Open Access Article
Open Access ArticleTo improve the key properties of nonlinear optical crystals assembled with tetrahedral functional building units†
Zhiyong
Bai
 ad,
Lehui
Liu
ad,
Lehui
Liu
 a,
Dongmei
Wang
a,
Chun-Li
Hu
*b and
Zhoubin
Lin
a,
Dongmei
Wang
a,
Chun-Li
Hu
*b and
Zhoubin
Lin
 *abc
*abc
aCAS Key Laboratory of Optoelectronic Materials Chemistry and Physics, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China. E-mail: lzb@fjirsm.ac.cn
bState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China. E-mail: clhu@fjirsm.ac.cn
cFujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou, Fujian 350108, P. R. China
dUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 26th January 2021
Abstract
Nonlinear optical (NLO) crystals assembled with conventional non-π-conjugated tetrahedral functional building units (FBUs), generally referring to [PO4] and [BO4], usually exhibit weak nonlinearity and poor birefringence. It is currently proposed that partially substituting oxygen atoms with fluoride atoms in these FBUs could enhance these crucial properties. Hence, we investigated for the first time the NLO-related properties of NH4BAsO4F (ABAF), which was constructed from tetrahedral [BO3F] and [AsO4] FBUs, and enhancements of these properties were observed in this material, that is large second-harmonic generation (SHG) response (2 × KDP) and improved birefringence (0.03 at 1064 nm). Notably, both SHG coefficient and birefringence of ABAF exceeded those of a great majority of phosphates, sulfates, or boron phosphates and achieved a preferable balance. It is interesting that ABAF shows vast structural similarities to the typical NLO crystals Sr2Be2B2O7 (SBBO) and KBe2BO3F2 (KBBF), which might be the partial reason why it showed improvement in these vital properties. This work may afford some inspiration for enhancing the key performances of NLO crystals assembled with non-π-conjugated tetrahedra.
Introduction
Nonlinear optical (NLO) crystals capable of switching specific coherent light sources generated by a solid-state laser to the ultraviolet (UV) and deep-UV spectral region are of great demand and have attracted considerable attention for their important applications in areas as diverse as photolithography, laser micromachining, and advanced instruments.1–6 To date, KBe2BO3F2 (KBBF) is the sole NLO material that can output the deep-UV coherent radiation by a direct SHG process. However, the practical application was restricted by its intrinsic drawbacks including toxic BeO and layered growth habit.7To be deep-UV NLO candidates, materials must satisfy three fundamental requirements: (1) a short absorption edge, less than 200 nm; (2) large SHG coefficient, >0.39 pm V−1; and (3) sufficient birefringence (0.05–0.10).8–10 It is widely agreed that tetrahedral FBUs, mainly referring to [PO4] and [BO4] tetrahedra, are very helpful for deep-UV transmittance, and thus they have been employed to design and synthesize deep-UV NLO materials.11 The unremitting efforts by researchers result in a series of NLO-active optical materials exclusively consisting of [BO4] or/and [PO4] tetrahedrons being discovered, such as SrB4O7,12 BPO4,13 Ba3P3O10X (X = Cl, Br),14 RbBa2(PO3)5,15 LiM2PO4 (M = Rb, Cs),16,17 RbNaMgP2O7,18 and M4Mg4(P2O7)2 (M = K, Rb).19 As reported, these materials are transparent down to the deep-UV spectral region very well with a short absorption edge, e.g., BPO4 (130 nm),13 Ba3P3O10Cl (180 nm),14 LiCs2PO4 (174 nm),16 and K4Mg4(P2O7)2 (170 nm).19 However, there also remain undesired issues that are often-weak SHG susceptibility and poor birefringence, especially the poor birefringence which vastly limits the phase-matched wavelengths reaching the deep-UV region.20 In fact, the recent research studies reveal that the SHG coefficient can be efficiently improved by adopting some strategies. For example, the edge-sharing tetrahedra make LiCs2PO4 and Li2BaSiO4 exhibit enlarged SHG response of 2.6 and 2.8 times that of KDP, respectively, which are even larger than that of many borate-based NLO crystals.16,21 Nonetheless, except for a few exceptions, most of these materials have a weak SHG response. Compared to SHG efficiency, improving the birefringence is much more challenging because of the small anisotropy of [BO4] and [PO4].20 On the basis of our statistics, the birefringence of almost all of the reported deep-UV transparent NLO materials in phosphates and boron phosphates is less than 0.02 which is too small to satisfy the phase-matching conditions in the deep-UV region (see Table 1). To address this, Pan's groups proposed that new tetrahedral FBUs, such as [BO3F] and [PO3F] tetrahedra, generated by partially substituting the oxygen atoms with fluoride atoms in [BO4] and [PO4], respectively, possess superior optical properties compared to the original ones and these new FBUs can efficiently enhance the birefringence of materials.20,22 Recent research demonstrated the usefulness of this proposition. For example, very recently reported (NH4)2PO3F20 and NaNH4PO3F·H2O,23 both of them exclusively composed of tetrahedral [PO3F] units, exhibit enhanced birefringence, that is 0.027 and 0.053, respectively. It should be noted that in particular, NaNH4PO3F·H2O exhibits the largest birefringence among phosphates which enable its shortest phase-matching wavelength reaching the deep-UV region (194 nm). Motivated by these achievements, a fluoroboron arsenate, NH4BAsO4F (ABAF),24 which was assembled with [BO3F] and [AsO4] tetrahedra, attracted our attention. As mentioned above, the occurrence of [BO3F] tetrahedra in this compound may induce an enhanced birefringence and [AsO4] tetrahedra may help in enhancement of SHG coefficient. In this article, we reported for the first time its linear and nonlinear optical properties and explored the structure–property relationships.
| Crystals | PSHG (×KDP) | SHG coefficient (pm V−1) | Absorption edge (nm) | Birefringence (Δn) | Ref. |
|---|---|---|---|---|---|
| a N. R.: not reported or not available. | |||||
| BPO4 | 2 | d 36 = 0.76 | 134 | 0.0056 @ 589 nm | 13 |
| Ba3P3O10Cl | 0.6 | d ave = 0.57 | 180 | 0.030 @ 532 nm | 14 |
| Ba3P3O10Br | 0.5 | d ave = 0.49 | <200 | 0.024 @ 532 nm | 14 |
| Ba5P6O20 | 0.8 | N. R. | 167 | N. R. | 29 |
| RbBa2(PO3)5 | 1.3 | d 11 = 0.066, d12 = 0.037, d13 = −0.591, d15 = 0.007, d24 = 0.197, d33 = 0.037 | 163 | 0.009 @ 1064 nm | 15 and 30 |
| KBa2(PO3)5 | 0.9 | d = 7.50–7.79 × 10−10 esu | 167 | N. R. | 31 |
| KPb2(PO3)5 | 0.5 | N. R. | 177 | 0.021 @ 1064 nm | 30 |
| KLa(PO3)4 | 0.9 | d 14 = 1.62 × 10−9, d16 = 1.58 × 10−9, d22 = d23 = 1.65 × 10−9 esu | 162 | 0.0084 @ 1064 nm | 32 |
| K4Mg4(P2O7)3 | 1.3 | N. R. | 170 | 0.0108 @ 1064 nm | 19 |
| Rb4Mg4(P2O7)3 | 1.4 | N. R. | <200 | 0.009 @ 1064 nm | 19 |
| RbNaMgP2O7 | 1.5 | N. R. | 185 | N. R. | 18 |
| CsNaMgP2O7 | 1.1 | d 31 = 0.368, d32 = −0.178, d33 = −0.321 | 180 | N. R. | 33 |
| LiCs2PO4 | 2.6 | d 15 = d31 = −0.65, d24 = d32 = 0.22, d33 = 0.61 | 174 | N. R. | 16 |
| LiRb2PO4 | 2.1 | d 15 = −0.46, d24 = 0.11, d33 = 0.28 | 170 | N. R. | 17 |
| KBPO4F | 1 | N. R. | <200 | 0.044 | 34 |
| CsLiCdP2O7 | 1.5 | N. R. | <200 | N. R. | 35 |
| Ba2NaClP2O7 | 1.3 | d ave = 1.11 | <176 | 0.017 @ 1064 nm | 36 |
| (NH4)2PO3F | 1 | d 15 = 0.14, d24 = 0.40, d33 = −0.35, d14 = 0.08 | <177 | 0.027 @ 1064 nm | 20 |
| K2SrP4O12 | 0.5 | d 14 = −0.534, d36 = 0.185 | <200 | 0.016 @ 1064 nm | 37 |
| KMg(H2O)PO4 | 1.14 | d 31 = 0.55, d32 = 0.48, d33 = 1.17 | <200 | 0.018 @ 1064 nm | 38 |
| NH4NaPO3F·H2O | 1.1 | d 11 = 0.727 | N. R. | 0.053 @ 589.3 nm | 23 |
| NH4NaLi2(SO4)2 | 1.1 | N. R. | 186 | N. R. | 39 |
| Li2BaSiO4 | 2.8 | d 15 = d31 = −0.26, d33 = 3.77 | <190 | N. R. | 21 |
| CsSiP2O7F | 0.7 | d 16 = −0.22, d14 = 0.10, d25 = 0.34, d23 = −0.35 | <190 | 0.009 @ 1064 nm | 40 |
| NH4BAsO4F | 2 | d 12 = 1.25 | <200 | 0.03 @ 1064 nm | This work |
Results and discussion
Crystal structure and structural comparison
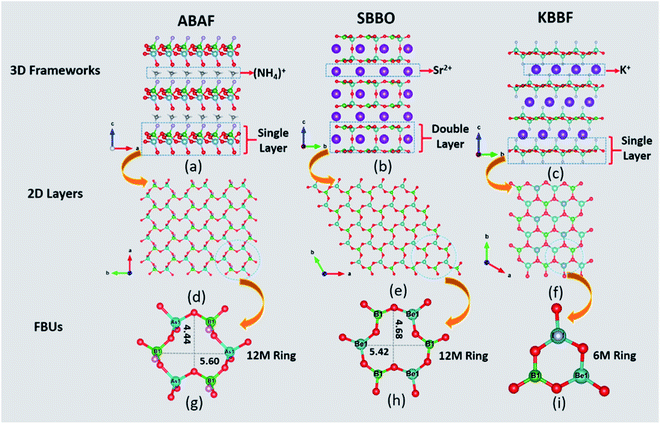
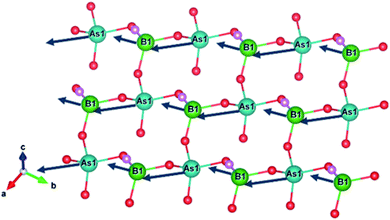
ABAF crystallizes in a non-centrosymmetric (NCS) and polar space group Cc (no. 9). Its asymmetric unit consists of one As, one B, one N, one F and four O atoms. The As atom is tetrahedrally coordinated with oxygen atoms, yielding the deformed [AsO4] tetrahedron with one shorter As–O bond length 1.632(14) Å and three longer As–O bond lengths ranging from 1.705(12) Å to 1.721(13) Å. The B atom forms a distorted [BO3F] tetrahedral configuration with a shorter B–F bond length 1.391(19) Å and three longer B–O bond lengths varying from 1.45(2) Å to 1.471 (19) Å. The B–F and B–O bond lengths in ABAF agree well with those of NLO crystals containing BO3F tetrahedra, which are summarized in Table S9.†Structurally, three [BO3F] and three [AsO4] tetrahedra are linked alternately with two longer B–O (or As–O) bonds to create ladybird-shaped [B3As3O15F3] functional building units (FBUs). The [B3As3O15F3] FBUs are further connected mutually through another longer B–O (or As–O) bond and then extended in the a–b plane to build a two-dimensional (2D) honeycomb-like [BAsO4F]∞ infinite layer, which stacked along the c-axis with NH4+ cations which are located at the interlayer as charge compensation to complete the three-dimensional framework construction. The terminal F atoms in [BO3F] and O atoms of the shorter As–O bond in [AsO4] formed a H-bond network with H atoms in NH4+ to reinforce the interlayer interactions. The H-bond calculation, realized by PLATON, shows that each NH4+ is bonded to six oxygen atoms and three fluorine atoms to create a N–H4⋯O6F3 H-bond network with a variety of H⋯O/F bond distances of 2.05 Å to 2.57 Å. It is widely thought that a H-bond network can efficiently strengthen the interlayer binding in layered crystals.25,26 Thus, ABAF probably has better crystal growth habit than KBBF along the c axis. The calculated bond valences are 4.92 and 3.08 for As and B atoms, respectively, which are in accordance with their normal oxidation states.27
It is interesting that ABAF “integrated” somehow the crystal structural characteristics of both SBBO and KBBF. For comparison, their crystal structural representations are graphically illustrated in Fig. 1 and then discussed below. Firstly, FBUs of ABAF and SBBO possess homogeneous ladybird-like 12-membered (12M) ring FBUs. In SBBO, three [BO3] triangles are linked with three [BeO4] tetrahedra alternately, yielding the [B3Be3O15] ladybird-shaped 12M FBU which can be described as (3Δ + 3T); similarly, the ladybird-shaped 12M ring FBU [As3B3O15F3] in ABAF, which can be described as (3T + 3T), is composed of three [BO3F] and three [AsO4] tetrahedra. The size of the 12M ring of SBBO and ABAF is very close; the tiny difference between them is that the ring of SBBO is slightly “thinner” and “higher” than that of ABAF. However, KBBF′s FBU, [Be2BO6F2], composed of one [BO3] triangle and two [BeO3F] tetrahedra and can be described as (1Δ + 2T), is a 6-membered ring and is distinct from SBBO and KBBF. Secondly, the likeness between ABAF and KBBF is mainly reflected in their 2D single layer structure. In KBBF and ABAF, the single layers were separated by K+ and NH4+ cations, respectively. The interlayer distance is 6.38 Å for ABAF which is slightly longer than that of KBBF (6.25 Å).25 However, the inverse [BeO4] tetrahedra in adjacent [Be3B3O12] layers are connected directly by bridging oxygen atoms, which leads to the [Be2B2O7] double layers in SBBO.
Linear and nonlinear optical properties
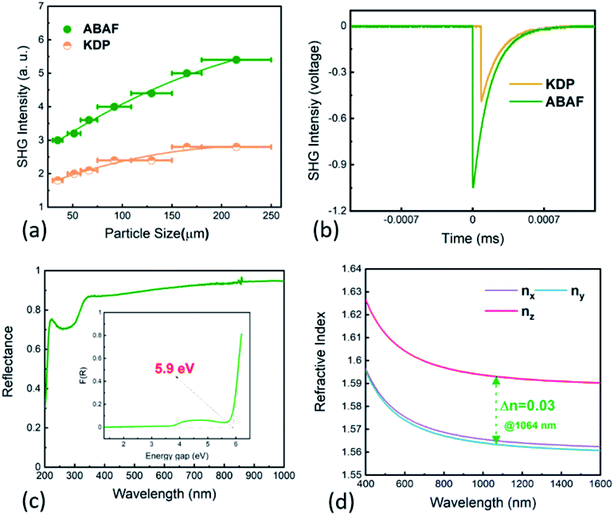
The UV-Vis-NIR diffuse reflectance spectrum was recorded to examine the UV transparency capacity and determine the band gap of ABAF (Fig. 2d). The spectrum implies that ABAF is transparent down to the deep-UV spectral region with 30% reflectance at 200 nm. Additionally, ABAF has a high reflectance, corresponding to high transparency, from 220 to 1000 nm (>75%). On the basis of the Kubelka function, the band gap of ABAF is determined to be 5.9 eV.Attributable to SHG-favorable structural features, mainly referring to the optimized arrangement of [BO3F] and [AsO4] groups in space, ABAF may display an enhanced SHG response. To examine this, the particle size dependent powder second harmonic generation (SHG) response was executed utilizing 1064 nm coherent light as an incident source via the method proposed by Kurtz and Perry.28 KH2PO4 (KDP) was used as a reference. As is clearly seen from Fig. 2a and b, ABAF exhibits SHG signals, about twice that of KDP in the particle size range of 180–250 μm at 1064 nm. The SHG response of ABAF is superior to or comparable to those deep-UV transparent NLO crystals exclusively composed of nonπ-conjugated tetrahedral FBUs, such as phosphates, sulfates, boron phosphates (see Table 1). In addition, the SHG intensity increased with increasing particle size and a decreasing tendency was not observed, manifesting that SHG is phase-matchable at 1064 nm, which was also verified using the calculated chromatic dispersion curves.
Birefringence, as a key parameter to achieve phase-matching, is essential for NLO crystals. Therefore, in order to know the phase-matching capability of ABAF, we next investigated its birefringence. According to the refractive index calculated using first-principles calculations, birefringence of ABAF at 1064 nm is determined to be 0.03. Obviously, such a value is larger than that of most of the reported SHG-active boron phosphates and phosphates (see Table 1). Notably, this value is also comparable to those of several newly discovered bismuth- and/or lead-based NLO phosphates, namely, A3BBi(P2O7)3 (A = Rb, Cs; B = Pb, Ba) (Δn = 0.025–0.03 at 1064 nm) which encompass highly distorted Bi–O and/or Pb–O polyhedra which easily induce an enhanced birefringence.41
It seems necessary to discuss the possible mechanism of birefringence enhancement observed in ABAF and we inferred that [BO3F] tetrahedra are mainly responsible for this. As shown in Table 1 and Fig. 3, those NLO crystals assembled with tetrahedra in which the central atoms are coordinated with all O atoms universally have smaller birefringence than those composed of tetrahedra in which the central atoms are coordinated with mixed O atoms and F atoms. This suggests that the all-oxygen-coordinated AsO4 geometry in ABAF is probably not the leading contributing one to birefringence enhancements. Therefore, it seems that such improvements are possibly due to the BO3F tetrahedron. Actually, as described earlier, based on theoretical computation, the occurrence of F atoms in tetrahedral geometric anions is in favor of strengthening birefringence, and desirable improvements are indeed discovered in some NLO materials, such as (NH4)2PO3F, and NaNH4PO3F·H2O (Table 1). It should be mentioned that it is difficult to quantitatively describe the contribution of BO3F tetrahedra to birefringence. The related discussions are just based on the previous work and reasonable speculation.
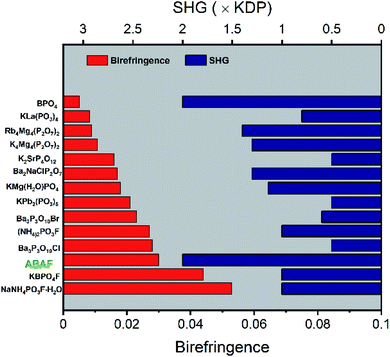 | ||
| Fig. 3 The SHG coefficient and birefringence summarization for NLO crystals assembled with tetrahedral units with deep-UV transmittance as a prerequisite. | ||
Optical property comparison
To more intuitively comprehend the key linear and nonlinear optical properties of ABAF, the SHG coefficient-, birefringence-, and SHG coefficient-birefringence plots of the recently discovered NLO crystals exclusively composed of nonπ-conjugated tetrahedral FBUs, are shown in Fig. S2† and 3, with deep-UV transparency window as the prerequisite. Firstly, the SHG coefficient of most of these crystals lies below the 2 × KDP line, and only a few crystals, including BPO4, LiCs2PO4, LiRb2PO4, and Li2BaSiO4 as well as ABAF can touch or exceed this line, indicating that ABAF has enhanced SHG response which is not only highly competitive to those crystals with SHG efficiency <2 × KDP, but also comparable with BPO4, LiCs2PO4, LiRb2PO4, and Li2BaSiO4 (Fig. S2(a)†). Secondly, as displayed in Fig. S2(b),† most of these crystals have poor birefringence less than 0.02, and only a few crystals including ABAF have an improved birefringence, larger than 0.03. Therefore, in the cases of birefringence and SHG response, ABAF achieved a preferable balance among these NLO crystals (Fig. 3).Thermal stability
DTA and TG analysis of ABAF were also performed to know its thermal stability. As shown in Fig. S5,† in the DTA curve, a sharp endothermic peak was observed at 389 °C, and simultaneously ABAF started to lose mass in the TG curve, revealing that ABAF could be stable up to 389 °C and above this temperature it starts to thermally decompose to other phases. The thermal stability of ABAF is better than those of NLO crystals containing NH4, such as NH4B4O6F (∼300 °C) and NH4Be2BO3F2 (∼350 °C).Structure–property relationships
We carried out first principles calculations by using the DFT method within the CASTEP package to help understand the intrinsic relationship between the structure and optical properties of ABAF (see Fig. S4†). The calculated band structure suggests that ABAF has a band gap of 4.93 eV, which is smaller than the experimental value of 5.9 eV. The band gap underestimation originated from the inherent limitation of the exchange and correlation functional in the DFT method. Therefore, to well match the experimental gap and accurately describe the optical properties, a “scissor” of 0.97 eV was used in our following calculations and analyses.The density of states (DOS), including the total DOS and partial DOS projected onto specific atoms is plotted in Fig. S7.† We focus on the energy zones in the vicinity of the forbidden band (−10 to 17 eV), which account for most of the chemical bonding characteristics in the compound. It is clear that in the region, the electronic states of N-2s 2p and H-1s and B-2s 2p and O-2p together with F-2p, as well as As-4s 4p and O-2p are well overlapped, inferring the strong bonding interactions of N–H, B–O, B–F and As–O bonds in the system. The upper part of the VB (−5 to 0 eV) is dominated by O-2p and F-2p states, with a bit of As-4p and B-2p states mixed; while the lower part of CB (5–10 eV) is composed of the unoccupied As-4s 4p, O-2p and small amount of B-2p states. Since the optical properties of a material are mainly dependent on the electronic states on both sides of the forbidden band, it can be predicted that the optical properties of ABAF are intimately associated with [BO3F] and [AsO4] tetrahedra in the [BAsO4F]∞ layers.
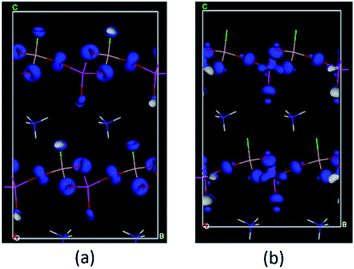
We next calculated the SHG coefficients. Among them, d12 is the largest tensor whose absolute value is 1.25 pm V−1 in the static limit. The magnitude of d12 is about 3.2 times that of KDP (d36 = 0.39 pm V−1), consistent with the measured powder SHG effect (2 times that of KDP). To elucidate the inherent origin of the SHG response of ABAF, we further performed the SHG-weighted electron density (SHG-density) analysis for d12, which can clearly disclose the SHG-contributed orbitals in the real space (Fig. 4): in the VB, the O-2p and F-2p nonbonding states in AsO4 and BO3F tetrahedra dominate the SHG effect, and in the CB, the empty orbitals of O-2p and As-4s 4p contribute principally to the SHG effect. Based on the SHG-density analysis, we also calculated the contribution percentages to the SHG effect from the constituent groups, which are 63.01% for AsO4, 32.96% for BO3F and 4.03% for NH4+. Obviously, the SHG-contribution of NH4+ is negligibly small. This can also be verified by the dipole moment calculations. The calculation was carried out by a bond-valence approach described previously.42 The results are listed in Table S10.† Obviously, the net dipole moment direction of BO3F and AsO4 pointed to the same direction (Fig. 5), suggesting their overlapping instead of cancelling out, which are favourable for the enhancement of the SHG effect. In addition, the magnitude of the net dipole moment for the AsO4 tetrahedron is about 2.05 Debye, larger than that of BO3F (1.17 Debye), revealing that AsO4 contributed more to the overall SHG effect than the BO3F group. Above all, it could be concluded that the cooperative interaction of BO3F and AsO4 tetrahedra enables ABAF exhibit good nonlinear optical performances.
Conclusions
In summary, the nonlinear optical and related properties of NH4BAsO4F were investigated for the first time in this article. This material achieved improvements in key properties including birefringence and second-order nonlinearity. The study on structure–property relationships suggests that the 2D [BAsO4F]∞ layers are mainly responsible for its optical properties. This work probably can provide some inspiration for identifying compounds with enhanced nonlinear optical properties by analysing structural features.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (no. XDB20000000).Notes and references
- P. Becker, Adv. Mater., 1998, 10(13), 979–992 CrossRef CAS
.
- W. Yao, R. He, X. Wang, Z. Lin and C. Chen, Adv. Opt. Mater., 2014, 2(5), 411–417 CrossRef CAS
.
- T. T. Tran, H. Yu, J. M. Rondinelli, K. R. Poeppelmeier and P. S. Halasyamani, Chem. Mater., 2016, 28(15), 5238–5258 CrossRef CAS
.
- Y. Shen, S. Zhao and J. Luo, Coord. Chem. Rev., 2018, 366, 1–28 CrossRef CAS
.
- M. Mutailipu, M. Zhang, Z. Yang and S. Pan, Acc. Chem. Res., 2019, 52(3), 791–801 CrossRef CAS
.
- M. Mutailipu and S. Pan, Angew. Chem., Int. Ed., 2020, 59(46), 20302–20317 CrossRef CAS
.
- P. S. Halasyamani and J. M. Rondinelli, Nat. Commun., 2018, 9(1), 1–4 CrossRef CAS
.
- L. Kang, S. Luo, G. Peng, N. Ye, Y. Wu, C. Chen and Z. Lin, Inorg. Chem., 2015, 54(22), 10533–10535 CrossRef CAS
.
- G. Shi, Y. Wang, F. Zhang, B. Zhang, Z. Yang, X. Hou, S. Pan and K. R. Poeppelmeier, J. Am. Chem. Soc., 2017, 139(31), 10645–10648 CrossRef CAS
.
- Y. Wang, B. Zhang, Z. Yang and S. Pan, Angew. Chem., Int. Ed., 2018, 57(8), 2150–2154 CrossRef CAS
.
- B. H. Lei, Z. Yang, H. Yu, C. Cao, Z. Li, C. Hu, K. R. Poeppelmeier and S. Pan, J. Am. Chem. Soc., 2018, 140(34), 10726–10733 CrossRef CAS
.
- F. Pan, G. Shen, R. Wang, X. Wang and D. Shen, J. Cryst. Growth, 2002, 241(1–2), 108–114 CrossRef CAS
.
- X. Zhang, L. Wang, S. Zhang, G. Wang, S. Zhao, Y. Zhu, Y. Wu and C. Chen, J. Opt. Soc. Am. B, 2011, 28(9), 2236–2239 CrossRef CAS
.
- P. Yu, L. M. Wu, L. J. Zhou and L. Chen, J. Am. Chem. Soc., 2014, 136(1), 480–487 CrossRef CAS
.
- S. Zhao, P. Gong, S. Luo, L. Bai, Z. Lin, C. Ji, T. Chen, M. Hong and J. Luo, J. Am. Chem. Soc., 2014, 136(24), 8560–8563 CrossRef CAS
.
- L. Li, Y. Wang, B. H. Lei, S. Han, Z. Yang, K. R. Poeppelmeier and S. Pan, J. Am. Chem. Soc., 2016, 138(29), 9101–9104 CrossRef CAS
.
- L. Li, Y. Wang, B. H. Lei, S. Han, Z. Yang, H. Li and S. Pan, J. Mater. Chem. C, 2017, 5(2), 269–274 RSC
.
- S. Zhao, X. Yang, Y. Yang, X. Kuang, F. Lu, P. Shan, Z. Sun, Z. Lin, M. Hong and J. Luo, J. Am. Chem. Soc., 2018, 140(5), 1592–1595 CrossRef CAS
.
- H. Yu, J. Young, H. Wu, W. Zhang, J. M. Rondinelli and P. S. Halasyamani, Chem. Mater., 2017, 29(4), 1845–1855 CrossRef CAS
.
- B. Zhang, G. Han, Y. Wang, X. Chen, Z. Yang and S. Pan, Chem. Mater., 2018, 30(15), 5397–5403 CrossRef CAS
.
- H. Wu, B. Zhang, H. Yu, Z. Hu, J. Wang, Y. Wu and P. S. Halasyamani, Angew. Chem., Int. Ed., 2020, 59(23), 8922–8926 CrossRef CAS
.
- G. Han, Y. Wang, B. Zhang and S. Pan, Chem.–Eur. J., 2018, 24(67), 17638–17650 CrossRef CAS
.
- J. Lu, J. N. Yue, L. Xiong, W. K. Zhang, L. Chen and L. M. Wu, J. Am. Chem. Soc., 2019, 141(20), 8093–8097 CAS
.
- A. Lieb and M. T. Weller, Z. Anorg. Allg. Chem., 2009, 635(12), 1877–1881 CrossRef CAS
.
- G. Peng, N. Ye, Z. Lin, L. Kang, S. Pan, M. Zhang, C. Lin, X. Long, M. Luo, Y. Chen, Y. H. Tang, F. Xu and T. Yan, Angew. Chem., Int. Ed., 2018, 57(29), 8968–8972 CrossRef CAS
.
- G. Yang, P. Gong, Z. Lin and N. Ye, Chem. Mater., 2016, 28(24), 9122–9131 CrossRef CAS
.
- I. D. Brown and D. Altermatt, Acta Crystallogr., 1985, 41(4), 244–247 CrossRef
.
- S. K. Kurtz and T. T. Perry, J. Appl. Phys., 1968, 39(8), 3798–3813 CrossRef CAS
.
- L. Qi, Z. Chen, X. Shi, X. Zhang, Q. Jing, N. Li, Z. Jiang, B. Zhang and M. H. Lee, Chem. Mater., 2020, 32(19), 8713–8723 CrossRef CAS
.
- S. Zhao, P. Gong, S. Luo, L. Bai, Z. Lin, Y. Tang, Y. Zhou, M. Hong and J. Luo, Angew. Chem., Int. Ed., 2015, 54(14), 4217–4221 CrossRef CAS
.
- M. Abudoureheman, S. Han, B. H. Lei, Z. Yang, X. Long and S. Pan, J. Mater. Chem. C, 2016, 4(45), 10630–10637 RSC
.
- P. Shan, T. Sun, H. Liu, S. Liu, S. Chen, X. Liu, Y. Kong and J. Xu, Cryst. Growth Des., 2016, 16(10), 5588–5592 CrossRef CAS
.
- P. Shan, T. Sun, H. Chen, H. Liu, S. Chen, X. Liu, Y. Kong and J. Xu, Sci. Rep., 2016, 6(1), 1–10 CrossRef
.
- S. Zhao, Y. Yang, Y. Shen, X. Wang, Q. Ding, X. Li, Y. Li, L. Li, Z. Lin and J. Luo, J. Mater. Chem. C, 2018, 6(15), 3910–3916 RSC
.
- J. H. Jiang, L. C. Zhang, Y. X. Huang, Z. M. Sun, Y. Pan and J. X. Mi, Dalton Trans., 2017, 46(5), 1677–1683 RSC
.
- Y. Shen, S. Zhao, B. Zhao, C. Ji, L. Li, Z. Sun, M. Hong and J. Luo, Inorg. Chem., 2016, 55(22), 11626–11629 CrossRef CAS
.
- J. Chen, L. Xiong, L. Chen and L. M. Wu, J. Am. Chem. Soc., 2018, 140(43), 14082–14086 CrossRef CAS
.
- Z. Bai, L. Liu, L. Zhang, Y. Huang, F. Yuan and Z. Lin, Chem. Commun., 2019, 55(58), 8454–8457 RSC
.
- Z. Bai, C. L. Hu, L. Liu, L. Zhang, Y. Huang, F. Yuan and Z. Lin, Chem. Mater., 2019, 31(22), 9540–9545 CrossRef CAS
.
- Y. Li, F. Liang, S. Zhao, L. Li, Z. Wu, Q. Ding, S. Liu, Z. Lin, M. Hong and J. Luo, J. Am. Chem. Soc., 2019, 141(9), 3833–3837 CrossRef CAS
.
- Q. Ding, X. Liu, S. Zhao, Y. Wang, Y. Li, L. Li, S. Liu, Z. Lin, M. Hong and J. Luo, J. Am. Chem. Soc., 2020, 142(14), 6472–6476 CrossRef CAS
.
- P. A. Maggard, T. S. Nault, C. L. Stern and K. R. Poeppelmeier, J. Solid State Chem., 2003, 175(1), 27–33 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Tables of crystal data and structure refinement, fractional atomic coordinates, anisotropic displacement parameters, selected bond lengths and bond angles, figures of EDS, DTA/TG, and DOS, and powder X-ray diffraction patterns. CCDC 2049585. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc00080b |
| This journal is © The Royal Society of Chemistry 2021 |