Open Access Article
Open Access ArticleRational design of iridium–porphyrin conjugates for novel synergistic photodynamic and photothermal therapy anticancer agents†
Liping
Zhang‡
a,
Yun
Geng‡
 a,
Lijuan
Li
a,
Xiaofan
Tong
a,
Shi
Liu
a,
Lijuan
Li
a,
Xiaofan
Tong
a,
Shi
Liu
 b,
Xingman
Liu
a,
Zhongmin
Su
b,
Xingman
Liu
a,
Zhongmin
Su
 a,
Zhigang
Xie
a,
Zhigang
Xie
 *b,
Dongxia
Zhu
*b,
Dongxia
Zhu
 *a and
Martin R.
Bryce
*a and
Martin R.
Bryce
 *c
*c
aKey Laboratory of Nanobiosensing and Nanobioanalysis at Universities of Jilin Province, Department of Chemistry, Northeast Normal University, 5268 Renmin Street, Changchun, Jilin Province 130024, P. R. China. E-mail: zhudx047@nenu.edu.cn
bState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022, P. R. China. E-mail: xiez@ciac.ac.cn
cDepartment of Chemistry, Durham University, Durham, DH1 3LE, UK. E-mail: m.r.bryce@durham.ac.uk
First published on 29th March 2021
Abstract
Near-infrared (NIR) emitters are important probes for biomedical applications. Nanoparticles (NPs) incorporating mono- and tetranuclear iridium(III) complexes attached to a porphyrin core have been synthesized. They possess deep-red absorbance, long-wavelength excitation (635 nm) and NIR emission (720 nm). TD-DFT calculations demonstrate that the iridium–porphyrin conjugates herein combine the respective advantages of small organic molecules and transition metal complexes as photosensitizers (PSs): (i) the conjugates retain the long-wavelength excitation and NIR emission of porphyrin itself; (ii) the conjugates possess highly effective intersystem crossing (ISC) to obtain a considerably more long-lived triplet photoexcited state. These photoexcited states do not have the usual radiative behavior of phosphorescent Ir(III) complexes, and they play a very important role in promoting the singlet oxygen (1O2) and heat generation required for photodynamic therapy (PDT) and photothermal therapy (PTT). The tetranuclear 4-Ir NPs exhibit high 1O2 generation ability, outstanding photothermal conversion efficiency (49.5%), good biocompatibility, low half-maximal inhibitory concentration (IC50) (0.057 μM), excellent photothermal imaging and synergistic PDT and PTT under 635 nm laser irradiation. To our knowledge this is the first example of iridium–porphyrin conjugates as PSs for photothermal imaging-guided synergistic PDT and PTT treatment in vivo.
Introduction
Transition metal complexes have made remarkable achievements in the clinical treatment of neuropathy, tumor therapy and diabetes.1 Phosphorescent iridium(III) complexes are especially attracting attention as photosensitizers (PSs) for phototherapy, owing to the excellent photothermal stability and high intersystem crossing (ISC) ability.2–8 Unfortunately, the currently reported PSs based on Ir(III) complexes are far from ideal, and suffer from some obvious drawbacks: (1) the inherent short excitation wavelength (ca. 450 nm) leads to autofluorescence interference, poor tissue penetration and a lack of selective uptake by neoplastic tissue;9–13 (2) their applications have mainly focused on photodynamic therapy (PDT), and consequently their therapeutic effect is highly subject to the oxygen content in the tumor, and tumor hypoxia limits the formation of reactive oxygen species (ROS) which ultimately leads to cancer cell death;14,15 (3) there are very few reports on their application in photothermal therapy (PTT), which seriously limits their applications in phototherapy.16–18 Therefore, there is an urgent need to design Ir(III) complexes as PSs that can overcome the above shortcomings and can achieve more optimal tumor therapies.PSs with long-wavelength excitation and deeper tissue penetration are crucial to guarantee effective clinical applications.19,20 Studies have sought to extend the phototherapeutic window by introducing fluorescent groups into Ir(III) complexes, such as boron dipyrromethene (BODIPY)21 and coumarin.22,23 This strategy merges the advantages of Ir(III) complexes with long-lived triplet metal–ligand charge transfer (3MLCT) states and fluorescent molecular π–π* transitions, leading to promising anticancer activity.12 Most of these reported PSs require excitation with short-wavelength light, which is ineffective for deep tumors.3,4,24 PTT is usually triggered by red light and the absorbed light energy can be converted into heat, which provides a thermal effect leading to the apoptosis of cancer cells.25–29 More importantly, PTT is independent of the oxygen concentration, and is thus an excellent candidate to treat hypoxic tumors, which will effectively make up for the deficiency of PDT. However, few research works have studied Ir(III) complexes as PSs for PTT, which is likely due to their short excitation wavelength (ca. 450 nm) caused by the typical 3MLCT process. Additionally, the photothermal effect is beneficial to promote the internalization of PSs and oxygen in the tumor tissues, thereby further improving the PDT effect.30–33 Hence, synergistic PDT and PTT treatment is a more effective prospect for tumor therapy in terms of concurrent metastasis than single PDT or PTT.34–40 Therefore, achieving long-wavelength excitation of PSs is a very important goal to realize PTT and to effectively overcome the oxygen dependence in PDT, and ultimately to improve the clinical outcome of phototherapy.
Porphyrin derivatives have been utilized as phototherapy reagents due to their absorption in the red-light region, although this absorption is of relatively low intensity.41 Specific varied examples include tetraphenylchlorin as a photosensitizer, a phosphatidylcholine-pyropheophorbide conjugate, a diphenylporphyrin conjugated polymer and a chlorin-based porous organic polymer.42–46 However, they possess the common drawbacks of the small organic molecular PSs, such as larger energy gap (ΔEST) between the first singlet excited state (S1) and triplet state (T1) and less triplet photoexcited state compared with transition metal complexes, which directly leads to reduced 1O2 production and low photothermal conversion efficiency.46–50 We asked the following question: Is it possible to combine the respective advantages of small organic molecules and transition metal complex PSs to obtain Ir(III)-porphyrin conjugates with long-wavelength excitation for high-efficiency synergistic PDT and PTT treatment?
We recently reported that multinuclear Ir(III) complexes encapsulated in nanoparticles (NPs) have good biocompatibility, and that they can increase 3MLCT transitions and molar absorption coefficients, resulting in excellent PDT effects.51 However, their short excitation wavelength (450 nm) is unsatisfactory for in vivo treatment due to poor tissue penetration. We hypothesized that combining the advantages of long-wavelength excitation and NIR emission of porphyrin itself, and highly effective long-lived triplet photoexcited state of transition metal complexes, inhibiting the phosphorescent emission from transition metal complexes simultaneously, would be a good strategy to enhance the photosensitizer-assisted (PS-assisted) phototherapy, PDT and PTT.
Results and discussion
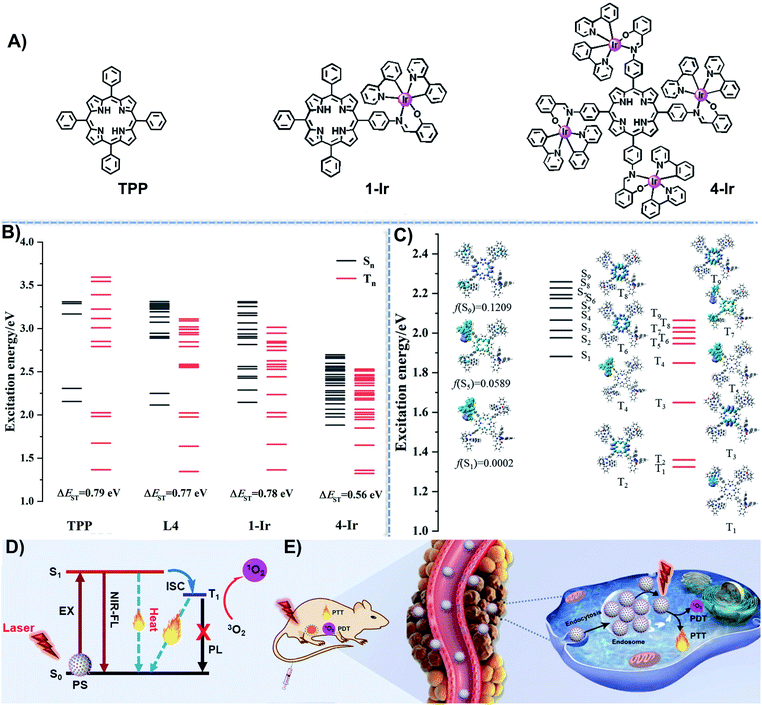
Recent promising metal–porphyrin conjugates include: porphyrinoid-Pt(II) conjugates;52 metal–organic frameworks with porphyrin ligands;53 a porphyrin–Ru complex conjugate;54 and a combination of cationic Cu–porphyrin with Schiff base Cu complexes.55 These covalent porphyrin-transition metal conjugates, used for PDT only, are structurally very different from the molecules reported herein for PDT and PTT. In this work, we synthesized two porphyrin derivatives substituted with mono- and tetra-Ir(III) complexes, named 1-Ir and 4-Ir, respectively (Fig. 1A), both of which can be excited with long-wavelength light, and their corresponding polymer-encapsulated NPs, named 1-Ir NPs and 4-Ir NPs. The iridium complexes have a stereogenic metal center and will exist as a mixture of Λ and Δ enantiomers. Separation of the enantiomers of an Ir complex is rarely accomplished and is beyond the scope of the present work.56 Tetraphenylporphyrin (TPP), a standard PS,42–46 is studied as a comparison to 1-Ir and 4-Ir. The detailed synthetic procedures and spectroscopic characterization of TPP, 1-Ir and 4-Ir are reported in the ESI (Fig. S1–S13†). The 4-Ir molecule has a smaller ΔEST and stronger spin–orbit couplings compared with TPP and 1-Ir, thereby significantly promoting the generation of abundant and effective triplet photoexcited state through intersystem crossing. 4-Ir NPs possess deep-red absorption, long-wavelength excitation (635 nm), near-infrared (NIR) emission, high 1O2 generation ability, outstanding photothermal conversion efficiency (49.5%) and good biocompatibility. More importantly, the temperature of tumors injected with 4-Ir NPs could reach to 61.9 °C after laser irradiation, and good photothermal imaging has been accomplished. The synergistic PDT and PTT effect of 4-Ir NPs gives high-efficiency inhibition of tumor growth in vivo.To study the molecular properties of TPP, L4, 1-Ir, and 4-Ir, density functional theory (DFT) and time-dependent (TD-DFT) calculations were performed in Gaussian 16. As shown in Fig. S14,† the HOMO and LUMO of TPP and L4 with a large overlap are delocalized on the π-conjugated porphyrin ring. On the contrary, 1-Ir and 4-Ir exhibit obvious HOMO–LUMO separation. The HOMO–LUMO energy gap decreases with the increasing number of Ir centers from TPP (2.72 eV) to 4-Ir (2.31 eV). The calculations show that both the HOMO and LUMO energy levels of L4 are raised somewhat compared to TPP (Fig. S14†). However, the energy gap of L4 (2.63 eV) is changed little compared with TPP (2.72 eV) and its energy gap is still significantly larger than that of Ir-4 (2.26 eV) (Fig. S14†). The relatively narrow gap suggests a long-wavelength absorption, especially for 4-Ir, which is promising for phototherapy. The attached Ir complexes filled the missing excited states of the porphyrin and did not affect the distribution of the excited states of the porphyrin itself (Fig. 1B). As shown in Fig. 1C, the strong absorptions in the long-wavelength region corresponding to singlet excited states S5 and S9, which are close to the absorptions of TPP, are derived from contributions of the porphyrin unit. The lowest triplet excited state (T1) was mainly contributed by the local charge-transfer transition in the ligands (3LC) of one cyclometalated Ir complex. The absence of orbital participation from the Ir atoms indicates that there is no phosphorescent emission in 4-Ir. This means that 4-Ir complex still retains long-wavelength excitation and near-infrared emission originating from porphyrin, while a more long-lived triplet photoexcited state is generated from the cyclometalated Ir(III) fragments. Thereby, the participation of the cyclometalated Ir(III) complexes at the singlet (Sn) and triplet (Tn) excited states is beneficial to the inter-system crossing (ISC) of 4-Ir. The energy gap between S1 and T1 is reduced from TPP (0.79 eV) to 4-Ir (0.56 eV) as shown in Fig. 1B. At the same time, the singlet and triplet photoexcited state with close energy levels also increased along with the additional number of Ir centers (Fig. 1B). The emission and transient decay of 4-Ir are shown in Fig. S22.† The short excited-state lifetime (τ 4.76 ns) indicates that the fluorescence of 4-Ir is from the porphyrin unit and not from the attached Ir complexes.
Due to the large molecular size of 4-Ir, we take the spin–orbit coupling values of TPP and 1-Ir as examples to find the contribution of the attached Ir complexes in increasing the ISC ability. Compared with TPP, the spin–orbit coupling constants between Sn and Tn of 1-Ir complex increased considerably from 0.297 to 148 cm−1, as shown in Table S1.† These data indicate that the spin–orbit coupling values in 4-Ir should be enhanced to an even greater extent. Meanwhile, a comparison of the geometrical structures at S0 and T1 states for TPP and 4-Ir, together with their respective root mean square deviation (RMSD) (0.16 Å and 0.46 Å) suggests a larger nonradiative vibrational relaxation of 4-Ir, as shown in Fig. S16.† The results indicate that the attached cyclometalated Ir complexes without phosphorescent emission play very important roles in: (i) greatly improving the highly effective ISC ability to ensure sufficient triplet photoexcited states; (ii) greatly promoting the nonradiative vibrational processes for the energy conversion. Therefore, theoretical results support our proposed design concept: namely, to realize an advantageous combination of the photophysical performance of porphyrin and iridium complexes.
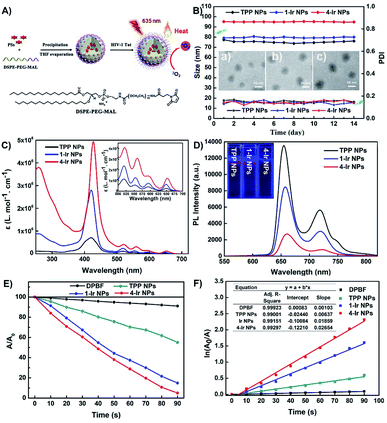
The TPP NPs, 1-Ir NPs and 4-Ir NPs were prepared by polymer-encapsulation (Fig. 2A).57–62 The introduced amphiphilic polymer chain (DSPE-PEG-MAL) was used to obviously change the biocompatibility of the compound.51,57 HIV-1 Tat as the cell penetrating peptide can effectively promote the entry of NPs into cells.51,57 Transmission electron microscopy (TEM) images confirmed that TPP NPs, 1-Ir NPs and 4-Ir NPs exhibit spherical morphologies with average diameters of about 44, 48, 57 nm, respectively (Fig. 2B). Similar features were observed in the scanning electron microscopy (SEM) images (Fig. S17†). The hydrodynamic diameters of TPP NPs, 1-Ir NPs and 4-Ir NPs determined by dynamic light scattering (DLS) are 74, 79 and 95 nm, respectively (Fig. S18; Table S3†). The larger sizes measured by DLS than by TEM can be attributed to a hydrated layer on the NPs. In addition, the zeta potential of the 4-Ir NPs was determined to be +27.8 mV, confirming that HIV-1 Tat was successfully conjugated to the surface of NPs.61,624-Ir NPs released a low amount of 4-Ir (≈1.69%) over 72 h in water (Fig. S19†). Such a low release of contents verifies that the PSs remain intact as the active species. Moreover, no obvious changes of the size or size distribution of TPP NPs, 1-Ir NPs and 4-Ir NPs were observed within 14 days suggesting the high stability of the NPs. In addition, the size of 4-Ir NPs in serum (118 nm) and PBS (106 nm) is stable within 14 days (Fig. S24†). These NPs with spherical morphology, appropriate size and excellent stability are suited for biological applications.
The UV-vis absorption and photoluminescence (PL) spectra of TPP NPs, 1-Ir NPs and 4-Ir NPs were recorded in water. The extended absorption band in the 500–700 nm region (Fig. 2C) is advantageous for matching long-wavelength excitation to enhance tissue penetration. The UV-Vis absorption spectra of the corresponding pure compounds, TPP, 1-Ir and 4-Ir, exhibit similar absorption bands in tetrahydrofuran (Fig. S20†). The analogous Ir(III) complex alone possesses absorption bands around 350–500 nm. Such a short absorption wavelength is not suitable for practical clinical applications.63 The molar absorption coefficient at 650 nm follows the sequence 4-Ir NPs (ε = 10 × 4.12 M−1 cm−1) > 1-Ir NPs (ε = 10 × 3.84 M−1 cm−1) > TPP NPs (ε = 10 × 3.38 M−1 cm−1) consistent with efficient intramolecular energy transfer between the porphyrin and the Ir centers. The simulated absorption spectra for TPP and 4-Ir in the long-wavelength region by TDDFT/B3LYP methods (Fig. S23†) also indicate that 4-Ir NPs possess stronger absorption than that of TPP NPs at 550 nm. The NPs all exhibited NIR emission (Fig. 2D). The emission intensity of TPP NPs at 720 nm is 1.84 and 7.14 times higher than 1-Ir NPs and 4-Ir NPs, respectively. In addition, L4 and TPP exhibit similar photophysical properties (Fig. S21†). The increased molar absorbance coefficient and the reduced emission intensity are conducive to accelerating the transfer of excited energy to the triplet state and to nonradiative vibrational processes, thereby improving the efficiency of both PDT and PTT.64
The photoluminescence quantum yields (PLQYs) in water of TPP NPs (29%) are higher than those of 1-Ir NPs and 4-Ir NPs (11% and 5%, respectively) (Table S2†). The fluorescent quantum efficiency and kr values decrease from TPP NPs, 1-Ir NPs to 4-Ir NPs, while knr gradually increases from TPP NPs, 1-Ir NPs to 4-Ir NPs. 4-Ir NPs are more likely to promote highly effective ISC which is beneficial to efficient utilization of the absorbed light energy for PDT and PTT. The excited-state lifetimes (τ) of TPP NPs (4.32 ns), 1-Ir NPs (4.67 ns) and 4-Ir NPs (4.82 ns) are all at the nanosecond level (Table S2†), meaning that the fluorescence of 1-Ir NPs and 4-Ir NPs is from the porphyrin unit and not from the phosphorescent Ir complexes. Meanwhile, the τ values gradually increase in the order TPP NPs< 1-Ir NPs < 4-Ir NPs, which is more conducive to the generation of a long-lived triplet photoexcited state from 4-Ir NPs. This favors the full energy transfer between 4-Ir NPs and oxygen molecules in the process of photodynamic therapy to obtain high-efficiency singlet oxygen. Together with the computational results, these experimental data demonstrate that 4-Ir NPs fully achieve our molecular design objective of iridium-porphyrin conjugate nanoparticles with long wavelength excitation and near-infrared emission for synergistic PDT and PTT treatment.
High 1O2 generation is a pre-requisite for efficient PDT.65,66 The 1O2 generation ability of TPP NPs, 1-Ir NPs and 4-Ir NPs was monitored by using 1,3-diphenylisobenzofuran (DPBF) as an indicator. Negligible spectral changes were observed in the control groups: (i) DPBF + irradiation (635 nm, 0.4 W cm−2, 90 s); (ii) PSs + irradiation; and (iii) DPBF + PSs, indicating excellent photostability. As expected, upon irradiation, a continuous and significant decrease of absorbance was observed for solutions of PSs and DPBF consistent with efficient 1O2 generation (Fig. 2E and S23†). In contrast, the absorption spectra were essentially unchanged with time for the following samples: (i) DPBF + NPs (Fig. S25†); (ii) NPs + irradiation (Fig. S26†) and (iii) DPBF + irradiation (Fig. S28†). The 1O2 generation of all the PSs follows first-order kinetics (Fig. 2F). The slopes are in the order of TPP NPs (0.00637) < 1-Ir NPs (0.01859) < 4-Ir NPs (0.02654). Notably, the slopes of 1-Ir NPs and 4-Ir NPs are 2.92 and 4.17 times higher than that of TPP NPs, respectively. The 1O2 quantum yields of TPP NPs, 1-Ir NPs and 4-Ir NPs are 54%, 72% and 89%, respectively, with methylene blue (MB) as the reference (ΦΔ = 52% in MeCN). These results confirmed that the 4-Ir NPs exhibit the highest 1O2 generation ability.
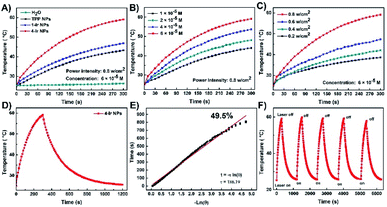
Subsequently, the photothermal activity of the NPs was evaluated. Upon laser irradiation (635 nm, 0.8 W cm−2, 300 s), the aqueous solution temperatures of TPP NPs, 1-Ir NPs and 4-Ir NPs (6 × 10−5 M) increased from room temperature to 43.3, 46.9 and 59.1 °C, respectively, demonstrating photothermal activity (Fig. 3A). In addition, the solution temperature of 4-Ir NPs increased with the increasing concentration and the laser power density (Fig. 3B and C). The photothermal conversion efficiencies (η) of TPP NPs, 1-Ir NPs and 4-Ir NPs are 31.2%, 37.8% and 49.5%, respectively (Fig. S29†). These data clearly indicate that the Ir centers significantly improve the photothermal effect. The data for 4-Ir NPs are shown in Fig. 3D–F, including five heating-cooling cycles which verify excellent photothermal stability. The thermal images of TPP NPs, 1-Ir NPs and 4-Ir NPs were observed upon 635 nm laser irradiation for 10 min; the temperature of 4-Ir NPs (71.5 °C) is higher than that of 1-Ir NPs (60.3 °C) and TPP NPs (47.4 °C) (Fig. S30†). Furthermore, both the absorbance and PL intensity of 4-Ir NPs retain more than 90% of their initial values after irradiation with a 635 nm laser (0.8 W cm−2, 5 min) for 7 days (Fig. S31†). These results confirm that PSs based on a TPP-Ir(III) system display prominent photothermal effects and excellent optical stability.
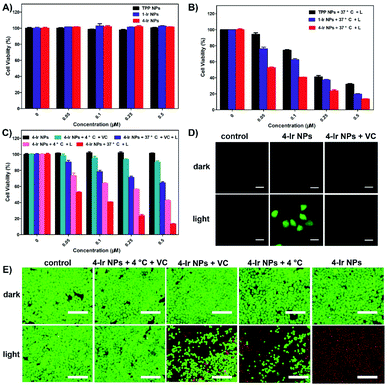
The phototherapeutic efficacy of TPP NPs, 1-Ir NPs and 4-Ir NPs was determined against HeLa cells by using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In the dark, the cell viability exceeded 95% after incubation with TPP NPs, 1-Ir NPs and 4-Ir NPs, revealing good cytocompatibility (Fig. 4A). In contrast, under laser irradiation (635 nm, 0.6 W cm−2, 5 min), TPP NPs, 1-Ir NPs and 4-Ir NPs showed a concentration-dependent cytotoxicity (Fig. 4B). The order of the half-maximal inhibitory concentration (IC50) is as follows: TPP NPs (0.225 μM) > 1-Ir NPs (0.145 μM) > 4-Ir NPs (0.057 μM). The lower IC50 value with 4-Ir NPs validates that more Ir centers can enhance the therapeutic effect. A similar effect was observed in HeLa cells after incubation with 4-Ir NPs for 72 h (Fig. S32†). Furthermore, the inhibition rate slightly reduced with 62.2% in the presence of Vitamin C, while the viability of cells decreased with 42.1% treated with a temperature of 4 °C (Fig. 4C). These results indicated that 4-Ir NPs possess synergetic PDT and PTT effects, and that the PDT effect is dominant.
The intracellular ROS generation has also been investigated by using 2′,7′-dichlorofluorescin diacetate (DCFH-DA) as a probe (Fig. 4D). Negligible green fluorescence was detected in the control groups indicating a lack of ROS generation: (i) DCFH-DA only (control); (ii) DCFH-DA + 4-Ir NPs; (iii) DCFH-DA + 4-Ir NPs + Vitamin C; (iv) DCFH-DA + irradiation. However, obvious green fluorescence was monitored from the cells cultured with 4-Ir NPs containing DCFH-DA under irradiation, whereas green fluorescence disappeared when the ROS capturer Vitamin C was added, indicating the effective generation of ROS in the cells. (Fig. S23†). Besides, bright field images show that the cells remain alive (Fig. S35†). The live/dead staining experiments further visually investigated the therapeutic effect of 4-Ir NPs in vitro (Fig. 4E). Upon laser irradiation, negligible green fluorescence was observed, implying that almost all of the cells were killed by 4-Ir NPs. In contrast, under the same conditions, significant green fluorescence was monitored from the cells treated at 4 °C and with Vitamin C. Compared with the 4 °C treatment, the cells with Vitamin C exhibited brighter green fluorescence, proving the inhibition rate evidently decreased. These results are in accordance with the MTT assay.
The intracellular uptake and distribution of TPP NPs, 1-Ir NPs and 4-Ir NPs were evaluated in HeLa cells. Confocal laser scanning microscopy (CLSM) showed that all the NPs exhibited comparable intracellular red emission, suggesting that they can be internalized by living cells (Fig. S33†). In addition, the relatively weak red fluorescence observed in HeLa cells treated with sodium azide which causes energy depletion (Fig. S34†) suggests that energy-mediated endocytosis is the primary uptake pathway for the NPs. These results convincingly verify the excellent biocompatibility and outstanding synergistic PDT and PTT treatment effect of 4-Ir NPs.
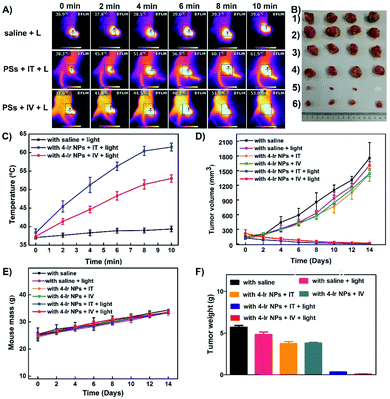
Inspired by the in vitro performance, 4-Ir NPs were assessed in vivo with U14 tumor-bearing mice. These murine models were randomized into six groups: (1) with saline; (2) with saline + light; (3) 4-Ir NPs + IT; (4) 4-Ir NPs + IV; (5) 4-Ir NPs + IT + light, (6) 4-Ir NPs + IV + light. As shown in Fig. S36,† the strong fluorescence intensity of U14 tumor-bearing mice after intravenous (IV) injection with IR780-labeled 4-Ir NPs into the tumor gradually increased within 12 h (with negligible residues in surrounding tissues) showing rapid and high accumulation of 4-Ir NPs. These data indicated that 12 h after injection is the optimal time for light irradiation. An infrared thermal camera was used to monitor the temperature changes at the tumor sites. As illustrated in Fig. 5A and C, the temperature reached a plateau of 61.9 °C and 53.0 °C with intratumoral (IT) and intravenous injection after 10 min irradiation, respectively, manifesting the superior photothermal imaging and treatment effect. The tumor volume increased swiftly during two weeks in groups 1 to 4 (Fig. 5D), demonstrating that neither laser irradiation alone, nor 4-Ir NPs alone, could suppress tumor growth. In sharp contrast, markedly reduced tumor volume and weight was observed in both groups 5 and 6 (Fig. 5B and F), indicating the outstanding tumor inhibition efficiency of 4-Ir NPs under irradiation. The white spot in Fig. 5B indicates that the tumor tissue had been completely killed. For the tumors in groups 5 and 6, 4-Ir NPs had a highly efficient tumor inhibition rate exceeding 90% (Fig. S37†). This can be attributed to the enhanced permeability and retention (EPR) effect of 4-Ir NPs for systematically enhanced tumor targeting. There was no abnormal murine body weight loss in groups 2 to 6 (Fig. 5E), validating the minimal systemic toxicity of 4-Ir NPs.
To study further the biodistribution of 4-Ir NPs, the ex vivo fluorescence images of the major organs and tumor were obtained (Fig. S38 and S39†). The strongest fluorescence intensity was observed from tumor, liver and kidney. H&E-stained images of heart, liver, spleen, lung, kidney and tumor from sacrificed mice in each group were collected and assessed. No noticeable abnormalities in major organs from the six treatment groups were discovered, implying that 4-Ir NPs are harmless in vivo. However, the tumor slices from groups 5 and 6 exhibit obvious cellular nuclei damage, suggesting that 4-Ir NPs caused cell apoptosis and necrosis. All these results indicated that 4-Ir NPs are eminently suitable for phototherapy in vivo.
Conclusions
In summary, this work has demonstrated that iridium–porphyrin conjugates encapsulated in nanoparticles can act as PSs for photothermal imaging-guided synergistic PDT and PTT therapy. Two deep-red absorbing, NIR-emitting compounds were rationally designed and synthesized. The small ΔEST, separation of HOMO–LUMO, abundant and effective photoexcited state and high spin–orbit coupling constant increases with the number of peripheral Ir centers, thereby increasing the molar absorption coefficient at long wavelengths (635 nm), 1O2 production capacity and photothermal activity. Especially, 4-Ir NPs are ideal PSs for cancer treatment because of the following advantages: deep-red absorption, long-wavelength excitation (635 nm), NIR emission, remarkable 1O2 generation ability, excellent photothermal conversion efficiency (49.5%), negligible dark toxicity and low IC50 (0.057 μM). Furthermore, both intravenous and intratumoral injections of 4-Ir NPs exhibited an obvious temperature rise and high-efficiency inhibitory effect for tumor growth in vivo. This study provides new insights in the design of multifunctional PSs with long excitation wavelength for clinical therapeutic applications.Author contributions
L. Z., Z. X. and D. Z. designed the experiments and the research. Y. G., L. Z., L. L., X. T., S. L. and X. L. performed the experiments. L.Z., Z. S., Z. X. and D. Z. analyzed the data. L. Z., Z. X., D. Z. and M. R. B. wrote the paper. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
All animal studies were performed in strict accordance with the NIH guidelines for the care and use of laboratory animals (NIH Publication No. 85-23 Rev. 1985) and were approved by the guidelines of the Committee on Animal Use and Care of the Chinese Academy of Sciences. The work was funded by NSFC (No. 52073045), the key scientific and technological project of Jilin province (20190701010 GH), the Development and Reform Commission of Jilin province (2020C035-5). D. X. Zhu thanks the support from Key Laboratory of Nanobiosensing and Nanobioanalysis at Universities of Jilin Province. M. R. B. thanks EPSRC grant EL/L02621X/1 for funding. The authors acknowledge the support from Jilin Provincial Department of Education.Notes and references
- E. J. Anthony, E. M. Bolitho, H. E. Bridgewater, O. W. L. Carter, J. M. Donnelly, C. Imberti, E. C. Lant, F. Lermyte, R. J. Needham, M. Palau, P. J. Sadler, H. Shi, F. Wang, W. Zhang and Z. Zhang, Chem. Sci., 2020, 11, 12888–12917 RSC.
- H. Huang, S. Banerjee, K. Qiu, P. Zhang, O. Blacque, T. Malcomson, M. J. Paterson, G. J. Clarkson, M. Staniforth and V. G. Stavros, Nat. Chem., 2019, 11, 1041–1048 CrossRef CAS PubMed.
- R. Bevernaegie, B. Doix, E. Bastien, A. Diman, A. Decottignies, O. Feron and B. Elias, J. Am. Chem. Soc., 2019, 141, 18486–18491 CrossRef CAS PubMed.
- W. Lv, Z. Zhang, K. Y. Zhang, H. Yang, S. Liu, A. Xu, S. Guo, Q. Zhao and W. Huang, Angew. Chem., Int. Ed., 2016, 55, 9947–9951 CrossRef CAS PubMed.
- L. He, C. P. Tan, R. R. Ye, Y. Z. Zhao, Y. H. Liu, Q. Zhao, L. N. Ji and Z. W. Mao, Angew. Chem., Int. Ed., 2014, 126, 12333–12337 CrossRef.
- H. Shi, X. Ma, Q. Zhao, B. Liu, Q. Qu, Z. An, Y. Zhao and W. Huang, Adv. Funct. Mater., 2014, 24, 4823–4830 CrossRef CAS.
- J. J. Conesa, A. C. Carrasco, V. Rodríguez-Fanjul, Y. Yang, J. L. Carrascosa, P. Cloetens, E. Pereiro and A. M. Pizarro, Angew. Chem., Int. Ed., 2020, 59, 1270–1278 CrossRef CAS PubMed.
- C. Jin, F. Liang, J. Wang, L. Wang, J. Liu, X. Liao, T. W. Rees, B. Yuan, H. Wang, Y. Shen, Z. Pei, L. Ji and H. Chao, Angew. Chem., Int. Ed., 2020, 59, 15987–15991 CrossRef CAS PubMed.
- J. S. Nam, M. Kang, J. Kang, S. Park, S. J. C. Lee, H. Kim, J. K. Seo, O. Kwon, M. H. Lim, H. Rhee and T. Kwon, J. Am. Chem. Soc., 2016, 138, 10968–10977 CrossRef CAS PubMed.
- S. M. King, S. Claire, R. I. Teixeira, A. N. Dosumu, A. J. Carrod, H. Dehghani, M. J. Hannon, A. D. Ward, R. Bicknell, S. W. Botchway, N. J. Hodges and Z. Pikramenou, J. Am. Chem. Soc., 2018, 140, 10242–10249 CrossRef CAS PubMed.
- J. Karges, S. Kuang, F. Maschietto, O. Blacque, I. Ciofini, H. Chao and G. Gasser, Nat. Commun., 2020, 11, 3262 CrossRef CAS PubMed.
- P. Wang, S. Guo, H. Wang, K. Chen, N. Zhang, Z. Zhang and T. Lu, Nat. Commun., 2019, 10, 3155 CrossRef PubMed.
- V. Novohradsky, A. Rovira, C. Hally, A. Galindo, G. Vigueras, A. Gandioso, M. Svitelova, R. Bresolí-Obach, H. Kostrhunova, L. Markova, J. Kasparkova, S. Nonell, J. Ruiz, V. Brabec and V. Marchán, Angew. Chem., Int. Ed., 2019, 58, 6311–6315 CrossRef CAS PubMed.
- X. Li, J. Wu, L. Wang, C. He, L. Chen, Y. Jiao and C. Duan, Angew. Chem., Int. Ed., 2020, 59, 6420–6427 CrossRef CAS PubMed.
- R. Guan, Y. Chen, L. Zeng, T. W. Rees, C. Jin, J. Huang, Z. Chen, L. Ji and H. Chao, Chem. Sci., 2018, 9, 5183–5190 RSC.
- X. Zheng, H. Tang, C. Xie, J. Zhang, W. Wu and X. Jiang, Angew. Chem., Int. Ed., 2015, 54, 8212–8217 CrossRef.
- S. Kuang, X. Liao, X. Zhang, T. W. Rees, R. Guan, K. Xiong, Y. Chen, L. Ji and H. Chao, Angew. Chem., Int. Ed., 2020, 59, 3315–3321 CrossRef CAS PubMed.
- J. Zhao, K. Yan, G. Xu, X. Liu, Q. Zhao, C. Xu and S. Gou, Adv. Funct. Mater., 2020, 2008325, DOI:10.1002/adfm.202008325.
- Z. Dong, L. Feng, Y. Hao, M. Chen, M. Gao, Y. Chao, H. Zhao, W. Zhu, J. Liu, C. Liang, Q. Zhang and Z. Liu, J. Am. Chem. Soc., 2018, 140, 2165–2178 CrossRef CAS PubMed.
- H. Min, J. Wang, Y. Qi, Y. Zhang, X. Han, Y. Xu, J. Xu, Y. Li, L. Chen, K. Cheng, G. Liu, N. Yang, Y. Li and G. Nie, Adv. Mater., 2019, 31, 1808200 CrossRef PubMed.
- E. Palao, R. Sola-Llano, A. Tabero, H. Manzano, A. R. Agarrabeitia, A. Villanueva, I. López-Arbeloa, V. Martínez-Martínez and M. J. Ortiz, Chem.–Eur. J., 2017, 23, 10139–10147 CrossRef CAS PubMed.
- X. Zhang, Y. Hou, X. Xiao, X. Chen, M. Hu, X. Geng, Z. Wang and J. Zhao, Coord. Chem. Rev., 2020, 417, 213371 CrossRef CAS.
- V. Novohradsky, A. Rovira, C. Hally, A. Galindo, G. Vigueras, A. Gandioso, M. Svitelova, R. Bresolí-Obach, H. Kostrhunova, L. Markova, J. Kasparkova, S. Nonell, J. Ruiz, V. Brabec and V. Marchán, Angew. Chem., Int. Ed., 2019, 58, 6311–6315 CrossRef CAS PubMed.
- M. H. Chen, Y. Zheng, X. J. Cai, H. Zhang, F. X. Wang, C. P. Tan, W. H. Chen, L. N. Ji and Z. W. Mao, Chem. Sci., 2019, 10, 3315–3323 RSC.
- Y. Liu, H. Wang, S. Li, C. Chen, L. Xu, P. Huang, F. Liu, Y. Su, M. Qi, C. Yu and Y. Zhou, Nat. Commun., 2020, 11, 1724 CrossRef CAS PubMed.
- Z. Zhang, W. Xu, M. Kang, H. Wen, H. Guo, P. Zhang, L. Xi, K. Li, L. Wang, D. Wang and B. Z. Tang, Adv. Mater., 2020, 32, 2003210 CrossRef CAS PubMed.
- S. B. Wang, C. Zhang, Z. X. Chen, J. J. Ye, S. Y. Peng, L. Rong, C. J. Liu and X. Z. Zhang, ACS Nano, 2019, 13, 5523–5532 CrossRef CAS PubMed.
- W. Wang, L. Wang, Y. Li, S. Liu, Z. Xie and X. Jing, Adv. Mater., 2016, 28, 9320–9325 CrossRef CAS PubMed.
- Y. Yang, X. Fan, L. Li, Y. Yang, A. Nuernisha, D. Xue, C. He, J. Qian, Q. Hu, H. Chen, J. Liu and W. Huang, ACS Nano, 2020, 14, 2509–2521 CrossRef CAS PubMed.
- Q. W. Chen, X. H. Liu, J. X. Fan, S. Y. Peng, J. W. Wang, X. N. Wang, C. Zhang, C. J. Liu and X. Z. Zhang, Adv. Funct. Mater., 2020, 30, 1909806 CrossRef CAS.
- K. Zhang, X. Meng, Y. Cao, Z. Yang, H. Dong, Y. Zhang, H. Lu, Z. Shi and X. Zhang, Adv. Funct. Mater., 2018, 28, 1804634 CrossRef.
- Z. He, L. Zhao, Q. Zhang, M. Chang, C. Li, H. Zhang, Y. Lu and Y. Chen, Adv. Funct. Mater., 2020, 30, 1910301 CrossRef CAS.
- C. Yang, Y. Chen, W. Guo, Y. Gao, C. Song, Q. Zhang, N. Zheng, X. Han and C. Guo, Adv. Funct. Mater., 2018, 28, 1706827 CrossRef.
- Q. Wan, R. Zhang, Z. Zhuang, Y. Li, Y. Huang, Z. Wang, W. Zhang, J. Hou and B. Z. Tang, Adv. Funct. Mater., 2020, 30, 2002057 CrossRef CAS.
- Y. Wang, N. Gong, Y. Li, Q. Lu, X. Wang and J. Li, J. Am. Chem. Soc., 2020, 142, 1735–1739 CrossRef CAS PubMed.
- W. Li, J. Yang, L. Luo, M. Jiang, B. Qin, H. Yin, C. Zhu, X. Yuan, J. Zhang, Z. Luo, Y. Du, Q. Li, Y. Lou, Y. Qiu and J. You, Nat. Commun., 2019, 10, 3349 CrossRef PubMed.
- E. S. Shibu, M. Hamada, N. Murase and V. Biju, J. Photochem. Photobiol., C, 2013, 15, 53–72 CrossRef CAS.
- Z. Yang, Z. Sun, Y. Ren, X. Chen, W. Zhang, X. Zhu, Z. Mao, J. Shen and S. Nie, Mol. Med. Rep., 2019, 20, 5–15 CAS.
- M. Rizwan, T. Rasheed, A. Raza, M. Bilal, R. Yahya, M. Yar and H. M. N. Iqbal, J. Drug Delivery Sci. Technol., 2019, 51, 70–82 CrossRef CAS.
- J. Oh, H. Yoon and J. H. Park, Biomed. Eng. Lett., 2013, 3, 67–73 CrossRef.
- R. D. Arasasingham and T. C. Bruice, Inorg. Chem., 1990, 29, 1422–1427 CrossRef CAS.
- H. S. Jung, P. Verwilst, A. Sharma, J. Shin, J. L. Sessler and J. S. Kim, Chem. Soc. Rev., 2018, 47, 2280–2297 RSC.
- X. Zheng, L. Wang, S. Liu, W. Zhang, F. Liu and Z. Xie, Adv. Funct. Mater., 2018, 28, 1706507 CrossRef.
- Q. Pei, X. Hu, X. Zheng, S. Liu, Y. Li, X. Jing and Z. Xie, ACS Nano, 2018, 12, 1630–1641 CrossRef CAS PubMed.
- M. Rajora, J. Lou and G. Zheng, Chem. Soc. Rev., 2017, 46, 6433–6469 RSC.
- X. Zheng, L. Wang, Y. Guan, Q. Pei, J. Jiang and Z. Xie, Biomaterials, 2020, 235, 119792 CrossRef CAS PubMed.
- X. Zheng, L. Wang, M. Liu, P. Lei, F. Liu and Z. Xie, Chem. Mater., 2018, 30, 6867–6876 CrossRef CAS.
- J. Jin, Y. Zhu, Z. Zhang and W. Zhang, Angew. Chem., Int. Ed., 2018, 57, 16354–16358 CrossRef CAS PubMed.
- W. Liu, Y. M. Wang, Y. H. Li, S. J. Cai, X. B. Yin, X. W. He and Y. K. Zhang, Small, 2017, 13, 1603459 CrossRef PubMed.
- W. Hou, J. W. Lou, J. Bu, E. Chang, L. Ding, M. Valic, H. H. Jeon, D. M. Charron, C. Coolens and D. Cui, Angew. Chem., Int. Ed., 2019, 58, 14974–14978 CrossRef CAS PubMed.
- L. Zhang, Y. Li, W. Che, D. Zhu, G. Li, Z. Xie, N. Song, S. Liu, B. Z. Tang and X. Liu, Adv. Sci., 2019, 6, 1802050 CrossRef PubMed.
- I. Toubia, C. Nguyen, S. Diring, L. M. A. Ali, L. Larue, R. Aoun, C. Frochot, M. Gary-Bobo, M. Kobeissi and F. Odobel, Inorg. Chem., 2019, 58, 12395–12406 CrossRef CAS PubMed.
- G. Lan, K. Ni, Z. Xu, S. S. Veroneau, Y. Song and W. Lin, J. Am. Chem. Soc., 2018, 140, 5670–5673 CrossRef CAS PubMed.
- J. Jiang, D. Liu, Y. Zhao, F. Wu, K. Yang and K. Wang, Appl. Organomet. Chem., 2018, 32, e4468 CrossRef.
- Q. Zhang, Q. Zhang, Z. Li, H. Liu and J. Liu, Dyes Pigm., 2020, 173, 107923 CrossRef CAS.
- D. L. Davies, K. Singh, S. Singh and B. Villa-Marcos, Chem. Commun., 2013, 49, 6546–6548 RSC.
- L. Zhang, W. Che, Z. Yang, X. Liu, S. Liu, Z. Xie, D. Zhu, Z. Su, B. Z. Tang and M. R. Bryce, Chem. Sci., 2020, 11, 2369–2374 RSC.
- J. Dai, Y. Li, Z. Long, R. Jiang, Z. Zhuang, Z. Wang, Z. Zhao, X. Lou, F. Xia and B. Z. Tang, ACS Nano, 2020, 14, 854–866 CrossRef CAS PubMed.
- B. Guo, Z. Huang, Q. Shi, E. Middha, S. Xu, L. Li, M. Wu, J. Jiang, Q. Hu and Z. Fu, Adv. Funct. Mater., 2020, 30, 1907093 CrossRef CAS.
- J. Karges, U. Basu, O. Blacque, H. Chao and G. Gasser, Angew. Chem., Int. Ed., 2019, 58, 14334–14340 CrossRef CAS PubMed.
- P. Zhang, X. Nie, M. Gao, F. Zeng, A. Qin, S. Wu and B. Z. Tang, Mater Chem Front, 2017, 1, 838–845 RSC.
- G. Lin, P. N. Manghnani, D. Mao, C. Teh, Y. Li, Z. Zhao, B. Liu and B. Z. Tang, Adv. Funct. Mater., 2017, 27, 1701418 CrossRef.
- Y. You, H. S. Huh, K. S. Kim, S. W. Lee, D. Kim and S. Y. Park, Chem. Commun., 2008, 3998–4000 RSC.
- M. H. Chen, Y. Zheng, X. J. Cai, H. Zhang, F. X. Wang, C. P. Tan, W. H. Chen, L. N. Ji and Z. W. Mao, Chem. Sci., 2019, 10, 3315–3323 RSC.
- Y. Gao, X. Wang, X. He, Z. He, X. Yang, S. Tian, F. Meng, D. Ding, L. Luo and B. Z. Tang, Adv. Funct. Mater., 2019, 29, 1902673 CrossRef.
- W. Sun, T. Shi, L. Luo, X. Chen, P. Lv, Y. Lv, Y. Zhuang, J. Zhu, G. Liu and X. Chen, Adv. Mater., 2019, 31, 1808024 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc00126d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |