Open Access Article
Open Access ArticleStabilization of hydrated AcIII cation: the role of superatom states in actinium-water bonding†
Yang
Gao
 ab,
Payal
Grover
b and
Georg
Schreckenbach
ab,
Payal
Grover
b and
Georg
Schreckenbach
 *b
*b
aInstitute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China, Chengdu, Sichuan 610054, China
bDepartment of Chemistry, University of Manitoba, Winnipeg, Manitoba R3T 2N2, Canada. E-mail: schrecke@cc.umanitoba.ca
First published on 4th January 2021
Abstract
225Ac-based radiopharmaceuticals have the potential to become invaluable in designated cancer therapy. However, the limited understanding of the solution chemistry and bonding properties of actinium has hindered the development of existing and emerging targeted radiotherapeutics, which also poses a significant challenge in the discovery of new agents. Herein, we report the geometric and electronic structural properties of hydrated AcIII cations in the [AcIII(H2O)n]3+ (n = 4–11) complexes in aqueous solution and gas-phase using density functional theory. We found that nine water molecules coordinated to the AcIII cation is the most stable complex due to an enhanced hydration Gibbs free energy. This complex adopts a closed-shell 18-electron configuration (1S21P61D10) of a superatom state, which indicates a non-negligible covalent character and involves H2O → AcIII σ donation interaction between s-/p-/d-type atomic orbitals of the Ac atom and 2p atomic orbitals of the O atoms. Furthermore, potentially existing 10-coordinated complexes need to overcome an energy barrier (>0.10 eV) caused by hydrogen bonding to convert to 9-coordination. These results imply the importance of superatom states in actinide chemistry generally, and specifically in AcIII solution chemistry, and highlight the conversion mechanism between different coordination numbers.
Introduction
Actinium (Ac), as the first member of the actinide (An) family, is often overlooked and considered to be a member of the d-block transition metals (TMs), due to it showing striking similarities to Sc (3d14s2), Y (4d15s2), and La (5d16s2) with respect to the electronic configuration.1–3 However, recent discoveries tend to support its reassignment as an actual f-block element.4–7 All isotopes of actinium are unstable and highly radioactive. Specifically, the Ac isotope 225Ac, as a promising anticancer therapeutic agent, makes targeted radionuclide therapy a powerful cancer treatment strategy.5–9 The main reasons are that (a) the short half-life of 10 days matches the pharmacokinetics of the final radioactive drug, (b) the isotope is a pure α-emitter with a high energy (100 keV μm−1) and short distance (<100 μm) in biological tissue, (c) the final product (209Bi) from the 225Ac decay chain leaves with small accumulated energy of 28 meV and is innocuous in vivo. Despite these advantages, many basic science problems are surrounding the coordination and solution chemistry of actinium, and the biological delivery of the 225Ac isotope remains unresolved.10,11From the viewpoint of electronic structure, the electronic configuration of the Ac atom (5f06d17s2) is indeed similar to that of TMs (it only involves s- and d-electrons). As a result, some established knowledge of TM chemistry also applies to light actinides, such as the electron-counting rule for characterization of superatomic stability.12,13 Recently, a host of TM clusters/complexes have been designed and constructed via specific electron-counting rules based on the “Jellium model”, which can mimic the chemical behavior of elements in the periodic table, and hence these clusters and complexes can be regarded as “superatoms”.14,15 The Jellium model is a critical guiding principle for evaluating new superatom candidates.15 In this system, all the charges of the nuclei and core electrons in the cluster are uniformly distributed throughout the cluster spheroid. As such, the energy levels for electrons interacting with such a charge distribution correspond to 1S2, 1P6, 1D10, 2S2, 1F14, 2P6, etc., where the resulting magic numbers are 2, 8, 18, 20, 32, 40, … (to distinguish the electronic shells of atoms, the super shells are depicted as capital letters).
Examples include the famous WAu12 superatom cluster,16,17 Fe(C5H5)2, Cr(C6H6)2, M(CO)6 (M = Cr, Mo, W) and M(CO)8 (M = Ca, Sr, or Ba) complexes,18–21 whose chemical stability is not only due to the high coordination number and corresponding high cohesive energy for surface atoms, but also mainly on account of the strong relativistic effects and the closed shell 18-electron rule (1S21P61D10). Focusing on the actinides, the An@Au14 (An = Ac−, Th, Pa+), Sg@Au12 clusters, [An(CO)8]+/− (An = Th, U) and [Ac(H2)n]3+ (n = 9–12) complexes22–25 were also found to be superatom clusters, obeying the 18-electron rule. Even though the 5f electrons of actinides prefer to localize, they were still found to be excited to 6d shells to satisfy the bonding of the 18-electron rule,26 which demonstrated the similarity in the electron-counting rules between the early actinides and transition metals. On the other hand, the An@C28 (An = Th, Pa+, U2+, Pu4+), [U@Si20]6− and U@B40 superatom clusters27–29 follow the 32-electron rule (1S21P61D101F14). Hence, when it comes to the development of thermodynamically stable and kinetically inert actinide cation complexes as part of radiopharmaceuticals, the superatom states play a major role in the coordination and solution chemistry.
It is well known that the Ac atom usually exists in the trivalent oxidation state (i.e. AcIII with the configuration 5f06d07s0), which leads to its invisible nature in spectroscopy and resulting difficulty to characterize.30 Further, because of the highly radioactive nature and limited availability of Ac isotopes, experimental works have been greatly hindered.31 For instance, the first Ac bond distance wasn't measured until 2016,30 which means that many basic chelate properties of AcIII have not been discovered in solution chemistry yet. In this work, a systematic investigation on the superatom chemistry of hydrated AcIII cations in the [AcIII(H2O)n]3+ (n = 4–11) complexes in gas-phase (gas, for short in the following) and aqueous solution (aq, for short in the following) conditions is presented by employing first-principles density functional theory (DFT). This study addresses the superatom states in the optimized geometries of AcIII aquo complexes, the change of coordination number of the AcIII cation in solution chemistry, as well as the nature of bonding between AcIII and oxygen. The analysis of such superatom states of the hydrated AcIII cation has broad potential to be implicated in the development of a predictive understanding of its solubility, stability, and reactivity.
Results and discussion
Geometry structures
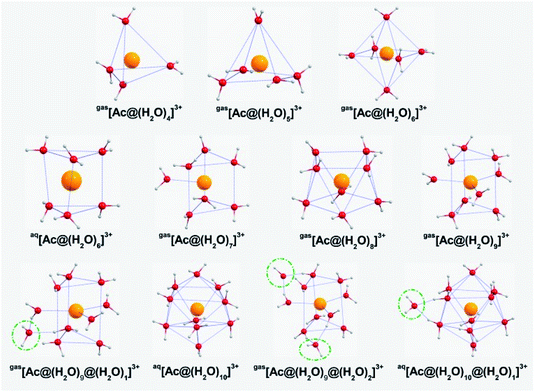
The stable AcIII aquo complexes were obtained via PBE-D3 optimization, followed by validation with the B3LYP-D3 functional, as shown in Fig. 1 (see the isomers of different initial structures in Table S1 of ESI†). When the coordination numbers (CN) in the first hydration shell are 4, 5, 7, 8, 9, the most stable structures obtained under both gas-phase and aqueous solution conditions are similar. However, when the numbers of water molecule in the first hydration shell of initial structures are 6, 10, 11, the most stable geometries are different between the gas-phase and aqueous solution. Specifically, the coordination geometries of the water molecules for CN = 4 and 5 are trigonal pyramidal and square pyramidal, respectively. For CN = 6, the most stable structures in the gas-phase and aqueous solution are octahedral and trigonal prism (TP), respectively. For CN = 7, the structure formed is equivalent to adding a cap to one side of the TP. Upon adding three caps to the three sides of the TP, the coordination geometry becomes a tricapped trigonal prism (TTP), which correspond to CN = 9. This TTP structure looks similar to those of [U(H2O)9]3+,32 and [La(H2O)9]3+.33 On the contrary, when CN = 8, the AcIII aquo complex possesses a square antiprism (SAP) structure, instead of a bicapped trigonal prism (BTP). The reason for the sharp change in geometries from BTP → SAP → TTP is that the BTP structure is an intermediate of both the 9 → 8 and 8 ← 9 reactions, whereas the CN = 8 structure rearranges itself to the SAP structure upon reaching a stable state.34Regarding the geometry of the first hydration shell, we further considered using initial starting structures containing 10 or 11 water molecules to coordinate an AcIII cation. The calculation results show the CNs in the optimized structures always tends to be 10 (Bicapped Square Antiprism) under aqueous solution conditions and 9 (TTP) under gas-phase conditions. Fig. 1 clearly shows that the water molecules marked with green circles have moved into the second hydration shell. Recently, it has been observed experimentally that there are approximately 10.0 ± 0.9 (ref. 36) and 10.9 ± 0.5 (ref. 35) water molecules in the first AcIII coordination sphere, at an average distance of ∼2.630 Å. Also, the MDDFT (accompanying that work:35 AcIII cation in a supercell of 64 water molecules), DFT,37 and classical MD38 studies predicted a CN of nine water molecules at a distance of 2.689 ± 0.110 Å, 2.66 ± 0.02 Å, and 2.699 Å, respectively. Based on the above works, it is possible for all three coordination numbers (9, 10 and 11) of Ac cations to actually exist simultaneously. Obviously, we cannot be restricted to the AcIII cation itself. Instead, we need to also include the hydration properties of other actinide trivalent cations to discuss the coordination chemistry of the AcIII cation.
Over the last half-century, both experimental and theoretical studies have been devoted to the understanding of the hydration properties of actinoid ions in water. Reported structural parameters of AnIII aquo complexes are summarized in Table 1. Compared with the systematic experimental investigations for aqueous trivalent lanthanoids, there are no comprehensive experimental studies in the actinoid series. This is not only because of the difference that arises from partial delocalization of 5f orbitals versus localization of 4f orbitals, but also the higher experimental difficulty in studying these systems owing to the redox instability of the lighter AnIII aqua ions. In short, the experimentally observed CNs of trivalent actinide cations (U, Np, Pu, Am, Cm, Bk, and Cf), range from 7.0 to 10.3, which is still not in complete agreement with the theoretically predicted hydration numbers (8 or 9). However, these differences become justified if the following practical issues are considered: (1) different metal and ligand concentrations in the experiments will result in different hydration spheres about the metal ion (see e.g. in “[PuIII(H2O)x]3+” Table 1);42,43 (2) there are many issues in accurately evaluating the coordination number of EXAFS, including the statistical limitations of the EXAFS fitting problem, empirical effects due to sample preparation, and the assumptions made about the physical structure surrounding the absorber in the course of data analysis;35–38,54 (3) a natural constraint on the quantum mechanics: an increase of the coordination number is directly associated with an An–O lengthening, while the two parameters (bond length and CN) can be fitted separately in EXAFS. In summary, the remaining deviations between theory and experiment appear reasonable, and further studies including the detailed analysis from the perspective of electronic structure and relaxed potential energy surface scanning certainly will help in figuring out the dynamic coordination (9 or 10) for the AcIII cation.
| Actinides cation | CN | An–Owater distances | Technique | Solution conditions |
|---|---|---|---|---|
| a Metal solution concentration: 20 mM. b Metal solution concentration: 20 mM. For [PuIII(H2O)x]3+, only one combination (10 mM + 0.01 M, LiCl) is the most reliable, the other two sets of values are listed here for comparison purposes. EXAFS (extended X-ray absorption fine structure); MD (molecular dynamics); CPCM (conductor-like polarizable continuum model); NEMO (non-empirical molecular orbital). | ||||
| [AcIII(H2O)x]3+ | 10.9 ± 0.5 | 2.63 ± 0.01 | EXAFS | 0.11 M, HO3SCF3 (ref. 35) |
| 10.0 ± 0.9 | 2.63 ± 0.02 | EXAFS | 0.05 M, HNO3 (ref. 36) | |
| 9.0 | 2.699 | GGA functional with COSMO37 | ||
| 8.9 | 2.66 ± 0.02 | Classical MD38 | ||
| 9.0 | 2.689 ± 0.110 | DFT-based MD with PBE functional35 | ||
| [ThIII(H2O)x]3+ | 9.0 | 2.682 | GGA functional with COSMO37 | |
| [PaIII(H2O)x]3+ | 9.0 | 2.656 | GGA functional with COSMO37 | |
| [UIII(H2O)x]3+ | 9.1 ± 0.6 | 2.52 ± 0.01 | EXAFS | pH 0 HCl39 |
| 9.0 | 2.636 | GGA functional with COSMO37 | ||
| 9.0 | 2.51 | Polarizable model40 | ||
| [NpIII(H2O)x]3+ | 10.0 ± 1.2 | 2.51 ± 0.01 | EXAFS | pH 0 HCl39 |
| 9.0 ± 1.0 | 2.48 ± 0.02 | EXAFS | 1 M, HClO4 (ref. 41) | |
| 9.0 | 2.617 | GGA functional with COSMO37 | ||
| 9.0 | 2.50 | Polarizable model40 | ||
| [PuIII(H2O)x]3+ | 9.9 ± 0.3 | 2.49 ± 0.01 | EXAFS | pH0 HCl39 |
| 10.2 ± 1.1 | 2.51 ± 0.06 | EXAFS | 0.01 M, LiCla42 | |
| 9.2 ± 0.33 | 2.51 ± 0.06 | EXAFS | 0.01 M, LiClb43 | |
| 5.2 ± 0.33 | 2.50 ± 0.06 | EXAFS | 12.3 M, LiClb43 | |
| 8.6 ± 0.2 | 2.50 ± 0.02 | EXAFS | 1 M, HClO4 (ref. 44) | |
| 9.0 (8.0) | 2.599 (2.561) | GGA functional with COSMO37 | ||
| 9.0 | 2.490 | Polarizable model40 | ||
| [AmIII(H2O)x]3+ | 9.5 ± 0.87 | 2.48 ± 0.01 | EXAFS | 0.11 M, HO3SCF3 (ref. 30) |
| 8.3 ± 0.4 | 2.473 ± 0.004 | EXAFS | 0.025 M, HClO4 (ref. 45) | |
| 10.3 ± 0.33 | 2.480 ± 0.06 | EXAFS | 0.25 M, HCl43 | |
| 8.9 ± 0.8 | 2.470 ± 0.01 | EXAFS | 0.05 M, HNO3 (ref. 36) | |
| 9.0 (8.0) | 2.582 (2.544) | GGA functional with COSMO37 | ||
| 9.0 | 2.480 | Polarizable model40 | ||
| [CmIII(H2O)x]3+ | 10.3 ± 0.33 | 2.450 ± 0.06 | EXAFS | 0.25 M, HCl43 |
| 7.0 ± 0.4 | 2.469 ± 0.007 | EXAFS | 1 M, HClO4 (ref. 46) | |
| 9.6 ± 0.7 | 2.470 ± 0.01 | EXAFS | 0.05 M, HNO3 (ref. 36) | |
| 8.5 ± 0.8 | 2.477 ± 0.005 | EXAFS | 0.1 M, HClO4 (ref. 47) | |
| 9.0 (8.0) | 2.566 (2.526) | GGA functional with COSMO37 | ||
| 9.0 (8.0) | 2.473 (2.445) | Hybrid GGA functional with CPCM48 | ||
| 9.0 | 2.460 | Polarizable model40 | ||
| 8.9 | 2.550 | Polarizable NEMO potential49 | ||
| 9.0 | 2.480 | DFT-based MD with PBE functional50 | ||
| 9.0 | 2.520–2.530 | Non-polarizable force field50 | ||
| [BkIII(H2O)x]3+ | 9.0 ± 0.6 | 2.430 ± 0.02 | EXAFS | 1 M, HClO4 (ref. 51) |
| 9.0 (8.0) | 2.551 (2.511) | GGA functional with COSMO37 | ||
| 9.0 | 2.430 | Polarizable model40 | ||
| [CfIII(H2O)x]3+ | 8.0 ± 0.0 | 2.420 ± 0.01 | EXAFS | 0.1 M, HClO4 (ref. 52) |
| 8.5 ± 1.5 | 2.420 ± 0.02 | EXAFS | 1 M, HCl53 | |
| 9.0 (8.0) | 2.537 (2.495) | GGA functional with COSMO37 | ||
| 8.9 | 2.420 | Polarizable model40 | ||
| [EsIII(H2O)x]3+ | 9.0 (8.0) | 2.524 (2.482) | GGA functional with COSMO37 | |
| 8.5 | 2.37 | Polarizable model40 | ||
| [FmIII(H2O)x]3+ | 9.0 (8.0) | 2.513 (2.469) | GGA functional with COSMO37 | |
| 8.3 | 2.35 | Polarizable model40 | ||
| [MdIII(H2O)x]3+ | 9.0 (8.0) | 2.502 (2.455) | GGA functional with COSMO37 | |
| 8.1 | 2.34 | Polarizable model40 | ||
| [NoIII(H2O)x]3+ | 9.0 (8.0) | 2.490 (2.443) | GGA functional with COSMO37 | |
| 8.0 | 2.32 | Polarizable model40 | ||
| [LrIII(H2O)x]3+ | 9.0 (8.0) | 2.478 (2.431) | GGA functional with COSMO37 | |
| 8.0 | 2.31 | Polarizable model40 | ||
In addition, the Ac–Owater distances for prismatically arranged (Oprism) and capping (Ocap) oxygen atoms in [AcIII(H2O)9]3+ (TTP) complex are shown in Table 2 and Table S2 of the ESI† together with those of the others AcIII aquo complexes. The Ac–Owater distances become longer as the CN increases which is mainly due to the steric effects. The 9- and 10-coordinated AcIII cation complexes show average distance values of 2.642/2.663 Å and 2.690/2.711 Å at the PBE-D3/B3LYP-D3 levels of theory, respectively. The differences compared with previously reported values are all of the order of ∼1 × 10−2 Å. For each complex, the average Ac–Owater bond length is shortened or lengthened by ∼1 × 10−3 Å when spin-orbit coupling-ZORA (SOC-ZORA) relativistic effects are included in the gas-phase calculations in comparison to scalar relativistic calculations (SR-ZORA). For the SR-ZORA calculations including water solvent effects, the overall average Ac–Owater distance is reduced by ∼4 × 10−2 Å compared to the corresponding gas-phase calculation. This difference is consistent with that of the equatorial U–OH2 bond lengths of the [UO2(H2O)5]2+·nH2O complexes (n = 0, 5, 7, 10, 12) in gas phase and solution.55
| Geometries | PBE-D3 | B3LYP-D3 | ||||
|---|---|---|---|---|---|---|
| gasSR | gasSOC | aqSR | gasSR | gasSOC | aqSR | |
| a Both structure a and b are 9-coordinated AcIII cation complexes and correspond to the c6 and d8 energy points in Fig. 5, respectively. | ||||||
| [AcIII(H2O)4]3+ | 2.524 | 2.523 | 2.478 | 2.545 | 2.543 | 2.505 |
| [AcIII(H2O)5]3+ | 2.563 | 2.563 | 2.518 | 2.584 | 2.584 | 2.541 |
| gas[AcIII(H2O)6]3+ | 2.599 | 2.599 | — | 2.620 | 2.618 | — |
| aq[AcIII(H2O)6]3+ | — | — | 2.552 | — | — | 2.572 |
| [AcIII(H2O)7]3+ | 2.629 | 2.631 | 2.580 | 2.648 | 2.647 | 2.600 |
| [AcIII(H2O)8]3+ | 2.657 | 2.662 | 2.610 | 2.678 | 2.676 | 2.630 |
| [AcIII(H2O)9]3+ | 2.690 | 2.695 | 2.642 | 2.706 | 2.706 | 2.663 |
| gas[AcIII(H2O)9(H2O)1]3+ | 2.688 | 2.691 | — | 2.704 | 2.702 | — |
| aq[AcIII(H2O)10]3+ | — | — | 2.690 | — | — | 2.711 |
| gas[AcIII(H2O)9(H2O)2]3+ | 2.690 | 2.687 | — | 2.701 | 2.700 | — |
| aq[AcIII(H2O)10(H2O)1]3+ | — | — | 2.693 | — | — | 2.701 |
| aq[AcIII(H2O)8(H2O)16]3+ | — | — | 2.640 | — | — | — |
| aq[AcIII(H2O)9(H2O)18]3+ | — | — | 2.648 | — | — | — |
| aq[AcIII(H2O)10(H2O)35]3+ | — | — | 2.726 | — | — | — |
| aq[AcIII(H2O)9(H2O)36]3+ | — | — | 2.669a | — | — | — |
| aq[AcIII(H2O)9(H2O)36]3+ | — | — | 2.679b | — | — | — |
Hydration Gibbs free energies
Gibbs free energies of appropriate reactions have always been a useful tool for identifying the optimal coordination number.56,57 Accordingly, the hydration Gibbs free energies of AcIII cations were calculated in aqueous solution at the PBE-D3 and B3LYP-D3 levels of theory using the following reactions:| Ac3+(gas) + n(H2O)(aq) ↔ [Ac(H2O)n]3+(aq), ΔG(Ac3+) = G([Ac(H2O)n]3+) − G0(Ac3+) − nG(H2O) | (R1) |
| Geometries | Reaction (1) | Reaction (2) | ||
|---|---|---|---|---|
| PBE-D3 | B3LYP-D3 | PBE-D3 | B3LYP-D3 | |
| [AcIII(H2O)4]3+ | 46.60 | 40.86 | −17.16 | −11.80 |
| [AcIII(H2O)5]3+ | 31.17 | 30.05 | −11.26 | −5.63 |
| [AcIII(H2O)6]3+ | 21.64 | 25.41 | −11.65 | −11.58 |
| [AcIII(H2O)7]3+ | 11.72 | 14.82 | −10.73 | −11.17 |
| [AcIII(H2O)8]3+ | 2.72 | 4.64 | −4.45 | −5.63 |
| [AcIII(H2O)9]3+ | 0.00 | 0.00 | 0.00 | 0.00 |
| [AcIII(H2O)10]3+ | 1.73 | 0.99 | −5.81 | −1.00 |
To further probe the preferences in terms of energy for accommodating one additional water molecule in the first coordination shell, we also examined the hydration Gibbs free energies in aqueous solution at the PBE-D3 and B3LYP-D3 levels of theory by using the following reactions:
| [Ac(H2O)n]3+(aq) + H2O(aq) ↔ [Ac(H2O)n+1]3+(aq), G([Ac(H2O)n]3+) = G([Ac(H2O)n+1]3+) − G(H2O) | (R2) |
Table 3 shows that the complexes with (n = 4 → 8, 10) have a stronger ability to accept one additional water molecule than the complex with n = 9. This means that the additional water molecule is most difficult to add to the first hydration shell of the [AcIII(H2O)9]3+ complex, i.e. the complex with nine water molecules coordinated to the AcIII cation is the most stable complex. This result is consistent with the coordination environment of other hydrated trivalent actinide cations.4,32,46
Electronic structures
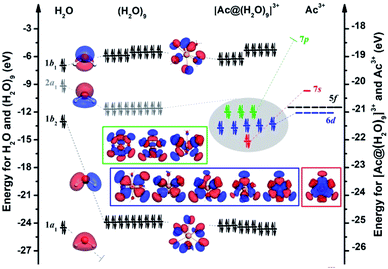
Free energy calculations can only determine the preferred CNs but do not explain the reason why this is the case. To achieve a better understanding of the electronic requirements of the AcIII aquo complexes, we chose the [AcIII(H2O)9]3+ complex as an example. A diagram of the interaction between the AcIII cation and the 9H2O ligands is shown in Fig. 2. For actinide complexes in which water molecules act as ligands, it is beneficial to start from the electronic structure of a simple H2O molecule. To reduce the complexity of the electronic structure, the 1s orbital of the O atom was frozen. As a result, there are only four occupied orbitals (1a1)2(1b2)2(2a1)2(1b1)2 for the isolated H2O molecule. The lowest energy orbital (1a1) is (mostly) contributed from the 2s orbitals of the O atom. The three highest occupied orbitals (1b2 σ-bonding, 2a1 lone pair electrons, 1b1 lone pair electrons) are resulting from the 2p atomic orbitals of the O atom and are orthogonal along the x, y, z directions of the oxygen atom. From the molecular orbital (MO) diagram of the [AcIII(H2O)9]3+ complex, we found that the 1a1, 1b2, 1b1 MOs of H2O are MOs without any interaction with the AcIII cation, and still maintain the character of the isolated H2O molecule. Contrary to that, the MOs of 2a1 symmetry of H2O molecules are hybridized with the AcIII cation, and all the lone pair (LP) electrons (similar to free electrons) are more or less pointing toward the AcIII cation, with noticeable deformation of the electron cloud. This implies that the AcIII–Owater interactions are not completely dominated by electrostatic interactions.It is well known that the electron configuration of an isolated Ac atom is 6d17s2, and the positive trivalent AcIII cation will certainly adopt the electronic configuration (6d07s0) of the inert-gas Rn atom, which means that the [AcIII(H2O)9]3+ complex could satisfy the closed-shell 18-electron rule (9 × 2 + 0) in the Jellium model. According to the nodal shape of the MOs, as well as the fact that only the 2a1 LP electrons of the nine water molecules participate in the chemical bonding, we can indeed identify a series of superatomic orbitals within the framework of the Jellium model in the sequence 1S, 1D, 1P that corresponds to the 9 occupied MOs with 18 electrons (see Fig. 2, for the detailed composition of the MOs see Table S3 of ESI†). In order to better understand the valence orbitals of [AcIII(H2O)9]3+ as a “18e-superatom” based on the Jellium model, we compared the molecular orbitals of the [AcIII(H2O)9]3+ complex and the [Ac@Au14]− cluster (see Fig. S1 of ESI†). Although [AcIII(H2O)9]3+ is not a metal cluster, its configuration can be similarly described as 1S21P61D10 based on the Jellium model. Very recent research applying the Jellium model also proved that the octacarbonyl metal cation complex [Ba(CO)8]2− and the metal cluster [BaBe8]2− strictly satisfy the closed-shell “20e-superatom” configuration of 1S21P61D101F2.58
Interestingly, the [AcIII(H2O)9]3+ “superatom” formulation is different from a number of gold-based superatom clusters, such as An@Au14 (An = Ac−, Th, Pa+),22 and also different from complexes with other small molecules as ligands, such as M(CO)8 (M = Ca, Sr, or Ba).21 All the electrons in its potential field come from the nine water molecules instead of from both, central atom and water cage. Further insight can be gained from charge transfer analysis, resulting in the charges of the AcIII cation based on the Voronoi deformation density (VDD), Hirshfeld, and Mulliken charge analysis methods with values of 0.24e, 0.84e, 2.11e and 0.19e, 0.79e, 2.11e under gas-phase and aqueous solution conditions respectively. It is important to note that it is not the absolute values of the charge populations that are important to our analyses here, but rather the trends between the An ions. This indicates that the positive trivalent AcIII cation has gained electrons from the nine water molecules and has thus attained a charge that is smaller than +3.
To provide detailed insight into the chemical bonding, we analyzed the nature of the Ac–Owater bonds with the natural orbitals for chemical valence (NOCV) combined with the energy decomposition analysis (EDA).59 The EDA-NOCV results for the interaction between the AcIII cation and the (H2O)9 ligand (Table 4) show that the attractive contribution to the total interaction energy ΔEint comes from the electrostatic term ΔEelstat (57.24%), the orbital term ΔEorb (42.54%), and the dispersion term ΔEdis (0.22%). The electrostatic attraction (ΔEelstat) is responsible for the overall trend, which is also in line with the long-held idea of the strong electrostatic character of the ion–water interaction.32,56,60–62 Further, since the ΔEorb term represents a mixture of orbitals between the two fragments, it can be identified as a covalent contribution to the chemical bond. It should be noted that the strength of the covalent bond is not determined by the value of the ΔEorb term alone, but rather by the balance between the attractive forces (electrostatic and covalent) and the repulsive exchange (Pauli) term. Therefore, when we considered the explicit second water shell for aq[AcIII(H2O)8]3+ and aq[AcIII(H2O)9]3+, the orbital interactions energy (ΔEorb) representing covalent characteristics still accounts for more than 30% of the total attractive energy (ΔEelstat+ΔEorb+ΔEdis), indicating that there is a non-negligible covalent character (ΔEorb) between the Ac cation and water molecules (see Table S4 of ESI†).
| Energy terms | Assignmentb | Interacting fragments | ||
|---|---|---|---|---|
| (H2O)9vs. AcIII | (NH3)9vs. AcIII | (PH3)9vs. AcIII | ||
| a ΔEpauli is the Pauli repulsion term; ΔEdis is the dispersion term; ΔEelstat is the classical electrostatic interaction term; ΔEorb is the orbitals interaction term. Within this energy decomposition scheme the attractive and repulsive terms are negative and positive, respectively. “x” denotes the valence acceptor atomic orbitals of Ac atom. b For the [AcIII(NH3)9]3+, and [AcIII(PH3)9]3+ complexes, we only list the percentage of the nine superatomic molecular orbitals that account for the majority of the total orbital interaction energy. | ||||
| ΔE int | −474.98 | −525.82 | −413.81 | |
| ΔE pauli | 136.70 | 134.49 | 113.25 | |
| ΔE dis | −1.35 (0.22%) | −2.34 (0.35%) | −3.12 (0.59%) | |
| ΔE elstat | −350.14 (57.24%) | −373.35 (56.55%) | −168.92 (32.05%) | |
| ΔE orb | −260.19 (42.54%) | −284.62 (43.10%) | −355.03 (67.36%) | |
| ΔEorb(1) | [9H2O(2p)] σ donation → [Ac(6d)] (1D) | −26.58 (10.22%) | −33.64 (11.82%) | −44.57 (12.55%) |
| ΔEorb(2) | [9H2O(2p)] σ donation → [Ac(6d)] (1D) | −26.45 (10.17%) | −33.56 (11.79%) | −44.38 (12.50%) |
| ΔEorb(3) | [9H2O(2p)] σ donation → [Ac(6d)] (1D) | −24.32 (9.35%) | −31.49 (11.06%) | −43.87 (12.36%) |
| ΔEorb(4) | [9H2O(2p)] σ donation → [Ac(6d)] (1D) | −24.06 (9.25%) | −30.57(10.74%) | −39.93 (11.25%) |
| ΔEorb(5) | [9H2O(2p)] σ donation → [Ac(6d)] (1D) | −23.34 (8.97%) | −28.42 (9.99%) | −39.43 (11.11%) |
| ΔEorb(6) | [9H2O(2p)] σ donation → [Ac(7s)] (1S) | −16.98 (6.53%) | −22.99 (8.08%) | −33.13 (9.33%) |
| ΔEorb(7) | [9H2O(2p)] σ donation → [Ac(7p)] (1P) | −9.53 (3.66%) | −11.29 (3.97%) | −14.46 (4.07%) |
| ΔEorb(8) | [9H2O(2p)] σ donation → [Ac(7p)] (1P) | −9.48 (3.64%) | −11.19 (3.93%) | −14.28 (4.02%) |
| ΔEorb(9) | [9H2O(2p)] σ donation → [Ac(7p)] (1P) | −6.28 (2.41%) | −11.11 (3.90%) | −14.21 (4.00%) |
| ΔEorb(10) | [9H2O(2p)] σ donation → [Ac(5f)] | −7.67 (2.95%) | — | — |
| ΔEorb(11) | [9H2O(2p)] σ donation → [Ac(5f)] | −8.14 (3.13%) | — | — |
| ΔEorb(12) | [9H2O(2p)] σ donation → [Ac(5f)] | −8.06 (3.10%) | — | — |
| ΔEorb(13) | [9H2O(2p)] σ donation → [Ac(5f)] | −7.92 (3.04%) | — | — |
| ΔEorb(14) | [9H2O(2p)] σ donation → [Ac(5f)] | −6.13 (2.36%) | — | — |
| ΔEorb(15) | [9H2O(2p)] π bonding ← [Ac(x)] | −7.17 (2.76%) | — | — |
| ΔEorb(16) | [9H2O(2p)] π bonding ← [Ac(x)] | −7.17 (2.76%) | — | — |
| ΔEorb(17) | 9H2O polarization | −37.94 (14.58%) | — | — |
| ΔEorb(18) | Rest | −2.97 (1.14%) | — | — |
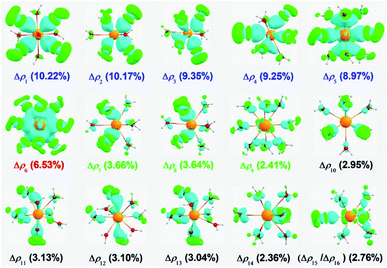
In particular, ΔEorb can be further split into pairwise orbital interactions. This has been proven very helpful, because it provides a quantitative expression of the frontier molecular orbital model of Fukui63 and the orbital symmetry rules of Woodward and Hoffman.64 The shapes of the deformation densities Δρ corresponding to the ΔEorb contributions are shown in Fig. 3. The first five deformation densities (Δρ1 to Δρ5) display a slightly larger charge flow in the direction [9H2O(2p)] → [Ac(6d)]; they correspond to the 1D orbitals of the [AcIII(H2O)9]3+ superatom. In a similar way, the following one (Δρ6) and three deformation densities (Δρ7 to Δρ9) correspond to the 1S and 1P orbitals of the [AcIII(H2O)9]3+ superatom. Interestingly, we also found that the 5f orbitals could also accept a small amount of 2p electrons (Δρ10 to Δρ14) and the valence acceptor electrons of the Ac atom might also go back via weak π bonding (Δρ15 and Δρ16). In general, the Ac–Owater bonding arises mainly from the [9H2O(2p)] to [Ac(x)] σ donation of the 2p atomic orbitals of the O atom into x, where x denotes the valence acceptor atomic orbitals of the AcIII cation. This also explains why the AcIII cation gains electrons, as mentioned above.
In order to confirm the superatomic formation mechanism of the Ac cation with such a monodentate ligand, we further calculated and analyzed the EDA-NOCV results of the interaction of the positive trivalent Ac cation with nine NH3 and nine PH3 molecules. We found the [AcIII(NH3)9]3+ and [AcIII(PH3)9]3+ complexes, like the [AcIII(H2O)9]3+ complex, both obey the 18-electron rule. This is mainly because NH3 and PH3 have strong polarity and also can provide LP electrons to coordinate with many metal ions to form coordination complexes (the detailed electronic structure diagrams are shown in Fig. S2 and S3 of ESI†). Specifically, the EDA-NOCV results are very interesting because they demonstrate that there is a non-negligible covalent character (ΔEorb) between the Ac cation and the monodentate ligands (see Table 4).
The ammonia ligands NH3 exhibit the largest interaction energy (−525.82 kcal mol−1) with the Ac cation out of all three monodentate ligands. The Pauli repulsion exhibited by the NH3 ligands (134.49 kcal mol−1) is slightly smaller than for H2O (136.70 kcal mol−1) and larger than for PH3 (113.25 kcal mol−1), which leaves the orbital term (ΔEorb) as a crucial component for the trend of the interaction energies (ΔEint). Table 4 shows that the ΔEorb value for PH3 (−355.03 kcal mol−1) is much larger than for H2O (−260.19 kcal mol−1) and NH3 (−284.62 kcal mol−1). The trend can be explained with the hybridization of the σ lone-pair orbital at the P atom, which has a much higher hybrid percentage in PH3 than in NH3 and H2O (see Table S5 of ESI†). The higher hybrid percentage makes the lone-pair orbital to be more delocalized, which yields a larger overlap with the metal nucleus, which in turn is the main component of the electrostatic attraction in Ac-monodentate ligands.
In addition, the electronic structure diagrams of the gas[AcIII(H2O)9(H2O)1]3+, aq[AcIII(H2O)10]3+, gas[AcIII(H2O)9(H2O)2]3+ and aq[AcIII(H2O)10(H2O)1]3+ complexes are shown in Fig. 4. All of these complexes obey the 18-electron rule. This includes the 10-coordinated aq[AcIII(H2O)10]3+ and aq[AcIII(H2O)10(H2O)1]3+ complexes (with a total of 20e) that have the valence electron configuration 1S21P61D101F2, where the 1F orbital is a ligand-only orbital (or nonbonding orbital), which satisfies the 18-electron rule perfectly. The electrons in the 1F2 orbital are stabilized by the field effect of the metal on the ligand cage because there is no valence atomic orbital of the s/p/d shells that possesses the same symmetry (geometric structure). Molecular orbital component analysis further proves this point, as the 1F2 orbitals in orange circles in Fig. 4 arise from the contribution of the mixing of 2p atomic orbitals of the oxygen atoms (∼99%) and slightly from the 5f shell of AcIII cation due to the polarization (∼1%). Moreover, the phenomenon of ligand-only superatom orbitals has also recently been discovered in the octacarbonyl complexes M(CO)8 (M = Ca, Sr, Ba, Sc−, Y−, La−).21,65 In order to explore the direct relationship between the coordination number of the AcIII cation hydration layer and the 18-electron rule, we also analyzed the electronic structure of the Ac cation with coordination numbers ranging from 7 to 10, and found that the decrease in the number of water molecules corresponds to the disappearance of the 1D superatom states (see Fig. S4 of ESI†). To be specific, the superatom states based on the Jellium model are 1S21P61D10 for 9-coordination (18e), 1S21P61D8 for 8-coordination (16e), 1S21P61D6 for 7-coordination (14e). Therefore, there is indeed a strong connection between coordination number 9 and the 18 electron rule in hydrated AcIII complexes.
At the conclusion of this section, it should be emphasized that one reviewer remarked that the existence of many known hydrated transition metal complexes is completely unrelated to the 18-electron rule, and this would similarly apply to the hydrated actinide complexes. However, here we summarize two traditional misunderstandings and use this to explain why the rule does indeed apply to the system we are studying: (a) the relevance of the individual atomic orbitals is not just limited to transition metal atoms, it also applies to some alkali earth metal elements and early lanthanide and actinide elements; (b) the “superatom concept” has nothing to do with whether the metal–ligand interactions are weak or strong. Specifically, within the spherical potential field of a superatom cluster, all electrons are separately confined in quantized molecular orbitals. Moreover, the symmetry of the MOs could prevent that the occupied ligand orbitals donate electronic charge into the valences orbitals of the metal and thus contribute to the electron count.66,67 Regardless of whether the ligand is H2O, CO or Au, they could all be regarded as a confined outer-shell structure that plays the same role, meaning that they all obey the 18-electron rule. In fact, both H2O and CO are 2-electron donor ligands, notwithstanding the fact that the latter is also a strong π-acceptor. The discussion of the superatoms concept herein for an actinide metal complex, combined with previous examples (see the above discussion), will facilitate the understanding of superatomic states in solution chemistry and coordination chemistry.
Changes in coordination numbers
As discussed above, despite the use of the same initial structures containing 10 (or more) water molecules and 1 AcIII cation, the CN always tends to be 10 in aqueous solution and 9 in gas phase. Therefore, considering this geometrical difference between gas phase and aqueous solution, we performed simulations for relaxed potential energy surface (PES) scans for the distance between an O atom and the AcIII cation in both the complexes. In Fig. 5a, the PES is displayed as a function of the distance between the O atom in the second hydration shell of the gas[AcIII(H2O)9(H2O)1]3+ complex and the AcIII cation. The initial O⋯Ac distance is 4.470 Å (see a1 in Fig. 5a and 6). This distance is scanned to both sides, to a minimum of 2.300 Å and a maximum of 5.500 Å. Between 4.470 Å and 2.300 Å, there is a significant and sudden drop in the energy, at geometries a2 (O⋯Ac distance: 3.096 Å) and a3 (O⋯Ac distance: 3.077 Å), Fig. 5a. By checking the corresponding structures, we found that the geometries of a2 and a3 have changed dramatically (see Fig. 6). In other words, if we force a water molecule of the second hydration shell to be added to the first hydration shell, it will push one of the nine water molecules in the first hydration shell to the second hydration shell, and bring the geometry of this complex back to the original geometry at the same time. Indeed, the geometries of a1 and a4 are almost similar, Fig. 6. This implies that the coordination geometry with 9 water molecules (CN = 9) is most stable under gas-phase conditions. Besides, we also found that when the O⋯Ac distance reaches 5.122 Å (see a5 in Fig. 5a and 6), the energy has a small discontinuity, which is caused by the breaking of a hydrogen bond.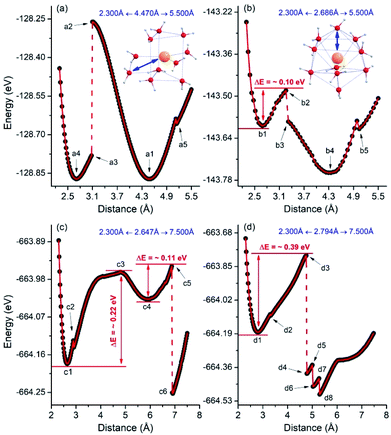 | ||
| Fig. 5 Relaxed potential energy surface scans for the distance between O atom and AcIII cation at the PBE-D3/TZ2P level of theory: (a) for gas[AcIII(H2O)9(H2O)1]3+, (b) for aq[AcIII(H2O)10]3+, (c and d) for aq[AcIII(H2O)10(H2O)35]3+. In the aq[AcIII(H2O)10(H2O)35]3+ complex, (c and d), two typical O atoms (O8 and O5, see the detailed structural information in Fig. S5 of ESI†) were chosen in the first hydration shell. | ||
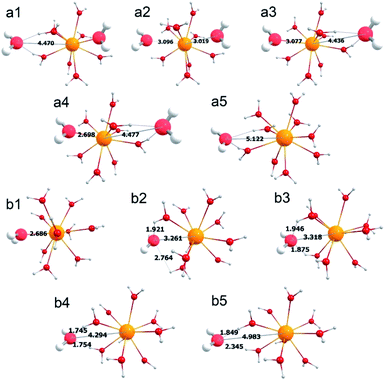 | ||
| Fig. 6 Typical geometries of the relaxed scan for the Ac–Owater bond length in the gas[AcIII(H2O)9(H2O)1]3+ and aq[AcIII(H2O)10]3+ complexes (see Fig. 5). | ||
For the aq[AcIII(H2O)10]3+ complexes, the initial distance between O atom and AcIII cation is 2.686 Å (see b1 in Fig. 5b and 6). The O⋯Ac distance is scanned in the ranges 2.300 Å ← 2.686 Å → 5.500 Å. As the O⋯Ac distance increases, there is an obvious instantaneous drop in energy, from b2 (O⋯Ac distance: 3.261 Å) to b3 (O⋯Ac distance: 3. 318 Å), which is mainly due to the hydrogen bond forming by the target O atom with a H atom in an adjacent water molecule. Furthermore, when the O⋯Ac distance reaches 4.294 Å (i.e. CN = 9, see b4 in Fig. 5b and 6), the energy of the complex is the lowest. Therefore, even under aqueous conditions, the CN = 9 is more stable than the CN = 10. However, the change in the CNs from 10 to 9 requires crossing an energy barrier of ∼0.10 eV caused by hydrogen bonds.
As is well-known, like all implicit solvent models, COSMO cannot account for explicit interactions and charge transfer between the solute and solvent.68,69 Herein, we consider an “explicit hydrogen bond model” (EHBM) for the implicit solvent model to simulate a real water environment, using 35 water molecules as the second hydration shell on the periphery of the 10 water molecules in the aq[AcIII(H2O)10]3+ complex, i.e.aq[AcIII(H2O)10(H2O)35]3+ (see the detailed structural information in Fig. S5 of ESI†). Two prototypical O atoms were chosen in the first hydration shell of aq[AcIII(H2O)10(H2O)35]3+. Similar relaxed PES scans as those above were performed, changing the O⋯Ac distance from 2.300 Å to 7.500 Å (see Fig. 5c and d). In Fig. 5c, the process of changing the CN from 10 to 9 requires crossing an energy barrier of ∼0.22 eV, but the energy of the potential well (see c4 in Fig. 5c) is higher (less negative) than that of the starting point (see c1 in Fig. 5c). Thus, an energy barrier of ∼0.11 eV must again be crossed to reach a more stable potential well (see c6 in Fig. 5c). In Fig. 5d, the process of transferring the CN from 10 to 9 only needs to cross an energy barrier of ∼0.39 eV. Evidently, when the AcIII cation is in a real water environment, despite the preferred CN of the first hydration of 9, the CN = 10 (or even larger complexes) are also possible to exist. All the various breakpoints of the energy (Fig. 5) are directly related to the breakage and formation of hydrogen bonds. Overall, our research results are consistent with those of a recent DFT-based MD study on the hydrated Th(IV) complexes.70 That is, the 9-coordinated complex is stable, and to achieve 10 coordination requires crossing of a non-negligible energy barrier.
Conclusions
In this work, we systematically studied the superatom states and the change of CN of the AcIII cation in the [AcIII(H2O)n]3+ (n = 4–11) complexes under gas-phase and aqueous solution conditions via first-principles DFT. From the geometrical point of view of the first hydration shell, the CN of the AcIII cation tends to be 9 under gas-phase conditions. This is further verified through PES scans by forcing a tenth water molecule into the first hydration shell which results in one water molecule always being knocked out. However, for the aqueous solution case, the CN tends to be 10 although the 9-coordination exhibits greater stability than the 10-coordination in the first hydration shell and PES scans show a crossing energy barrier of 0.10 eV for the change of CN from 10 to 9. These results are strongly supported by both implicit (COSMO) and explicit (EHBM) solvation models. Moreover, electronic structure analysis reveals that the chemical stability of the [AcIII(H2O)9]3+ complex can be attributed to the 18-electron rule (1S21P61D10). It primarily involves H2O → AcIII σ donation interaction between s-/p-/d-type atomic orbitals of the Ac atom and 2p atomic orbitals of the O atom.Currently, due to the lack of a deep understanding of the physical and chemical properties of Ac, lanthanides and heavier actinides are often used as stable Ac surrogates.30,71 However, our and other results show that Ac chemistry cannot be simply predicted from their behavior under comparable circumstances. In these cases, advanced quantum-chemical methods are supposed to help understand and define the bonding properties of actinide valence electrons, thus determine the possible type of bonding (covalent bond, ionic bond, or both). The nature of the Ac–O/N bond is the core of chelating agents, and one can expect similar behavior (18-electron rule, electron donation from ligand to metal) when the Ac cation interacts with oxygen atoms of other ligands. However, more details need to be considered regarding possible similarities and differences when oxygen and nitrogen atoms coordinate together. Hopefully, the results obtained herein for monodentate ligands, combined with previous studies of multidentate ligands (such as DOTA and its derivatives, EDTA, DTPA, PEPA, etc.),72–74 in this context will facilitate future clinical applications.
Methods
For hydrated complexes containing actinide ions, the Perdew–Burke–Ernzerhof (PBE)75 functional can reasonably reproduce the results of high-level ab initio wave-function theory.55,76 Thus, the spin-polarized generalized gradient approximation (GGA) with empirical dispersion77 corrections, PBE-D3, was used throughout this work. Both scalar relativistic (SR) and spin-orbit coupling (SOC) effects within the zeroth-order regular approximation (ZORA) have been considered.78–81 Moreover, we also performed re-optimized energy calculations at the empirical dispersion corrected Becke-3-Lee-Yang-Parr82,83 (B3LYP-D3) hybrid functional level based on the PBE-D3 geometries to provide additional evidence for the validity of our method.84 A triple-ζ with two polarization functions (TZ2P) uncontracted Slater-type orbital (STO) basis set was used,85 with a [1s2–4f10] frozen core for Ac, a [1s2] frozen core for O, and all electrons for H atoms, respectively.Considering the effect of water solvent, all the complexes obtained under gas-phase conditions were re-optimized at both the PBE-D3/TZ2P and B3LYP-D3/TZ2P levels by applying the conductor-like screening solvation model (COSMO).86–89 Since the free energy for each complex was computed at 1 atm pressure, an entropy correction for higher pressure is a simple way to model translational degrees of freedom in the solvent. An effective pressure of p = 1354 atm was used to mimic the condensed phase, which is the pressure obtained from p = ρwRT, corresponding to the experimental density of liquid water ρw = 997.02 kg m−3 at the temperature of 298 K.55,90
The nature of the Ac–Owater interactions has been investigated employing the EDA-NOCV method, which combines the energy decomposition analysis (EDA) with the natural orbitals for chemical valence (NOCV) approach.59 The interaction energy ΔEint between the fragments is divided into four components:
| ΔEint = ΔEelstat + ΔEPauli + ΔEorb + ΔEdisp |
In addition, for the aq[AcIII(H2O)10(H2O)35]3+ complex in aqueous solution, we considered an “explicit hydrogen bond model” (EHBM) to simulate a real water environment for the implicit COSMO solvent model. Specific steps are as follows: (a) centering on the PBE-based aq[AcIII(H2O)10]3+ complex, we constructed an initial structure of a larger water droplet with a radius of 12 Å. (b) In the Molecular Orbital PACkage (MOPAC 2012),92 because there are no available parameters for actinides, the AcIII cation was temporarily removed. To further obtain a reasonable hydrogen bond network between the first and the second hydration shells, as well as to avoid errors caused by the removal of the AcIII cation, all ten water molecules in the first hydration shell were frozen, while the remaining water molecules in the droplet were free to move. This large water droplet without AcIII cation was initially optimized via a semi-empirical method (PM6-D3H4)93 containing water solvent effects using the MOPAC 2012 program. (c) With the idea of being able to completely coat the aq[AcIII(H2O)10]3+ complex but save computational cost, we removed excess water molecules (such that 35 water molecules were left in the second hydration shell) and performed the same optimization as in step (b) again. (d) The aq[(H2O)10(H2O)35] structure optimized at the semi-empirical method was used and the AcIII cation was added back as in the initial structure. (e) The final stable complex aq[AcIII(H2O)10(H2O)35]3+ was then optimized without any constraints at the PBE-D3/TZ2P level of theory (ADF 2017).
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors would like to thank Prof. Gernot Frenking for his input and comments. YG acknowledges the support of the National Science Foundation of China (under grant number 11904049) and the Project funded by China Postdoctoral Science Foundation (2019M653365). GS acknowledges funding from the Natural Sciences and Engineering Research Council of Canada (NSERC, Discovery Grant) and the Government of Canada through the New Frontiers in Research Fund – Exploration (NFRF-E) program.References
- G. J. P. Deblonde and R. J. Abergel, Nat. Chem., 2016, 8, 1084 CrossRef CAS.
- https://iupac.org/what-we-do/periodic-table-of-elements/ .
- W. H. Xu and P. Pyykkö, Phys. Chem. Chem. Phys., 2016, 18, 17351–17355 RSC.
- L. M. Thierer and N. C. Tomson, ACS Cent. Sci, 2017, 3, 153–155 CrossRef CAS.
- Y. S. Kim and M. W. Brechbiel, Tumor. Biol, 2012, 33, 573–590 CrossRef CAS.
- M. W. Brechbiel, Dalton Trans., 2007, 4918–4928 RSC.
- D. E. Milenic, K. E. Baidoo, Y. S. Kim, R. Barkley and M. W. Brechbiel, Dalton Trans., 2017, 46, 14591–14601 RSC.
- M. Miederer, D. A. Scheinberg and M. R. McDevitt, Adv Drug Deliver Rev, 2008, 60, 1371–1382 CrossRef CAS.
- N. A. Thiele and J. J. Wilson, Canc. Biother. Rad., 2018, 33, 336–348 Search PubMed.
- A. K. H. Robertson, C. F. Ramogida, P. Schaffer and V. Radchenko, Curr. Rad., 2018, 11, 156–172 CrossRef.
- NSAC Isotopes Subcommittee, Meeting Isotope Needs and Capturing Opportunities for the Future: The 2015 Long Range Plan for the DOE-NP Isotope Program, July 2015 Search PubMed.
- P. Jena and Q. Sun, Chem. Rev., 2018, 118, 5755–5870 CrossRef CAS.
- Y. Gao and Z. Wang, Chin. Phys. B, 2016, 25, 083102 CrossRef.
- S. N. Khanna and P. Jena, Phys. Rev. Lett., 1992, 69, 1664–1667 CrossRef CAS.
- W. D. Knight, K. Clemenger, W. A. Deheer, W. A. Saunders, M. Y. Chou and M. L. Cohen, Phys. Rev. Lett., 1984, 52, 2141–2143 CrossRef CAS.
- X. Li, B. Kiran, J. Li, H. J. Zhai and L. S. Wang, Angew. Chem. Int. Ed., 2002, 41, 4786–4789 CrossRef CAS.
- P. Pyykkö and N. Runeberg, Angew. Chem. Int. Ed., 2002, 41, 2174–2176 CrossRef.
- T. J. Kealy and P. L. Pauson, Nature, 1951, 168, 1039–1040 CrossRef CAS.
- R. Pandey, B. K. Rao, P. Jena and M. A. Blanco, J. Am. Chem. Soc., 2001, 123, 3799–3808 CrossRef CAS.
- R. Arratia-Perez and C. Y. Yang, J. Chem. Phys., 1985, 83, 4005–4014 CrossRef CAS.
- X. Wu, L. Zhao, J. Jin, S. Pan, W. Li, X. Jin, G. Wang, M. Zhou and G. Frenking, Science, 2018, 361, 912–916 CrossRef CAS.
- Y. Gao, B. Wang, Y. Y. Lei, B. K. Teo and Z. G. Wang, Nano Res., 2016, 9, 622–632 CrossRef CAS.
- G. J. Cao, W. H. Schwarz and J. Li, Inorg. Chem., 2015, 54, 3695–3701 CrossRef CAS.
- C. Chi, S. Pan, J. Jin, L. Meng, M. Luo, L. Zhao, M. Zhou and G. Frenking, Chem.–Eur. J, 2019, 25, 11772–11784 CrossRef CAS.
- M. Joshi and T. K. Ghanty, Chem. Commun., 2019, 55, 7788–7791 RSC.
- Y. Gao, W. Jiang, D. Xu and Z. Wang, Adv. Theory Simul., 2018, 1, 1700038 CrossRef.
- J. P. Dognon, C. Clavaguera and P. Pyykkö, Chem. Sci., 2012, 3, 2843–2848 RSC.
- J. P. Dognon, C. Clavaguéra and P. Pyykkö, J. Am. Chem. Soc., 2009, 131, 238–243 CrossRef CAS.
- T. R. Yu, Y. Gao, D. X. Xu and Z. G. Wang, Nano Res., 2018, 11, 354–359 CrossRef CAS.
- M. G. Ferrier, E. R. Batista, J. M. Berg, E. R. Birnbaum, J. N. Cross, J. W. Engle, H. S. La Pierre, S. A. Kozimor, J. S. Lezama Pacheco, B. W. Stein, S. C. Stieber and J. J. Wilson, Nat. Commun., 2016, 7, 12312 CrossRef CAS.
- M. Salvatores and G. Palmiotti, Prog. Part. Nucl. Phys., 2011, 66, 144–166 CrossRef CAS.
- C. Apostolidis, B. Schimmelpfennig, N. Magnani, P. Lindqvist-Reis, O. Walter, R. Sykora, A. Morgenstern, E. Colineau, R. Caciuffo, R. Klenze, R. G. Haire, J. Rebizant, F. Bruchertseifer and T. Fanghänel, Angew. Chem. Int. Ed., 2010, 49, 6343–6347 CrossRef CAS.
- P. D'Angelo and R. Spezia, Chem.–Eur. J, 2012, 18, 11162–11178 CrossRef.
- M. Duvail, R. Spezia and P. Vitorge, ChemPhysChem, 2008, 9, 693–696 CrossRef CAS.
- M. G. Ferrier, B. W. Stein, E. R. Batista, J. M. Berg, E. R. Birnbaum, J. W. Engle, K. D. John, S. A. Kozimor, J. S. L. Pacheco and L. N. Redman, ACS Cent. Sci, 2017, 3, 176–185 CrossRef CAS.
- M. G. Ferrier, B. Stein, S. E. Bone, S. K. Cary, A. S. Ditter, S. A. Kozimor, J. S. Lezama Pacheco, V. Mocko and G. T. Seidler, Chem. Sci., 2018, 9, 7078–7090 RSC.
- J. Wiebke, A. Moritz, X. Cao and M. Dolg, Phys. Chem. Chem. Phys., 2007, 9, 459–465 RSC.
- R. R. Pappalardo, D. Z. Caralampio, J. M. Martinez and E. S. Marcos, Inorg. Chem., 2019, 58, 2777–2783 CrossRef CAS.
- B. Brendebach, N. L. Banik, C. M. Marquardt, J. Rothe, M. A. Denecke and H. Geckeis, Radiochim. Acta, 2009, 97, 701–708 CAS.
- M. Duvail, F. Martelli, P. Vitorge and R. Spezia, J. Chem. Phys., 2011, 135, 044503 CrossRef.
- M. R. Antonio, L. Soderholm, C. W. Williams, J. P. Blaudeau and B. E. Bursten, Radiochim. Acta, 2001, 89, 17–25 CAS.
- P. G. Allen, J. J. Bucher, D. K. Shuh, N. M. Edelstein and T. Reich, Inorg. Chem., 1997, 36, 4676–4683 CrossRef CAS.
- P. G. Allen, J. J. Bucher, D. K. Shuh, N. M. Edelstein and I. Craig, Inorg. Chem., 2000, 39, 595–601 CrossRef CAS.
- R. Kirsch, D. Fellhauer, M. Altmaier, V. Neck, A. Rossberg, T. Fanghanel, L. Charlet and A. C. Scheinost, Environ. Sci. Technol., 2011, 45, 7267–7274 CrossRef CAS.
- T. Stumpf, C. Hennig, A. Bauer, M. A. Denecke and T. Fanghanel, Radiochim. Acta, 2004, 92, 133–138 CAS.
- S. Skanthakumar, M. R. Antonio, R. E. Wilson and L. Soderholm, Inorg. Chem., 2007, 46, 3485–3491 CrossRef CAS.
- P. Lindqvist-Reis, C. Apostolidis, J. Rebizant, A. Morgenstern, R. Klenze, O. Walter, T. Fanghanel and R. G. Haire, Angew. Chem. Int. Ed., 2007, 46, 919–922 CrossRef CAS.
- T. Yang and B. E. Bursten, Inorg. Chem., 2006, 45, 5291–5301 CrossRef CAS.
- D. Hagberg, E. Bednarz, N. M. Edelstein and L. Gagliardi, J. Am. Chem. Soc., 2007, 129, 14136–14137 CrossRef CAS.
- R. Atta-Fynn, E. J. Bylaska, G. K. Schenter and W. A. de Jong, J. Phys. Chem. A, 2011, 115, 4665–4677 CrossRef CAS.
- M. R. Antonio, C. W. Williams and L. Soderholm, Radiochim. Acta, 2002, 90, 851–856 CAS.
- E. Galbis, J. Hernandez-Cobos, C. den Auwer, C. Le Naour, D. Guillaumont, E. Simoni, R. R. Pappalardo and E. S. Marcos, Angew. Chem. Int. Ed., 2010, 49, 3811–3815 CrossRef CAS.
- R. Revel, C. Den Auwer, C. Madic, F. David, B. Fourest, S. Hubert, J. F. Le Du and L. R. Morss, Inorg. Chem., 1999, 38, 4139–4141 CrossRef CAS.
- B. Ravel and S. D. Kelly, AIP Conf. Proc., 2007, 882, 150–152 CrossRef CAS.
- G. A. Shamov and G. Schreckenbach, J. Phys. Chem. A, 2006, 110, 12072 CrossRef CAS.
- J. Kuta and A. E. Clark, Inorg. Chem., 2010, 49, 7808–7817 CrossRef CAS.
- J. Zhang, N. Heinz and M. Dolg, Inorg. Chem., 2014, 53, 7700–7708 CrossRef CAS.
- K. Wang, C. Xu, D. Li and L. Cheng, Commun. Chem., 2020, 3, 39 CrossRef CAS.
- M. P. Mitoraj, A. Michalak and T. Ziegler, J. Chem. Theory Comput., 2009, 5, 962–975 CrossRef CAS.
- C. Terrier, P. Vitorge, M. P. Gaigeot, R. Spezia and R. Vuilleumier, J. Chem. Phys., 2010, 133, 044509 CrossRef.
- M. Duvail, P. Vitorge and R. Spezia, J. Chem. Phys., 2009, 130, 104501 CrossRef.
- G. Dupouy, I. Bonhoure, S. D. Conradson, T. Dumas, C. Hennig, C. Le Naour, P. Moisy, S. Petit, A. C. Scheinost, E. Simoni and C. Den Auwer, Eur. J. Inorg. Chem., 2011, 2011, 1560–1569 CrossRef.
- K. Fukui, Theory of Orientation and Stereoselection, Springer Verlag: Berlin, 1975 Search PubMed.
- R. B. Woodward and R. Hoffmann, Angew. Chem. Int. Ed., 1969, 8, 781–853 CrossRef CAS.
- J. Jin, T. Yang, K. Xin, G. Wang, X. Jin, M. Zhou and G. Frenking, Angew. Chem. Int. Ed., 2018, 57, 6236–6241 CrossRef CAS.
- R. Hoffmann, J. M. Howell and E. L. Muetterties, J. Am. Chem. Soc., 1972, 94, 3047–3058 CrossRef CAS.
- J. Jin, S. Pan, X. Jin, S. Lei, L. Zhao, G. Frenking and M. Zhou, Chem.–Eur. J, 2019, 25, 3229–3234 CrossRef CAS.
- I. S. Ufimtsev, N. Luehr and T. J. Martinez, J. Phys. Chem. Lett., 2011, 2, 1789–1793 CrossRef CAS.
- A. van der Vaart and K. M. Merz, J. Am. Chem. Soc., 1999, 121, 9182–9190 CrossRef CAS.
- R. Spezia, Y. Jeanvoine, C. Beuchat, L. Gagliardi and R. Vuilleumier, Phys. Chem. Chem. Phys., 2014, 16, 5824–5832 RSC.
- S. Cotton, Lanthanide and actinide chemistry, John Wiley & Sons, Ltd, Uppingham, Rutland, UK, 2006 Search PubMed.
- I. A. Davis, K. A. Glowienka, R. A. Boll, K. A. Deal, M. W. Brechbiel, M. Stabin, P. N. Bochsler, S. Mirzadeh and S. J. Kennel, Nucl. Med. Biol., 1999, 26, 581–589 CrossRef CAS.
- K. A. Deal, I. A. Davis, S. Mirzadeh, S. J. Kennel and M. W. Brechbiel, J. Med. Chem., 1999, 42, 2988–2992 CrossRef CAS.
- N. A. Thiele, V. Brown, J. M. Kelly, A. Amor-Coarasa, U. Jermilova, S. N. MacMillan, A. Nikolopoulou, S. Ponnala, C. F. Ramogida, A. K. H. Robertson, C. Rodriguez-Rodriguez, P. Schaffer, C. Williams, J. W. Babich, V. Radchenko and J. J. Wilson, Angew. Chem. Int. Ed., 2017, 56, 14712–14717 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS.
- M. P. Kelley, J. Su, M. Urban, M. Luckey, E. R. Batista, P. Yang and J. C. Shafer, J. Am. Chem. Soc., 2017, 139, 9901–9908 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef.
- E. van Lenthe, A. Ehlers and E.-J. Baerends, J. Chem. Phys., 1999, 110, 8943–8953 CrossRef CAS.
- E. van Lenthe, E. J. Baerends and J. G. Snijders, J. Chem. Phys., 1993, 99, 4597–4610 CrossRef CAS.
- E. van Lenthe, J. G. Snijders and E. J. Baerends, J. Chem. Phys., 1996, 105, 6505–6516 CrossRef CAS.
- E. van Lenthe, E. J. Baerends and J. G. Snijders, J. Chem. Phys., 1994, 101, 9783–9792 CrossRef CAS.
- C. T. Lee, W. T. Yang and R. G. Parr, Phys. Rev. B, 1988, 37, 785–789 CrossRef CAS.
- A. D. Becke, J. Chem. Phys, 1993, 98, 5648–5652 CrossRef CAS.
- G. A. Shamov, G. Schreckenbach and T. N. Vo, Chem.–Eur. J, 2007, 13, 4932–4947 CrossRef CAS.
- E. van Lenthe and E. J. Baerends, J. Comput. Chem, 2003, 24, 1142–1156 CrossRef CAS.
- A. Klamt and G. Schüürmann, J. Chem. Soc., Perkin Trans. 2, 1993, 799–805 RSC.
- A. Klamt, J. Chem. Phys, 1995, 99, 2224–2235 CrossRef CAS.
- A. Klamt and V. Jonas, J. Chem. Phys, 1996, 105, 9972–9981 CrossRef CAS.
- C. C. Pye and T. Ziegler, Theor. Chem. Acc, 1999, 101, 396–408 Search PubMed.
- R. L. Martin, P. J. Hay and L. R. Pratt, J. Phys. Chem. A, 1998, 102, 3565–3573 CrossRef CAS.
- G. Te Velde, F. M. Bickelhaupt, E. J. Baerends, C. Fonseca Guerra, S. J. A. Van Gisbergen, J. G. Snijders and T. Ziegler, J. Comput. Chem, 2001, 22, 931–967 CrossRef CAS.
- J. J. Stewart, J. Comput. Aided Mol. Des., 1990, 4, 1–105 CrossRef CAS.
- J. Rezac and P. Hobza, J. Chem. Theory Comput, 2012, 8, 141–151 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Part 1. Different isomers of AcIII aquo complexes; part 2. Ac–Owater bond lengths of AcIII aquo complexes; part 3. Detailed composition of superatom MOs of AcIII(H2O)9]3+ complexes; part 4. Molecular orbitals of [AcIII(H2O)9]3+ and [Ac@Au14]− based on the Jellium model; part 5. EDA-NOCV results with and without the explicit second water shell; part 6. Electronic structure of [AcIII(NH3)9]3+ and [AcIII(PH3)9]3+ complexes; part 7. Electronic structure diagrams for the Ac cation with coordination numbers from 7 to 10; part 8. Optimized geometry of the aq[AcIII(H2O)10(H2O)35]3+ complex. See DOI: 10.1039/d0sc02342f |
| This journal is © The Royal Society of Chemistry 2021 |