Open Access Article
Open Access ArticleTowards the object-oriented design of active hydrogen evolution catalysts on single-atom alloys†
Chuan
Zhou
 a,
Jia Yue
Zhao
b,
Peng Fei
Liu
a,
Jia Yue
Zhao
b,
Peng Fei
Liu
 b,
Jianfu
Chen
a,
Sheng
Dai
b,
Jianfu
Chen
a,
Sheng
Dai
 c,
Hua Gui
Yang
c,
Hua Gui
Yang
 b,
P.
Hu
b,
P.
Hu
 ad and
Haifeng
Wang
ad and
Haifeng
Wang
 *a
*a
aKey Laboratory for Advanced Materials, Centre for Computational Chemistry, Research Institute of Industrial Catalysis, East China University of Science and Technology, Shanghai, 200237, China. E-mail: hfwang@ecust.edu.cn
bKey Laboratory for Ultrafine Materials of Ministry of Education, Shanghai Engineering Research Center of Hierarchical Nanomaterials, East China University of Science and Technology, Shanghai, 200237, China
cKey Laboratory for Advanced Materials, Feringa Nobel Prize Scientist Joint Research Center, Institute of Fine Chemicals, East China University of Science and Technology, Shanghai, 200237, China
dSchool of Chemistry and Chemical Engineering, The Queen's University of Belfast, Belfast BT9 5AG, UK
First published on 1st July 2021
Abstract
Given a desired property, locating relevant materials is always highly desired but very challenging in a range of areas, including heterogeneous catalysis. Obviously, object-oriented design/screening is an ideal solution to this problem. Herein, we develop an inverse catalyst design workflow in Python (CATIDPy) that utilizes a genetic-algorithm-based global optimization method to guide on-the-fly density functional theory calculations, successfully realizing the highly accelerated location of active single-atom alloy (SAA) catalysts for the hydrogen evolution reaction (HER). 70 binary and 752 ternary SAA candidate catalysts are identified for the HER. Furthermore, via considering the segregation stability and cost of materials, we extracted 6 binary and 142 ternary SAA candidate catalysts that are recommended for experimental synthesis. Remarkably, guided by these theoretical identifications, homogeneously dispersed Ni-based bimetallic catalysts (e.g., NiMo, NiAl, Ni3Al, NiGa, and NiIn) were synthesized experimentally to test the reliability of the CATIDPy workflow, and they showed superior HER performance to bare Ni foam, indicating huge potential for use in real-world water electrolysis techniques. Perhaps more importantly, these results demonstrate the capacity of such a proposed approach for investigating unexplored chemical spaces to efficiently design promising catalysts without knowledge from the expert domain, which has far-reaching implications.
Introduction
The rapid development of density functional theory (DFT) has led to an unprecedented atomic-scale understanding of catalytic processes and catalyst screening in recent decades. Currently, a rational catalyst design strategy usually consists of two subtasks: one is the development of a descriptor-based approach1–3 for reducing the dimensionality of the parameter space to a few descriptors (preferably 1–2 adsorption energies) using so-called scaling relations and Brønsted–Evans–Polanyi relations,4–8 which illustrate the relation between these descriptors and activity, resulting in volcano plots.8,9 The other one still remains, however, a grand challenge: finding the specific materials meeting the requirement of the activity descriptor. Currently, the approaches to achieve this target include: searching databases,10–20 identifying the structure–reactivity relationship21–24 and machine learning methods.25–27 Thereinto, (i) database construction requires a lot of early effort and is not friendly to the unexplored catalytic systems; (ii) the establishment of structure–reactivity relationship is inseparable from the support of experts' domain knowledge; (iii) although machine learning methods have attracted extensive attention, much effort is usually needed for the feature extraction with expert knowledge and training the model still requires a certain amount of pre-processing data. Essentially, searching a target material can be regarded as a discrete optimization problem in the tremendous material/configuration space; meanwhile, the inherently catalytic information could be automatically learned by an optimization algorithm. Therefore, developing an automated, efficient and oriented catalyst screening strategy is extremely desirable, and should constitute a basis for achieving a rational catalyst design.Exploring clean and renewable energy sources is currently an urgent issue due to the ascending consumption of fossil fuels and environmental contamination.28,29 Water splitting, especially the hydrogen evolution reaction (HER),25,30–34 is one of the most promising reactions in electrochemistry and the simplest way to produce hydrogen efficiently and in an environment-friendly manner. Generally, Pt-based electrocatalysts with a highly catalytic activity are recognized as superior HER catalysts in acid media to achieve favorable reaction kinetics.35–39 However, their scarcity, as well as high cost, limit their industrial-scale applications. While in alkaline media, the Ni–Mo alloy is universally acknowledged as a highly active HER catalyst due to its similar surface electronic state to that of platinum.40–43 Nevertheless, designing cheaper, stable and active HER catalysts has been one of the main goals in the field of renewable energy research.
Single-atom alloys (SAAs)44–48 are a class of single-atom catalysts in which an atomically active metal is generally dispersed in a less-active metal host, resulting in well-defined local coordination environments of active sites and unique catalytic properties from the individual components.49–56 Currently, a number of studies focusing on SAA catalysts are reported in this field. For example, Li et al.57 identified Cu–Pt dual sites on Pd nanorings, exhibiting a high catalytic activity for HERs. Du et al.58 synthesized a RuAu SAA using laser ablation in liquid, which gives a high stability and a low overpotential for the HER in alkaline media. Sautet and co-workers59 showed single-nickel-atom-modified Pt nanowires with an enhanced activity towards HERs. Despite the progress, reports on the rational design of highly active SAA catalysts for HERs are rare. Perhaps more importantly, an efficient and automatic approach to achieve this aim is still lacking. One conceivable reason is that the sample search space is too large to be explored exhaustively and expert knowledge of the structure–composition activity remains far from sufficient. For instance, considering the situation of one site of one specific surface of ternary SAAs (consisting of one host metal and two guest metals), the search space could form 8000 possibilities with a combination of 20 different elements, resulting in a great obstacle in rationally screening high-performance catalysts by resorting to DFT calculations.
To this end, in this work a Python-based software for the inverse design of high-performance catalyst (namely CATIDPy) is developed, which enables an automated and object-oriented search, instructing the on-the-fly DFT calculations by virtue of a genetic-algorithm-based (GA) global optimization method. By taking the SAA-catalyzed HER system as a case study, we efficiently screen out 70 binary and 752 ternary SAA catalysts superior for HERs without expert domain knowledge and a portion of these were verified experimentally. Subsequently, the stability and cost of materials are further considered, further shortening the SAAs to a list of 6 binary and 142 ternary SAAs towards HERs. The experimental results also demonstrate the applicability of the CATIDPy workflow for rapidly screening superior HER electrocatalysts. Remarkably, this workflow can be easily extended to other catalytic materials and reactions.
Results and discussion
Framework of the oriented design of SAAs for HERs
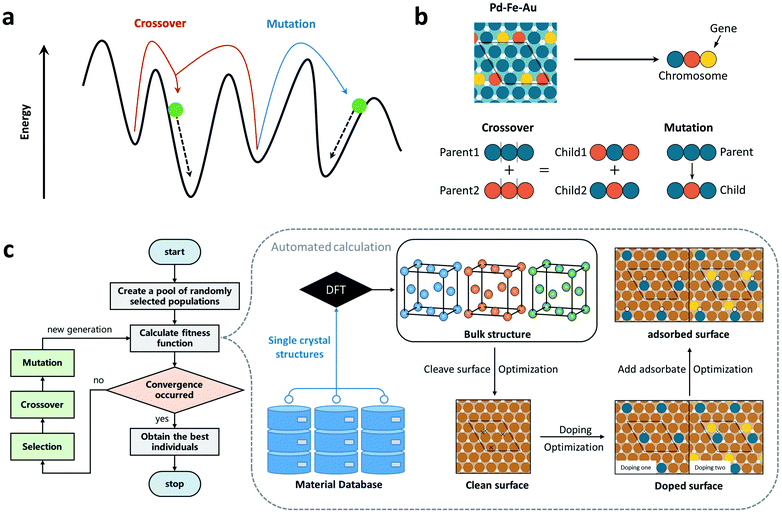
To design the superior SAA catalysts for HER, the goal of the optimization problem is to locate the surface composition corresponding to the proper activity descriptor EH (the adsorption energy of H atom; see discussions later). As shown in Fig. 1a, in the developed CATIDPy software, we adopted the genetic-algorithm-based (GA) global optimization method to determine the search direction in the structural or compositional space, which is based on evolutionary principles such as selection, crossover and mutation, and a relatively small modification or mutation of the structure could be sufficient to surpass the barrier and find a nearby local minimum. In the evolution process, each structural unit was initially encoded into a string (see Theoretical methods in the ESI†), i.e., a chromosome, and a wide variety of potential catalytic materials were subsequently generated via evolutionary operators (see Fig. 1b). Fig. 1c illustrates the whole automated and object-oriented workflow in CATIDPy for HERs on SAA catalysts, in which the on-the-fly DFT calculations can be automatically performed to investigate the activity of SAA catalysts on the basis of GA. Specifically, this process can be described as follows: (i) the unit cells of host metal can be obtained from material database according to the structural-gene-represented string, generated by GA; (ii) after relaxing the bulk unit cells of these host metals, the fcc hollow site of the (111) facet is chosen and one or two host atoms closest to the center of site are substituted by guest atoms, forming different local active-site environments (denoted as HHG1 or HG1G2, where H, G1/G2 correspond to the host and guest atoms, respectively); (iii) the adsorption structure of atomic H is optimized on the designed active site configuration, and the DFT results are stored in a database for calculating EH; (iv) the guest atoms are automatically selected and updated following the direction determined by GA, and this yields a closed feedback loop of high-throughput screening. Notably, the fitness function, which largely determines the convergence speed of GA towards the optimal solution, is defined as fitness(x) = 1/|EH(x) − Eopt| in this application for the HER, where EH(x) and Eopt represent the adsorption energies of atomic hydrogen (H*) of the individual (x) and target value, respectively. It is worth emphasizing that CATIDPy provides a general workflow to realize object-oriented catalyst design, which can perform on-the-fly DFT calculations for extending to other adsorbates, bulk materials, surfaces and adsorption sites with various descriptors accessible from DFT calculations.Determination of the activity descriptor for the HER
Regarding the assignment of Eopt, a descriptor–activity relationship was utilized to predict the catalyst activity towards a HER prior to invoking CATIDPy, which gives an adsorption free energy of hydrogen (ΔGH) at 0 eV (corresponding to Eopt = −0.27 eV) for the optimal activity at the working voltage of a standard hydrogen electrode.25,30 We also considered the effect of surface coverage of the H* intermediate on the volcano-type activity trend in reality,60–62 as shown in Fig. S1,† to obtain these potential HER catalysts more accurately. The coverage effect has little impact on the right side of the volcano curve due to the weak adsorption of the adsorbate, while a stronger adsorption of the adsorbate on the left side of volcano curve would increase the coverage, which would result in greater interactions between the adsorbates, thereby weakening the adsorption energy and boosting the reaction activity. Therefore, on the basis of such considerations, a range of −0.57 to −0.17 eV was utilized to discriminate the potential catalysts.Location of active binary SAAs
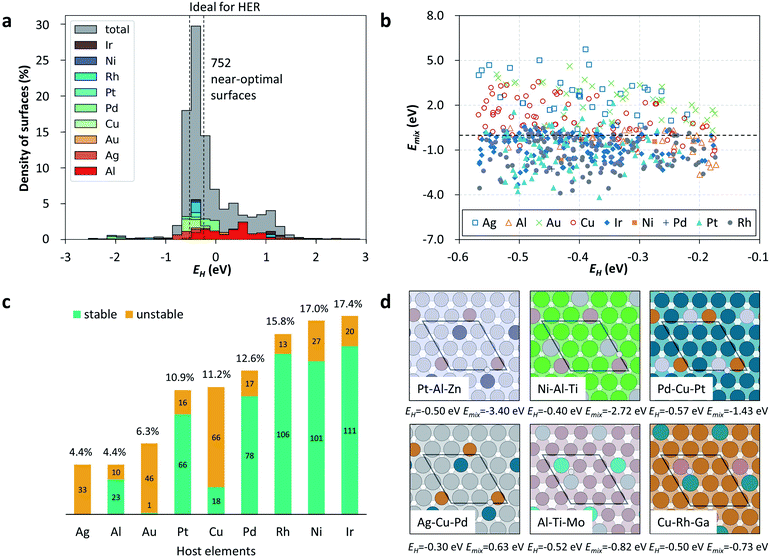
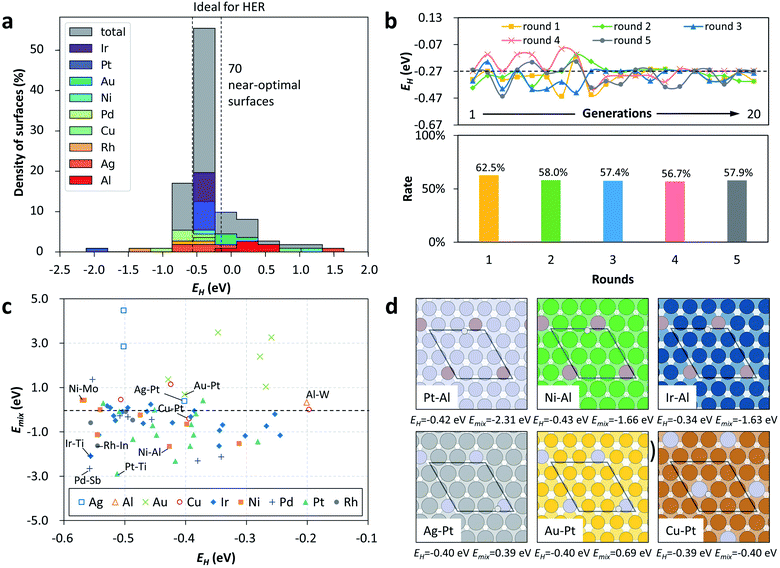
Twenty-nine guest elements, of which 69% are d-block elements and 31% are p-block elements, were employed to combine with a specific host metal (i.e., Cu, Ag, Au, Pt, Pd, Rh, Ir, Ni and Al) on the surface, yielding a large set of SAAs for the HERs, as shown in Fig. S2.† After one round of GA evolution to search the target combinations, which consists of 20 generations with a population size of 10 configurations for each generation, 112 binary SAAs were automatically examined by DFT calculations and stored in the search space. Fig. 2a illustrates that the EH values of these 112 materials have a large energy range (over 3.5 eV) and uneven energy distribution. Interestingly, most of them are inside the range of (−0.7, 0) eV, being around the targeted range of (−0.57, −0.17) eV, which preliminarily demonstrates the efficiency of such a GA-guided search. We further explored the potential SAAs with a near-optimal EH; it was found that there were as many as 70 candidate SAA catalysts identified by these 112 DFT calculations, which are listed in detail in Fig. 2a and Table S1.† Such a high probability (62.5%) indicates the excellent performance of our workflow for an object-oriented catalyst design, compared to that of the pure exhaustive DFT calculations (see Fig. S3 and S4†). Remarkably, some of these 70 SAA catalysts have already been reported to be active for HERs. For example, Ni-based catalysts were shown to be active for HERs,40–43,59 and a theoretical calculation indicated that the ΔGH values of Pt/Au(111), Rh/Au(111) and Pt/Ag(111) are near zero, being potential HER catalysts.63 Therefore, those that have not been tried yet are highly recommended for experimental work for HERs in the future.The reliability of this framework is also addressed; a total of five rounds of the GA search starting from different initial conditions were independently performed, as shown in Fig. 2b. This demonstrates the high search success rate of five rounds of random tests, and especially the highly overlapped active SAA-composition space from these five parallel tests. Moreover, we retrospectively tested the iterative efficiency via evaluation of the best EH at each generation. It was found that the best EH at each generation gradually converges to the criteria (−0.27 eV). More specifically, the best EH at each generation before the tenth generation fluctuates greatly, and then gradually converges, although there are still some small fluctuations caused by mutation. Overall, these results demonstrate the significant performance of the automation of the DFT workflow towards an object-oriented design of active hydrogen evolution catalysts on SAAs.
To further screen out the thermodynamically easily synthesized catalytic materials in experiments from these 70 candidates, we also explored the relative stability of each SAA by evaluating the surface mixing energies (Emix), which are expressed as follows:
| Emix = E(An−1B) + Eatom(A) − [E(An) + Eatom(B)] | (1) |
| Emix = E(An−2BC) + 2Eatom(A) − [E(An) + Eatom(B) + Eatom(C)] | (2) |
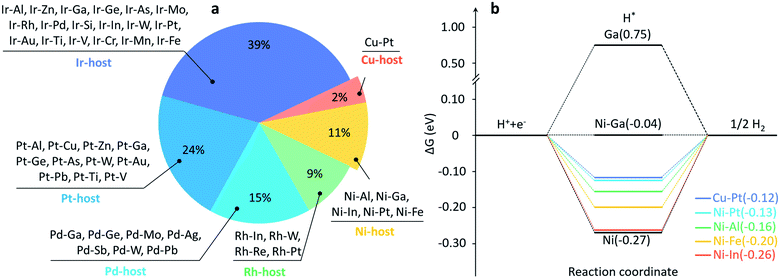
In addition, it is worth discussing that one can infer some basic rules on the composition pattern of these identified active and stable binary SAA candidates, which can be divided into three categories. (i) The first category is the noble host metals (Pt, Ir, Pd, Rh) that are originally active for HERs and finely modified with promising guest atoms, e.g., Pt–Al, Rh–In, Pd–Ga and Ir–Al, as shown in Fig. 2d and 3a. The majority of candidates are in this category, implying the more dominating role of the host metal in determining EH. However, the expensive cost of these materials still limits their large-scale application. (ii) The second one is the inactive coinage metals (Au, Ag) as hosts doped with approximately a single guest atom, e.g., Ag–Pt, Au–Pt (see Fig. 2d). (iii) The third category is a very promising and attractive group, which comprises the cost-effective Ni and Cu as the host metals, including one Cu-based (Cu–Pt) and five Ni-based SAAs (i.e., Ni–Al, Ni–Ga, Ni–In, Ni–Pt and Ni–Fe; see Fig. 3a). Noteworthily, with the established dataset of SAA above, one can also extract some host–guest-interaction induced variation trends of EH. Specifically, a stronger/weaker binding ability of the metal with a weaker/stronger one will produce a moderate binding ability towards the adsorption of H*. For example, ΔGH values on pure Ga(111) and Ni(111) are 0.75 eV and −0.27 eV, respectively, while Ni–Ga SAA possesses a moderate ΔGH of −0.04 eV (see Fig. 3b). In addition, ΔGH values on these 6 cost-effective SAA catalysts are near zero, showing a potential activity for HERs, especially under alkaline conditions. These findings demonstrate some basic activity–composition relations in SAA-catalysis, which are conducive to the rational design or optimization of SAA-catalysts.
HER performance evaluation of the Ni-based category
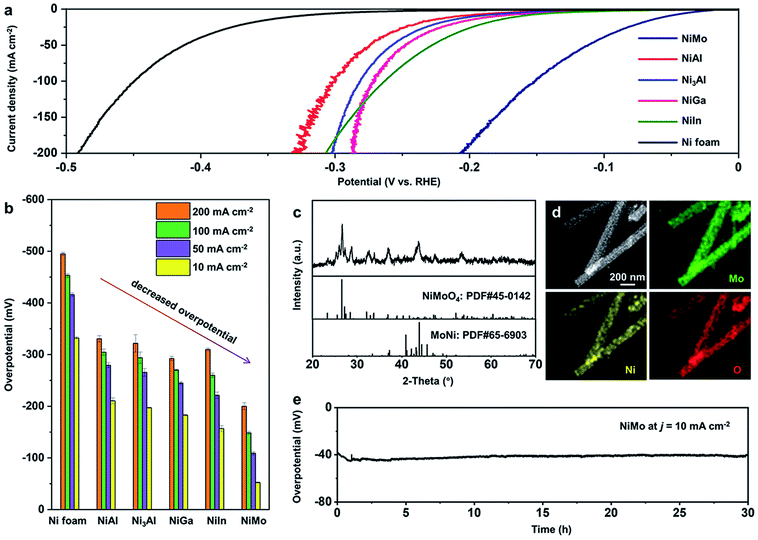
To test the reliability of the CATIDPy workflow, we chose to fabricate the cost-effective Ni-based category catalysts and evaluated their HER performances. The homogeneously dispersed Ni-based binary alloys (e.g., NiMo, NiAl, Ni3Al, NiGa and NiIn) were synthesized via a thermal hydrogen treatment method on a Ni foam substrate.64–67 Notably, despite the fact that the prepared samples were Ni-based alloys instead of the ideal model of Ni-based single atom alloys, they featured local active sites like SAAs, with an atomically dispersed guest metal on the Ni surface layer (see details in the ESI, Fig. S8–S13†). The HER performances were evaluated in a three-electrode H-type cell with the synthesized bimetallic alloy on the Ni foam as the working electrode, an Ag/AgCl (3.5 M KCl) electrode as the reference electrode and a graphite rod as the counter electrode, in a 1.0 M KOH alkaline electrolyte (see Experimental methods in the ESI, Fig. S14†). In Fig. 4a, the linear sweep voltammetric (LSV) curves show that all the fabricated Ni-based bimetallic electrocatalysts, which were inspired by the CATIDPy workflow, exhibit superior HER performances to that of the bare Ni foam, with obvious lower overpotentials (η) to achieve the same current densities (j). Furthermore, the corresponding η of the electrocatalysts at a j of 10, 50, 100 and 200 mA cm−2 were also collected from at least 3 independent tests (see Fig. 4b, S15–S20 and Table S2†), which indicates the decreased η10, η50, η100 and η200 trend of Ni foam, NiAl, Ni3Al, NiGa, NiIn and NiMo bimetallic electrocatalysts. It is worth mentioning that Ni3Al displays a lower η10, η50, η100 and η200 at −197.22 ± 0.58, −265.69 ± 7.17, −293.98 ± 11.04 and −321.72 ± 16.82 mV than those of NiAl at −210.69 ± 5.80, −279.52 ± 4.84, −304.83 ± 5.72 and −330.61 ± 6.01 mV (Table S2†). This indicates that the Ni3Al electrocatalyst features higher activities in both low and high overpotential regions than those of the NiAl sample, which may be attributed to more isolated Al atoms in the Ni3Al bimetallic electrocatalyst with more SAA active sites (see Fig. S13†), and this is also consistent with the theoretical results.Particularly, the NiMo-based bimetallic electrocatalyst exhibits the optimal alkaline HER performance among the synthesized samples, showing η10 and η200 at −52.32 ± 1.12 and −200.06 ± 6.69 mV along with Tafel slopes of 37.84 ± 3.39 and 102.05 ± 1.16 mV dec−1 in the low and high overpotential regions (see Fig. 4b and S15†), respectively, which shows an excellent alkaline HER performance like those of the reported NiMo-based electrocatalysts.64–66 Also, the superior catalytic performance of the NiMo catalyst can be seen by comparing with that of the common Pt catalyst (see Fig. S6†). Structural characterizations were further conducted to analyze this typical NiMo-based electrocatalyst. In Fig. 4c, the X-ray diffraction (XRD) analysis shows that the NiMo sample consists of crystalline NiMoO4 and the MoNi alloy. The high angle annular dark field scanning transmission electron microscopy (HAADF-STEM) image of the NiMo sample displays a nanorod morphology with a diameter of ∼150 nm (see Fig. 4d). The corresponding mappings also suggest a uniform distribution of the Mo, Ni and O elements in the whole region (see Fig. 4d). The above XRD and STEM analyses unambiguously prove the existence of bimetallic NiMo alloy in the as-prepared sample. The stability of the NiMo-based electrocatalyst was examined at a constant j of 10 mA cm−2 with η around −40 mV, and exhibited no obvious degradation for more than 30 h of testing (see Fig. 4e), which proves its robustness in long-term electrolysis.
Taking these experimental results together, we have demonstrated the effectiveness of the CATIDPy workflow for rapidly screening efficient HER electrocatalysts. Notably, although the fabricated Ni-based bimetallic electrocatalysts do not necessarily correspond to the well-defined SAAs, the guest atoms of Al, Ga, In and Mo along with the host Ni atoms are homogeneously dispersed, decorated with active binary SAA sites in the samples. With this in mind, we anticipate well-defined SAA electrocatalysts (e.g., NiAl, NiGa, NiIn and NiMo SAAs), which could be constructed via advanced synthesis methods, to homogeneously isolate the active SAA motifs with abundant exposed active sites to achieve extraordinary HER performances, featuring a huge potential in real-world water electrolysis techniques.
Location of more complex active ternary SAAs
Our approach has successfully achieved the screening of binary SAA catalysts, but the restriction of the binary combination resulted in only a limited number of candidate catalysts being identified on some host metals. For example, only one Cu-based binary SAA catalyst (Cu–Pt) was identified inside the range of (−0.57, −0.17) eV. Thus, we further expand the material search space to ternary SAA systems to test more potential SAA catalysts on a specific host metal. Herein, we performed our workflow with ten rounds of GA to investigate ternary SAA catalysts. It is worth emphasizing that after ten rounds of GA (a total of 2000 structures generated by 10 × 10 × 20), non-repetitive 1447 SAAs were explored with the DFT calculations guided by the GA, and 752 near-optimal surfaces out of these 1447 SAA catalysts were identified (see Fig. 5a for details), corresponding to a success rate of 0.52, which is a more efficient way compared to the pure exhaustive method (see Fig. S4†). Likewise, we also considered Emix of these 752 promising catalysts to narrow the material search space (see Table S3†). Noteworthily, ternary SAAs show more diverse combinations and a denser distribution in comparison with the binary ones, as shown in Fig. 5b. More specifically, the range of Emix is from −4.17 to 5.74 eV for these 1447 SAAs and there are 504 combinations of SAAs with Emix less than zero, which can be expected to be active for HERs and synthesized by experiments in the future.Furthermore, subsequent statistical analyses also reveal the difference in the distribution of the number of SAAs in various host metals according to their Emix, as illustrated by Fig. 5c. It is found that most of the Ag-, Cu- and Au-based ternary SAAs are unstable, which add up to 21.9%, and they might not be favorable to experimental synthesis due to their relatively high Emix. In contrast, Pt-, Pd-, Rh-, Ni- and Ir-based ternary SAAs are the thermodynamically more stable ones, and account for a total of 73.7%. Moreover, considering the earth-abundant and cheap Al, Ni or Cu as the host metals of SAAs, 142 ternary SAA catalysts can be further identified (see Table S3†), on which further experimental work is deserved. For example, some promising ternary SAA surface configurations, e.g., Ni–Ai–Ti, Al–Ti–Mo and Cu–Rh–Ga, are illustrated in Fig. 5d. In particular, it is worth mentioning that the Pd–Cu–Pt ternary SAA catalyst identified in this work, which possesses an EH of −0.57 eV and Emix of −1.43 eV, was explicitly verified experimentally.57
Conclusions
In summary, we investigated the HER on binary/ternary SAA catalysts, in which an automated and object-oriented CATIDPy workflow without user intervention and knowledge from the expert domain was proposed. This workflow achieves on-the-fly DFT calculations guided by a generic algorithm, and it is capable of screening/designing SAA catalysts for the HER, with 70 binary and 752 ternary catalyst candidates. On the basis of stability and cost, 6 cost-effective binary and 142 ternary SAA catalysts were further identified, which are recommended for the experimental verification of their activities. In particular, based on these theoretical guidelines, homogeneously dispersed Ni-based binary SAAs were synthesized and they were verified to have superior HER performance, confirming the applicability of the CATIDPy workflow. Perhaps more importantly, such a workflow could be expandable and have significant application prospects for designing other types of catalytic materials with one or more different criteria.Data availability
Most of the data that support the fndings of this study are present in the article and its ESI. The corresponding author will provide additional data not available in these documents upon reasonable request.Author contributions
H. F. W. conceived the project and P. H. and J. F. C. contributed to the design of the calculations. C. Z. made the workflow, performed the calculations, and wrote the paper. P. F. L., J. Zhao, S. D., and H. G. Y. carried out the experimental work. All the authors discussed the results and commented on the manuscript. We also thank Prof. M. H. Zhu, Prof. Y. Guo, Dr X. H. Liu, and Dr L. Wang for helpful discussions relating to experimental characterization.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was supported by the National Key R&D Program of China (2018YFA0208602), NSFC (21873028, 91945302, 21703067), National Ten Thousand Talent Program for Young Top-notch Talents in China, Shanghai ShuGuang project (17SG30), and the Fundamental Research Funds for the Central Universities. S. D. acknowledges support from the Shanghai Rising-Star Program (20QA1402400). Additional support was provided by the Feringa Nobel Prize Scientist Joint Research Center.Notes and references
- F. Calle-Vallejo, O. A. Díaz-Morales, M. J. Kolb and M. T. M. Koper, ACS Catal., 2015, 5, 869–873 CrossRef CAS.
- A. J. Medford, C. Shi, M. J. Hoffmann, A. C. Lausche, S. R. Fitzgibbon, T. Bligaard and J. K. Nørskov, Catal. Lett., 2015, 145, 794–807 CrossRef CAS.
- Y. Ding, Y. Xu, Y. Mao, Z. Wang and P. Hu, Chem. Commun., 2020, 56, 3214–3217 RSC.
- J. E. Sutton and D. G. Vlachos, J. Catal., 2016, 338, 273–283 CrossRef CAS.
- F. Abild-Pedersen, J. Greeley, F. Studt, J. Rossmeisl, T. R. Munter, P. G. Moses, E. Skúlason, T. Bligaard and J. K. Nørskov, Phys. Rev. Lett., 2007, 99, 016105 CrossRef CAS PubMed.
- A. Michaelides, Z.-P. Liu, C. Zhang, A. Alavi, D. A. King and P. Hu, J. Am. Chem. Soc., 2003, 125, 3704–3705 CrossRef CAS PubMed.
- E. M. Fernandez, P. G. Moses, A. Toftelund, H. A. Hansen, J. I. Martinez, F. Abild-Pedersen, J. Kleis, B. Hinnemann, J. Rossmeisl, T. Bligaard and J. K. Nørskov, Angew. Chem., Int. Ed., 2008, 47, 4683–4686 CrossRef CAS PubMed.
- T. Bligaard, J. K. Nørskov, S. Dahl, J. Matthiesen, C. H. Christensen and J. Sehested, J. Catal., 2004, 224, 206–217 CrossRef CAS.
- B. Meyer, B. Sawatlon, S. Heinen, O. A. von Lilienfeld and C. Corminboeuf, Chem. Sci., 2018, 9, 7069–7077 RSC.
- A. Jain, S. P. Ong, G. Hautier, W. Chen, W. D. Richards, S. Dacek, S. Cholia, D. Gunter, D. Skinner and G. Ceder, APL Mater., 2013, 1, 011002 CrossRef.
- G. Pizzi, A. Cepellotti, R. Sabatini, N. Marzari and B. Kozinsky, Comput. Mater. Sci., 2016, 111, 218–230 CrossRef.
- C. Draxl and M. Scheffler, MRS Bull., 2018, 43, 676–682 CrossRef.
- J. E. Saal, S. Kirklin, M. Aykol, B. Meredig and C. Wolverton, JOM, 2013, 65, 1501–1509 CrossRef CAS.
- S. Curtarolo, W. Setyawan, S. Wang, J. Xue, K. Yang, R. H. Taylor, L. J. Nelson, G. L. Hart, S. Sanvito and M. Buongiorno-Nardelli, Comput. Mater. Sci., 2012, 58, 227–235 CrossRef CAS.
- D. Landis, J. Hummelshøj, S. Nestorov, J. Greeley, M. D. lak, T. Bligaard, J. Nørskov and K. Jacobsen, Comput. Sci. Eng., 2012, 14, 51–57 Search PubMed.
- S. Haastrup, M. Strange, M. Pandey, T. Deilmann, P. S. Schmidt, N. F. Hinsche, M. N. Gjerding, D. Torelli, P. M. Larsen and A. C. Riis-Jensen, 2D Mater., 2018, 5, 042002 CrossRef CAS.
- F. H. Allen, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 380–388 CrossRef PubMed.
- A. Belsky, M. Hellenbrandt, V. L. Karen and P. Luksch, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 364–369 CrossRef PubMed.
- S. Gražulis, A. Daškevič, A. Merkys, D. Chateigner, L. Lutterotti, M. Quiros, N. R. Serebryanaya, P. Moeck, R. T. Downs and A. Le Bail, Nucleic Acids Res., 2012, 40, D420–D427 CrossRef PubMed.
- Y. Xu, M. Yamazaki and P. Villars, Jpn. J. Appl. Phys., 2011, 50, 11RH02 CrossRef.
- J. K. Nørskov and B. Hammer, Adv. Catal., 2000, 31, 71–129 Search PubMed.
- X. Ma and H. Xin, Phys. Rev. Lett., 2017, 118, 036101 CrossRef.
- F. Calle-Vallejo, J. I. Martinez, J. M. Garcia-Lastra, P. Sautet and D. Loffreda, Angew. Chem., Int. Ed., 2014, 53, 8316–8319 CrossRef CAS PubMed.
- C. Zhou, B. Zhang, P. Hu and H. Wang, Phys. Chem. Chem. Phys., 2020, 22, 1721–1726 RSC.
- K. Tran and Z. W. Ulissi, Nat. Catal., 2018, 1, 696–703 CrossRef CAS.
- C. Chen, W. Ye, Y. Zuo, C. Zheng and S. P. Ong, Chem. Mater., 2019, 31, 3564–3572 CrossRef CAS.
- S. Back, K. Tran and Z. W. Ulissi, ACS Catal., 2019, 9, 7651–7659 CrossRef CAS.
- J. Wang, H. Wang and P. Hu, Sci. China: Chem., 2018, 61, 336–343 CrossRef CAS.
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Nørskov and T. F. Jaramillo, Science, 2017, 355, eaad4998 CrossRef PubMed.
- J. K. Nørskov, T. Bligaard, A. Logadottir, J. Kitchin, J. G. Chen, S. Pandelov and U. Stimming, J. Electrochem. Soc., 2005, 152, J23–J26 CrossRef.
- E. Skúlason, G. S. Karlberg, J. Rossmeisl, T. Bligaard, J. Greeley, H. Jónsson and J. K. Nørskov, Phys. Chem. Chem. Phys., 2007, 9, 3241–3250 RSC.
- S. Fang, X. Zhu, X. Liu, J. Gu, W. Liu, D. Wang, W. Zhang, Y. Lin, J. Lu and S. Wei, Nat. Commun., 2020, 11, 1029–1036 CrossRef CAS PubMed.
- R. B. Wexler, J. M. P. Martirez and A. M. Rappe, J. Am. Chem. Soc., 2018, 140, 4678–4683 CrossRef CAS PubMed.
- D. D. V. Anićijević, V. M. Nikolić, M. P. Marčeta-Kaninski and I. A. Pašti, Int. J. Hydrogen Energy, 2013, 38, 16071–16079 CrossRef.
- Y. H. Li, J. Xing, Z. J. Chen, Z. Li, F. Tian, L. R. Zheng, H. F. Wang, P. Hu, H. J. Zhao and H. G. Yang, Nat. Commun., 2013, 4, 2500 CrossRef PubMed.
- J. Xing, H. B. Jiang, J. F. Chen, Y. H. Li, L. Wu, S. Yang, L. R. Zheng, H. F. Wang, P. Hu, H. J. Zhao and H. G. Yang, J. Mater. Chem. A, 2013, 1, 15258–15264 RSC.
- J. Xing, J. F. Chen, Y. H. Li, W. T. Yuan, Y. Zhou, L. R. Zheng, H. F. Wang, P. Hu, Y. Wang, H. J. Zhao, Y. Wang and H. G. Yang, Chem.–Eur. J., 2014, 20, 2138–2144 CrossRef CAS PubMed.
- J. Kibsgaard and I. Chorkendorff, Nat. Energy, 2019, 4, 430 CrossRef.
- J. Wang, F. Xu, H. Jin, Y. Chen and Y. Wang, Adv. Mater., 2017, 29, 1605838 CrossRef PubMed.
- I. A. Raj and K. Vasu, J. Appl. Electrochem., 1990, 20, 32–38 CrossRef CAS.
- C. Fan, D. Piron, A. Sleb and P. Paradis, J. Electrochem. Soc., 1994, 141, 382 CrossRef CAS.
- W. F. Chen, K. Sasaki, C. Ma, A. I. Frenkel, N. Marinkovic, J. T. Muckerman, Y. Zhu and R. R. Adzic, Angew. Chem., Int. Ed., 2012, 51, 6131–6135 CrossRef CAS PubMed.
- Q. Zhang, P. Li, D. Zhou, Z. Chang, Y. Kuang and X. Sun, Small, 2017, 13, 1701648 CrossRef PubMed.
- R. T. Hannagan, D. A. Patel, L. A. Cramer, A. C. Schilling, P. T. Ryan, A. M. Larson, V. Çınar, Y. Wang, T. A. Balema and E. C. H. Sykes, ChemCatChem, 2020, 12, 488–493 CrossRef CAS.
- M. T. Darby, R. Réocreux, E. C. H. Sykes, A. Michaelides and M. Stamatakis, ACS Catal., 2018, 8, 5038–5050 CrossRef CAS.
- M. D. Marcinkowski, M. T. Darby, J. Liu, J. M. Wimble, F. R. Lucci, S. Lee, A. Michaelides, M. Flytzani-Stephanopoulos, M. Stamatakis and E. C. H. Sykes, Nat. Chem., 2018, 10, 325 CrossRef CAS PubMed.
- M. T. Greiner, T. Jones, S. Beeg, L. Zwiener, M. Scherzer, F. Girgsdies, S. Piccinin, M. Armbrüster, A. Knop-Gericke and R. Schlögl, Nat. Chem., 2018, 10, 1008–1015 CrossRef CAS PubMed.
- G. Kyriakou, M. B. Boucher, A. D. Jewell, E. A. Lewis, T. J. Lawton, A. E. Baber, H. L. Tierney, M. Flytzani-Stephanopoulos and E. C. H. Sykes, Science, 2012, 335, 1209–1212 CrossRef CAS PubMed.
- M. T. Darby, M. Stamatakis, A. Michaelides and E. C. H. Sykes, J. Phys. Chem. Lett., 2018, 9, 5636–5646 CrossRef CAS PubMed.
- G. Sun, Z.-J. Zhao, R. Mu, S. Zha, L. Li, S. Chen, K. Zang, J. Luo, Z. Li and S. C. Purdy, Nat. Commun., 2018, 9, 4454–4462 CrossRef PubMed.
- F. Xing, J. Jeon, T. Toyao, K.-i. Shimizu and S. Furukawa, Chem. Sci., 2019, 10, 8292–8298 RSC.
- P. Aich, H. Wei, B. Basan, A. J. Kropf, N. M. Schweitzer, C. L. Marshall, J. T. Miller and R. Meyer, J. Phys. Chem. C, 2015, 119, 18140–18148 CrossRef CAS.
- J. Shan, J. Liu, M. Li, S. Lustig, S. Lee and M. Flytzani-Stephanopoulos, Appl. Catal., B, 2018, 226, 534–543 CrossRef CAS.
- G. Rupprechter, Nat. Chem., 2017, 9, 833–834 CrossRef CAS PubMed.
- F. R. Lucci, J. Liu, M. D. Marcinkowski, M. Yang, L. F. Allard, M. Flytzani-Stephanopoulos and E. C. H. Sykes, Nat. Commun., 2015, 6, 8550–8557 CrossRef PubMed.
- J. Liu, F. R. Lucci, M. Yang, S. Lee, M. D. Marcinkowski, A. J. Therrien, C. T. Williams, E. C. H. Sykes and M. Flytzani-Stephanopoulos, J. Am. Chem. Soc., 2016, 138, 6396–6399 CrossRef CAS PubMed.
- T. Chao, X. Luo, W. Chen, B. Jiang, J. Ge, Y. Lin, G. Wu, X. Wang, Y. Hu, Z. Zhuang, Y. Wu, X. Hong and Y. Li, Angew. Chem., Int. Ed., 2017, 56, 16047–16051 CrossRef CAS PubMed.
- C. H. Chen, D. Wu, Z. Li, R. Zhang, C. G. Kuai, X. R. Zhao, C. K. Dong, S. Z. Qiao, H. Liu and X. W. Du, Adv. Energy Mater., 2019, 9, 1803913 CrossRef.
- M. Li, K. Duanmu, C. Wan, T. Cheng, L. Zhang, S. Dai, W. Chen, Z. Zhao, P. Li, H. Fei, Y. Zhu, R. Yu, J. Luo, K. Zang, Z. Lin, M. Ding, J. Huang, H. Sun, J. Guo, X. Pan, W. Goddard III, P. Sautet, Y. Huang and X. Duan, Nat. Catal., 2019, 2, 495–503 CrossRef CAS.
- L. C. Grabow, B. Hvolbæk and J. K. Nørskov, Top. Catal., 2010, 53, 298–310 CrossRef CAS.
- L. Qi and J. Li, J. Catal., 2012, 295, 59–69 CrossRef CAS.
- A. C. Lausche, A. J. Medford, T. S. Khan, Y. Xu, T. Bligaard, F. Abild-Pedersen, J. K. Nørskov and F. Studt, J. Catal., 2013, 307, 275–282 CrossRef CAS.
- M.-J. Cheng, E. L. Clark, H. H. Pham, A. T. Bell and M. Head-Gordon, ACS Catal., 2016, 6, 7769–7777 CrossRef CAS.
- Y. Y. Chen, Y. Zhang, X. Zhang, T. Tang, H. Luo, S. Niu, Z. H. Dai, L. J. Wan and J. S. Hu, Adv. Mater., 2017, 29, 1703311 CrossRef PubMed.
- J. Zhang, T. Wang, P. Liu, Z. Liao, S. Liu, X. Zhuang, M. Chen, E. Zschech and X. Feng, Nat. Commun., 2017, 8, 15437–15444 CrossRef CAS PubMed.
- W. Du, Y. Shi, W. Zhou, Y. Yu and B. Zhang, Angew. Chem., Int. Ed., 2021, 60(13), 7051–7055 CrossRef CAS PubMed.
- G. Meng, J. Sun, L. Tao, K. Ji, P. Wang, Y. Wang, X. Sun, T. Cui, S. Du, J. Chen, D. Wang and Y. Li, ACS Catal., 2021, 11, 1886–1896 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc01018b |
| This journal is © The Royal Society of Chemistry 2021 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: