 475004, P. R. China. E-mail: jiangzhiyong@htu.edu.cn
bCollege of Biological, Chemical Sciences and Engineering, Jiaxing University, Jiaxing, Zhejiang, P. R. China 314001
cSchool of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan 453007, P. R. China. E-mail: caoshanshan@htu.edu.cn
First published on 29th March 2021
Abstract
Totally different functionalization and construction as two fundamental synthetic protocols have long been applied to furnish azaarene variants. Here, a novel radical-based functionalization-oriented construction strategy by exploiting the electronic properties of azaarenes and the high reactivity of radicals is developed. Under a photoredox catalysis platform, the robust ability of such an artful combination of functionalization with construction is disclosed in the synthesis of valuable 3-azaarene-substituted densely functionalized pyrroles. In addition to the ability to use the readily accessible feedstocks, the high synthetic efficiency and the good functional group tolerance, the substrate scope is broad (81 examples) resulting from the capability to flexibly replace the types of azaarenes and other substituents. Control experiments and density functional theory (DFT) calculations elucidate the plausible mechanism involving the reaction pathways and the important role of NaH2PO4 as an additive in the reaction.
Introduction
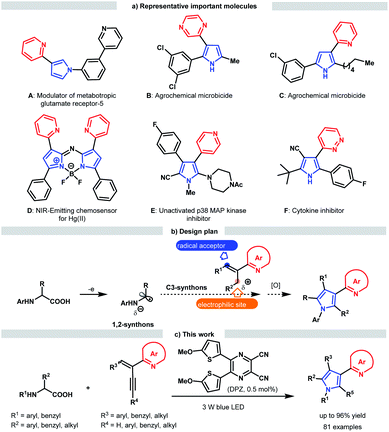
Nitrogen-containing aromatic heterocycles (azaarenes) are ubiquitous in natural products, pharmaceuticals, functional materials, catalysts and ligands, and notably, a number of these valuable compounds often possess at least two linked azaarenes in their structure. As an example, pyrroles connected with imine-bearing azaarenes at the 3-position through a C–C bond are the privileged structural motifs of an enormous variety of bioactive molecules and chemosensors (Scheme 1a).1 Structurally, the representative molecules A–F robustly indicate that distinct azaaryl groups and substituents as well as diverse substitution patterns would endow various important application potentials for such special pyrrole derivatives. To date, several direct functionalization examples via coupling pyrroles and derivatives with azaarene-based substrates have been described by Buchwald, Ellman and Bergman et al., which represent efficient approaches to afford simple 3-azaarene-substituted pyrroles.2 To provide more complex variants with other C-substituents, many construction strategies have been developed, and most of them are operative in introducing only an extra substituent.1d,3 Notably, rare examples have been established to access highly functionalized 3-azaaryl pyrroles; in addition to tedious multistep chemical transformations, these methods lack the ability to flexibly tune the azaarene and substituent types.1e–hFor more than a century, the prominent importance of pyrrole derivatives in many scientific and industrial fields has inspired numerous direct synthetic methodologies, especially those involving three classical protocols: the Knorr, Paal–Knorr and Hantzsch reactions.4 However, these methods have not yet been used to assemble valuable highly functionalized 3-azaaryl pyrroles. This dilemma is likely attributable to the challenge in the synthesis of viable starting substrates or incompatible reactivity when the corresponding functional groups are replaced by azaarenes. Accordingly, we envisaged exploring the viability of directly triggering transformations by exploiting the electron-deficient properties of these azaarenes and performing radical pathways, which have been verified to be a powerful functionalization tool for the synthesis of azaarene derivatives owing to the demonstrated considerable potential of using simple azaarene-containing feedstocks and their high reactivity.5–7 To this end, we were intrigued in taking advantage of versatile radical addition to alkenylazaarenes6 and using α-amino radicals as the reaction partners (Scheme 1b). In addition to having a robust ability to undergo addition to these inert alkenes,7 α-amino radicals can be facilely generated from abundant α-amino acid derivatives7d,8 through sustainable photoredox catalysis.9 Moreover, α-amino radicals might serve as bis-nucleophilic C–N fragments.8e,10 As such, if alkenylazaarene-based substrates could further accept the addition of the nucleophilic amine moiety within α-amino radicals after the first radical addition step, formal [3 + 2] cycloaddition would afford the precursors of pyrroles; through subsequent oxidative aromatization, the desired pyrroles should be achieved.
Herein, we report the development of a sequential redox-neutral radical addition, cyclization and aerobic oxidative aromatization reaction of N-aryl α-amino acids with 2-azaarene-substituted 1,3-enynes under visible light-driven photoredox catalysis (Scheme 1c). By using a dicyanopyrazine-derived chromophore (DPZ) as the sensitizer,11 this novel radical-based functionalization-oriented construction strategy offers a direct, efficient and modular approach to construct a variety of densely functionalized 3-azaarene-based pyrroles in high yields and with a broad substrate scope.
Results and discussion
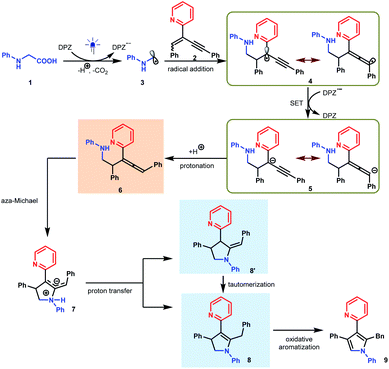
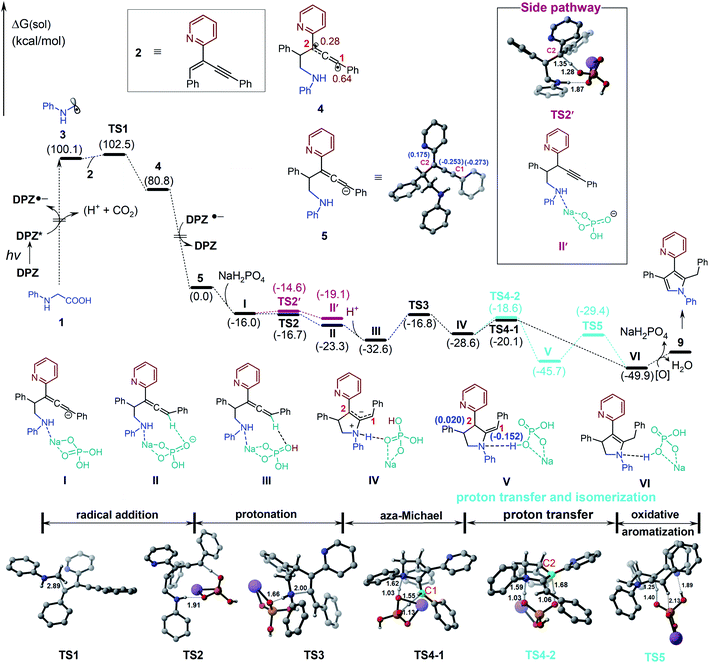
We set out our investigation by exploring the model reaction between N-phenyl glycine (1) and 2-(2-enynyl)pyridine (2) with DPZ as the photoredox catalyst (Scheme 2 and Table 1). Mechanistically, the photoactivated DPZ [Et(S*/S˙−) = +1.42 V vs. SCE, Ered1/2 = −1.07 V vs. SCE in CH3CN]12 could oxidize 1 (Ep = +0.52 vs. Ag/AgCl in CH3CN) via outer-sphere single-electron transfer (SET) to furnish α-amino radical 3 (Scheme 2). 1,3-Enyne 2 is a conveniently available compound and has been extensively used to synthesize indolizines.13 We conceived that radical 4 would be readily formed via the addition of α-amino radical 3 to the olefin moiety of 2, which could be further reduced by DPZ˙− to complete the photoredox catalytic cycle. After protonation of the resulting anion 5, the desired electrophilic functional group, i.e., allene 6, would be generated. A putative challenge in this scenario is the direct generation of an alkyne from 5, given that the poor electron-withdrawing capability of pyridine would impede the deprotonation of the alkyne intermediate. Notably, since the allene of 6 is not a terminal olefin, the ability to accept intramolecular addition by the amine moiety remains elusive.14 If it is feasible, this aza-Michael addition can offer zwitterion 7, which would further lead to 8′ and/or the key intermediate 8 after proton transfer. This process is putatively crucial, since tautomerization of the exocyclic double bond of 8′ potentially constitutes a formidable challenge owing to the weak activation ability of pyridine. Oxidative aromatization of 8 would finally furnish the target pyrole 9.| Entry | Additive (equiv.) | Solvent | Yieldb (%) |
|---|---|---|---|
| a Reactions were performed with 1 (0.015 mmol), 2 (0.01 mmol), and DPZ (5.0 × 10−5 mmol) in solvent (0.3 mL) at 25 °C within 14 h and then purification was conducted. b The yield was determined by GC using dodecane as an internal standard. HOAc = acetic acid. TFA = trifluoroacetic acid. TfOH = trifluoromethanesulfonic acid. TsOH = p-toluenesulfonic acid. DCE = 1,2-dichloroethane. | |||
| 1 | No additive | THF | 0 |
| 2 | (PhO)2P(O)OH (0.2) | THF | 15 |
| 3 | (PhO)2P(O)OH (0.2) | CH2Cl2 | 14 |
| 4 | (PhO)2P(O)OH (0.2) | CHCl3 | 10 |
| 5 | (PhO)2P(O)OH (0.2) | Toluene/CH3CN | 0 |
| 6 | (PhO)2P(O)OH (0.2) | DMSO | Trace |
| 7 | K2CO3/Na2HPO4 (1.0) | THF | 0 |
| 8 | NaH2PO4 (1.0) | THF | 20 |
| 9 | HOAc (1.0) | THF | Trace |
| 10 | TFA/TfOH/TsOH (1.0) | THF | 0 |
| 11 | NaH2PO4 (1.0) | THF/CH2Cl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
52 |
| 12 | NaH2PO4 (1.0) | THF/DCE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
93 |
| 13 | NaH2PO4 (1.0) | THF/CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
36 |
| 14 | NaH2PO4 (1.0) | THF/DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
52 |
| 15 | LiH2PO4 (1.0) | THF/DCE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
86 |
| 16 | NaH2PO4 (0.5) | THF/DCE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
84 |
With these considerations in mind, we first attempted the transformation using only 0.5 mol% DPZ in THF as the solvent at 25 °C and under irradiation with a 3 W blue LED (entry 1, Table 1). It was found that only a small amount of E-2 transformed to its Z-configuration. Given the recognized activation capability of Brønsted acids in radical additions to alkenylazaarenes,5d 20 mol% diphenylphosphate was tested; while the conversion was rather poor (approximately 20%), GC-MS analysis suggested that the desired product 9 with intermediates 8 and/or 8′ was obtained (entry 2).15 Subsequent careful examination also revealed that oxidative aromatization could work smoothly when the reaction mixture was exposed to the ambient atmosphere, and after approximately 10 minutes, only product 9 was detected,16 of which the isolated yield was determined to be 15%. Solvent evaluation was performed next (entries 3–6). A lower yield was obtained when using CH2Cl2 and CHCl3, and toluene, CH3CN and DMSO could not produce adduct 9. The use of stoichiometric diphenylphosphate was futile to improve the yield. As such, a variety of bases and acids with 1.0 equivalents were examined (entries 7–10). The results showed that only weakly acidic NaH2PO4 led to a slightly increased yield (20%, entry 8). When using the strong Brønsted acids, i.e., TFA, TfOH and TsOH, no reaction was found (entry 10), which is most likely due to the generation of insoluble pyridinium salts from 1 with acids, thus hampering the reaction proceeding. Subsequently, a series of mixed solvents were evaluated (entries 11–14); this evaluation was engaged in enhancing the solubility of NaH2PO4 in the reaction system. To our delight, 9 was obtained in 93% yield when using mixed THF/CH2Cl2 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio as the solvent system (entry 12). A similar acidic inorganic salt, LiH2PO4, was then used instead of NaH2PO4, and a good yield was achieved (86%, entry 15). When the amount of NaH2PO4 was reduced to 0.5 equivalents, an 84% yield of 9 was obtained (entry 16). Finally, several control experiments on the effect of distinct reaction parameters were carried out, and the results supported that DPZ, NaH2PO4, visible light and an oxygen-free environment were indispensable for this transformation.17
1 ratio as the solvent system (entry 12). A similar acidic inorganic salt, LiH2PO4, was then used instead of NaH2PO4, and a good yield was achieved (86%, entry 15). When the amount of NaH2PO4 was reduced to 0.5 equivalents, an 84% yield of 9 was obtained (entry 16). Finally, several control experiments on the effect of distinct reaction parameters were carried out, and the results supported that DPZ, NaH2PO4, visible light and an oxygen-free environment were indispensable for this transformation.17
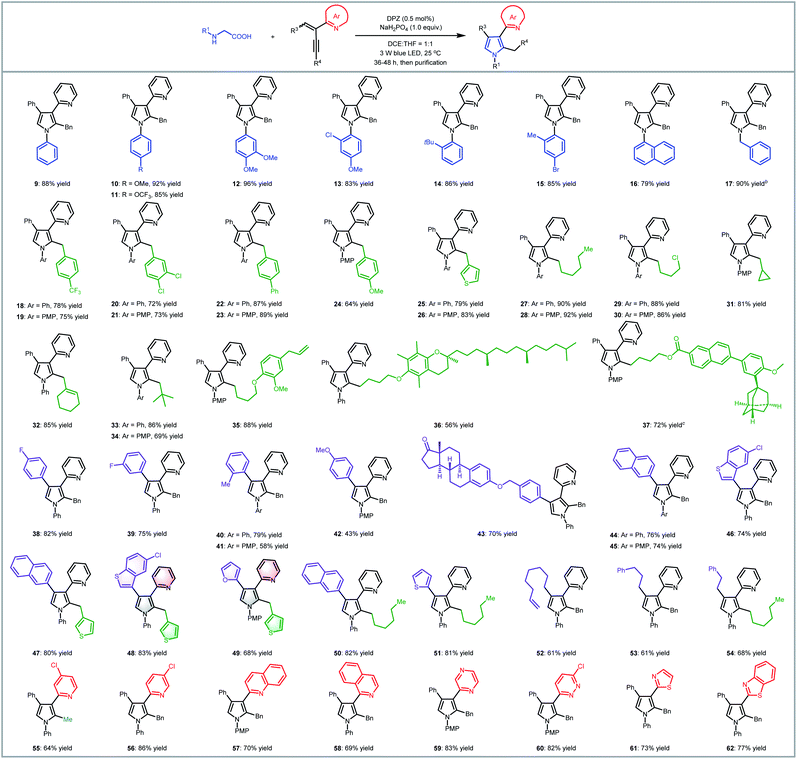
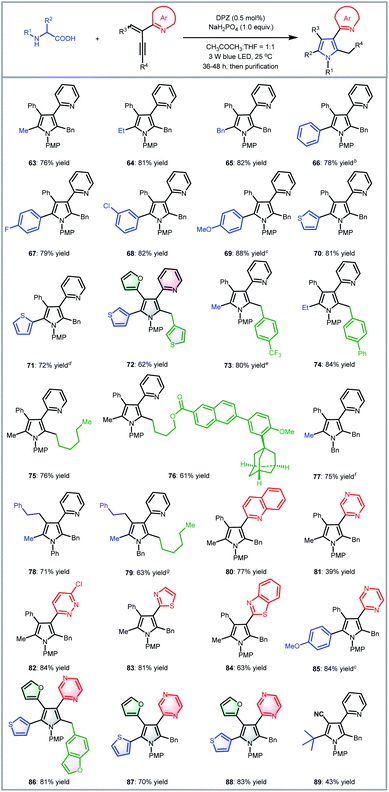
To examine the scope of this radical-based functionalization-oriented construction strategy, optimal conditions were first evaluated with a wide range of N-aryl/benzyl glycines and 2-azaarene-substituted 1,3-enynes for the synthesis of N-aryl/benzyl-3-azaaryl pyrroles containing two other substituents at the 2- and 4-positions (Table 2). With respect to the reactions of N-aryl glycines with 2-(2-enynyl)pyridine (2), products 9–16 were obtained in 79 to 96% yields. Electron-donating substituents on the N-aryl ring of glycines (10–13) were beneficial to chemoselectivity, presenting good to excellent yields. Moreover, although electron-withdrawing groups attenuated reactivity, introducing an extra electron-donating substituent could afford products 13 and 15 with equally satisfactory results. It is worth mentioning that the method was also effective for the glycine derivative (14), which contains a tert-butyl on the ortho-position of the N-phenyl group, offering high steric hindrance. N-Benzyl glycine was then evaluated for reaction with 1,3-enyne 2; since DPZ could not trigger the transformation, another decarboxylative method using Ir[dF(CF3)ppy]2(dtbbpy)PF6 as the photoredox catalyst and stoichiometric caesium carbonate was utilized,7d leading to the corresponding product 17 in 90% yield. Subsequently, the reactions between N-phenyl/PMP (para-methoxy-phenyl)glycine and various 2-(2-enynyl)pyridines that contain diverse aromatic, fused aromatic, heteroaromatic and alkyl substituents on the alkyne and olefin were carried out, leading to a series of 3-(2-pyridyl)pyrroles bearing 2-benzyl/4-phenyl (18–26), 2-alkyl/4-phenyl (27–37), 2-benzyl/4-(hetero)aryl (38–49), 2-alkyl/4-(hetero)aryl (50–51), 2-benzyl/4-alkyl (52–53) and 2-alkyl/4-alkyl (54) in 43–92% yields. In addition to the broad substrate scope, the success of flexibly integrating several complex bioactive molecules, such as vitamin E (36), adapalene (37), and estrone (43), onto the 2- or 4-position of the pyrrole ring, as well as the high yield of product 35, which features an allyl, further robustly underscores the generality and good functional group tolerance of this catalytic system. Notably, the method could collect four distinct aromatic heterocycles into a molecule (e.g., 48 and 49), which always represents an attractive but highly challenging task in pyrrole and even heterocyclic chemistry. Finally, by using 1,3-enynes bearing other azaaryl groups at the 2-position, a range of valuable but difficult-to-access pyrroles were obtained in 69–86% yields, in which multifarious azaarenes, including 4-chloro-2-pyridyl (55), 5-chloro-2-pyridyl (56), 2-quinolyl (57), 1-isoquinolyl (58), 2-pyrazinyl (59), 6-chloro-3-pyridazinyl (60), 2-thiazolyl (61) and 2-benzothiazolyl (62), were successfully introduced onto the 3-position of pyrroles. Among them, the terminal alkyne (55) was demonstrated to be compatible, providing an efficient pathway to assemble a methyl group on the 2-position of such important pyrroles.
We next attempted the direct construction of fully substituted pyrroles by using a variety of N-aryl/benzyl α-substituted α-amino acids as reaction partners (Table 3). It was found that diverse alkyl (63, 64, 73–84 and 89), benzyl (65) and aryl groups (66–72, 85–88) were successfully introduced onto the last remaining positions of the obtained pyrroles in Table 2, thus leading to a series of attractive densely functionalized 3-azaarene-based pyrroles 63–89 in 39 to 88% yields. A strong ability to flexibly alter substituents was perfectly exhibited, given that the diversity of the newly recommended substituents from α-amino acids was perfectly tolerant of the multiformity of the other three substituents derived from various tested 2-azaarene-substituted 1,3-enynes in Table 2. The attempt to integrate such fully substituted pyrroles into bioactive molecules was also successful; a representative product 76 was obtained in 61% yield. In addition to the ability to embed different imine-containing azaarenes at the 3-position (e.g., products 81–88), notably, the method was verified to be a powerful tool to precisely deliver attractive pyrroles that concentrate four different (87 and 88) as well as five partially identical (72) and even totally distinct (86) aromatic heterocycles. Furthermore, adduct 89, an analogue of cytokine inhibitor F (Scheme 1), could be synthesized when using a 1,3-enyne that features a nitrile group-substituted olefin.
 | (1) |
 | (2) |
a The reaction was performed on a 0.1 mmol scale. Yields were determined based on the isolated material after chromatographic purification.
b Trifluoroacetic acid instead of NaH2PO4 was used.
c LiH2PO4 instead of NaH2PO4 was used.
d DCE as the solvent for 60 h.
e THF/DCE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the solvent.
f 2.0 mol% Ir[dF(CF3)ppy]2(dtbbpy)PF6 and 1.0 equiv. of Cs2CO3 were used in THF/DCE (1 1) as the solvent.
f 2.0 mol% Ir[dF(CF3)ppy]2(dtbbpy)PF6 and 1.0 equiv. of Cs2CO3 were used in THF/DCE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the solvent at 30 °C and under irradiation with 3 × 3 W blue LEDs.
g 2.0 mol% Ir[dF(CF3)ppy]2(dtbbpy)PF6 and 1.0 equiv. of Cs2CO3 were used in THF/acetone (1 1) as the solvent at 30 °C and under irradiation with 3 × 3 W blue LEDs.
g 2.0 mol% Ir[dF(CF3)ppy]2(dtbbpy)PF6 and 1.0 equiv. of Cs2CO3 were used in THF/acetone (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the solvent at 25 °C and under irradiation with 3 × 3 W blue LEDs. 1) as the solvent at 25 °C and under irradiation with 3 × 3 W blue LEDs.
|
|---|
|
To further reveal the synthetic utility of this radical-based sustainable method, fully substituted pyrrole 64 was synthesized on a 3.0 mmol scale, and a similar yield was obtained (eqn (1)). It is worth mentioning that we attempted to directly use α-amino acids to react with these 1,3-enynes, but no reaction was detected, although many possible reaction conditions were applied; the lack of reaction was likely due to the infeasibility of generating α-free amine-substituted alkyl radicals from these feedstocks. In this context, the method cannot escape the limitation that all products must have an N-substituent on the pyrrole ring, which is derived from N-protected α-amino acids. Although these products should have diverse potential important applications, we still anticipated obtaining unprotected pyrroles, further expanding the utility of this synthetic strategy. To this end, treatment of N-benzyl pyrrole 17 with sodium and naphthalene was carried out, and to our delight, the desired product 90 was readily obtained in 84% yield (eqn (2)).
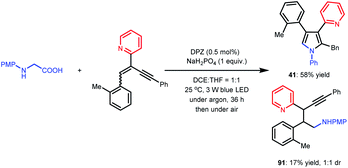 | (3) |
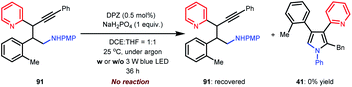 | (4) |
In previous works,7d,8b–e we demonstrated that N-aryl α-amino acids can be oxidized by photoactivated DPZ (*DPZ). The Stern–Volmer experiment also showed no measurable luminescence quenching of *DPZ by 1,3-enyne 2. Accordingly, the transformations should be triggered by the reductive quenching of *DPZ with N-aryl α-amino acids, thus leading to α-amino radicals (Scheme 2). With respect to the subsequent processes, notably, the transformation of product 41 provided several important revelations. As shown in eqn (3), in addition to 41, 91 as a by-product was obtained in 17% yield. Furthermore, 91 could not continue to transform to pyrrole 41 under the established reaction conditions, even when adding an extra 1.0 equivalent of K2CO3, Cs2CO3 or KOH to carry on deprotonation (eqn (4)). The results suggest that the α-amino radicals would first attack the olefin moiety of 1,3-enynes and that the produced allenes (6, Scheme 2) should be the key intermediates. In addition, the formation of stable alkynes is a competitive reaction in the transformations.
To further understand the plausible mechanism of this multistep tandem reaction, we carried out density functional theory (DFT) calculations to study the representative reaction; the results are given in Table 1, and the DFT-calculated reaction energy profiles are illustrated in Fig. 1 (see the ESI† for computational details). The catalytic cycle is initiated by the excitation of the photocatalyst DPZ to DPZ*, which subsequently oxidizes 1 through an outer-sphere single-electron transfer to give α-amino radical 3. Radical 3 undergoes addition to 2 (TS1), leading to activated radical 4 with a quite low barrier (ΔΔG≠ = 2.4 kcal mol−1), and the high exergonicity (19.3 kcal mol−1) indicates that this step is kinetically and thermodynamically favourable. It should be noted that the spin density in radical 4 is delocalized on carbon C1 (0.64) and carbon C2 (0.28). Then, radical 4 could be further reduced by DPZ˙− to complete the photoredox catalytic cycle and generate anion 5. From anion 5, we considered two plausible reaction pathways invoking the protonation of either the C1 site or C2 site by acidic NaH2PO4. The calculations indicate that the protonation of the C1 site viaTS2 (ΔG = −16.7 kcal mol−1) is more favourable than the protonation of the C2 site viaTS2′ (ΔG = −14.6 kcal mol−1), which could also be supported by the more negative charge on C1 (−0.273) than C2 (0.175). Therefore, the C1 site on anion 5 prefers to be protonated by NaH2PO4 through a barrierless transition state TS2 to give allene intermediate II with NaHPO4 anion. Considering the acidic solution in this reaction, the generated NaHPO4 anion could easily abstract a proton (from carboxylic acid) in the solution and convert to NaH2PO4. Subsequently, the intramolecular aza-Michael addition of an amine –NH– to the allenyl group occurs via the five-membered ring transition state TS3 with an energy barrier of 15.8 kcal mol−1, which is the rate-determining step of the full catalytic cycle, leading to the zwitterionic intermediate IV (Scheme 2). The next step is [1,3]-proton transfer from ammonium ions to the C1 or C2 negative site through TS4-1 (ΔΔG = 8.5 kcal mol−1) or TS4-2 (ΔG = 10.0 kcal mol−1) assisted by NaH2PO4 to give intermediate VI or isomer V, and NaH2PO4 acts as a proton shuttle to assist the proton transfer. As depicted in Fig. 1, the energy barrier of TS4-1 is 1.5 kcal mol−1, slightly lower than that of TS4-2; thus, both intermediates V and VI could be generated under this reaction condition. In addition, V could further convert to VI through the 1,3-proton transfer transition state TS5 assisted by NaH2PO4, the natural population analysis (NPA) charge results indicate that the C1 (−0.152) site possesses more charge than the C2 (0.020) site, which is the driving force for the tautomerization of intermediate V to VI. Finally, the oxidative aromatization of VI leads to the production of pyrrole 9.
Conclusions
In summary, we have developed a general, expedient and efficient photoredox catalytic synthetic method to access valuable 3-imine-containing azaarene-substituted highly functionalized pyrroles. Readily accessible and abundant α-amino acids and 2-azaarene-substituted 1,3-enynes were verified to be viable feedstocks. Through smoothly experiencing tandem redox-neutral radical addition, cyclization and aerobic oxidative aromatization, the transformations led to a variety of pyrroles bearing three and four substituents at the carbon positions in high yields (up to 96%). In addition to the good functional group tolerance, the excellent merits include the robust capability to flexibly replace the types of both azaarenes and substituents. The linchpin of this success is exploitation of the electron-withdrawing properties of azaarenes and the highly reactive radical pathway, which creates a new synthetic strategy for azaarene derivatives, that is, radical-based functionalization-oriented construction, an ingenious combination of functionalization with construction. Moreover, control experiments and theoretical studies via DFT calculations not only suggested a plausible mechanism with respect to the reaction pathways but also indicated the important role of NaH2PO4 in protonation and cyclization. We anticipate that this versatile radical-based strategy will encourage the development of more efficient and modular methods to offer known and unprecedented important multi-azaarene derivatives.Author contributions
W. H. and Q. Z. synthesized and characterized the compounds; W. H., Q. Z. and S. C. collected and analysed the spectroscopic data; S. C. performed DFT calculation; Z. J., S. C. and H. Z. wrote the manuscript. All authors discussed the results and commented on the manuscript. Z. J. directed the project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Grants from the National Natural Science Foundation of China (21925103), Henan Normal University and Henan University are gratefully acknowledged.Notes and references
- (a) N. D. P. Cosford, D. Huang, J. R. Roppe, N. D. Smith and L. R. Tehrani, PCT Int. Appl., WO 2004089308 A2 20041021, 2004; (b) H. Saso, K. Hagiwara, S. Sano and Y. Yokota, Jpn. Kokai Tokkyo Koho, JP 10298179 A 19981110, 1998; (c) K. Hagiwara, M. Maruyama, S. Sano and Y. Yokota, Jpn. Kokai Tokkyo Koho, JP 09194477 A 19970729, 1997; (d) A. Coskun, M. D. Yilmaz and E. U. Akkaya, Org. Lett., 2007, 9, 607 CrossRef CAS PubMed; (e) J. Bullington, D. Argentieri, K. Averill, D. Carter, D. Cavender, B. Fahmy, X. Fan, D. Hall, G. Heintzelman, P. Jackson, W.-P. Leung, X. Li, P. Ling, G. Olini, T. Razler, M. Reuman, K. Rupert, R. Russell, J. Siekierka, S. Wadsworth, R. Wolff, B. Xiang and Y.-M. Zhang, Bioorg. Med. Chem. Lett., 2006, 16, 6102 CrossRef CAS PubMed; (f) J. L. Bullington, X. Fan, P. F. Jackson and Y.-M. Zhang, PCT Int. Appl., WO 2004029040 A1 20040408, 2004; (g) S. E. De Laszlo, N. J. Liverton, G. S. Ponticello, H. G. Selnick and N. B. Mantlo, US Pat., US 5792778 A 19980811, 1998 Search PubMed; (h) S. E. De Laszlo and N. B. Mantlo, PCT Int. Appl., WO 9716426 A1 19970509, 1997.
- (a) K. L. Billingsley, K. W. Anderson and S. L. Buchwald, Angew. Chem., Int. Ed., 2006, 45, 3484 CrossRef CAS PubMed; (b) K. Billingsley and S. L. Buchwald, J. Am. Chem. Soc., 2007, 129, 3348 CrossRef PubMed; (c) M. Brasse, J. A. Ellman and R. G. Bergman, Chem. Commun., 2011, 47, 5019 RSC; (d) J. Roppe, N. D. Smith, D. Huang, L. Tehrani, B. Wang, J. Anderson, J. Brodkin, J. Chung, X. Jiang, C. King, B. Munoz, M. A. Varney, P. Prasit and N. D. P. Cosford, J. Med. Chem., 2004, 47, 4645 CrossRef CAS PubMed.
- (a) A. R. Katritzky, J. Jiang and J. V. Greenhill, J. Org. Chem., 1993, 58, 1987 CrossRef CAS; (b) A. R. Katritzky and J. Jiang, J. Org. Chem., 1994, 59, 4551 CrossRef CAS; (c) A. R. Katritzky, L. Zhu, H. Lang, O. Denisko and Z. Wang, Tetrahedron, 1995, 51, 13271 CrossRef CAS; (d) N. D. Smith, D. Huang and N. D. P. Cosford, Org. Lett., 2002, 4, 3537 CrossRef CAS PubMed.
- For selected reviews, see: (a) V. Estévez, M. Villacampa and J. C. Menéndez, Chem. Soc. Rev., 2010, 39, 4402 RSC; (b) V. Estévez, M. Villacampa and J. C. Menéndez, Chem. Soc. Rev., 2014, 43, 4633 RSC; (c) M. Leonardi, V. Estévez, M. Villacampa and J. C. Menéndez, Synthesis, 2019, 51, 816 CrossRef CAS; For selected recent examples, see: (d) G. Bharathiraja, S. Sakthivel, M. Sengoden and T. Punniyamurthy, Org. Lett., 2013, 15, 4996 CrossRef CAS PubMed; (e) J. Xuan, X.-D. Xia, T.-T. Zeng, Z.-J. Feng, J.-R. Chen, L.-Q. Lu and W.-J. Xiao, Angew. Chem., Int. Ed., 2014, 53, 5653 CrossRef CAS PubMed; (f) C. Huang, Y. Zeng, H. Cheng, A. Hu, L. Liu, Y. Xiao and J. Zhang, Org. Lett., 2017, 19, 4968 CrossRef CAS PubMed; (g) H.-C. Chiu, X. Y. See and I. A. Tonks, ACS Catal., 2019, 9, 216 CrossRef CAS PubMed; (h) R. N. Ram, S. D. Sadanandan and K. Gupta, Adv. Synth. Catal., 2019, 361, 5661 CrossRef CAS; (i) J. Ge, Q. Ding, X. Wang and Y. Peng, J. Org. Chem., 2020, 85, 7658 CrossRef CAS PubMed; (j) Y. Zhou, L. Zhou, L. T. Jesikiewicz, P. Liu and S. L. Buchwald, J. Am. Chem. Soc., 2020, 142, 9908 CrossRef CAS PubMed; (k) S. Dutta, B. Prabagar, R. Vanjari, V. Gandon and A. K. Sahoo, Green Chem., 2020, 22, 1113 RSC; (l) L. Wei, S.-M. Xu, Z. Jia, H.-Y. Tao and C.-J. Wang, Chem. Commun., 2020, 56, 9691 RSC.
- For selected reviews, see: (a) R. S. J. Proctor and R. J. Phipps, Angew. Chem., Int. Ed., 2019, 58, 13666 CrossRef CAS PubMed; (b) A. C. Sun, R. C. McAtee, E. J. McClain and C. R. J. Stephenson, Synthesis, 2019, 51, 1063 CrossRef CAS; (c) Y. Zhao and W. Xia, Org. Biomol. Chem., 2019, 17, 4951 RSC; (d) Y. Yin, X. Zhao and Z. Jiang, ChemCatChem, 2020, 12, 4471 CrossRef CAS.
- (a) K. Miyazawa, Y. Yasu, T. Koike and M. Akita, Chem. Commun., 2013, 49, 7249 RSC; (b) M. Nakajima, Q. Lefebvre and M. Rueping, Chem. Commun., 2014, 50, 3619 RSC; (c) F. Lima, U. K. Sharma, L. Grunenberg, D. Saha, S. Johannsen, J. Sedelmeier, E. V. Van der Eycken and S. V. Ley, Angew. Chem., Int. Ed., 2017, 56, 15136 CrossRef CAS PubMed.
- (a) H. B. Hepburn and P. Melchiorre, Chem. Commun., 2016, 52, 3520 RSC; (b) L. Capaldo, M. Fagnoni and D. Ravelli, Chem.–Eur. J., 2017, 23, 6527 CrossRef CAS PubMed; (c) K. N. Lee, Z. Lei and M.-Y. Ngai, J. Am. Chem. Soc., 2017, 139, 5003 CrossRef CAS PubMed; (d) Y. Yin, Y. Dai, H. Jia, J. Li, L. Bu, B. Qiao, X. Zhao and Z. Jiang, J. Am. Chem. Soc., 2018, 140, 6083 CrossRef CAS PubMed; (e) A. Trowbridge, D. Reich and M. J. Gaunt, Nature, 2018, 561, 522 CrossRef CAS PubMed; (f) K. Cao, S. M. Tan, R. Lee, S. Yang, H. Jia, X. Zhao, B. Qiao and Z. Jiang, J. Am. Chem. Soc., 2019, 141, 5437 CrossRef CAS PubMed; (g) Y. Yin, Y. Li, T. P. Gonçalves, Q. Zhan, G. Wang, X. Zhao, B. Qiao, K.-W. Huang and Z. Jiang, J. Am. Chem. Soc., 2020, 142, 19451 CrossRef CAS PubMed.
- (a) L. Chen, C. S. Chao, Y. Pan, S. Dong, Y. C. Teo, J. Wang and C.-H. Tan, Org. Biomol. Chem., 2013, 11, 5922 RSC; (b) J. Li, M. Kong, B. Qiao, R. Lee, X. Zhao and Z. Jiang, Nat. Commun., 2018, 9, 2445 CrossRef PubMed; (c) Y. Liu, X. Liu, J. Li, X. Zhao, B. Qiao and Z. Jiang, Chem. Sci., 2018, 9, 8094 RSC; (d) T. Shao, Y. Yin, R. Lee, X. Zhao, G. Chai and Z. Jiang, Adv. Synth. Catal., 2018, 360, 1754 CrossRef CAS; (e) X. Liu, Y. Yin and Z. Jiang, Chem. Commun., 2019, 55, 11527 RSC.
- For selected reviews, see: (a) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed; (b) D. M. Schultz and T. P. Yoon, Science, 2014, 343, 1239176 CrossRef PubMed; (c) J. W. Beatty and C. R. J. Stephenson, Acc. Chem. Res., 2015, 48, 1474 CrossRef CAS PubMed; (d) M. H. Shaw, J. Twilton and D. W. C. MacMillan, J. Org. Chem., 2016, 81, 6898 CrossRef CAS PubMed; (e) N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075 CrossRef CAS PubMed.
- J. A. Leitch, T. Rogova, F. Duarte and D. Dixon, Angew. Chem., Int. Ed., 2020, 59, 4121 CrossRef CAS PubMed.
- For a selected review, see: (a) X. Lv, H. Xu, Y. Yin, X. Zhao and Z. Jiang, Chin. J. Chem., 2020, 38, 1480 CrossRef CAS; For selected examples, see: (b) Y. Zhao, C. Zhang, K. F. Chin, O. Pytela, G. Wei, H. Liu, F. Bureš and Z. Jiang, RSC Adv., 2014, 4, 30062 RSC; (c) X. Liu, X. Ye, F. Bureš, H. Liu and Z. Jiang, Angew. Chem., Int. Ed., 2015, 54, 11443 CrossRef CAS PubMed; (d) C. Zhang, S. Li, F. Bureš, R. Lee, X. Ye and Z. Jiang, ACS Catal., 2016, 6, 6853 CrossRef CAS.
- Z. Hloušková, J. Tydlitát, M. Kong, O. Pytela, T. Mikysek, M. Klikar, N. Almonasy, M. Dvořák, Z. Jiang, A. Růžička and F. Bureš, ChemistrySelect, 2018, 3, 4262 CrossRef.
- For a selected review, see: (a) F.-D. Lu, X. Jiang, L.-Q. Lu and W.-J. Xiao, Acta Chim. Sin., 2019, 77, 803 CrossRef CAS; For selected examples, see: (b) R.-R. Liu, Z.-Y. Cai, C.-J. Lu, S.-C. Ye, B. Xiang, J. Gao and Y.-X. Jia, Org. Chem. Front., 2015, 2, 226 RSC; (c) R.-R. Liu, C.-J. Lu, M.-D. Zhang, J.-R. Gao and Y.-X. Jia, Chem.–Eur. J., 2015, 21, 7057 CrossRef CAS PubMed; (d) R.-R. Liu, S.-C. Ye, C.-J. Lu, B. Xiang, J. Gao and Y.-X. Jia, Org. Biomol. Chem., 2015, 13, 4855 RSC; (e) P. N. Bagle, M. V. Mane, K. Vanka, D. R. Shinde, S. R. Shaikh, R. G. Gonnade and N. T. Patil, Chem. Commun., 2016, 52, 14462 RSC; (f) S. Mahesh and R. V. Anand, Eur. J. Org. Chem., 2017, 2698 CrossRef CAS; (g) S. Mahesh, D. K. Paluru, F. Ahmad, S. Patil, G. Kant and R. V. Anand, Asian J. Org. Chem., 2017, 6, 1857 CrossRef CAS; (h) S. R. Pathipati, A. van der Werf and N. Selander, Org. Lett., 2018, 20, 3691 CrossRef CAS PubMed; (i) D. K. Paluru, S. Mahesh, F. Ahmad and R. V. Anand, Chem.–Asian J., 2019, 14, 4688 CrossRef CAS PubMed.
- C. Xu, C. W. Muir, A. G. Leach, A. R. Kennedy and A. J. B. Watson, Angew. Chem., Int. Ed., 2018, 57, 11374 CrossRef CAS PubMed.
- The Rf values of 8 and/or 8′ with 9 were same, and thus they could not be directly differentiated through TLC analysis..
- The aromatization rate could be drastically enhanced when removing solvents under rotary evaporation..
- When in the absence of DPZ, NaH2PO4 or visible light, no reaction was observed. When the transformation was performed under the ambient atmosphere, 1 was exhausted but no product 9 was detected..
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2049838. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc01470f |
| ‡ The authors made equal contributions. |
| This journal is © The Royal Society of Chemistry 2021 |