Open Access Article
Open Access ArticleEffect of charge-transfer enhancement on the efficiency and rotary mechanism of an oxindole-based molecular motor†
Daisy R. S.
Pooler‡
 a,
Robin
Pierron‡
a,
Robin
Pierron‡
 b,
Stefano
Crespi‡
b,
Stefano
Crespi‡
 a,
Romain
Costil
a,
Romain
Costil
 a,
Lukas
Pfeifer
a,
Lukas
Pfeifer
 a,
Jérémie
Léonard
a,
Jérémie
Léonard
 *b,
Massimo
Olivucci
*b,
Massimo
Olivucci
 *cd and
Ben L.
Feringa
*cd and
Ben L.
Feringa
 *a
*a
aStratingh Institute for Chemistry, Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands. E-mail: b.l.feringa@rug.nl
bInstitut de Physique et Chimie des Matériaux de Strasbourg, Université de Strasbourg, CNRS, UMR 7504, F-67034 Strasbourg, France. E-mail: jeremie.leonard@ipcms.unistra.fr
cDipartimento di Biotecnologie, Chimica e Farmacia, Università di Siena, 53100 Siena, Italy. E-mail: massimo.olivucci@unisi.it
dChemistry Department, Bowling Green State University, Bowling Green, Ohio 43403, USA. E-mail: molivuc@bgsu.edu
First published on 29th April 2021
Abstract
Harvesting energy and converting it into mechanical motion forms the basis for both natural and artificial molecular motors. Overcrowded alkene-based light-driven rotary motors are powered through sequential photochemical and thermal steps. The thermal helix inversion steps are well characterised and can be manipulated through adjustment of the chemical structure, however, the insights into the photochemical isomerisation steps still remain elusive. Here we report a novel oxindole-based molecular motor featuring pronounced electronic push–pull character and a four-fold increase of the photoisomerization quantum yield in comparison to previous motors of its class. A multidisciplinary approach including synthesis, steady-state and transient absorption spectroscopies, and electronic structure modelling was implemented to elucidate the excited state dynamics and rotary mechanism. We conclude that the charge-transfer character of the excited state diminishes the degree of pyramidalisation at the alkene bond during isomerisation, such that the rotational properties of this oxindole-based motor stand in between the precessional motion of fluorene-based molecular motors and the axial motion of biomimetic photoswitches.
Introduction
The first molecular-level step in vision1 represents a reference model for the design of functional photo-activated molecules2–14 often called photoactuators: this is the photochemical double-bond isomerisation of the protonated Schiff base of 11-cis-retinal (rPSB, see Fig. 1A), the co-factor of the scotopic visual pigment Rhodopsin (Rho). Nature has engineered this paradigmatic process to react quickly (<200 fs)15 and efficiently (quantum yield φ = 67%)16 to a light stimulus, initiating a cascade of protein conformational changes ultimately resulting in the stimulation of the optic nerve. Such isomerisation displays two basic mechanistic features. First, only one specific double bond isomerises, i.e. C11![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C12, making the reaction diastereoselective. Second, the reactive process occurs via population of the charge-transfer, first singlet (S1) excited state9 of rPSB, effectively turning the rotationally locked double bond into a freely rotating single bond.17 One additional notable feature associated with the Rho light-induced isomerisation is the presence of sub-picosecond to picosecond spectroscopic signatures of the vibrational motion driving the C11
C12, making the reaction diastereoselective. Second, the reactive process occurs via population of the charge-transfer, first singlet (S1) excited state9 of rPSB, effectively turning the rotationally locked double bond into a freely rotating single bond.17 One additional notable feature associated with the Rho light-induced isomerisation is the presence of sub-picosecond to picosecond spectroscopic signatures of the vibrational motion driving the C11![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C12 twist of rPSB.18–23 These observations were rationalised by computational chemistry studies which provided a mechanistic description of the photoreaction showing that the Rho quantum yield (QY) is controlled by the precise progression along three rPSB vibrational modes, driven by the topographical features of the potential energy surface (PES) along the isomerisation coordinate.24–27
C12 twist of rPSB.18–23 These observations were rationalised by computational chemistry studies which provided a mechanistic description of the photoreaction showing that the Rho quantum yield (QY) is controlled by the precise progression along three rPSB vibrational modes, driven by the topographical features of the potential energy surface (PES) along the isomerisation coordinate.24–27
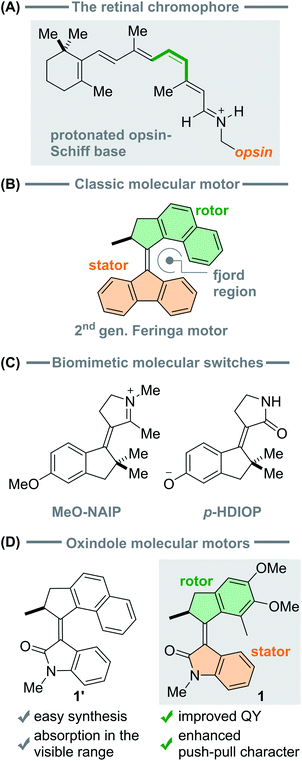 | ||
| Fig. 1 (A) Structure of the retinal chromophore. (B) A second generation Feringa motor based on the fluorene scaffold. (C) Biomimetic molecular switches characterised by a charged structure, either positive (MeO-NAIP) or negative (p-HDIOP). (D) Originally reported52 (1′) and novel (1) structures of the oxindole based molecular motor and their characteristic features. | ||
The Rho photoisomerisation inspired the preparation of a plethora of photoactuators to harvest light energy and convert it into mechanical energy at the molecular scale.28–31 Among those, single-molecules featuring a single exocyclic double-bond and, therefore, undergoing a diastereoselective rPSB-like photochemical E–Z isomerisation, allow a rotor moiety to spin with respect to a stator (see Fig. 1B).32–35 These systems have been employed to control light-actuated molecular muscles,36,37 photoresponsive gels,31,38 and mechanical motion of a macroscopic object in liquid crystal polymers.39 In particular, recent literature reviews the amplification of single molecular motion into macroscopic effects.2,34,40–43
While most synthetic photoactuators derive from modifications of well-known cores, e.g. azobenzenes, overcrowded alkenes and stiff-stilbenes, other research efforts aimed to more closely mimic the photoisomerisation of Rho. The family of the cationic N-alkylated indanylidene-pyrrolinium (NAIP) molecular switches were synthesised to emulate the rPSB π-electron system and S1 charge-transfer character that contributes the unique light-induced dynamics and quantum yield of Rho (see Fig. 1C).44–49 The design of this system was guided by the consideration that a precise, mechanistic-level understanding and control on the directionality and properties of the rotational motion generated by the reaction33 is crucial for the development of effective molecular machines based on double bond isomerisation.
This biomimetic strategy was successful for engineering molecular switches undergoing photoreaction dynamics similar to that of rPSB in Rho,46 but their photoisomerisation QY does not exceed 35%, and a rational approach remains to be devised for further optimising their efficiency.48 This strategy was also recently extended to replicate the photochemical isomerisation of the chromophore of the green fluorescent protein in para-hydroxydimethylindanylidene-oxopyrroline (p-HDIOP) anions (see Fig. 1C), affording more straightforward chemical synthesis.50,51 Both NAIP cations and p-HDIOP anions are characterised by similar electronic structures and PESs, including a biomimetic charge-transfer character facilitating the isomerisation via S1.50
The use of NAIP or p-HDIOP scaffolds in unidirectional molecular motors has also been proposed,53–55 through the incorporation of a stereogenic centre.56,57
For a full control of the directionality of the rotational movement generated by the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond isomerisation, light-driven molecular motors based on chiral overcrowded alkenes (e.g.Fig. 1b) operate through sequential excited state photochemical E–Z isomerisations and thermal helix inversion (THI) steps.58 Recently, a novel direction in the quest of advancing unidirectional photoactuators has been pursued by developing molecular motors based on the oxindole core (1′, in Fig. 1D).52 These structures are synthetically accessible through a one-pot Knoevenagel-type condensation and, more interestingly, their rotation can be triggered with visible light, while the dimension of the ring connected to the alkene bond controls the speed of their thermal step.52 However, it was found that the oxindole-based motors prepared so far have low (2–3%) photoisomerisation quantum yields.
C double bond isomerisation, light-driven molecular motors based on chiral overcrowded alkenes (e.g.Fig. 1b) operate through sequential excited state photochemical E–Z isomerisations and thermal helix inversion (THI) steps.58 Recently, a novel direction in the quest of advancing unidirectional photoactuators has been pursued by developing molecular motors based on the oxindole core (1′, in Fig. 1D).52 These structures are synthetically accessible through a one-pot Knoevenagel-type condensation and, more interestingly, their rotation can be triggered with visible light, while the dimension of the ring connected to the alkene bond controls the speed of their thermal step.52 However, it was found that the oxindole-based motors prepared so far have low (2–3%) photoisomerisation quantum yields.
Here, we explore a new version of the oxindole motor enhancing its push–pull (i.e. charge-transfer) character to possibly approach an electronic structure and isomerisation mechanism mimicking that of biological chromophores – aiming to improve the photoisomerisation QY – and provide insight into the rotary mechanism. We report the synthesis of the novel motor 1 (see Fig. 1D), and the detailed investigation of its photochemical and thermal steps. We show that 1 preserves the advantageous features of its class,52i.e. facile synthesis, visible light addressability, fast thermal steps, but also achieves higher quantum yields in the range of 10%. Combined transient absorption spectroscopy and computational studies allow us to reveal its ultrafast photoreaction dynamics and mechanism. In particular, we observe spectroscopic signatures of excited-state vibrational motions driving the system out of the Franck–Condon region, towards a dark state and subsequently to a region comprising a conical intersection (CInt) funnel9,59 where the decay to the ground state (S0) takes place. These findings are in line with previous observations reported for second-generation Feringa molecular motors.60
Concomitant to the observation of the improved QY, the computational investigation of the electronic structure and photoreaction mechanism of 1 concludes that the engineered push–pull character also affects the isomerisation mechanism – which may be categorised as intermediate between the precessional, stilbene-like rotary motion characteristic of the fluorene motors, and the axial, Rho-like rotation typical of the biomimetic molecular switches, as we will discuss below.
Results
Synthesis
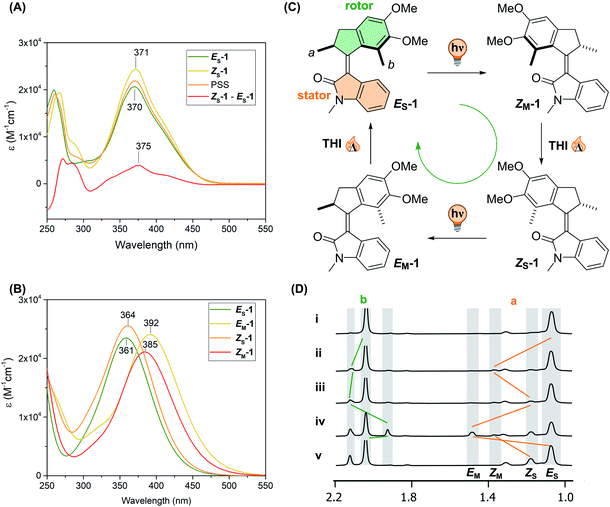
Motor 1 was prepared in two steps from commercially available materials (see ESI†). The first step involves a tandem Friedel–Crafts acylation/Nazarov cyclisation of 2,3-dimethoxytoluene and methacrylic acid, facilitated by polyphosphoric acid (PPA). Next, the ketone was subjected to a Knoevenagel condensation with N-methyl oxindole, mediated by TiCl4 as a Lewis acid and DBU as a base, yielding motor 1 in 44%, exclusively as the stable E isomer (ES-1). Single crystals suitable for X-ray diffraction were grown from a saturated solution of ES-1 in EtOAc (see ESI†). The structure obtained confirms a C(O)–N bond length of 1.3764(18) Å, which is in line with values for typical oxindole systems,61 and a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of 1.3593(19) Å. Irradiation of a dichloromethane solution of ES-1 (0.01 M) under ambient light at room temperature for 24 h afforded the ZS-1 isomer that could be isolated using flash column chromatography combined with recrystallisation from EtOAc (23% yield). The enantiomers of ES-1 and ZS-1 were separated by HPLC and were irradiated with a 400 nm LED. The CD spectra of the enantiopure samples before and after irradiation show that no racemisation occurs during the photoisomerisation (see ESI†).
C bond of 1.3593(19) Å. Irradiation of a dichloromethane solution of ES-1 (0.01 M) under ambient light at room temperature for 24 h afforded the ZS-1 isomer that could be isolated using flash column chromatography combined with recrystallisation from EtOAc (23% yield). The enantiomers of ES-1 and ZS-1 were separated by HPLC and were irradiated with a 400 nm LED. The CD spectra of the enantiopure samples before and after irradiation show that no racemisation occurs during the photoisomerisation (see ESI†).
Steady-state spectroscopy and assessment of the rotational cycle
The UV-vis spectra in methanol of ES-1 and ZS-1 show a broad absorption band with λmax = 370 nm (ε = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1 cm−1) and λmax = 371 nm (ε = 24
800 M−1 cm−1) and λmax = 371 nm (ε = 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 M−1 cm−1), respectively (see Fig. 2). Irradiation at room temperature with either 365 or 390 nm LEDs in methanol afforded in both cases a photostationary state (PSS) composed of a mixture solely consisting of ES-1 and ZS-1 in a 2
500 M−1 cm−1), respectively (see Fig. 2). Irradiation at room temperature with either 365 or 390 nm LEDs in methanol afforded in both cases a photostationary state (PSS) composed of a mixture solely consisting of ES-1 and ZS-1 in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (see Fig. 2A). The absence of metastable states in the photostationary distribution is in line with our previous reports on similar scaffolds52 and with the simulated spectra of the four species involved in the isomerisation (Fig. 2B). Indeed, the metastable states are predicted to display a marked red-shift compared to their respective stable forms, a feature not observed in the steady-state UV-vis spectrum of the PSS at room temperature. The photoisomerisation quantum yield of both isomers was measured following either the method of Börjesson and coworkers62 or the initial slope method63 (see ESI†). Both methods were in excellent agreement, revealing QY values of 8% to 9% for the ES-1 and 12% for ZS-1.
1 ratio (see Fig. 2A). The absence of metastable states in the photostationary distribution is in line with our previous reports on similar scaffolds52 and with the simulated spectra of the four species involved in the isomerisation (Fig. 2B). Indeed, the metastable states are predicted to display a marked red-shift compared to their respective stable forms, a feature not observed in the steady-state UV-vis spectrum of the PSS at room temperature. The photoisomerisation quantum yield of both isomers was measured following either the method of Börjesson and coworkers62 or the initial slope method63 (see ESI†). Both methods were in excellent agreement, revealing QY values of 8% to 9% for the ES-1 and 12% for ZS-1.
The rotational cycle (Fig. 2C) was first studied by in situ irradiation with 1H NMR spectroscopy (Fig. 2D). Upon irradiation of an NMR sample of stable ES-1 in CD2Cl2 at −90 °C with 365 nm light, a new set of signals appeared, corresponding to the metastable Z isomer (ZM-1). A significant shift of the methyl group protons at the stereogenic centre (Ha), and the protons of the methylene group (Hb/b′) allowed the detection of this newly formed isomer. The deshielding of Ha is typically observed when the methyl at the stereogenic centre assumes a pseudo-equatorial conformation, which is a fingerprint for the formation of the metastable isomer in Feringa-type motors.64
Even at low temperature, ZM-1 undergoes slow thermal helix inversion (THI) to form the corresponding stable isomer ZS-1 which can perform a second photoisomerisation to form the metastable EM-1. Consequently, under continuous irradiation for ca. 130 min at −90 °C, the photostationary state (PSS) between all isomers of the motor was obtained. The ratio between the isomers at the PSS365 at −90 °C is 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 (ES/ZM/ZS/EM). Raising the temperature (−45 °C, 15 min), EM-1 undergoes thermal helix inversion, too, to reform ES-1, completing the 360° rotation and affording the same PSS observed with UV/Vis spectroscopy at room temperature.
15 (ES/ZM/ZS/EM). Raising the temperature (−45 °C, 15 min), EM-1 undergoes thermal helix inversion, too, to reform ES-1, completing the 360° rotation and affording the same PSS observed with UV/Vis spectroscopy at room temperature.
The directionality of the motor52 was confirmed by the sequential appearance of ZM, ZS and EM when irradiating a pure sample of ES-1 (see Fig. 2D) and when starting the irradiation from ZS-1 (see Fig. S2, ESI†).
The thermal isomerisation behaviour of 1 was investigated in more detail in CD3OD, following the decay and appearance of new peaks for the protons at the methyl group of the stereogenic centre (Ha). A sample of ES-1 was irradiated with 365 nm and the kinetics of the interconversion of the metastable states back to their relative stable states were followed at temperatures ranging from −90 to −45 °C (see ESI†). Eyring analysis of the decays provided the Gibbs free energy barriers for the thermal steps of the rotation cycle. The metastable ZM-1 isomer is the most short-lived, with a ΔG‡ = 11.8 kcal mol−1. The corresponding E metastable isomer EM-1 is slightly more stable, with a ΔG‡ = 17.4 kcal mol−1. The THI energy barriers were also computed at the DFT level of theory with implicit solvation to be between 14.4–15.1 kcal mol−1 for ZM-1, 16.2–16.9 kcal mol−1 for EM-1, reproducing the trend observed experimentally (see Table S3†). These computations also provide information about the transition state structure (see Fig. S53–S56†). In particular, the difference between the two THI barriers comes from the benzylic methyl in the rotor interacting with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the stator in the ZM-1 → ZS-1 transition state or with the bulkier aromatic ring of the oxindole during the EM-1 → ES-1 step. (see Fig. 2 and S53–S56†). The free energy barriers for both THI steps of motor 1 are nearly identical to those found for the structurally related oxindole motor previously reported (1′ in Fig. 1).52 Hence, the electron-donating MeO substitutions do not affect the THI steps.
O in the stator in the ZM-1 → ZS-1 transition state or with the bulkier aromatic ring of the oxindole during the EM-1 → ES-1 step. (see Fig. 2 and S53–S56†). The free energy barriers for both THI steps of motor 1 are nearly identical to those found for the structurally related oxindole motor previously reported (1′ in Fig. 1).52 Hence, the electron-donating MeO substitutions do not affect the THI steps.
Transient absorption spectroscopy
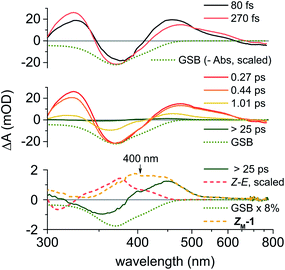
Fig. 3 displays a selection of transient absorption (TA) spectra recorded upon 400 nm excitation of a methanol solution of ES-1 into S1. About 80 fs after excitation, the early S1 signature is characterised by two excited state absorption (ESA, S1 → Sn transitions) bands peaking at around 455 nm (Vis ESA) and 325 nm (UV ESA). A stimulated emission (SE, S1 → S0 transition) band is also observed as a weak, negative signal at λ > 625 nm. The ground state bleach (GSB, S0 → S1 transition) appears around 375 nm. Within the first 270 fs (Fig. 3, top panel), the intensities of the GSB and UV ESA bands increase, which is due to the dynamic blue shift of the positive UV ESA band spectrally overlapping the negative GSB band, most likely characterising the early motion of the S1 population away from the Franck Condon (FC) region. On the same time scale, the Vis ESA and the red SE bands slightly weaken and red-shift. Between 270 fs and 440 fs (Fig. 3, middle panel), no GSB recovery – hence no decay to S0 – is observed, while the UV ESA intensity starts decreasing and the SE completely decays or further red-shifts outside of the observation window. This observation indicates further evolution on the S1 PES, towards conformations of lower S1–S0 oscillator strength and lower S1–S0 energy gaps.Then, by 1 ps all the S1 signatures and the GSB have decayed significantly, indicating S1 to S0 decay. Further spectral relaxation, attributed to vibrational cooling in S0, occurs until a quasistationary spectrum is reached by 25 ps (weak amplitude, see green line in the bottom panel of Fig. 3), which remains unchanged until the maximum delay of 5 ns achievable in this pump-probe experiment. The >25 ps spectrum does not overlap with the Z–E difference of steady-state spectra (displayed in Fig. 2A), demonstrating that the state formed upon the decay to S0 is not ZS-1. The latter must be formed subsequently on a time scale longer than 5 ns. The >25 ps TA spectrum is composed of a positive photoproduct absorption band in the visible range, and a negative, residual GSB, corresponding to 8% of the initial GSB amplitude. By subtracting this GSB contribution from the >25 ps spectrum, we infer the photoproduct absorption spectrum (Fig. 3, yellow dashed line in bottom panel) with maximum around 400 nm. We assign it to the metastable ZM-1 conformer, formed upon C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond isomerisation of the photoexcited ES-1 with an estimated 8% photoisomerisation quantum yield, in agreement with the QY values measured independently, as described above.
C double bond isomerisation of the photoexcited ES-1 with an estimated 8% photoisomerisation quantum yield, in agreement with the QY values measured independently, as described above.
TD-DFT calculations predict that the UV-Vis absorption maximum for ZM-1 is 31 nm red-shifted compared to ES-1 (see Fig. 2B and S57–S58†), consistently with the ZM-1 spectrum extrapolated here from TA data (Fig. 3, bottom panel). In related oxindole motors featuring higher THI energy barriers,52 the absorption spectra of their long-lived metastable species could be measured by steady-state spectroscopy and displayed similar spectral shifts with respect to the stable species, in line with the present results.
The TA data recorded with a methanol solution of ZS-1 under the same conditions are displayed in the ESI Fig. S26† and reveal nearly identical signatures, with a slightly slower blue-shift of the UV ESA and red-shift of the SE up to ∼360 fs after excitation. For ZS-1, the GSB does not start recovering before 0.6 ps, and significant S1 decay is observed at slightly later delays compared to ES-1. A quasistationary spectrum is also reached after 25 ps, and interpreted as the superposition of the photoproduct – i.e. the metastable EM-1 – absorption, peaking close to 400 nm, and a residual GSB contribution evaluated to ∼10% of the early (<0.6 ps) GSB amplitude, therefore indicative of a 10% photoisomerisation QY, consistent with the 12% value reported above, and with the ZS-1 and EM-1 spectra predicted by TD-DFT calculations (Fig. S59 and S60†).
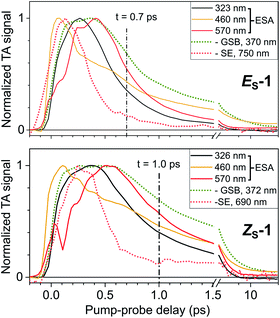
For both isomers, the early ESA and SE spectral shifts and the lack of GSB recovery reveal S1 population dynamics illustrated in Fig. 4 with a selection of TA kinetic traces. In both cases, the TA signal rises the fastest (close to the experimental time resolution of ∼60 fs) at 460 nm (yellow traces), corresponding to the maximum of the early Vis ESA. The UV ESA traces (black) have a slower rise time and reach their maxima at 270 fs and 360 fs for ES-1 and ZS-1, respectively. On the same time scale, the (very weak) SE signals (red dashed traces) already decay. The low-energy side of the Vis ESA bands monitored at 570 nm in Fig. 4 show the slowest rise times reaching their maxima at 415 fs and ∼550 fs for ES-1 and ZS-1, respectively. At these time delays, the SE signal has already decayed by 80–90%, while the GSB barely starts recovering in both isomers. Altogether, this suggests that within 400 fs (ES-1) to 600 fs (ZS-1) – which are also typical time scales for solvent relaxation65 – the S1 population evolves to a region of the S1 PES characterised by a red-shifted and weak S1 → S0 optical transition (vanishing SE), a blue-shifted UV ESA and a red-shifted Vis ESA which both remain relatively intense (transitions from S1 to higher-lying states). This is reminiscent of the transient, so-called dark, excited state already reported for related fluorene molecular motors.60 Hence, the S1 lifetime may be estimated from the decay kinetics of the ESA signals. However, no mulitexponential functional form can be used to fit the beginning (first ps) of the observed decay kinetics (see Fig. S25†). This sometimes occurs when electronic populations evolve on time scales faster than vibrational relaxation, in which case rate equation models do not apply (see e.g.66). We instead propose to evaluate the S1 lifetime by specifying the Vis ESA signal half-life, which is in the order of 0.6–0.7 ps for ES-1 and 0.9–1 ps for ZS-1 (see black vertical dashed lines in Fig. 4).
Fig. S27† displays the TA spectroscopy data of ES-1 in non-polar n-hexane. The observations are very similar to those made in polar methanol. Hence, the early spectral relaxations result from S1 conformational change – rather than solvation dynamics – resulting in a transient, dark S1 species characterised by a vanishing SE, a red-shifted Vis ESA with an almost identical half-life of 0.5–0.6 ps. A quasistationary spectrum is also reached by 25 ps, revealing the ZM-1 spectrum characterised by two absorption maxima peaking at 380 nm and 440 nm in n-hexane (see Fig. S27†).
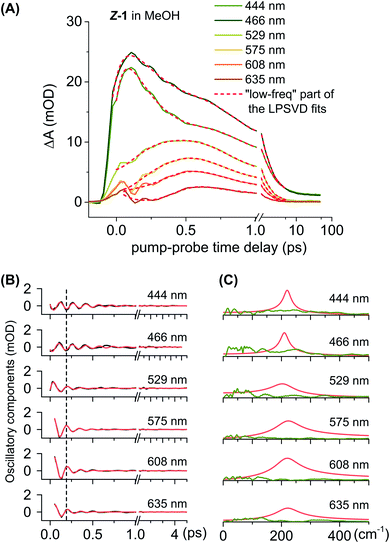
Notably, the oscillations observed in the Vis ESA band (at 460 nm and 570 nm in Fig. 4, most pronounced in the ZS-1 case) are indicative of vibrational motion in S1 away from the FC region during the first 0.5 ps. Fig. 5 illustrates these oscillatory signals in the case of ZS-1 in methanol and their quantitative analysis, performed with Linear Prediction and Singular Value Decomposition (LPSVD).67,68 An oscillatory component is clearly observed at 215 ± 8 cm−1 with a signal-to-noise ratio of ∼5 (see Fig. 5C), and a π phase shift (see black vertical dashed line, in Fig. 5B) in the red side with respect to the blue side of the Vis ESA signature of S1 (see further discussion in the ESI, Section 8†). In the case of ES-1, characterised by slightly faster S1 dynamics (faster ESA red-shift and shorter ESA half-life), similar S1 oscillations are observed – only in the red side of the Vis ESA band, i.e. λ > 500 nm – at frequencies of 190 ± 10 cm−1 in methanol and 185 ± 12 cm−1 in n-hexane (see the ESI Fig. S28, S29 and Table S2†).
Excited-state electronic structure modelling
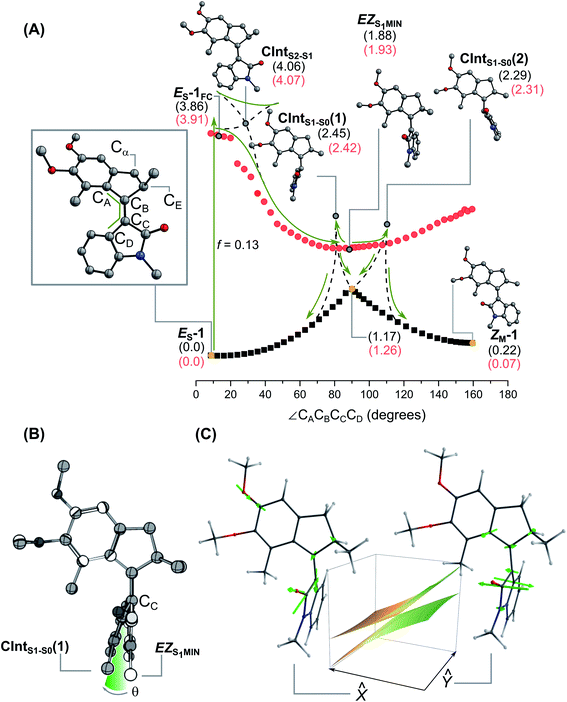
The topography of the PES of 1 was studied at the SA4-XMS-CASPT2/6-31G*//SA3-CASSCF(12,12)/6-31G* level (see ESI†) following a relaxed scan along the rotation coordinate identified by the dihedral angle CACBCCCD (see Fig. 6A). More specifically, to account for the missing dynamic electron correlation energy at the SA3-CASSCF(12,12)/6-31G* level of theory employed to follow the S1 relaxation, the energy is re-evaluated via multistate multi-configurational second-order perturbation theory through single point SA4-XMS-CASPT2 computations.While this protocol represents, arguably, the most robust excited state calculation that can be afforded for the system under investigation, the results remain semi-quantitative due to the different level of theory used for the geometry and energy calculations as well as for the use of a basis set of a limited size. The electronic structures computed for both isomers are very similar, hence we report here on the results for the E isomer and refer to the ESI (Section 9†) for the Z isomer.
The computed S0 → S1 transition of ES-1 is allowed with an oscillator strength f = 0.13, while the S0 → S2 transition is much weaker with f = 3.9 10−3. TD-DFT at the PCM(MeOH)-TD-ωB97X-D/6-311+G(2d,p)//MP2/6-31G(d) level shows the same trend, corroborating the hypothesis that S1 is the state populated directly after the photoexcitation. TD-DFT affords slightly higher oscillator strengths for both S0 → S1 and S0 → S2 (f = 0.56 and 0.15, respectively), due to the different type of limitations associated to these quantum chemical approaches (see ESI†).
At the CASSCF level of theory, we identify an S1 energy minimum (ES-1FC) in a flat region lying near the Franck–Condon (FC) point and characterised by a locally excited – rather than a charge-transfer – electronic character. Indeed, ES-1FC shows a very limited charge separation with the stator accommodating a Mulliken charge of +0.16, versus +0.17 at the same geometry in S0 (see ESI†). ES-1FC is predicted to be optically bright, with the same oscillator strength f = 0.13 for emission, as computed for ground state absorption. We propose that the observed SE in the TA experiments (see Fig. 5) originates from this region of the S1 PES.
The initial S1 movement from the Franck–Condon point to ES-1FC follows a rocking motion of the rotor (see ESI animated figure†). After calculation of the vibrational frequencies of the stationary ES-1FC point, we identified a specific mode, ν16 at 200 cm−1, which best corresponds to the aforementioned deformation (see ESI animated figure†). Hence, we assign the oscillatory signals observed in the Vis ESA band to this mode (see ESI† for a more detailed discussion). The same conclusions hold for the other isomer, where the ν16 mode characterising the stationary point ZS-1FC is predicted at 210 cm−1, in line with the slightly higher frequency observed for the oscillatory TA signals of Z-1. We note that prominent S1 vibrational activity was already reported in cis-stilbene69 at 216 cm−1, in cis-stiff-stilbene at 194 cm−1,70 and in molecular motors at somewhat lower frequencies.60,71
Progression along the double bond isomerisation coordinate leads towards the region of a CInt with the S2 state (CIntS2-S1; see Fig. 6A and B). At higher values of ∠CACBCCCD dihedral angle, the PES has a steep decrease in energy, resulting in a sloped path towards a global S1 minimum at ca. 90° (45.5 kcal mol−1 downhill compared to ES-1FC at the XMS-CASPT2 level; all the energies described in the following paragraphs are from the same level of theory), that we label EZS1-MIN and refer to as the perpendicular state in the following discussion. EZS1-MIN features a substantially planarised oxindole ring, with no pyramidalisation in CB or CC either (θ = 1°, see Fig. 6B). More accurate energy evaluation at the XMS-CASPT2 level shifts CIntS2-S1 to higher energies, replacing it with a sloped avoided crossing. Such a refined topography does however not change the general reaction mechanism. In particular, both levels of theory predict a qualitative change in electronic character at the (avoiding) crossing with the negative charge on the stator increasing progressively from +0.16 Mulliken units at ES-1FC to −0.66 at EZS1-MIN.
Hence, the perpendicular state displays a zwitterionic character due to an almost complete charge transfer between stator and rotor. Finally, we notice that EZS1-MIN is characterised by an extremely weak S1 to S0 oscillator strength (f = 6.3 10−4), which makes it optically dim.
Two CInts (CIntS1-S0(1) and CIntS1-S0(2)) connect S1 and S0 (see Fig. 6). Compared to EZS1-MIN these funnels are energetically uphill by +13.0 and +9.3 kcal mol−1, and slightly more pyramidalised, with θ = 13° and 12° for CIntS1-S0(1) and CIntS1-S0(2), respectively (see Fig. 6B and ESI†). Both CInts have an even stronger charge-transfer character, with an almost unitary negative charge delocalised on the stator (−0.91 Mulliken units for CIntS1-S0(1) and −0.93 for CIntS1-S0(2)). Interestingly, the addition of an implicit solvent contribution (MeOH using the PCM method, see red values in Fig. 6A), has only a limited effect on the relative energies of the excited state species, while stabilising more effectively the metastable species at the ground state. While it overlooks explicit hydrogen bonding between solvent and solute, this evaluation of the MeOH solvent effect is simplistic but already in line with the observed similarity of the photoreaction dynamics in MeOH and n-hexane. We note, that a similar lack of solvent polarity effect was previously reported in Feringa motors.72,73
The branching space vectors74![[x with combining circumflex]](https://www.rsc.org/images/entities/i_char_0078_0302.gif) and ŷ (i.e. the ortho-normalised g and h branching plane vectors) are displayed in Fig. 6C for CIntS1-S0(1). They reveal the nature of motions which lift the CInt's degeneracies, and therefore govern the excited state decay mechanism. For both CInts, vector ŷ describes the CB
and ŷ (i.e. the ortho-normalised g and h branching plane vectors) are displayed in Fig. 6C for CIntS1-S0(1). They reveal the nature of motions which lift the CInt's degeneracies, and therefore govern the excited state decay mechanism. For both CInts, vector ŷ describes the CB![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC bond twisting associated with the reaction coordinate. In contrast, vector
CC bond twisting associated with the reaction coordinate. In contrast, vector ![[x with combining circumflex]](https://www.rsc.org/images/entities/i_char_0078_0302.gif) features the pyramidalisation distortion typical of the alkene branching plane,75 as well as a bond-length alternation (BLA) component typical instead of the Rho branching plane. Notably, in addition to the central CB
features the pyramidalisation distortion typical of the alkene branching plane,75 as well as a bond-length alternation (BLA) component typical instead of the Rho branching plane. Notably, in addition to the central CB![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC double stretching, the BLA component involves a substantial stretching of the Ar–OMe and C
CC double stretching, the BLA component involves a substantial stretching of the Ar–OMe and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds (see also the animated figures in ESI†), showcasing the influence of the push–pull system on the CInt topography. As we will discuss below, these results suggest that the character of the branching plane motions lies in between the two limiting cases of twist-pyramidalisation and twist-BLA previously associated to stilbene-like and Rho-like isomerisation mechanisms, respectively.33
O bonds (see also the animated figures in ESI†), showcasing the influence of the push–pull system on the CInt topography. As we will discuss below, these results suggest that the character of the branching plane motions lies in between the two limiting cases of twist-pyramidalisation and twist-BLA previously associated to stilbene-like and Rho-like isomerisation mechanisms, respectively.33
It is also worth mentioning that, given the sloped topography of the CIntS1-S0, the decay would actually occur in the region extending from the conical intersection towards the orthogonal intermediate EZS1-MIN. Notice that, if this is the case, then the decay “funnel” would feature a further diminished pyramidalization with respect to the CIntS1-S0.
Discussion
As compared to the previously reported oxindole motors,521 carries two electron-rich methoxy substituents allowing us to fine tune the electronic structure of the motor. Tuning of the S1 electronic structure and photoreaction properties of neutral stilbenoid molecular motors was already explored via introducing electron-donating or electron-withdrawing substituents.76In particular, the grafting of an electron-poor CN group on the rotor moiety improved the isomerisation QY up to 20% for a 2nd generation Feringa-type motor also characterized by a longer-lived (∼10 ps) S1dark state proposed to feature enhanced pyramidalization.72,77 Here, we observe that the isomerisation QY of 1 increases four-fold compared to the originally reported oxindoles (QYs ca. 2–3%). Besides, owing to the inherent structural resemblance of biomimetic p-HDIOP switches and 2nd generation oxindole-based molecular motors, we aim to explore whether the enhanced push–pull character of 1 allows us to approach an S1 electronic structure and photoreaction mechanism mimicking that of biological chromophores – with the expectation that it will help to guide and rationalise the chemical synthetic design of molecular motors showing further QY enhancement. First, we recall that distinct CInt electronic structures and topographies lead to qualitatively distinct mechanistic pictures.33
Stilbenoid molecular motors and the corresponding parent stilbene are neutral in their ground state but acquire a charge-transfer – or zwitterionic – character in their S1 perpendicular (e.g.EZS1-MIN) state.75 More precisely, in transient structures of this minimum, the isomerising bond acquires local polarisation with one of the two C atoms of the olefinic bond carrying significant negative partial charge and therefore acquiring a pronounced pyramidalisation distortion (see angle θ in Fig. 7C), especially at the CInt. The mechanistic consequences are the following: (i) the branching plane vectors associated to the S1 → S0 CInt, featuring degenerate diradical/charge-transfer characters, describe the so-called twist-pyramidalisation motion (see Fig. 7A), (ii) the path going from the low energy region of the S1 PES to the CInt is dominated by a charge-transfer electronic character and (iii) the motion leading towards the CInt and therefore molecular-scale rotary motion is precessional (see Fig. 7B).33 Conversely, in Rho and biomimetic switches, (i) the branching plane vectors associated to the same type of S1 → S0 CInt describe a so-called twist-BLA (see Fig. 7A), (ii) the path going from the low energy region of the S1 PES to the CInt is dominated by a mixed diradical-zwitterionic character, but displaying a much more pronounced charge translocation with respect to the one occurring upon excitation to the FC state,17,50 and (iii) no pyramidalisation occurs, the rotary motion becomes purely axial (see Fig. 7B).33
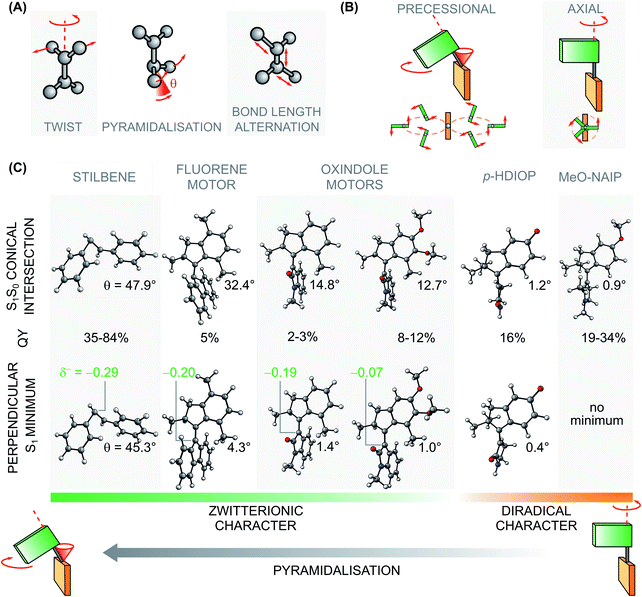 | ||
| Fig. 7 Mechanistic features of the rotation. (A) Modes associated to the excited state rotational movement of overcrowded alkenes and Rho-like compounds. (B) Paradigmatic rotational motions: on the left the precessional (or hippopede-like) type, typical of switches and motors with zwitterionic character around the S1 global minimum/S1 → S0 CInt; on the right the axial type, typical of photoactuators with diradical character at the S1 global minimum/S1 → S0 CInt.33 (C) QYs of isomerisation and pyramidalisation (θ, in degrees) of the perpendicular (phantom or dark) state and selected S1 → S0 CInts for different photoactuators characterised by zwitterionic (i.e. charge-transfer) or diradical character. The Löwdin charge (δ−) at the at the pyramidalised carbon of the perpendicular, zwitterionic S1 minimum of the motor was calculated at the SF-BH&HLYP/cc-pVDZ level on the optimised geometries present in the literature.72,78,79 The unsubstituted oxindole motor was optimized at the CASSCF(12,12)/6-31G* level of theory, with an active space comparable to the one used for 1. It has to be noted that MeO-NAIP does not present a minimum distinct from the CInt. | ||
The combined experimental and computational investigation reported above allows us to sum up the photoreaction dynamics of 1 as follows. We observe that electronic excitation to S1 triggers a fast isomerisation around the unlocked stilbenoid C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond, with S1 lifetimes in the 1 ps range, like for the unsubstituted fluorene motor of Fig. 1B.60,72
C double bond, with S1 lifetimes in the 1 ps range, like for the unsubstituted fluorene motor of Fig. 1B.60,72
More precisely, the initially bright, locally-excited S1 state evolves within 400 to 600 fs into a transient S1 state where SE is no longer present. This early S1 evolution is accompanied by an oscillatory behaviour observed in the Vis ESA for both isomers. We tentatively ascribe this vibration to the excited state ν16 mode which best corresponds to the conformational deformation from the initial Franck–Condon point to the nearby local minimum of the bright region.
XMS-CASPT2//CASSCF calculations suggest an almost barrierless evolution in the vicinity of CIntS2-S1 connecting the initial locally-excited and bright FC state to a region of the S1 PES featuring a strong charge-transfer character between the rotor and stator halves. This region presents a global minimum characterised by a perpendicular arrangement of the alkene bond (see Fig. 7) and a low S1 to S0 oscillator strength. Hence, we assign the SE decay and ESA spectral shifts – observed in the first 400 to 600 fs – to the formation of this perpendicular state, reminiscent of the phantom state described in the isomerisation of stilbene derivatives80,81 and of the dark state in molecular motors.60,82 However, despite its pronounced charge-transfer character the perpendicular state of 1 does not display the highly pyramidalised carbon expected for such states.75,83 Instead, EZS1-MIN features a substantially planarised oxindole ring and CB atom and its pyramidalisation angle at CC is only θ = 1° whereas it is θ = 45° for stilbene (see Fig. 7C).75
After reaching the S1 perpendicular state, 1 decays to S0 in the region of two sloped CInts, eventually populating the metastable isomer of the opposite configuration. When comparing CIntS1-S0(1) and CIntS1-S0(2) to the analogous CInt of stilbene and stilbenoid photoactuators, it is apparent that the degree of pyramidalisation at CC is strongly diminished (θ = 12–13°) with respect to the related carbon (θ = 48°) in stilbene75 and in a fluorene motor (θ = 32–35°).79 Comparably, the unsubstituted oxindole motor possesses a very similar pyramidalisation in the minimum (θ = 1.4) and in the CInt (θ = 14–16°) compared to 1. We argue that the diminished pyramidalisation of the perpendicular minimum and related CInts of the oxindole motors is the consequence of the presence of the amide group. However, the enhanced push–pull effect of two methoxy substituents in compound 1 favours even more the delocalisation of the negative charge on the ring, as suggested by the comparison of the partial charge δ− residing on the pyramidalised carbon in the different photoactuators featuring a zwitterionic perpendicular S1 minimum (see Fig. 7C).
Altogether, we propose that due to the reduced pyramidalisation and enhanced BLA contribution of the branching plane motions, the actual rotary motion of 1 must be intermediate between the precessional movement of fluorene motors33 or stilbene,75 and the axial rotation expected for non-pyramidalised – e.g. biomimetic (NAIPs or p-HDIOP) – compounds (see Fig. 7).50
For the paradigmatic example of the Rho photoreaction, a detailed description of a statistical ensemble of molecular trajectories from the FC state to the S1 → S0 CInt was shown to enable quantitative prediction of the outstanding value of the isomerisation QY.25,27 Here, we have focussed on describing the excited state PES topography and electronic character as well as the branching plane motions of 1. Since the details of the trajectory actually followed by the system through the CInt region are required but still missing, this is not enough to provide a univocal rationalisation of the observed photoisomerisation QY. However, we demonstrate and rationalise how electronic structures may be engineered to tune qualitatively and, in principle, continuously the nature of the molecular motions – i.e. from precessional to axial rotary motion – which ultimately governs the S1 decay at the CInt.
Conclusions
The full disclosure of a mechanistic thread connecting apparently similar photoactuators, in particular rotary motors, remains a challenge. Taking inspiration from Nature and biomimetic switches, the introduction of electronic push–pull substituents in 1 is shown (i) to increase the quantum efficiency of the isomerisation process four-fold compared to the previous oxindole motors design, (ii) to maintain the positive features associated to oxindole motors, i.e. the synthetic accessibility and the visible light addressability, and (iii) to demonstrate a rationalised strategy for tuning the nature of the motion governing the S1 decay at the CInt.In conclusion, while motor 1 cannot yet be categorised as a genuine biomimetic scaffold, it exploits some attributes derived from natural photoswitches, such as engineered electronic effects to tune adequately the critical molecular motions driving the excited state decay and possibly improve the QY. Although the system itself is still far from reaching the QYs of Rho or the biomimetic switches, it paves the way to new structures aiming to go beyond the present push–pull design.
Author contributions
MO and BLF designed the study. DRSP synthesised the compounds investigated, performed NMR and steady-state UV-Vis experiments. RP and JL performed transient absorption data acquisition and analysis. SC performed quantum chemical calculations, CD measurements, part of the steady-state UV-Vis, and NMR experiments, quantum yield determination and related analysis. RC purified the enantiomers of the compounds investigated. LP performed the X-Ray measurements and analysed the data. MO, JL and BLF supervised the work. SC, DRSP and JL wrote the paper. All authors discussed and commented on the manuscript. SC, JL, MO, LP and BLF acquired funding.Conflicts of interest
The authors declare there to be no conflicts of interest.Acknowledgements
D. R. S. P. gratefully acknowledges Dr M. Kathan for fruitful discussions and P. van der Meulen for NMR assistance and maintenance. Financial support from the Horizon 2020 Framework Programme (ERC Advanced Investigator Grant No. 694345 to B. L. F.), the Netherlands Ministry of Education, Culture and Science (Gravitation Programme 024.001.035 to B. L. F.) and the Marie Skłodowska-Curie Action (Individual Fellowship No. 838280 for S. C. and 793082 for L. P.) is gratefully acknowledged. We thank the Centre for Information Technology of the University of Groningen for their support and for providing access to the Peregrine high performance computing cluster. M. O. is grateful to Fondazione Banca d'Italia and the MIUR (Department of Excellence 2018 grant) and the NSF (CHE-CLP-1710191) for partial financial support. RP and JL acknowledge support from the Interdisciplinary Thematic Institute QMat, as part of the ITI 2021–2028 program of the University of Strasbourg, CNRS and Inserm, via the IdEx Unistra (ANR 10 IDEX 0002), SFRI STRAT’US (ANR 20 SFRI 0012), EUR QMAT (ANR-17-EURE-0024) and Labex NIE (ANR-11-LABX-0058_NIE) projects of the French Investments for the Future Program.References
- G. Wald, Nature, 1968, 219, 800–807 CrossRef CAS PubMed.
- B. L. Feringa, Angew. Chem., Int. Ed., 2017, 56, 11060–11078 CrossRef CAS PubMed.
- W. R. Browne and B. L. Feringa, Nat. Nanotechnol., 2006, 1, 25–35 CrossRef CAS PubMed.
- M. Schildhauer, F. Rott, S. Thumser, P. Mayer, R. de Vivie-Riedle and H. Dube, ChemPhotoChem, 2019, 3, 365–371 CrossRef CAS.
- R. Wilcken, M. Schildhauer, F. Rott, L. A. Huber, M. Guentner, S. Thumser, K. Hoffmann, S. Oesterling, R. de Vivie-Riedle, E. Riedle and H. Dube, J. Am. Chem. Soc., 2018, 140, 5311–5318 CrossRef CAS PubMed.
- R. Wilcken, L. Huber, K. Grill, M. Guentner, M. Schildhauer, S. Thumser, E. Riedle and H. Dube, Chem.–Eur. J., 2020, 26, 13507–13512 CrossRef CAS PubMed.
- V. Balzani, A. Credi and M. Venturi, Chem. Soc. Rev., 2009, 38, 1542–1550 RSC.
- S. Kassem, T. Van Leeuwen, A. S. Lubbe, M. R. Wilson, B. L. Feringa and D. A. Leigh, Chem. Soc. Rev., 2017, 46, 2592–2621 RSC.
- S. Gozem, F. Melaccio, H. L. Luk, S. Rinaldi and M. Olivucci, Chem. Soc. Rev., 2014, 43, 4019–4036 RSC.
- D. Dattler, G. Fuks, J. Heiser, E. Moulin, A. Perrot, X. Yao and N. Giuseppone, Chem. Rev., 2020, 120, 310–433 CrossRef CAS PubMed.
- L. Greb and J. M. Lehn, J. Am. Chem. Soc., 2014, 136, 13114–13117 CrossRef CAS PubMed.
- C. J. Bruns and J. F. Stoddart, The Nature of the Mechanical Bond, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2016 Search PubMed.
- M. Baroncini, S. Silvi and A. Credi, Chem. Rev., 2020, 120, 200–268 CrossRef CAS PubMed.
- M. Guentner, M. Schildhauer, S. Thumser, P. Mayer, D. Stephenson, P. J. Mayer and H. Dube, Nat. Commun., 2015, 6, 8406 CrossRef CAS PubMed.
- R. Schoenlein, L. Peteanu, R. Mathies and C. Shank, Science, 1991, 254, 412–415 CrossRef CAS PubMed.
- H. J. A. Dartnall, Vision Research, 1968, 8, 339–358 CrossRef CAS.
- S. Gozem, H. L. Luk, I. Schapiro and M. Olivucci, Chem. Rev., 2017, 117, 13502–13565 CrossRef CAS PubMed.
- Q. Wang, R. Schoenlein, L. Peteanu, R. Mathies and C. Shank, Science, 1994, 266, 422–424 CrossRef CAS PubMed.
- D. W. McCamant, P. Kukura and R. A. Mathies, J. Phys. Chem. B, 2005, 109, 10449–10457 CrossRef CAS PubMed.
- D. W. McCamant, J. Phys. Chem. B, 2011, 115, 9299–9305 CrossRef CAS PubMed.
- D. Polli, P. Altoè, O. Weingart, K. M. Spillane, C. Manzoni, D. Brida, G. Tomasello, G. Orlandi, P. Kukura, R. A. Mathies, M. Garavelli and G. Cerullo, Nature, 2010, 467, 440–443 CrossRef CAS PubMed.
- C. Schnedermann, M. Liebel and P. Kukura, J. Am. Chem. Soc., 2015, 137, 2886–2891 CrossRef CAS PubMed.
- P. J. M. Johnson, A. Halpin, T. Morizumi, V. I. Prokhorenko, O. P. Ernst and R. J. D. Miller, Nat. Chem., 2015, 7, 980–986 CrossRef CAS PubMed.
- B. G. Levine and T. J. Martínez, Annu. Rev. Phys. Chem., 2007, 58, 613–634 CrossRef CAS PubMed.
- I. Schapiro, M. N. Ryazantsev, L. M. Frutos, N. Ferré, R. Lindh and M. Olivucci, J. Am. Chem. Soc., 2011, 133, 3354–3364 CrossRef CAS PubMed.
- S. Hahn and G. Stock, J. Phys. Chem. B, 2000, 104, 1146–1149 CrossRef CAS.
- C. Schnedermann, X. Yang, M. Liebel, K. M. Spillane, J. Lugtenburg, I. Fernández, A. Valentini, I. Schapiro, M. Olivucci, P. Kukura and R. A. Mathies, Nat. Chem., 2018, 10, 449–455 CrossRef CAS PubMed.
- Molecular Switches, ed. B. L. Feringa and W. R. Browne, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2011 Search PubMed.
- C. Pezzato, C. Cheng, J. F. Stoddart and R. D. Astumian, Chem. Soc. Rev., 2017, 46, 5491–5507 RSC.
- M. Kathan and S. Hecht, Chem. Soc. Rev., 2017, 46, 5536–5550 RSC.
- Q. Li, G. Fuks, E. Moulin, M. Maaloum, M. Rawiso, I. Kulic, J. T. Foy and N. Giuseppone, Nat. Nanotechnol., 2015, 10, 161–165 CrossRef CAS PubMed.
- J. Michl and E. C. H. Sykes, ACS Nano, 2009, 3, 1042–1048 CrossRef CAS PubMed.
- M. Filatov and M. Olivucci, J. Org. Chem., 2014, 79, 3587–3600 CrossRef CAS PubMed.
- D. Roke, S. J. Wezenberg and B. L. Feringa, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 9423–9431 CrossRef CAS PubMed.
- R. D. Astumian, Chem. Sci., 2017, 8, 840–845 RSC.
- J. Chen, F. K. C. Leung, M. C. A. Stuart, T. Kajitani, T. Fukushima, E. Van Der Giessen and B. L. Feringa, Nat. Chem., 2018, 10, 132–138 CrossRef CAS PubMed.
- F. K. C. Leung, T. Van Den Enk, T. Kajitani, J. Chen, M. C. A. Stuart, J. Kuipers, T. Fukushima and B. L. Feringa, J. Am. Chem. Soc., 2018, 140, 17724–17733 CrossRef CAS PubMed.
- J. T. Foy, Q. Li, A. Goujon, J. R. Colard-Itté, G. Fuks, E. Moulin, O. Schiffmann, D. Dattler, D. P. Funeriu and N. Giuseppone, Nat. Nanotechnol., 2017, 12, 540–545 CrossRef CAS PubMed.
- R. Eelkema, M. M. Pollard, J. Vicario, N. Katsonis, B. S. Ramon, C. W. M. Bastiaansen, D. J. Broer and B. L. Feringa, Nature, 2006, 440, 163 CrossRef CAS PubMed.
- D. Bléger, Macromol. Chem. Phys., 2016, 217, 189–198 CrossRef.
- F. Lancia, A. Ryabchun and N. Katsonis, Nat. Rev. Chem., 2019, 3, 536–551 CrossRef CAS.
- A. Credi, M. Venturi and V. Balzani, ChemPhysChem, 2010, 11, 3398–3403 CrossRef CAS PubMed.
- R. J. D. Miller, Nat. Chem., 2012, 4, 523–525 CrossRef CAS PubMed.
- F. Lumento, V. Zanirato, S. Fusi, E. Busi, L. Latterini, F. Elisei, A. Sinicropi, T. Andruniów, N. Ferré, R. Basosi and M. Olivucci, Angew. Chem., Int. Ed., 2007, 46, 414–420 CrossRef CAS PubMed.
- A. Melloni, R. R. Paccani, D. Donati, V. Zanirato, A. Sinicropi, M. L. Parisi, E. Martin, M. Ryazantsev, W. J. Ding, L. M. Frutos, R. Basosi, S. Fusi, L. Latterini, N. Ferre and M. Olivucci, J. Am. Chem. Soc., 2010, 132, 9310–9319 CrossRef CAS PubMed.
- M. Gueye, M. Manathunga, D. Agathangelou, Y. Orozco, M. Paolino, S. Fusi, S. Haacke, M. Olivucci and J. Léonard, Nat. Commun., 2018, 9, 313 CrossRef PubMed.
- J. Léonard, I. Schapiro, J. Briand, S. Fusi, R. R. Paccani, M. Olivucci and S. Haacke, Chem.–Eur. J., 2012, 18, 15296–15304 CrossRef PubMed.
- M. Gueye, M. Paolino, E. Gindensperger, S. Haacke, M. Olivucci and J. Léonard, Faraday Discuss., 2020, 221, 299–321 RSC.
- I. V. Rubtsov and K. Yoshihara, J. Phys. Chem. A, 1999, 103, 10202–10212 CrossRef CAS.
- M. Paolino, M. Gueye, E. Pieri, M. Manathunga, S. Fusi, A. Cappelli, L. Latterini, D. Pannacci, M. Filatov, J. Léonard and M. Olivucci, J. Am. Chem. Soc., 2016, 138, 9807–9825 CrossRef CAS PubMed.
- C. McLaughlin, M. Assmann, M. A. Parkes, J. L. Woodhouse, R. Lewin, H. C. Hailes, G. A. Worth and H. H. Fielding, Chem. Sci., 2017, 8, 1621–1630 RSC.
- D. Roke, M. Sen, W. Danowski, S. J. Wezenberg and B. L. Feringa, J. Am. Chem. Soc., 2019, 141, 7622–7627 CrossRef CAS PubMed.
- A. Nikiforov, J. A. Gamez, W. Thiel and M. Filatov, J. Phys. Chem. Lett., 2016, 7, 105–110 CrossRef CAS PubMed.
- J. Wang and B. Durbeej, ChemistryOpen, 2018, 7, 583–589 CrossRef CAS PubMed.
- G. Marchand, J. Eng, I. Schapiro, A. Valentini, L. M. Frutos, E. Pieri, M. Olivucci, J. Léonard and E. Gindensperger, J. Phys. Chem. Lett., 2015, 6, 599–604 CrossRef CAS PubMed.
- I. Schapiro, M. Gueye, M. Paolino, S. Fusi, G. Marchand, S. Haacke, M. E. Martin, M. Huntress, V. P. Vysotskiy, V. Veryazov, J. Léonard and M. Olivucci, Photochem. Photobiol. Sci., 2019, 18, 2259–2269 CrossRef CAS PubMed.
- M. Paolino, T. Giovannini, M. Manathunga, L. Latterini, G. Zampini, R. Pierron, J. Léonard, S. Fusi, G. Giorgi, G. Giuliani, A. Cappelli, C. Cappelli and M. Olivucci, J. Phys. Chem. Lett., 2021, 3875–3884 CrossRef CAS PubMed.
- N. Koumura, R. W. J. Zijistra, R. A. Van Delden, N. Harada and B. L. Feringa, Nature, 1999, 401, 152–155 CrossRef CAS PubMed.
- I. Schapiro, F. Melaccio, E. N. Laricheva and M. Olivucci, Photochem. Photobiol. Sci., 2011, 10, 867 CrossRef CAS PubMed.
- J. Conyard, K. Addison, I. A. Heisler, A. Cnossen, W. R. Browne, B. L. Feringa and S. R. Meech, Nat. Chem., 2012, 4, 547–551 CrossRef CAS PubMed.
- M. N. G. James and G. J. B. Williams, Can. J. Chem., 1972, 50, 2407–2412 CrossRef CAS.
- K. Stranius and K. Börjesson, Sci. Rep., 2017, 7, 41145 CrossRef CAS PubMed.
- J. Otsuki, K. Suwa, K. K. Sarker and C. Sinha, J. Phys. Chem. A, 2007, 111, 1403–1409 CrossRef CAS PubMed.
- Molecular Switches, ed. B. L. Feringa and W. R. Browne, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2011 Search PubMed.
- M. L. Horng, J. A. Gardecki, A. Papazyan and M. Maroncelli, J. Phys. Chem., 1995, 99, 17311–17337 CrossRef CAS.
- J. Briand, O. Bräm, J. Réhault, J. Léonard, A. Cannizzo, M. Chergui, V. Zanirato, M. Olivucci, J. Helbing and S. Haacke, Phys. Chem. Chem. Phys., 2010, 12, 3178 RSC.
- H. Barkhuijsen, R. de Beer, W. M. M. Bovée and D. van Ormondt, J. Magn. Reson., 1985, 61, 465–481 CAS.
- H. Barkhuijsen, R. de Beer and D. van Ormondt, J. Magn. Reson., 1986, 67, 371–375 Search PubMed.
- A. L. Dobryakov, I. Ioffe, A. A. Granovsky, N. P. Ernsting and S. A. Kovalenko, J. Chem. Phys., 2012, 137, 244505 CrossRef CAS PubMed.
- M. Quick, F. Berndt, A. L. Dobryakov, I. N. Ioffe, A. A. Granovsky, C. Knie, R. Mahrwald, D. Lenoir, N. P. Ernsting and S. A. Kovalenko, J. Phys. Chem. B, 2014, 118, 1389–1402 CrossRef CAS PubMed.
- S. Amirjalayer, A. Cnossen, W. R. Browne, B. L. Feringa, W. J. Buma and S. Woutersen, J. Phys. Chem. A, 2016, 120, 8606–8612 CrossRef CAS PubMed.
- J. Conyard, A. Cnossen, W. R. Browne, B. L. Feringa and S. R. Meech, J. Am. Chem. Soc., 2014, 136, 9692–9700 CrossRef CAS PubMed.
- C. R. Hall, J. Conyard, I. A. Heisler, G. Jones, J. Frost, W. R. Browne, B. L. Feringa and S. R. Meech, J. Am. Chem. Soc., 2017, 139, 7408–7414 CrossRef CAS PubMed.
- I. F. Galván, M. G. Delcey, T. B. Pedersen, F. Aquilante and R. Lindh, J. Chem. Theory Comput., 2016, 12, 3636–3653 CrossRef PubMed.
- I. N. Ioffe and A. A. Granovsky, J. Chem. Theory Comput., 2013, 9, 4973–4990 CrossRef CAS PubMed.
- L. Pfeifer, M. Scherübl, M. Fellert, W. Danowski, J. Cheng, J. Pol and B. L. Feringa, Chem. Sci., 2019, 10, 8768–8773 RSC.
- P. Roy, A. S. Sardjan, A. Cnossen, W. R. Browne, B. L. Feringa and S. R. Meech, J. Phys. Chem. Lett., 2021, 12, 3367–3372 CrossRef CAS PubMed.
- J. Saltiel and S. Gupta, J. Phys. Chem. A, 2018, 122, 6089–6099 CrossRef CAS PubMed.
- A. Kazaryan, Z. Lan, L. V. Schäfer, W. Thiel and M. Filatov, J. Chem. Theory Comput., 2011, 7, 2189–2199 CrossRef CAS PubMed.
- M. Quick, A. L. Dobryakov, I. N. Ioffe, A. A. Granovsky, S. A. Kovalenko and N. P. Ernsting, J. Phys. Chem. Lett., 2016, 7, 4047–4052 CrossRef CAS PubMed.
- J. Saltiel, J. Am. Chem. Soc., 1967, 89, 1036–1037 CrossRef CAS.
- C. R. Hall, W. R. Browne, B. L. Feringa and S. R. Meech, Angew. Chem., Int. Ed., 2018, 57, 6203–6207 CrossRef CAS PubMed.
- N. Minezawa and M. S. Gordon, J. Phys. Chem. A, 2011, 115, 7901–7911 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2064588. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc01105g |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2021 |