Open Access Article
Open Access ArticleLithium silicate nanosheets with excellent capture capacity and kinetics with unprecedented stability for high-temperature CO2 capture†
Rajesh
Belgamwar
a,
Ayan
Maity
a,
Tisita
Das
d,
Sudip
Chakraborty
b,
Chathakudath P.
Vinod
 c and
Vivek
Polshettiwar
c and
Vivek
Polshettiwar
 *a
*a
aDepartment of Chemical Sciences, Tata Institute of Fundamental Research (TIFR), Mumbai, India. E-mail: vivekpol@tifr.res.in
bMaterials Theory for Energy Scavenging (MATES) Lab, Department of Physics, Indian Institute of Technology, Simrol, Indore, India
cCatalysis and Inorganic Chemistry Division, CSIR-National Chemical Laboratory (NCL), Pune, India
dHarish-Chandra Research Institute, HBNI, Allahabad, Uttar Pradesh, India
First published on 15th February 2021
Abstract
An excessive amount of CO2 is the leading cause of climate change, and hence, its reduction in the Earth's atmosphere is critical to stop further degradation of the environment. Although a large body of work has been carried out for post-combustion low-temperature CO2 capture, there are very few high temperature pre-combustion CO2 capture processes. Lithium silicate (Li4SiO4), one of the best known high-temperature CO2 capture sorbents, has two main challenges, moderate capture kinetics and poor sorbent stability. In this work, we have designed and synthesized lithium silicate nanosheets (LSNs), which showed high CO2 capture capacity (35.3 wt% CO2 capture using 60% CO2 feed gas, close to the theoretical value) with ultra-fast kinetics and enhanced stability at 650 °C. Due to the nanosheet morphology of the LSNs, they provided a good external surface for CO2 adsorption at every Li-site, yielding excellent CO2 capture capacity. The nanosheet morphology of the LSNs allowed efficient CO2 diffusion to ensure reaction with the entire sheet as well as providing extremely fast CO2 capture kinetics (0.22 g g−1 min−1). Conventional lithium silicates are known to rapidly lose their capture capacity and kinetics within the first few cycles due to thick carbonate shell formation and also due to the sintering of sorbent particles; however, the LSNs were stable for at least 200 cycles without any loss in their capture capacity or kinetics. The LSNs neither formed a carbonate shell nor underwent sintering, allowing efficient adsorption–desorption cycling. We also proposed a new mechanism, a mixed-phase model, to explain the unique CO2 capture behavior of the LSNs, using detailed (i) kinetics experiments for both adsorption and desorption steps, (ii) in situ diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy measurements, (iii) depth-profiling X-ray photoelectron spectroscopy (XPS) of the sorbent after CO2 capture and (iv) theoretical investigation through systematic electronic structure calculations within the framework of density functional theory (DFT) formalism.
Introduction
Fossil fuels provide greater than 85% of the world's energy needs.1 An excessive amount of CO2 is the main cause of climate change. If the use of fossil fuel is to be continued in the coming years, the CO2 emission needs to be dramatically reduced by developing sustainable sorbents to capture and separate CO2 from various industrial processes. For CO2 capture, in general, two protocols can be used, post-combustion and pre-combustion.2–4 Low-temperature sorbents (based on amines, silica, zeolites, carbon, and MOFs) are mainly used for post-combustion CO2 capture from flue gas at low temperatures (25 to 75 °C), while high-temperature sorbents are specifically developed for pre-combustion capture between 500 and 700 °C.2–4 “Pre-combustion capture” is one of the most challenging but best ways to tackle climate change as CO2 is captured during the process at high temperatures with ideally zero release into the environment.5–7 Pre-combustion capture is potentially less expensive than post-combustion capture, as CO2 gets captured as soon as it is produced during the process without releasing it into the environment.5–7Although a large body of work has been carried out for post-combustion low-temperature CO2 capture,2 there are very few high temperature pre-combustion CO2 capture processes. Lithium silicate (Li4SiO4) is one of the best high-temperature CO2 capture sorbents due to its relatively high capture capacities as compared to other alkali metal-containing ceramics.5–7 Although lithium silicates display excellent CO2 sorption capabilities at high temperatures, they face two main limitations, slow capture kinetics and poor stability–recyclability of the sorbents.5–7 Formation of a lithium carbonate product layer limits CO2 diffusion, thus limiting kinetic performance; second, sintering reduces the cycling stability necessary for practical application. Recently, nanowires of lithium silicates and lithium tungstate showed good kinetics of CO2 capture.8,9 However, these nanowires were not stable against agglomeration/sintering during the high-temperature CO2 adsorption–desorption process, with a loss of 50% of their capture performance (capacity and kinetics) in the first three cycles. Thus, there was a need for a sorbent that has the following properties: (i) high CO2 capture at elevated temperatures (between 600 and 750 °C), (ii) faster rate of adsorption/desorption, and (iii) cycling stability for hundreds of CO2 capture–release cycles.
In continuation of our work on CO2 capture and conversion,10–13 here we report lithium silicate nanosheets (LSNs) as a high-temperature CO2 sorbent. The LSNs showed a high CO2 capture capacity of 35.3 wt% CO2 (96.18% efficiency, in comparison with the theoretical capacity of 36.7 wt%) and ultra-fast kinetics (0.22 g g−1 min−1). Notably, the LSNs were stable even after 200 cycles without any loss in their capture capacity or kinetics.
Results and discussion
LSNs were synthesized using dendritic fibrous nanosilica (DFNS).12 The DFNS surface area is highly accessible by virtue of its fibrous nature instead of tubular pores like in MCM-41 and SBA-15.12 After several synthetic optimizations, in terms of lithium precursor, heating temperature and time (Tables S1 to S3†), the desired LSNs were prepared by thermal treatment of physically mixed DFNS and lithium nitrate with a Li![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si molar ratio of 4.66
Si molar ratio of 4.66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
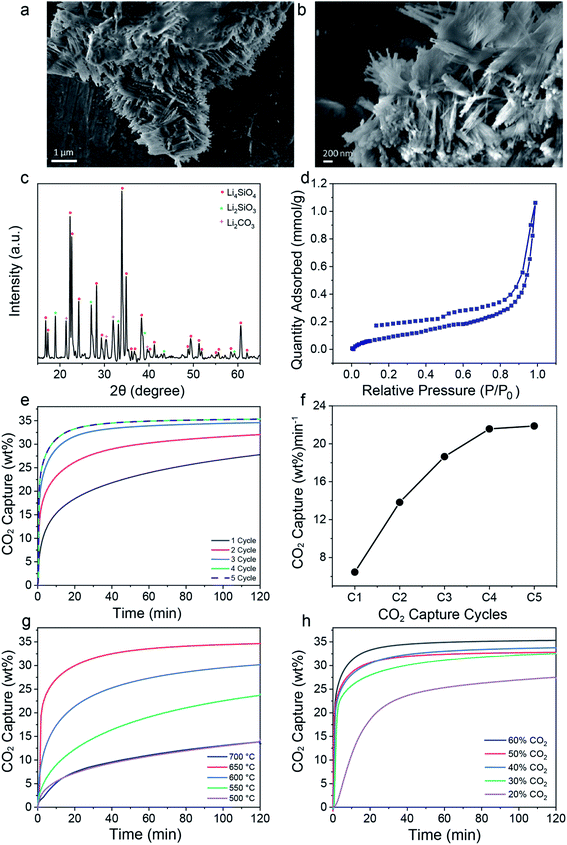
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 650 °C for 6 h in air. Scanning electron microscopy (SEM) images indicated that the LSNs are made up of nanosheets randomly stacked on each other with overall sheet widths of a few microns (Fig. 1a and b). Powder X-ray diffraction (PXRD) of the LSNs showed the presence of Li4SiO4 as a major phase, with Li2SiO3 and Li2CO3 as minor phases (Fig. 1c). These impurities were due to the reaction of the LSNs with trace amounts of CO2 present (∼400 ppm) in the environment during LSN synthesis. Synthesis of the pure phase of Li4SiO4 was possible by using an inert atmosphere (Fig. S1†); however, since a small amount of minor phases did not affect the CO2 performance of the LSNs, we chose sustainable synthesis conditions in an air environment. The N2 sorption isotherm (Fig. 1d) displayed type IV characteristics with hysteresis and a low surface area of 8 m2 g−1.
1 at 650 °C for 6 h in air. Scanning electron microscopy (SEM) images indicated that the LSNs are made up of nanosheets randomly stacked on each other with overall sheet widths of a few microns (Fig. 1a and b). Powder X-ray diffraction (PXRD) of the LSNs showed the presence of Li4SiO4 as a major phase, with Li2SiO3 and Li2CO3 as minor phases (Fig. 1c). These impurities were due to the reaction of the LSNs with trace amounts of CO2 present (∼400 ppm) in the environment during LSN synthesis. Synthesis of the pure phase of Li4SiO4 was possible by using an inert atmosphere (Fig. S1†); however, since a small amount of minor phases did not affect the CO2 performance of the LSNs, we chose sustainable synthesis conditions in an air environment. The N2 sorption isotherm (Fig. 1d) displayed type IV characteristics with hysteresis and a low surface area of 8 m2 g−1.
The CO2 capture performance of the LSNs was evaluated using thermogravimetric analysis (TGA). First, the CO2 adsorption/desorption of the LSNs was studied at 650 °C using 60% CO2 (balance nitrogen) with 25 mg of sorbent (Fig. 1e and f, and S2† for the effect of sorbent weight). Notably, we observed an increase in capture capacity from 0.277 to 0.353 g g−1 as well as initial kinetics from 0.06 to 0.22 g g−1 min−1 up to the 4th cycle. The increase in capture capacity and kinetics up to the 4th cycle was due to systematic purification of the as-prepared Li4SiO4, which had Li2SiO3, and Li2CO3 as minor phases. With every cycle of CO2 adsorption–desorption, these minor phases were converted to Li4SiO4 and at the 4th cycle, their concentrations were significantly reduced (Fig. 2a). The PXRD pattern indicated the reaction of Li4SiO4 with CO2 to form Li2SiO3 and Li2CO3 during the adsorption step followed by the conversion of Li2SiO3 and Li2CO3 phases to Li4SiO4 during the CO2 desorption step (eqn (1)). This process was reversible (Fig. 2a), which was the reason for the better cycling stability of the LSNs.
| Li4SiO4 + CO2 ⇌ Li2SiO3 + Li2CO3 | (1) |
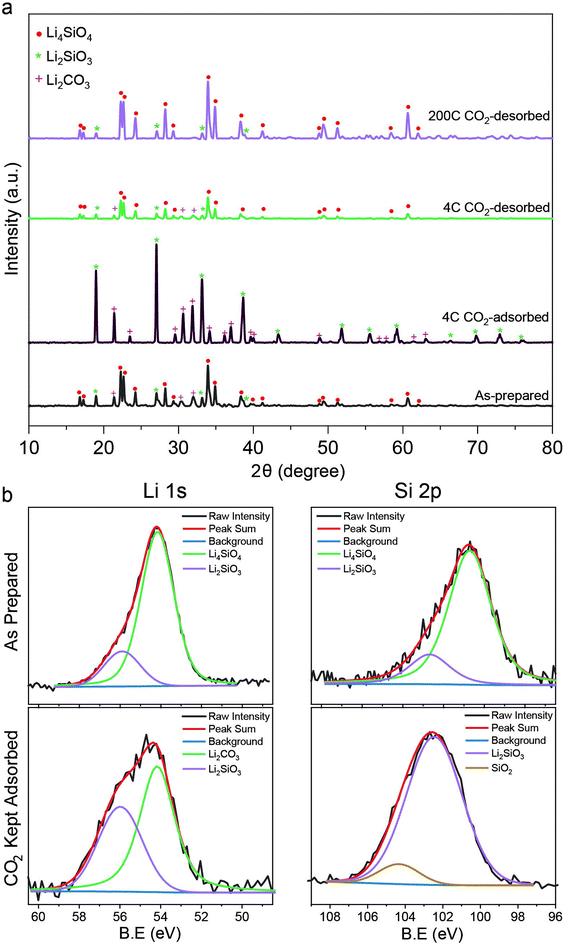 | ||
| Fig. 2 (a) PXRD pattern and (b) XPS spectra of lithium silicates after various CO2 adsorption–desorption cycles. | ||
The LSNs' CO2 capture performance was then studied at different temperatures using a 4-cycle activated sample using 60% CO2 with 25 mg of sorbent (Fig. 1g). The CO2 sorption capacity first increased from 500 to 650 °C and then decreased at 700 °C. At 500 °C, the diffusion process was kinetically controlled, and the diffusion barrier is the main reason for the low CO2 sorption capacity. With the increase in temperature to 650 °C, the diffusion and chemisorption processes were accelerated and hence the CO2 capture rate was much faster. However, at 700 °C, the desorption kinetics of CO2 was faster than the adsorption kinetics, reducing the overall capture capacity and kinetics. Thus, 650 °C was found to be the optimized temperature in terms of CO2 capture capacity. At this temperature, using these 4-cycle activated LSNs, the effect of CO2 concentration was also studied, with 20, 30, 40, 50 and 60% CO2, all of which (except 20% CO2) showed good capture capacities and kinetics (Fig. 1h and S3†), indicating their usability in various pre-combustion processes where different amounts of CO2 are generated. We then studied the effect of gas flow using 150, 100, and 50 mL min−1 of 100% CO2 (Fig. S4†). The LSNs showed good capture rates with all the flows, indicating their usability in various processes with different flows.
X-ray photoelectron spectroscopy (XPS) was performed to study the LSNs and how they change during CO2 adsorption–desorption steps (Fig. 2b). The XPS spectra contained two peaks for Li (1s) at binding energies (BEs) of ∼54 eV and ∼55.9 eV. The peak at ∼54 eV was assigned to Li4SiO4 (major phase)14 as well as Li2CO3 (minor phase).15 Although their deconvolution was not possible due to their very close binding energy (BE), based on PXRD, this peak at ∼54 eV was assigned mainly to Li4SiO4. The signal at ∼55.9 eV was assigned to Li2SiO3.14,15 After CO2 capture, in the adsorbed 4-cycle activated sample, the area under the peak of Li2SiO3 at ∼55.9 eV increased due to the conversion of Li4SiO4 to Li2SiO3 and Li2CO3, as also observed in PXRD (Fig. 2a). The formation of Li2CO3 was observed with the signal at ∼54 eV BE (overlapping with the Li4SiO4 signal), but clearly differentiable in PXRD. The Si (2p) spectra of the LSNs showed two Si signals, at BEs of ∼101 eV for Li4SiO4 and ∼102 eV for Li2SiO3 (Fig. 2a).14,15 After CO2 adsorption, ideally, there should be only one Si site, as PXRD indicates the presence of Li2SiO3 and Li2CO3 only (Fig. 2a). However, we observed two signals, at ∼102 and ∼104 eV. This indicates that the signal at ∼104 eV was for SiO2.16
The LSNs' stability and cyclability were then studied and the results indicated that the LSNs could be used for at least 200 cycles without any loss in their capture capacity or kinetics (Fig. 3a). We compared the recyclability of the LSNs with that of the best lithium silicates reported in the literature (Fig. 3b and Table S4†). The LSNs were found to be stable and did not lose their capture capacity or kinetics during the adsorption–desorption cycling. In general, conventional lithium silicates systematically lose their capture capacity with every cycle with a significant loss in the first few cycles (Fig. 3b);5–7,17–29 however, the LSNs were found to be stable even after 200 cycles. The LSNs were even better than one of the best recently reported sorbents, lithium silicate nanowires (NWs)8 (Fig. 3c). The NW sorbent lost more than 50% of its capture capacity, which fell from 0.35 to 0.15 g g−1, in just four cycles, while the LSNs capture capacity (0.35 g g−1) did not change even after 200 cycles. The kinetics of the NWs reduced to 0.1 g g−1 min−1 in 4 cycles, while the LSNs kinetics remained at 0.22 g g−1 min−1 even after 200 cycles. Thus, we found that the LSNs outperform the NWs and other reported sorbents in terms of capture capacity and kinetics as well as cycling stability. Notably, the LSNs were even stable in the presence of a CO2 feed containing water vapor (Fig. S5†).
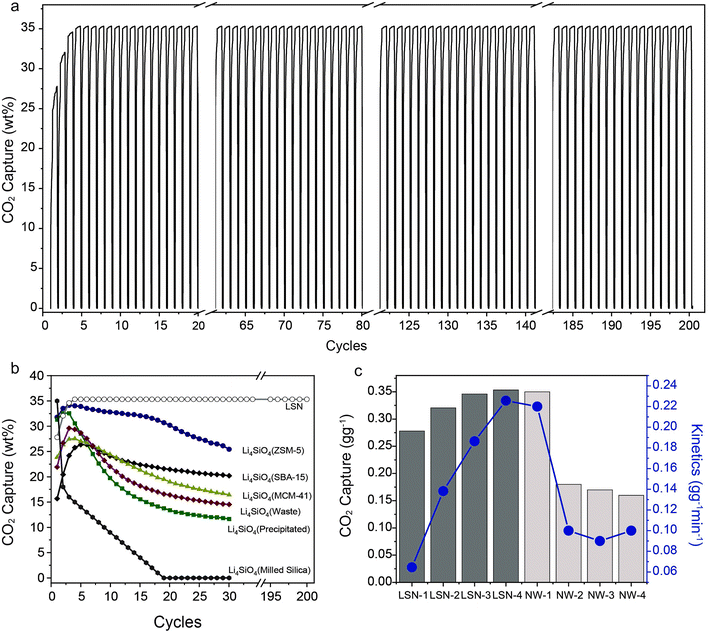 | ||
| Fig. 3 (a) Stability and cyclability of the LSNs for 200 cycles of CO2 adsorption–desorption, (b) comparison with various best-known lithium silicate sorbents, and (c) cycle-by-cycle comparison of the LSNs with lithium silicate nanowires (NWs)8 at 650 °C using 60% CO2. | ||
For most of the reported Li4SiO4, due to its dense morphology and large particle size (Table S4†), CO2 capture took place mainly at the surface, while the interior surface cannot fully interact with CO2, which limits the capture capacity of the conventional lithium silicate material. Also, as pore size has a critical impact on the CO2 capture performance of Li4SiO4,5 due to the formation of Li2SiO3 and Li2CO3, which have a higher molar volume than Li4SiO4, the pores get blocked due to this volumetric expansion, causing further degradation in the sorbent's CO2 capture performance (capacity, kinetics, and recyclability). Our synthesized sorbent LSNs, due to their thin sheets, provided a good external surface and allowed CO2 diffusion to every Li-site without any hindrance. Although the LSNs aggregated after 4 cycles, these sheets did not undergo sintering even after 200 cycles, possibly because they were covalently interlinked with each other between the sheets (Fig. S6 and S7†), and remained stable for 200 cycles. This was the reason for the excellent CO2 sorption performance of this sorbent (with CO2 capture capacity close to the theoretical value).
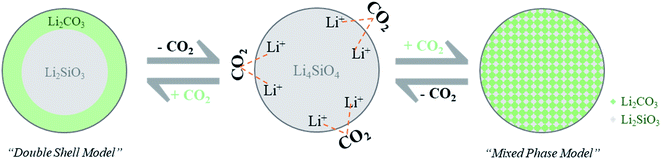
According to the double-shell model5 (Fig. 4, left), first Li4SiO4 reacts with CO2 at the sorbent surface to generate an external shell made up of Li2CO3. Once this shell is formed, the diffusion of CO2 and Li+ through this external shell dictates the sorbent performance. The formation of thick carbonate shells was the main reason for the degradation of capture performance for the conventional lithium silicates.5 In the case of the LSNs, the sheet thickness is a few nanometers, and hence CO2 and Li+ can easily diffuse across the entire sheet, and CO2 reacts with Li+ without the formation of the external shell. The rate of the CO2 capture is generally limited by the diffusion of CO2 and Li+ through the external shell; however, since no such shell was formed in the LSNs, they showed extremely fast CO2 capture kinetics (0.22 g g−1 min−1). Based on these observations, we propose that the LSNs adsorb/desorb CO2via a mixed-phase model and not the double-shell model (Fig. 4).
To study this process and understand the effect on CO2 chemisorption and Li+ diffusion, isotherms at different temperatures were fitted to the double-exponential equation (eqn (2); Fig. S8†)
y = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−k1x) + B exp(−k1x) + B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−k2x) + C exp(−k2x) + C | (2) |
Table 1 summarizes the kinetic parameters obtained after this fitting from the LSNs' CO2 adsorption isotherms (Fig. S4†). At the optimized sorption temperature, 650 °C, k1 was 0.065421 s−1, far better than the reported values for conventional silicates,5–7 indicating extremely fast CO2 chemisorption by interaction with Li-ions on the surface of the LSNs (Fig. 4). This confirms the role of nanosheet morphology, which allowed more external surface and less core, causing efficient CO2 adsorption and indicating the mixed-phase model of CO2 capture. In conventional silicates, in the 2nd step, the Li-ion has to diffuse through carbonate shells (as per the double-shell model, Fig. 4), and this slows down the CO2 capture kinetics (k2). In the case of LSNs, k2 was 0.00164 s−1, similar to reported values. However, in the case of LSNs, more than 90% of the total CO2 capture happened in the first 3 minutes, indicating that k1 (CO2 chemisorption) is the rate-limiting step and not k2 (Li+ diffusion). This again indicates that the LSNs' CO2 capture follows the proposed mixed-phase model.
| T (°C) | k 1 (s−1) | k 2 (s−1) | R 2 |
|---|---|---|---|
| 500 | 0.003510 | 0.00093 | 0.99878 |
| 550 | 0.003783 | 0.00028 | 0.99943 |
| 600 | 0.006214 | 0.00048 | 0.99771 |
| 650 | 0.065421 | 0.00164 | 0.99068 |
| 700 | 0.001788 | 0.00015 | 0.99960 |
In order to gain more insight into the mixed-phase model, we compared the LSN sorbent (prepared from DFNS) with a conventional sorbent prepared from Stöber silica, named Stöber lithium silicate (SLS), which is known to follow the double-shell model of CO2 adsorption–desorption. When we compared the CO2 capture performance of the LSNs and SLS using feed with various CO2 concentrations, in all cases, the LSNs showed far better CO2 capture capacity and kinetics as compared to SLS (Fig. S9†). For further comparison, we chose a feed with a 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 ratio of CO2
40 ratio of CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 and observed that in 2 h, the LSNs capture 35.3 wt% CO2 (22.04 wt% in the first minute) while SLS captures only 26.2 wt% (16.49 wt% in the first minute) (Fig. 5a). The LSNs showed faster kinetics of 5.8 wt% per minute, compared to the 3.1 wt% per minute for SLS (Fig. 5b), indicating faster Li diffusion throughout the LSNs, supporting the mixed-phase model. This model should also significantly affect desorption kinetics, as kinetics should be faster in the case of a mixed-phase model compared to the double-shell model, which is known for slow desorption due to the thick shell. The LSNs showed very fast desorption, 6.8 wt% per minute, while SLS desorbed 5.1 wt% per minute with respect to their total capture capacity. A similar trend was observed under all three flow conditions (Fig. S10†). This confirms the presence of a mixed-phase model in the LSNs but the double-shell model in SLS.
N2 and observed that in 2 h, the LSNs capture 35.3 wt% CO2 (22.04 wt% in the first minute) while SLS captures only 26.2 wt% (16.49 wt% in the first minute) (Fig. 5a). The LSNs showed faster kinetics of 5.8 wt% per minute, compared to the 3.1 wt% per minute for SLS (Fig. 5b), indicating faster Li diffusion throughout the LSNs, supporting the mixed-phase model. This model should also significantly affect desorption kinetics, as kinetics should be faster in the case of a mixed-phase model compared to the double-shell model, which is known for slow desorption due to the thick shell. The LSNs showed very fast desorption, 6.8 wt% per minute, while SLS desorbed 5.1 wt% per minute with respect to their total capture capacity. A similar trend was observed under all three flow conditions (Fig. S10†). This confirms the presence of a mixed-phase model in the LSNs but the double-shell model in SLS.
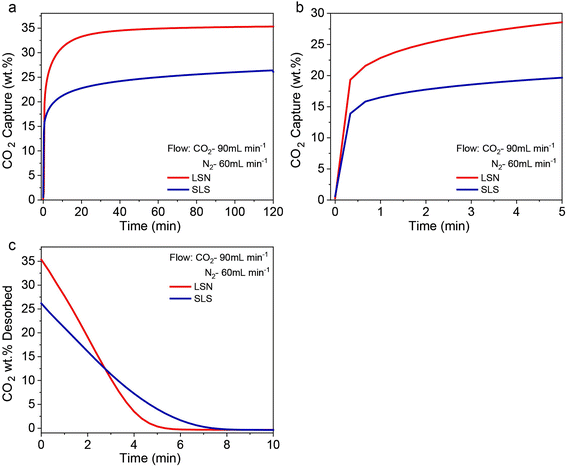 | ||
| Fig. 5 CO2 capture by the LSNs and SLS at 650 °C using 60% CO2: (a) CO2 adsorption thermograms of the LSNs and SLS; (b) CO2 capture kinetics in the first five minutes; (c) CO2 desorption kinetics. | ||
If the proposed mixed-phase model is correct, CO2 chemisorption should be a rate-determining step and not lithium-ion diffusion. The extremely high value of k1 and low value of k2 (in Table 1) clearly indicate that chemisorption is a major path of CO2 adsorption, while the lithium-ion diffusion path is insignificant. We then fitted the adsorption kinetics to a single exponential equation (eqn (S1)†) and found that k1 was 0.00285 s−1 for the LSNs and 0.00093 s−1 for SLS (Fig. S11 and Table S5†). In addition to adsorption kinetics, desorption kinetics should also be accelerated by the mixed-phase model. We hence fitted the desorption (Fig. 5c) to the single exponential equation (Fig. S12†) and found that k1 was 0.002027 s−1 for the LSNs as compared to only 0.001652 s−1 for SLS. This also confirms the presence of a mixed-phase model in the case of LSNs.
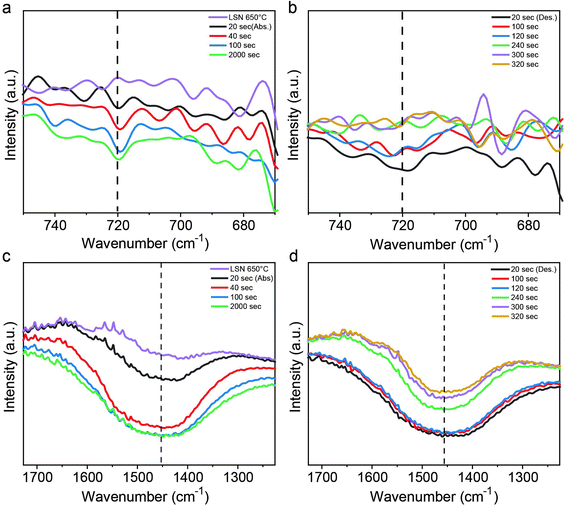
To gain mechanistic insights into the CO2 capture process of CO2 adsorption on the Li4SiO4 surface and the formation of Li2CO3 and Li2SiO3, we carried out in situ diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy measurements of the LSNs under similar CO2 adsorption and desorption conditions. For the adsorption study, in a reactor chamber with a heater and gas flow controller, the LSNs were treated with CO2 (60%) at 650 °C, while for the desorption study, the LSNs, after CO2 treatment, were treated with N2 at 650 °C and an IR spectrum was recorded at various time intervals. The band at 2342 cm−1 was assigned to the linearly chemisorbed CO2 molecule on the lithium silicate surface (Fig. S13a and b†),30 while the band in the region of 3500 to 3900 cm−1 was assigned to Si–OH10 of lithium silicates (Fig. S13c and d†).
The intensity of the band in the region of 650–750 cm−1, assigned to the stretching vibration of Si–O–Si of the SiO3 chain present in Li2SiO3 (ref. 31), increased with time (Fig. 6a) as the LSNs on exposure to CO2 (adsorption step) converted to Li2SiO3 and Li2CO3. When CO2 was replaced by a N2 flow (desorption step), the intensity of these bands decreased with time (Fig. 6b), indicating that Li2SiO3 and Li2CO3 converted back into Li4SiO4. The desorption took place in just 240 s (4 minutes), and such fast desorption indicates the mixed-phase model, as the double-shell model had very slow desorption kinetics. Along similar lines, the intensity of the band in the region of 1300–1600 cm−1, assigned to the stretching vibration of C–O in the carbonate ion (CO32−) present in Li2CO3 (ref. 32), increased with time (Fig. 6c) as the LSNs on exposure to CO2 (adsorption step) converted to Li2SiO3 and Li2CO3. During the desorption (Fig. 6d), this signal intensity decreased with time. Since complete disappearance was not observed, the as-synthesized LSNs have the impurity of lithium carbonate.
To further study the proposed mixed-phase model of CO2 adsorption–desorption, an XPS depth-profiling study of the LSNs was carried out by etching at various depths of the LSN sample after the CO2 adsorption step (Fig. 7). If it is a conventional double-shell model, one should see lithium carbonate only at the surface (and not in the core) in XPS, while if it is the proposed mixed-phase model, lithium carbonate should be present at the surface as well as inside the core. The XPS depth profile of the LSN sample after CO2 adsorption is shown in Fig. S14.† We calculated the area under the peak for Li 1s corresponding to Li2SiO3 and Li2CO3 (Fig. 7a) and observed that at all depths from 0 to 255 nm, there was a presence of both Li2CO3 and Li2SiO3, clearly confirming the mixed-phase model. This was further confirmed by comparing the area under the peak for Si 2p and C 1s at various depths (Fig. 7b and c) and notably, Si 2p and C 1s also followed the same trend as Li 1s, confirming the presence of Li2CO3 and Li2SiO3 across the LSNs. This further supports the proposed mixed-phase model of CO2 adsorption and desorption by the LSNs.
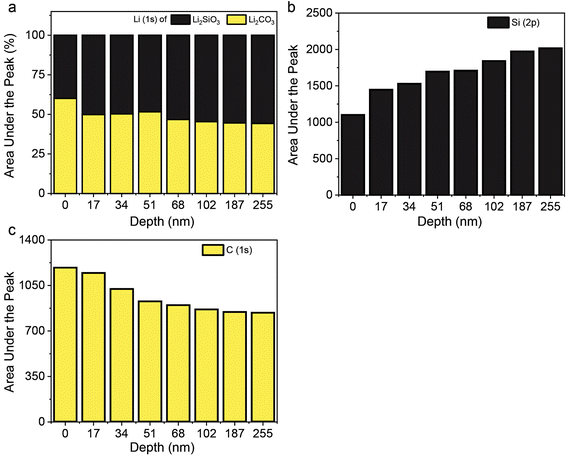 | ||
| Fig. 7 Area under the peak of (a) Li 1s for Li2SiO3 and Li2CO3, and (b) Si 2p and (c) C 1s of the LSN sample in the CO2 adsorbed state, from XPS fitting (Fig. S13†) at different etching depths. | ||
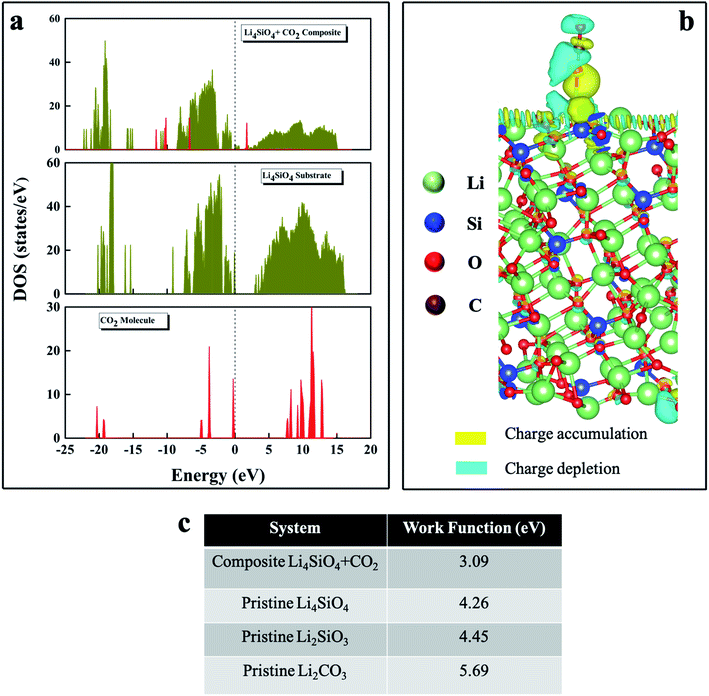
In order to gain further insight into the adsorption/desorption kinetics of the LSN sorbent, a theoretical investigation was carried out through systematic electronic structure calculations within the framework of density functional theory (DFT) formalism. In our DFT modeling, we primarily highlighted the correlation between adsorption characteristics and the corresponding charge transfer mechanism for the sorbent and adsorbate along with the adsorbate induced work function change, which are immensely instrumental fundamental parameters for any surface investigation. We analyzed the electronic density of states (DOS) of the composite (CO2 adsorbed on the LSNs) as well as the individual systems after and before the adsorption process to elucidate the salient features of the electronic structures, as depicted in Fig. 8. The middle panel of Fig. 8a illustrates the total DOS of Li4SiO4 before CO2 adsorption, exhibiting a wide bandgap semiconducting nature. But as soon as CO2 gets adsorbed on top of the LSNs, finite states are found to emerge near the Fermi level (EF) due to the interaction between Li4SiO4 and the CO2 molecule, as shown in the top panel of Fig. 8a. Interestingly, we observed that the density of states corresponding to an isolated CO2 molecule (as depicted in the bottom panel of Fig. 8a) has been shifted substantially in the vicinity of EF under the influence of the LSN surface, which is also manifested through the charge transfer mechanism representation in the subsequent section. It is worth mentioning that we observed the finite density of states appear near the Fermi region after adsorption in the composite system. This reveals the fact that the adsorbate in the form of CO2 is tuning the bandgap of the surface, which is Li4SiO4, which has also been reflected in the substantial charge transfer process. As we investigated the interaction of an incoming CO2 with the Li4SiO4 surface, the noticeable change in the electronic structure through the appearance of finite states after adsorption signifies the fact that CO2 is chemisorbed on the Li4SiO4 surface. The composite system represents the left-hand side of the reaction mechanism of CO2 adsorption by Li4SiO4 before converting to the product form, while our theoretical analysis focused on the surface adsorption, whereas in the experimental section, the CO2 adsorption occurred within the nanosheets of Li4SiO4.
The charge density distribution is illustrated in Fig. 8b, where it is observed that the charge is mainly accumulated around the Li–O bond region, indicating a valuable observation and validation of this work, as reflected in the covalent bond formation between the LSN surface and CO2. This particular theoretical finding has shed light not only on the CO2 capture but also on the subsequent desorption possibility, as found in the experimental outcome. Bader charge analysis indicates that 0.85 e− Å−3 (amount of charge) is transferred from a Li atom of LSNs to an O atom of CO2 during the adsorption process. During the electron transfer process from the Li atom to the O atom of CO2, covalent bond formation occurred, which eventually helps in the CO2 adsorption/capture. This particular covalent bond formation also supports the adsorption/desorption process, as desorption would be favorable in the absence of a strong ionic bond between the surface and adsorbate. This is due to the higher electronegativity of oxygen atoms as compared to lithium and carbon, as both of them share their electrons with oxygen, which leads to charge depletion around the lithium and carbon atoms.
The work function values (Fig. 8c) for Li2SiO3 and Li2CO3 are higher as compared to those of both LSNs and LSN–CO2 composite systems, suggesting that a larger amount of energy is required to extract an electron from Li2SiO3 and Li2CO3 surfaces as compared to the Li4SiO4 system. Therefore, we could infer the favorable capture of a CO2 molecule by the LSNs, while the subsequent desorption of CO2 had been reflected in the previously discussed Bader charge analysis. This observation has also been verified theoretically from the reaction energy value of the reaction (Li4SiO4 + CO2 ⇌ Li2SiO3 + Li2CO3), which has been calculated as −1.36 eV from DFT total energy calculations. Here, the negative reaction energy signifies the exothermic, spontaneous CO2 capture process. Thus, we have theoretically validated the experimental observation of not only the CO2 capture process but also the CO2 desorption. The adsorption of CO2 has also been confirmed from the elongated C–O bond being closer to the LSN surface as compared to that in the pre-adsorbed state, while the corresponding adsorption energy is found to be −1.54 eV.
The cycling stability of the sorbents is determined by the efficiency of CO2 desorption, which is known to be difficult from an interior (core) portion of the double-shell model in conventional lithium silicates, causing a reduction in CO2 capture performances with every cycle for reported lithium silicates (Fig. 3b).5 In addition to the formation of thick carbonate shells, the sintering of sorbent particles was another reason for the fast decline in capture capacity and kinetics in conventional silicates.8,9 In the LSN sorbent, due to the absence of any carbonate shell, CO2 desorption was very efficient (nearly 100%, Fig. 3a). This allowed efficient regeneration and reuse of the LSNs, and even after 200 cycles, they remained active, without even a minute loss of CO2 capture capacity or kinetics. Also, although the sheets of LSNs have nano-dimensions, they are stacked and their overall particle size is in microns, making them sinter-proof and stable. Thus, the LSN sorbent neither generated the carbonate shell nor underwent sintering. This made CO2 adsorption–desorption a completely reversible process and hence the LSNs were stable and recyclable even after 200 cycles.
Conclusions
In this work, we synthesized lithium silicate nanosheets by solid-state thermal treatment of DFNS and lithium nitrate. The LSNs showed CO2 capture capacity close to the theoretical value, with a high rate of CO2 adsorption. The nanosheet morphology of the LSNs provided a good external surface for efficient CO2 interaction with every Li-site, yielding high CO2 capture capacity (35.3 wt% CO2 using 60% CO2 feed gas). The rate of CO2 capture is generally limited by the diffusion of CO2 and Li+ through the external carbonate shell, but since no such shell was formed in the LSNs, they showed extremely fast CO2 capture kinetics (0.22 g g−1 min−1). Notably, the LSNs show dramatic stability (even after 200 cycles without any loss in their capture capacity or kinetics), as compared to reported sorbents, which are known to systematically lose their capture capacity significantly. The LSN sorbent neither formed a carbonate shell nor underwent sintering, causing efficient adsorption–desorption with no loss in capture performance. Thus, the LSNs have all three desirable properties (high capture capacity, fast kinetics and cycling stability for more than 200 cycles) of a CO2 sorbent. Various kinetics and spectroscopic studies (XPD depth profiling and in situ DRIFT) were performed to prove the proposed mechanism of the mixed-phase model, which explained the unique CO2 capture behavior of lithium silicate nanosheets in terms of good capture kinetics and prolonged stability.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Department of Atomic Energy (DAE), Government of India (under project no. R&D-TIFR-RTI4003). We acknowledge the use of the EM and XRD facilities of TIFR, Mumbai.References
- H. Yang, Z. Xu, M. Fan, R. Gupta, R. B. Slimane, A. E. Bland and I. Wright, Progress in carbon dioxide separation and capture: a review, J. Environ. Sci., 2008, 20, 14–27 CrossRef CAS.
- J. G. Vitillo, B. Smit and L. Gagliardi, Introduction: Carbon capture and separation, Chem. Rev., 2017, 117, 9521–9523 CrossRef CAS.
- V. O. Özçelik, K. Gong and C. E. White, Highly surface-active Ca(OH)2 monolayer as a CO2 capture material, Nano Lett., 2018, 18, 1786–1793 CrossRef.
- M. Bui, et al., Carbon capture and storage (CCS): the way forward, Energy Environ. Sci., 2018, 11, 1062–1176 RSC.
- Y. Zhang, Y. Gao, H. Pfeiffer, B. Louis, L. Sun, D. O'Hare and Q. Wang, Recent advances in lithium containing ceramic based sorbents for high-temperature CO2 capture, J. Mater. Chem. A, 2019, 7, 7962–8005 RSC.
- Y. Hu, W. Liu, Y. Yang, M. Qu and H. Li, CO2 capture by Li4SiO4 sorbents and their applications: current developments and new trends, Chem. Eng. J., 2019, 359, 604–625 CrossRef CAS.
- M. Kato, S. Yoshikawa and K. Nakagawa, Carbon dioxide absorption by lithium orthosilicate in a wide range of temperature and carbon dioxide concentrations, J. Mater. Sci. Lett., 2002, 21, 485–487 CrossRef CAS.
- A. Nambo, J. He, T. Q. Nguyen, V. Atla, T. Druffel and M. Sunkara, Ultrafast carbon dioxide sorption kinetics using lithium silicate nanowires, Nano Lett., 2017, 17, 3327–3333 CrossRef CAS.
- M. Z. Akram, V. Atla, A. Nambo, B. P. Ajayi, J. B. Jasinski, J. He, J. R. Gong and M. Sunkara, Low-temperature and fast kinetics for CO2 sorption using Li6WO6 nanowires, Nano Lett., 2018, 18, 4891–4899 CrossRef CAS.
- A. K. Mishra, R. Belgamwar, R. Jana, A. Datta and V. Polshettiwar, Defects in nanosilica catalytically convert CO2 to methane without any metal and ligand, Proc. Natl. Acad. Sci. U. S. A., 2020, 24, 6383–6390 CrossRef.
- M. Dhiman, A. Maity, A. Das, R. Belgamwar, B. Chalke, Y. Lee, K. Sim, J.-W. Nam and V. Polshettiwar, Plasmonic colloidosomes of black gold for solar energy harvesting and hotspots directed catalysis for CO2 to fuel conversion, Chem. Sci., 2019, 10, 6594–6603 RSC.
- A. Maity, R. Belgamwar and V. Polshettiwar, Facile synthesis protocol to tune size, textural properties and fiber density of dendritic fibrous nanosilica (DFNS): Applications in catalysis and CO2 capture, Nat. Protoc., 2019, 14, 2177–2204 CrossRef CAS.
- A. Maity, S. Chaudhari, J. J. Titman and V. Polshettiwar, Catalytic nanosponges of acidic aluminosilicates for plastic degradation and CO2 to fuel conversion, Nat. Commun., 2020, 11, 3828 CrossRef.
- P. V. Korake and A. G. Gaikwad, Capture of carbon dioxide over porous solid adsorbents lithium silicate, lithium aluminate and magnesium aluminate at pre-combustion temperatures, Front. Chem. Sci. Eng., 2011, 5, 215–226 CrossRef CAS.
- J. F. Moulder, W. F. Stickle, P. E. Sobol and K. D. Bomben, Handbook of X-ray photoelectron spectroscopy, ed. J. Chastain, Perkin-Elmer Corporation Physical Electronics Division, 1992, pp. 34–35 Search PubMed.
- A. Kaur, P. Chahal and T. Hogan, Selective Fabrication of SiC/Si Diodes by Excimer Laser under Ambient Conditions, IEEE Electron Device Lett., 2016, 37, 142–145 CAS.
- H. Kim, H. D. Jang and M. Choi, Facile synthesis of macroporous Li4SiO4 with remarkably enhanced CO2 adsorption kinetics, Chem. Eng. J., 2015, 280, 132–137 CrossRef CAS.
- M. T. Izquierdo, A. Turan, S. Garcia and M. M. Maroto-Valer, Optimization of Li4SiO4 synthesis conditions by a solid state method for maximum CO2 capture at high temperature, J. Mater. Chem. A, 2018, 6, 3249–3257 RSC.
- P. V. Subha, B. N. Nair, P. Hareesh, A. P. Mohamed, T. Yamaguchi, K. G. K. Warrier and U. S. Hareesh, Enhanced CO2 absorption kinetics in lithium silicate platelets synthesized by a sol–gel approach, J. Mater. Chem. A, 2014, 2, 12792–12798 RSC.
- P. V. Subha, B. N. Nair, A. P. Mohamed, G. M. Anilkumar, K. G. K. Warrier, T. Yamaguchi and U. S. Hareesh, Morphologically and compositionally tuned lithium silicate nanorods as high-performance carbon dioxide sorbents, J. Mater. Chem. A, 2016, 4, 16928–16935 RSC.
- N. A. Zubbri, A. R. Mohamed and M. Mohammadi, Parametric study and effect of calcination and carbonation conditions on the CO2 capture performance of lithium orthosilicate sorbent, Chin. J. Chem. Eng., 2018, 26, 631–641 CrossRef CAS.
- K. Wang, X. Wang, P. Zhao and X. Guo, High-temperature capture of CO2 on lithium based sorbents prepared by a water-based sol–gel technique, Chem. Eng. Technol., 2014, 37, 1552–1558 CrossRef CAS.
- M. E. Bretado, V. Guzman Velderrain, D. Lardizabal Gutierrez, V. Collins-Martinez and A. L. Ortiz, A new synthesis route to Li4SiO4 as CO2 catalytic/sorbent, Catal. Today, 2005, 4, 863–867 CrossRef.
- L. Ortiz, M. A. E. Bretado, V. G. Velderrain, M. M. Zaragoza, J. S. Gutierrez, D. L. Gutierrez and V. Collins-Martinez, Experimental and modeling kinetic study of the CO2 absorption by Li4SiO4, Int. J. Hydrogen Energy, 2014, 39, 16656–16666 CrossRef.
- S. Shan, S. Li, Q. Jia, L. Jiang, Y. Wang and J. Peng, Impregnation precipitation preparation and kinetic analysis of Li4SiO4-based sorbents with fast CO2 adsorption rate, Ind. Eng. Chem. Res., 2013, 52, 6941–6945 CrossRef CAS.
- X. Yang, W. Liu, J. Sun, Y. Hu, W. Wang, H. Chen, Y. Zhang, X. Li and M. Xu, Preparation of novel Li4SiO4 sorbents with superior performance at low CO2 concentration, ChemSusChem, 2016, 9, 1607–1613 CrossRef CAS.
- Y. Hu, W. Liu, Y. Yang, X. Tong, Q. Chen and Z. Zhou, Synthesis of highly efficient, structurally improved Li4SiO4 sorbents for high-temperature CO2 capture, Ceram. Int., 2018, 44, 16668–16677 CrossRef CAS.
- G. J. Rao, R. Mazumder, S. Bhattacharyya and P. Chaudhuri, Synthesis, CO2 absorption property and densification of Li4SiO4 powder by glycine-nitrate solution combustion method and its comparison with solid state method, J. Alloys Compd., 2017, 725, 461–471 CrossRef CAS.
- Y. Hu, W. Liu, Z. Zhou and Y. Yang, Preparation of Li4SiO4 sorbents for carbon dioxide capture via a spray-drying technique, Energy Fuels, 2018, 32, 4521–4527 CrossRef CAS.
- J. A. Lercher, C. Colombier and H. Noller, Acid–base properties of alumina–magnesia mixed oxides. Part 4. Infrared study of adsorption of carbon dioxide, J. Chem. Soc., Faraday Trans. 1, 1984, 80, 949–959 RSC.
- M. L. Grassoa, P. Arneodo Larochettea and F. C. Gennari, CO2 capture properties of Li4SiO4 after aging in air at room temperature, J. CO2 Util., 2020, 38, 232–240 CrossRef.
- K. Coenen, F. Gallucci, B. Mezari, E. Hensen and M. V. Annaland, An in situ IR study on the adsorption of CO2 and H2O on hydrotalcites, J. CO2 Util., 2018, 24, 228–239 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental, the effect of mole ratio, the effect of thermal treatment temperature and time, the effect of CO2 flow, XPS data, and kinetic data. See DOI: 10.1039/d0sc06843h |
| This journal is © The Royal Society of Chemistry 2021 |