Open Access Article
Open Access ArticleA window-space-directed assembly strategy for the construction of supertetrahedron-based zeolitic mesoporous metal–organic frameworks with ultramicroporous apertures for selective gas adsorption†
Lei
Zhang
*abcd,
Fangfang
Li
ad,
Jianjun
You
ad,
Nengbin
Hua
ad,
Qianting
Wang
ad,
Junhui
Si
ad,
Wenzhe
Chen
ad,
Wenjing
Wang
 b,
Xiaoyuan
Wu
b,
Xiaoyuan
Wu
 b,
Wenbin
Yang
b,
Wenbin
Yang
 b,
Daqiang
Yuan
b,
Daqiang
Yuan
 b,
Canzhong
Lu
b,
Canzhong
Lu
 *be,
Yanrong
Liu
*be,
Yanrong
Liu
 f,
Abdullah M.
Al-Enizi
f,
Abdullah M.
Al-Enizi
 g,
Ayman
Nafady
g and
Shengqian
Ma
g,
Ayman
Nafady
g and
Shengqian
Ma
 *c
*c
aCollege of Materials Science and Engineering, Fujian University of Technology, Fuzhou 350118, China. E-mail: leizhang@fjut.edu.cn
bCAS Key Laboratory of Design and Assembly of Functional Nanostructures, Fujian Provincial Key Laboratory of Nanomaterials, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China. E-mail: czlu@fjirsm.ac.cn
cDepartment of Chemistry, University of North Texas, Denton 76201, USA. E-mail: Shengqian.Ma@unt.edu
dCollaborative Innovation Center for Intelligent and Green Mold and Die of Fujian Province, Fuzhou 350118, China
eXiamen Institute of Rare Earth Materials, Chinese Academy of Sciences, Xiamen 361021, China
fEnergy Engineering, Division of Energy Science, Luleå University of Technology, Luleå 97187, Sweden
gDepartment of Chemistry, College of Science, King Saud University, Riyadh 11451, Saudi Arabia
First published on 5th March 2021
Abstract
Despite their scarcity due to synthetic challenges, supertetrahedron-based metal–organic frameworks (MOFs) possess intriguing architectures, diverse functionalities, and superb properties that make them in-demand materials. Employing a new window-space-directed assembly strategy, a family of mesoporous zeolitic MOFs have been constructed herein from corner-shared supertetrahedra based on homometallic or heterometallic trimers [M3(OH/O)(COO)6] (M3 = Co3, Ni3 or Co2Ti). These MOFs consisted of close-packed truncated octahedral cages possessing a sodalite topology and large β-cavity mesoporous cages (∼22 Å diameter) connected by ultramicroporous apertures (∼5.6 Å diameter). Notably, the supertetrahedron-based sodalite topology MOF combined with the Co2Ti trimer exhibited high thermal and chemical stability as well as the ability to efficiently separate acetylene (C2H2) from carbon dioxide (CO2).
Introduction
Metal–organic frameworks (MOFs),1 which are typically obtained via the self-assembly of metal ions/clusters and organic ligands through coordination bonds, have been a topic of research due to their great potential for various applications, including gas storage and separation,2,3 heterogeneous catalysis,4,5 enzyme immobilization6,7 and others. The properties of MOFs are highly dependent on the inherent network topologies, which, in turn, are strongly influenced by the coordination geometry of the metal nodes and the shape of the component organic ligands.8,9 These fascinating MOF network topologies are designed and constructed using numerous rational approaches,10–12 of which the strategy employing supermolecular building blocks (SBBs) is a most popular one; here, metal–organic polyhedra are used as building blocks to assemble MOFs with large cavities and high-connectivity network topologies.13,14 Supertetrahedron (ST),15–17 which is an enlarged SiO4 and AlO4 tetrahedral building unit of traditional inorganic zeolites, is a widely utilized SBB in MOF synthesis. Two of the most extensively studied mesoporous MOFs (MIL-100 and MIL-101) possess the same mtn zeolite topology, which results from corner-sharing STs whose vertices are occupied by chromium trimers.15,16 The presence of metal trimers in MOFs are known to accommodate single or multiple metal ions that exhibit excellent catalytic performance and exceptional gas adsorption and separation abilities.18–21 Of the numerous zeolitic network topologies reported, only two types can be categorized as zeolitic MOFs due to augmentation by STs based on metal trimers, namely, the mtn15,16,22 and β-cristobalite networks.17,23,24 This could be presumably due to the lack of large, high-quality crystals for single-crystal X-ray diffraction (SCXRD) analysis and the complexity of the results obtained via powder X-ray diffraction (PXRD). Despite the associated challenges, the deliberate introduction of corner-shared STs based on metal trimers into MOFs with other zeolitic network topologies, especially those containing suitably sized single-crystals for SCXRD, is a crucial step toward understanding these materials.The most thoroughly studied zeolitic imidazole framework to date is ZIF-8, which possesses a sodalite (sod) topology with a Brunauer–Emmett–Teller (BET) surface area of 1630 m2 g−1.25,26 ZIF-8 contains large β-cavities of approximately 11.6 Å in diameter that are accessible through narrow hexagonal windows. The square window of the β-cavity has a negligible diameter, whereas the hexagonal window is 3.4 Å in diameter. Significantly, ZIF-8 has a high uptake capacity as a result of its higher surface area and pore volume when compared with traditional inorganic zeolites. Furthermore, the presence of the ultramicroporous aperture in ZIF-8 facilitates its application for hydrocarbon separation via a molecular sieving effect.27,28 On the other hand, MOFs with the ultra-micropore scale (i.e., <7 Å) pore sizes are endowed with strong van der Waals interactions with adsorbed gas molecules.29,30 Therefore, the combination of ultramicroporous aperture, high surface area and pore volume could lead to the high gas storage and separation ability of MOFs. The adsorption capacity can be enhanced by extending the pore diameters of the β-cavities from the micro-to the mesoscale. However, this process is often compensated by enlarging the windows of the sod cage to afford pore apertures that are beyond the ultra-micropore scale, thereby weakening the interactions with gas molecules. We speculate that this issue can be addressed by partially closing the windows of the sod cage to retain ultramicroporous aperture thus strengthening the interactions with gas molecules during gas separation procedures. In principle, a sod cage with mesoporous β-cavities can be built by closing the large hexagonal window while keeping the small square window open. Such modification to the sod cage can maintain exceptionally large β-cavities for improved adsorption meanwhile reduce the pore aperture size to below 7 Å for the efficient separation of mixtures. Therefore, it is anticipated that this approach can circumvent the issues arising from the trade-off between higher adsorption capacity and better selectivity of the respective adsorbents.
The pore-space-partition (PSP) approach conceived by Bu features the division of a large cage or channel space into smaller segments by inserting pore-partitioning agents.31,32 The PSP approach significantly increases the stability of the framework and improves gas adsorption and separation.31–35 The C3 symmetric 2,4,6-tri(4-pyridyl)-1,3,5-triazine (tpt) ligand is often used as a pore-partitioning ligand that can be arranged within the windows of various crystalline molecular cages or cage-based MOFs.33,36–38
In this study, an alternative strategy based on the window-space-directed assembly (WSDA) approach is proposed; herein, the windows of the large cage are partially blocked to afford ultramicropore apertures and enhance the host–guest interactions that exist between the host frameworks and the guest molecules. As a proof-of-concept, we designed and synthesized a series of mixed-linker ST-based sod-topology MOFs [M3(OH/O)(H2O)(btc)2(tpt)2/3] (ST-sod-MOFs) containing 1,3,5-benzenetricarboxylate (btc), 2,4,6-tri(4-pyridyl)-1,3,5-triazine (tpt), homometallic trimer clusters (M3) such as Co3 (ST-sod-Co) and Ni3 (ST-sod-Ni), and a heterometallic trimer cluster composed of Co2Ti (ST-sod-Co/Ti); the MOFs generated featured ultramicroporous square windows and a mesoporous sod cage. In these MOFs, STs serve as corner-sharing SBBs to produce a sod net topology, and the paired tpt ligands act as window-space-directing agents located on the large hexagonal windows of the sod cage. Notably, the mesoporous ST-sod-Co/Ti product exhibited high thermal/chemical stability and demonstrated good performance in the separation of acetylene from carbon dioxide.
Results and discussion
The synthesis of the ST-sod-MOFs series were conducted by mixing of inorganic salts (CoSO4·7H2O or Ni(NO3)2·6H2O or CoCl2·6H2O and Cp2TiCl2), btc, and tpt with a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 stoichiometric ratio under solvothermal reaction. All three compounds are isostructural frameworks with homo- or heterometallic trimer clusters. The introduction of highly charged Ti4+ metals in the metal trimers is anticipated to render high thermal and chemical stability due to thermodynamic parameters.19,35 The use of tetravalent Ti4+ precursor and divalent Co2+ precursor afforded heterometallic [Co2Ti(μ3-O)(COO)6] clusters, which were determined by site occupancy refinement and further confirmed by energy dispersive X-ray spectroscopy analysis with the Co/Ti molar ratio (2
6 stoichiometric ratio under solvothermal reaction. All three compounds are isostructural frameworks with homo- or heterometallic trimer clusters. The introduction of highly charged Ti4+ metals in the metal trimers is anticipated to render high thermal and chemical stability due to thermodynamic parameters.19,35 The use of tetravalent Ti4+ precursor and divalent Co2+ precursor afforded heterometallic [Co2Ti(μ3-O)(COO)6] clusters, which were determined by site occupancy refinement and further confirmed by energy dispersive X-ray spectroscopy analysis with the Co/Ti molar ratio (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Fig. S6†). The [Co3(OH)(H2O)(btc)2(tpt)2/3] (ST-sod-Co) is discussed in detail here (Fig. 1). The SCXRD data analysis revealed that ST-sod-Co crystallized in the cubic space group Im
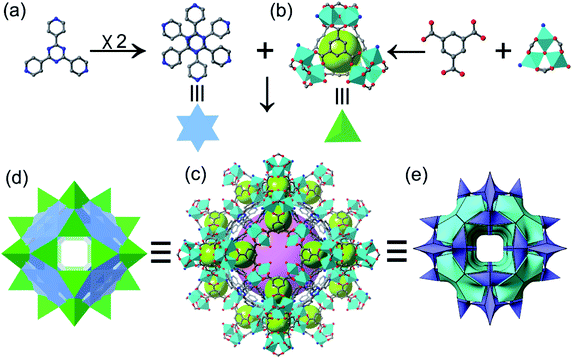
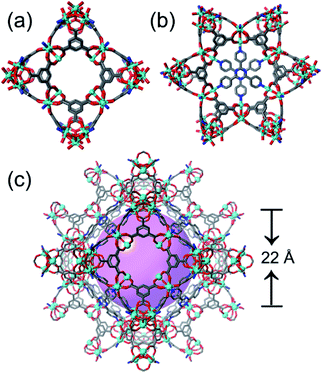
1) (Fig. S6†). The [Co3(OH)(H2O)(btc)2(tpt)2/3] (ST-sod-Co) is discussed in detail here (Fig. 1). The SCXRD data analysis revealed that ST-sod-Co crystallized in the cubic space group Im![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. The lattice parameter of a = b = c = 33.2828(3) Å was much longer than that of ZIF-8 (ca. 16.9910(1) Å). The secondary building units of ST-sod-Co are classic cobalt trimers [Co3(OH)(COO)6] that are connected by four btc ligands to form a ST with a cavity diameter of approximately 4.8 Å; here, the metal trimers and btc ligands were located at the vertices and four faces, respectively (Fig. 1b). The corner-sharing approach caused the STs to assemble into a three-periodic zeolitic sod topology framework featuring a large truncated octahedral sod cage with a diameter of about 22 Å (Fig. 1c and 2c) that is almost double the size of that observed in ZIF-8 (ca. 11.6 Å). Additionally, the larger hexagonal windows of the sod cages were occupied by π–π interaction paired tpt ligands, thereby leaving the relatively smaller square windows open (Fig. 1d, 2a and b). The large β-cavities are accessible via six square windows with ultramicroporous aperture dimensions of 5.57 × 5.57 Å2 (Fig. 2a). Thus, the overall structure of ST-sod-Co could be viewed as close-packed, large truncated octahedral sod cages, each of which possessed eight closed hexagonal windows and six open square windows (Fig. 1d and e).
m. The lattice parameter of a = b = c = 33.2828(3) Å was much longer than that of ZIF-8 (ca. 16.9910(1) Å). The secondary building units of ST-sod-Co are classic cobalt trimers [Co3(OH)(COO)6] that are connected by four btc ligands to form a ST with a cavity diameter of approximately 4.8 Å; here, the metal trimers and btc ligands were located at the vertices and four faces, respectively (Fig. 1b). The corner-sharing approach caused the STs to assemble into a three-periodic zeolitic sod topology framework featuring a large truncated octahedral sod cage with a diameter of about 22 Å (Fig. 1c and 2c) that is almost double the size of that observed in ZIF-8 (ca. 11.6 Å). Additionally, the larger hexagonal windows of the sod cages were occupied by π–π interaction paired tpt ligands, thereby leaving the relatively smaller square windows open (Fig. 1d, 2a and b). The large β-cavities are accessible via six square windows with ultramicroporous aperture dimensions of 5.57 × 5.57 Å2 (Fig. 2a). Thus, the overall structure of ST-sod-Co could be viewed as close-packed, large truncated octahedral sod cages, each of which possessed eight closed hexagonal windows and six open square windows (Fig. 1d and e).
From a topological viewpoint, the [Co3(OH)(COO)6] clusters and the ligands (btc and tpt) could be reduced to yield 8-, 3-, 3-connected nodes, respectively. As a result, the entire ST-sod-Co framework could be defined as a very rare trinodal (3,3,8)-connected network with the point symbol (43)6(46·615·87)3(63)2 (Fig. S4†). However, this initial simplification cannot clearly explain the entire structure of ST-sod-Co. Alternatively, we could ignore the pair of staggered tpt ligands and consider the four cobalt trimers connected via four btc ligands as the crux for ST formation, which was subsequently linked to the other STs in a corner-sharing fashion; as a result, the topology of the sod zeolitic network could be represented as (42·64) (Fig. 1d and S5†). To the best of our knowledge, this is the first example of a sod zeolitic network using corner-sharing, metal trimer-based supertetrahedra.39,40
For a better understanding of the role of tpt ligand on the assembly, a series of prototypical zeolitic ST-sod-MOFs were prepared. Like MIL-100 materials, the metal trimers are interconnected by the tricarboxylate linkers located in the faces to form ST, that extend into a three-dimensional zeolitic mtn topology with pentagonal and hexagonal pore windows. We also tried best to utilize such only ST vertices to obtain sod type MOFs with all open windows but without success. The reactions were carried out using the same protocol but without the use of tpt ligand; the self-assembly of single inorganic salt CoSO4·7H2O or Ni(NO3)2·6H2O and btc ligand led to the formation of unknown crystalline phases (Fig. S10†); while using mixed inorganic salts CoCl2·6H2O and Cp2TiCl2 with btc ligand produced a previously reported porous Co-BTC framework without Ti4+ cations (Fig. S11†).41 These experiments suggest that there appears to be a dominant structure-determining driving force of a pair of staggered tpt ligands acting as the window-space-directed agent leading to the formation of zeolitic mesoporous ST-sod-MOFs.
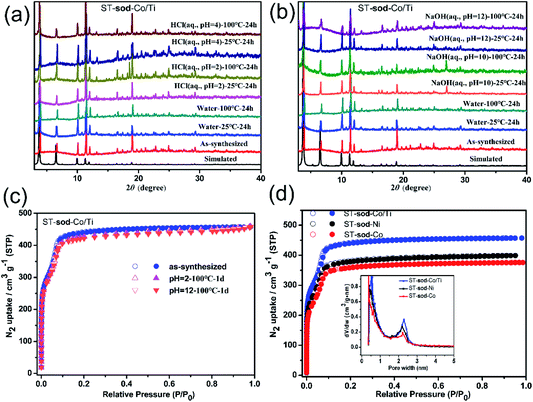
Thermogravimetric analysis revealed that ST-sod-Co, ST-sod-Ni, and ST-sod-Co/Ti were thermally stable up to 320, 350, and 380 °C in N2 stream, respectively (Fig. S15†). Furthermore, in situ variable-temperature PXRD patterns of ST-sod-Ni and ST-sod-Co/Ti under air confirmed the maintenance of their framework integrity up to 300 and 320 °C, respectively (Fig. S12†). The higher thermal stability of ST-sod-Co/Ti was probably due to the presence of stronger Ti(IV)–O coordination bonds. The phase purities of the ST-sod-MOFs were confirmed via PXRD (Fig. S13†), which was also employed to assess the chemical stability of samples treated in water, hydrochloric acid solution (pH = 2), and sodium hydroxide solution (pH = 12) at 25 and 100 °C for 24 h. Notably, the PXRD patterns of ST-sod-Co/Ti remained intact after various treatments and were in good agreement with the calculated patterns obtained from single crystal data, indicating the retention of crystallinity and structural integrity of ST-sod-Co/Ti (Fig. 3a and b). In addition, the stability of ST-sod-Co/Ti was also confirmed by N2 adsorption measurements. The adsorption capacities after water treatment under different conditions showed negligible changes as compared to the pristine sample (Fig. 3c). These results suggested that the Ti4+/Co2+ cooperative crystallization strategy increased the resistance of the framework against hydrolysis. Similar features have also been reported in heterometallic crystalline porous materials.19,20,35
The porosity of the ST-sod-MOFs was assessed by performing N2 sorption measurements at 77 K (Fig. 3d). Both the adsorption and desorption curves of all samples exhibited a reversible Type-I adsorption behavior with a stepwise N2 adsorption isotherm, which is indicative of a mesoporous cage in the framework.42 The saturated uptake values of 376, 400, and 457 cm3 g−1 were obtained for ST-sod-Co, ST-sod-Ni, and ST-sod-Co/Ti, respectively, with corresponding BET surface areas of 1767, 1783, and 2362 m2 g−1, respectively (Fig. S16–S18†). In particular, the BET surface area of ST-sod-Co/Ti was superior to those of other sod-type MOFs such as ZIF-8,25,26 IFMC-1,43 TTF-4,44 CPM-8S,45 and M-BTT (M = Mn, Fe, Co, Cu, Cd; BTT = 1,3,5-benzenetristetrazolate);46 the value is also slightly larger than those of well-known ST-based porous materials such as MIL-143,23 CAU-42,24 PCN-777,47 MOF-808,48 MOF-818,49 and sph-MOF-4.50 The pore volumes of ST-sod-Co, ST-sod-Ni, and T-sod-Co/Ti were as 0.62, 0.62, and 0.71 cm3 g−1, respectively, which were in good agreement with the corresponding theoretical values of 0.65, 0.73, and 0.65 cm3 g−1, respectively, calculated using the PLATON program. Furthermore, the pore size distributions of the ST-sod-MOFs were calculated using the Horvath–Kawazoe (H–K) model assuming the sphere pore geometry, whose result indicates two types of pores with diameters of approximately 5.0 and 22.0 Å (Fig. 3d). These calculated values were in agreement with the effective cavity diameters of the small tetrahedral cage and the large truncated octahedral cage, respectively, as observed in the crystal structure via SCXRD.
The permanent porosity and distinctive cage structure of the three isostructural MOFs in this study encouraged us to examine their selective gas adsorption performance toward acetylene (C2H2), ethane (C2H6), ethylene (C2H4), carbon dioxide (CO2), and methane (CH4). First, single-component adsorption isotherms of the five gases were obtained at 273 and 298 K, where the adsorption capacities of all three isostructural MOFs followed the trend of C2H2 > C2H6 > C2H4 ≈ CO2 ≫ CH4 under the same conditions (Fig. S19–S21†).
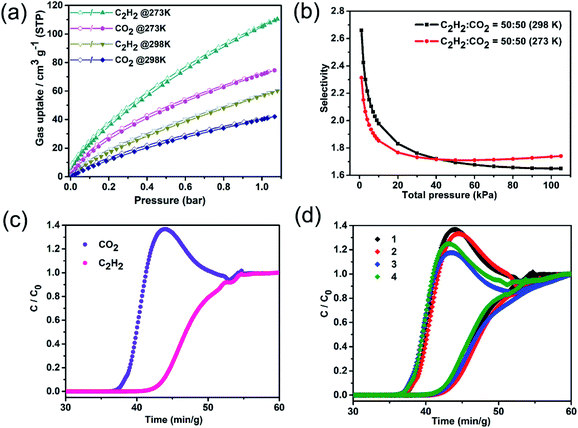
Considering that the removal of CO2 impurities to obtain high-purity C2H2 is highly desirable in industrial applications, the process of C2H2/CO2 separation is an important albeit difficult industrial separation process because of the similarities in molecular size and physicochemical characteristics of C2H2 and CO2.51Fig. 4 shows the results of separation using ST-sod-Co/Ti, which was chosen from the three MOF types examined because it exhibited the highest BET surface area and thermal/chemical stability. The adsorption capacity values of ST-sod-Co/Ti for C2H2 and CO2 at 1.0 bar and 273 K were 105 and 72 cm3 g−1, respectively; at 298 K and 1.0 bar, these values were 57 and 40 cm3 g−1, respectively (Fig. 4a). The resulting C2H2/CO2 uptake ratio of 1.42 for ST-sod-Co/Ti at 298 K and 1.0 bar was comparable to the results obtained for UTSA-74a (1.52)52 and higher than those of benchmark MOFs such as TIFSIX-2-Cu-i (0.95),53 SIFSIX-3-Ni (1.2),54 and KMOF-1-Ni (1.19)55 under the same conditions. The adsorption selectivity of ST-sod-Co/Ti for equimolar binary C2H2/CO2 gas mixtures was evaluated using the ideal adsorbed solution theory based on a single-site Langmuir–Freundlich simulation on the pure-component isotherms of C2H2 and CO2 at 273 and 298 K (Fig. 4b). The adsorption selectivity value of ST-sod-Co/Ti at 298 K was 2.66 at 0.01 bar, which decreased with increasing pressure to 1.65 at 1.0 bar. We noted that the C2H2/CO2 selectivity value of 1.65 for ST-sod-Co/Ti at 298 K and 1.0 bar was lower than those of prototypic MOFs such as FJU-90 (4.3),34 TIFSIX-2-Cu-i (6.5),53 and UTSA-74a (9.0),52 but was comparable to that of Zn-MOF-74 (2.0)52 under similar conditions. The adsorption isotherms of C2H2 and CO2 at 273 and 298 K were fitted using the virial equation (Fig. S22–S27†), and the isosteric heat (Qst) was calculated using the Clausius–Clapeyron equation. The zero-coverage Qst value of C2H2 for ST-sod-Co/Ti was calculated to be 42.4 kJ mol−1, which was much larger than that for CO2 of 33.6 kJ mol−1 (Fig. S28†). This discrepancy between the Qst values may be attributed to the synergistic effects exerted by the ultramicroporous apertures, the open metal sites, and the uncoordinated triazine N-atoms lined on the pores surfaces. Notably, the zero-coverage Qst value for C2H2 in ST-sod-Co/Ti (42.4 kJ mol−1) was at the higher end of the scale for MOF-based solid adsorbents (>40 kJ mol−1)55 and was much higher than those observed for ST-sod-Ni (25.2 kJ mol−1) and ST-sod-Co (29.1 kJ mol−1) as well as the values associated with many other benchmark MOFs, including UTSA-74a (32 kJ mol−1),52 JCM-1 (36.9 kJ mol−1),56 and DICRO-4-Ni-i (37.7 kJ mol−1),57 albeit significant lower than an acetylene nanotrap Cu-ATC (79.1 kJ mol−1) reported very recently.58 However, the Qst value for CO2 in ST-sod-Co/Ti (33.6 kJ mol−1) was similar to that observed for ST-sod-Ni (31.1 kJ mol−1), ST-sod-Co (32.1 kJ mol−1), JCM-1 (33.4 kJ mol−1),56 and DICRO-4-Ni-i (33.9 kJ mol−1).57 These results showed that there were more extensive interactions with C2H2 than CO2, thereby rendering the potential application of ST-sod-Co/Ti for C2H2/CO2 separation.
The C2H2/CO2 separation capability of ST-sod-Co/Ti in practical applications was investigated via mixed-gas breakthrough experiments conducted at 298 K. In these breakthrough experiments, a mixed gas of C2H2/CO2 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) at a total flow of 1 mL min−1 was injected into a column packed with activated ST-sod-Co/Ti. The resulting breakthrough curve indicated that the CO2 gas was the first eluted through the packed column after about 36.6 min g−1, whereas the C2H2 gas was not detected until 42.5 min g−1 (Fig. 4c). This result demonstrated that activated ST-sod-Co/Ti could effectively capture C2H2 from an equimolar C2H2/CO2 mixture via a packed column. Furthermore, the stability and recyclability of activated ST-sod-Co/Ti were examined using recycling breakthrough experiments. As shown in Fig. 4d, the activated ST-sod-Co/Ti was reusable for four cycles with no loss in its adsorption capacity, indicating that the MOF exhibited good regenerability for C2H2/CO2 separation. Thus, ST-sod-Co/Ti holds some promise for the challenging application of C2H2/CO2 separation.
50, v/v) at a total flow of 1 mL min−1 was injected into a column packed with activated ST-sod-Co/Ti. The resulting breakthrough curve indicated that the CO2 gas was the first eluted through the packed column after about 36.6 min g−1, whereas the C2H2 gas was not detected until 42.5 min g−1 (Fig. 4c). This result demonstrated that activated ST-sod-Co/Ti could effectively capture C2H2 from an equimolar C2H2/CO2 mixture via a packed column. Furthermore, the stability and recyclability of activated ST-sod-Co/Ti were examined using recycling breakthrough experiments. As shown in Fig. 4d, the activated ST-sod-Co/Ti was reusable for four cycles with no loss in its adsorption capacity, indicating that the MOF exhibited good regenerability for C2H2/CO2 separation. Thus, ST-sod-Co/Ti holds some promise for the challenging application of C2H2/CO2 separation.
Conclusions
In conclusion, we present a novel window-space-directed assembly strategy for the synthesis of zeolitic mesoporous MOFs with ultramicroporous apertures based on supertetrahedral building units. Three isostructural ST-based ST-sod-MOFs, namely, ST-sod-Co, ST-sod-Ni, and ST-sod-Co/Ti, with homo- or heterometallic trimers were fabricated and characterized via SCXRD. Notably, the ST-sod-MOFs possessed a sod topology whose STs served as nodes. In these ST-sod-MOFs, the large β-cavity mesoporous pores (∼22 Å diameter) were accessible via six square windows with ultramicroporous apertures (∼5.6 Å diameter), whereas their eight hexagonal windows were closed by the paired tpt ligands acting as window-space-directed agents. Remarkably, ST-sod-Co/Ti exhibited good thermal stability, high acid/base-proof stability, and the ability to effectively separate C2H2/CO2 mixtures. Like other heterometallic MOFs with variable metal trimers, this ST-sod-MOFs platform also exhibited enhanced chemical and functional complexity by tuning the metal cations. Thus, our study provides an effective and innovative strategy for building ST-based zeolitic mesoporous MOFs with ultramicroporous apertures and paves the way for the future design and synthesis of functional, highly connected materials for various applications.Author contributions
L. Z., C. L., and S. M conceived the research. H. Y. synthesized samples. L. Z., F. L., J. Y., N. H., Q. W., and J. S., performed the syntheses and structural characterizations of MOFs. W. C., W. W., X. W., W. Y. and D. Y. performed the gas sorption and breakthrough experiments. Y. L. performed the SEM characterizations. L. Z. A. N., A. M. A. and S. M. wrote the manuscript. All authors contributed to the discussion.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Key Research Program of Frontier Science, CAS (QYZDJ-SSW-SLH033), the National Natural Science Foundation of China (21521061, 21701024, 21875252, 51401053), the Central Government Research Program to Guide the Local Scientific and Technological Development (2018L3001), the Natural Science Foundation of Fujian Province (2018J05075, 2018J01629, 2018I0001), the Foundation for Distinguished Young Talents in Higher Education of Fujian Province (GY-Z17067), Projects from CAS Key Laboratory of Design and Assembly of Functional Nanostructures (2013DP173231), and the Scientific Research Foundation of Fujian University of Technology (GY-Z18175, GY-Z17001, GY-Z17152). Partial supports from the China Scholarship Council (CSC, 201909360002) (L. Z.), Carl Tryggers Stiftelse (Y. R. L.), the Researchers Supporting Program project no. (RSP-2021/79) at King Saud University, Riyadh, Saudi Arabia, and the Robert A. Welch Foundation (B-0027) (S. M.) are also acknowledged.References
- H. C. Zhou and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415–5418 RSC.
- X. Zhao, Y. Wang, D. S. Li, X. Bu and P. Feng, Adv. Mater., 2018, 30, 1705189 CrossRef PubMed.
- R.-B. Lin, S. Xiang, W. Zhou and B. Chen, Chem, 2020, 6, 337–363 CAS.
- J. Liu, L. Chen, H. Cui, J. Zhang, L. Zhang and C. Y. Su, Chem. Soc. Rev., 2014, 43, 6011–6061 RSC.
- Z. Niu, W. D. C. Bhagya Gunatilleke, Q. Sun, P. C. Lan, J. Perman, J.-G. Ma, Y. Cheng, B. Aguila and S. Ma, Chem, 2018, 4, 2587–2599 CAS.
- Y. Chen, V. Lykourinou, C. Vetromile, T. Hoang, L. J. Ming, R. W. Larsen and S. Ma, J. Am. Chem. Soc., 2012, 134, 13188–13191 CrossRef CAS PubMed.
- X. Lian, Y. P. Chen, T. F. Liu and H. C. Zhou, Chem. Sci., 2016, 7, 6969–6973 RSC.
- M. Li, D. Li, M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2014, 114, 1343–1370 CrossRef CAS PubMed.
- Y. Wang, L. Feng, W. Fan, K. Y. Wang, X. Wang, X. Wang, K. Zhang, X. Zhang, F. Dai, D. Sun and H. C. Zhou, J. Am. Chem. Soc., 2019, 141, 6967–6975 CrossRef CAS PubMed.
- X. J. Kong, T. He, Y. Z. Zhang, X. Q. Wu, S. N. Wang, M. M. Xu, G. R. Si and J. R. Li, Chem. Sci., 2019, 10, 3949–3955 RSC.
- H. Jiang, J. Jia, A. Shkurenko, Z. Chen, K. Adil, Y. Belmabkhout, L. J. Weselinski, A. H. Assen, D.-X. Xue, M. O'Keeffe and M. Eddaoudi, J. Am. Chem. Soc., 2018, 140, 8858–8867 CrossRef CAS PubMed.
- B. Ortin-Rubio, H. Ghasempour, V. Guillerm, A. Morsali, J. Juanhuix, I. Imaz and D. Maspoch, J. Am. Chem. Soc., 2020, 142, 9135–9140 CrossRef CAS PubMed.
- J. J. T. Perry, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400–1417 RSC.
- V. Guillerm, D. Kim, J. F. Eubank, R. Luebke, X. Liu, K. Adil, M. S. Lah and M. Eddaoudi, Chem. Soc. Rev., 2014, 43, 6141–6172 RSC.
- G. Férey, C. Serre, C. Mellot-Draznieks, F. Millange, S. Surblé, J. Dutour and I. Margiolaki, Angew. Chem., Int. Ed., 2004, 116, 6456–6461 CrossRef.
- G. Ferey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surble and I. Margiolaki, Science, 2005, 309, 2040–2042 CrossRef CAS PubMed.
- A. C. Sudik, A. P. Côté, A. G. Wong-Foy, M. O'Keeffe and O. M. Yaghi, Angew. Chem., Int. Ed., 2006, 45, 2528–2533 CrossRef CAS PubMed.
- A. Schoedel and M. J. Zaworotko, Chem. Sci., 2014, 5, 1269–1282 RSC.
- Q. G. Zhai, X. Bu, C. Mao, X. Zhao, L. Daemen, Y. Cheng, A. J. Ramirez-Cuesta and P. Feng, Nat. Commun., 2016, 7, 13645 CrossRef CAS PubMed.
- Q. G. Zhai, X. Bu, C. Mao, X. Zhao and P. Feng, J. Am. Chem. Soc., 2016, 138, 2524–2527 CrossRef CAS PubMed.
- S. Abednatanzi, P. Gohari Derakhshandeh, H. Depauw, F. X. Coudert, H. Vrielinck, P. Van Der Voort and K. Leus, Chem. Soc. Rev., 2019, 48, 2535–2565 RSC.
- D. Feng, T. F. Liu, J. Su, M. Bosch, Z. Wei, W. Wan, D. Yuan, Y. P. Chen, X. Wang, K. Wang, X. Lian, Z. Y. Gu, J. Park, X. Zou and H. C. Zhou, Nat. Commun., 2015, 6, 5979 CrossRef PubMed.
- H. Chevreau, T. Devic, F. Salles, G. Maurin, N. Stock and C. Serre, Angew. Chem., Int. Ed., 2013, 52, 5056–5060 CrossRef CAS PubMed.
- S. Leubner, V. E. G. Bengtsson, A. K. Inge, M. Wahiduzzaman, F. Steinke, A. Jaworski, H. Xu, S. Halis, P. Ronfeldt, H. Reinsch, G. Maurin, X. Zou and N. Stock, Dalton Trans., 2020, 49, 3088–3092 RSC.
- X.-C. Huang, Y.-Y. Lin, J.-P. Zhang and X.-M. Chen, Angew. Chem., Int. Ed., 2006, 45, 1557–1559 CrossRef CAS PubMed.
- K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- C. Zhang, R. P. Lively, K. Zhang, J. R. Johnson, O. Karvan and W. J. Koros, J. Phys. Chem. Lett., 2012, 3, 2130–2134 CrossRef CAS PubMed.
- D. J. Babu, G. He, J. Hao, M. T. Vahdat, P. A. Schouwink, M. Mensi and K. V. Agrawal, Adv. Mater., 2019, 31, e1900855 CrossRef PubMed.
- K. Adil, Y. Belmabkhout, R. S. Pillai, A. Cadiau, P. M. Bhatt, A. H. Assen, G. Maurin and M. Eddaoudi, Chem. Soc. Rev., 2017, 46, 3402–3430 RSC.
- S. Shalini, S. Nandi, A. Justin, R. Maity and R. Vaidhyanathan, Chem. Commun., 2018, 54, 13472–13490 RSC.
- S. T. Zheng, J. T. Bu, Y. Li, T. Wu, F. Zuo, P. Feng and X. Bu, J. Am. Chem. Soc., 2010, 132, 17062–17064 CrossRef CAS PubMed.
- Q. G. Zhai, X. Bu, X. Zhao, D. S. Li and P. Feng, Acc. Chem. Res., 2017, 50, 407–417 CrossRef CAS PubMed.
- X. Zhao, X. Bu, Q. G. Zhai, H. Tran and P. Feng, J. Am. Chem. Soc., 2015, 137, 1396–1399 CrossRef CAS PubMed.
- Y. Ye, Z. Ma, R. B. Lin, R. Krishna, W. Zhou, Q. Lin, Z. Zhang, S. Xiang and B. Chen, J. Am. Chem. Soc., 2019, 141, 4130–4136 CrossRef CAS PubMed.
- H. Yang, Y. Wang, R. Krishna, X. Jia, Y. Wang, A. N. Hong, C. Dang, H. E. Castillo, X. Bu and P. Feng, J. Am. Chem. Soc., 2020, 142, 2222–2227 CrossRef CAS PubMed.
- M. Fujita, D. Oguro, M. Miyazawa, H. Oka, K. Yamaguchi and K. Ogura, Nature, 1995, 378, 469–471 CrossRef CAS.
- L. Zhang, J. Qian, W. Yang, X. Kuang, J. Zhang, Y. Cui, W. Wu, X.-Y. Wu, C.-Z. Lu and W.-Z. Chen, J. Mater. Chem. A, 2015, 3, 15399–15402 RSC.
- X. T. Liu, K. Wang, Z. Chang, Y. H. Zhang, J. Xu, Y. S. Zhao and X. H. Bu, Angew. Chem., Int. Ed., 2019, 58, 13890–13896 CrossRef CAS PubMed.
- M. Eddaoudi, D. F. Sava, J. F. Eubank, K. Adil and V. Guillerm, Chem. Soc. Rev., 2015, 44, 228–249 RSC.
- Y. X. Tan, F. Wang and J. Zhang, Chem. Soc. Rev., 2018, 47, 2130–2144 RSC.
- C. N. Dzesse T, E. N. Nfor and S. A. Bourne, Polyhedron, 2020, 189, 114724 CrossRef CAS.
- N. Klein, I. Senkovska, K. Gedrich, U. Stoeck, A. Henschel, U. Mueller and S. Kaskel, Angew. Chem., Int. Ed., 2009, 48, 9954–9957 CrossRef CAS PubMed.
- J. S. Qin, J. C. Zhang, M. Zhang, D. Y. Du, J. Li, Z. M. Su, Y. Y. Wang, S. P. Pang, S. H. Li and Y. Q. Lan, Adv. Sci., 2015, 2, 1500150 CrossRef PubMed.
- Y. H. Tang, F. Wang, J. X. Liu and J. Zhang, Chem. Commun., 2016, 52, 5625–5628 RSC.
- Y. Gai, X. Chen, H. Yang, Y. Wang, X. Bu and P. Feng, Chem. Commun., 2018, 54, 12109–12112 RSC.
- E. D. Bloch, W. L. Queen, M. R. Hudson, J. A. Mason, D. J. Xiao, L. J. Murray, R. Flacau, C. M. Brown and J. R. Long, Angew. Chem., Int. Ed., 2016, 55, 8605–8609 CrossRef CAS PubMed.
- D. Feng, K. Wang, J. Su, T. F. Liu, J. Park, Z. Wei, M. Bosch, A. Yakovenko, X. Zou and H. C. Zhou, Angew. Chem., Int. Ed., 2015, 54, 149–154 CrossRef CAS PubMed.
- H. Furukawa, F. Gandara, Y. B. Zhang, J. Jiang, W. L. Queen, M. R. Hudson and O. M. Yaghi, J. Am. Chem. Soc., 2014, 136, 4369–4381 CrossRef CAS PubMed.
- Q. Liu, Y. Song, Y. Ma, Y. Zhou, H. Cong, C. Wang, J. Wu, G. Hu, M. O'Keeffe and H. Deng, J. Am. Chem. Soc., 2019, 141, 488–496 CrossRef CAS PubMed.
- H. Jiang, J. Jia, A. Shkurenko, Z. Chen, K. Adil, Y. Belmabkhout, L. J. Weselinski, A. H. Assen, D. X. Xue, M. O'Keeffe and M. Eddaoudi, J. Am. Chem. Soc., 2018, 140, 8858–8867 CrossRef CAS PubMed.
- C. R. Reid and K. M. Thomas, J. Phys. Chem. B, 2001, 105, 10619–10629 CrossRef CAS.
- F. Luo, C. Yan, L. Dang, R. Krishna, W. Zhou, H. Wu, X. Dong, Y. Han, T. L. Hu, M. O'Keeffe, L. Wang, M. Luo, R. B. Lin and B. Chen, J. Am. Chem. Soc., 2016, 138, 5678–5684 CrossRef CAS PubMed.
- K.-J. Chen, H. S. Scott, D. G. Madden, T. Pham, A. Kumar, A. Bajpai, M. Lusi, K. A. Forrest, B. Space, J. J. Perry and M. J. Zaworotko, Chem, 2016, 1, 753–765 CAS.
- Y. L. Peng, T. Pham, P. Li, T. Wang, Y. Chen, K. J. Chen, K. A. Forrest, B. Space, P. Cheng, M. J. Zaworotko and Z. Zhang, Angew. Chem., Int. Ed., 2018, 57, 10971–10975 CrossRef CAS PubMed.
- S. Mukherjee, Y. He, D. Franz, S. Q. Wang, W. R. Xian, A. A. Bezrukov, B. Space, Z. Xu, J. He and M. J. Zaworotko, Chem.–Eur. J., 2020, 26, 4923–4929 CrossRef CAS PubMed.
- J. Lee, C. Y. Chuah, J. Kim, Y. Kim, N. Ko, Y. Seo, K. Kim, T. H. Bae and E. Lee, Angew. Chem., Int. Ed., 2018, 57, 7869–7873 CrossRef CAS PubMed.
- H. S. Scott, M. Shivanna, A. Bajpai, D. G. Madden, K. J. Chen, T. Pham, K. A. Forrest, A. Hogan, B. Space, J. J. Perry Iv and M. J. Zaworotko, ACS Appl. Mater. Interfaces, 2017, 9, 33395–33400 CrossRef CAS PubMed.
- Z. Niu, X. Cui, T. Pham, G. Verma, P. C. Lan, C. Shan, X. Xing, K. A. Forrest, S. Suepaul, B. Space, A. M. Al-Enizi, A. Nafady and S. Ma, Angew. Chem., Int. Ed., 2021, 60, 5283–5288 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2026913–2026915. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc06841a |
| This journal is © The Royal Society of Chemistry 2021 |