Using metavisualization to revise an explanatory model regarding a chemical reaction between ions
Solange Wagner
Locatelli
 *a and
Bette
Davidowitz
*a and
Bette
Davidowitz
 b
b
aFederal University of ABC, Brazil. E-mail: solange.locatelli@ufabc.edu.br
bUniversity of Cape Town, South Africa. E-mail: bette.davidowitz@uct.ac.za
First published on 22nd January 2021
Abstract
The objective of this work was to evaluate the implementation of a metavisual strategy for students to revise and self-regulate concepts arising in a study of a chemical reaction between ions. For this purpose, two chemistry education undergraduate students at a Brazilian public university carried out an investigative activity, involving metavisual steps, to revise explanatory models at the submicro level. Students were given a problem, namely a reaction between ions drawn from a real-life situation and were provided with clay to construct an explanatory model of the submicro level for the initial and final stages of the reaction. The students were asked to compare their clay model with an example of a scientifically correct figure of the submicro level of the reaction generated by the researchers. At this stage students were given the option to reconstruct their model. Data were captured via photographs of the clay models and students’ verbal discussions as they proceeded through the activity. The findings reveal evidence of self-regulation of mental models at the submicro level, from the interaction of prior knowledge, chemical diagrams and discussions and reflections by the pair of students. Difficulties regarding chemical formulae were also observed in relation to the symbolic level. Finally, there are implications for teaching chemistry, since teachers in training need to experience metavisual strategies for future application in their classrooms.
Introduction
In 1976, the psychologist Flavell described metacognition as referring “to one's knowledge concerning one's own cognitive processes or anything related to them” (Flavell, 1976, p. 232) and proposed that metacognition is key to helping children solve problems. Since that time there has been a growing interest in understanding what metacognition is and, above all, its relationship to improved learning. Although there is no single definition for metacognition, Kuhn and Dean (2004), in an article describing psychology and education, define it as the process of raising awareness and proper management of the own thoughts of an individual. This is in line with what we consider encompassing the main aspects of metacognition.In a study of students’ conceptions and science learning, Driver (1989) associated metacognition with the learning of concepts in science. This view was supported by Schraw (1998) who described the role of metacognitive strategies as being associated with better learning outcomes. According to Schraw (1998, p. 123) metacognition is crucial for teachers to “help students construct explicit knowledge about when and where to use strategies”. Lai (2011) wrote a review of metacognition and pointed out that “although individual developmental models vary, most postulate significant improvements in metacognition during the first 6 years of life”, (p. 2). In addition, there is a possibility that aspects of this construct can be taught to students. Metacognition can be considered as fundamental to gain a better understanding of the creation of knowledge; thus, metacognition should be enabled in the classroom, with intentionality. Linked to this, Blank (2000) proposed a revised learning cycle model, the metacognitive learning cycle, which emphasized that metacognitive moments should be provided via formal opportunities in the classroom. This follows, since metacognitive processes can happen at any time. However, as proposed in the current study, metacognitive strategies are the approaches that intentionally provide moments for metacognitive skills to be contemplated in a chemistry class. Moments of reflection, monitoring and self-regulation should be provided for students to revise aspects linked to representational levels, which in turn makes it possible for them to understand the chemistry content.
Johnstone (1993) proposed the idea of the chemistry triplet i.e. three levels of representation, namely macro, micro and representation and suggested that they provide a useful framework for understanding and teaching chemistry. Gilbert and Treagust (2009) used the terms submicro, macro and symbolic to designate the three representational levels that underlie explanations about chemical concepts. These authors list the words or phrases which have been used to describe the three levels of representation in the chemical education literature. While there are variations on the first two terms listed above, Gilbert and Treagust's (2009) third representational level is currently referred to as the symbolic level. They suggested using the terms submicro, macro and symbolic to avoid confusion. Also, according to these authors, the macro level is phenomenological; it is visible. For example, when we observe the burning of coal, we can feel the heat emanating from the chemical reaction. The submicro level encompasses the particles, atoms, molecules and ions involved in the process and provides qualitative explanations. Finally, with respect to the symbolic level, it refers to symbols and balanced chemical equations associated with the studied chemical phenomena (Gilbert and Treagust, 2009).
In a study with undergraduate students, Chittleborough and Treagust (2008) pointed out that the distinction between macro and submicro levels is due to the scale on which they exist. Therefore, it is important to work with chemical diagrams that will serve as a visual stimulus of the submicro level for students. They concluded by recommending the use of a metavisual strategy to link the various representational levels, as well as their explanations (Chittleborough and Treagust, 2008).
In another study with aspects similar to the current research, Kelly (2017) investigated the understanding of 17 undergraduate students about a concept in chemistry, namely redox reactions. First, students observed the chemical phenomenon and had to draw an explanation about the three macroscopically observable changes, writing down important keywords in the explanations. Then, using two different animations – one simpler and the other more complex, both containing scientific inaccuracies – the students had the task of identifying the conceptual errors present. The findings indicated a preference for the simpler and more familiar animation, as well as an explicit link with aspects of the macroscopic level.
Another research study conducted by Kelly et al. (2010) investigated the misconceptions that students demonstrated when proposing explanatory models for three precipitation reactions at the submicro level. The first one is the same as in our study (precipitation of silver chloride), the second one is not actually a reaction, because the ions do not react to form a precipitate, and the last is a more complex precipitation reaction related to the charges on the ions. Kelly et al.'s (2010) findings indicated that the students were not clear about the meaning of an aqueous solution. They also had misconceptions related to the formation of molecules from ions in aqueous solution, as well as believing that the precipitate consists of molecules.
Hansen (2014) researched how undergraduates interacted with multiple representations involving visual problem-solving in chemistry. Various data collection instruments were used, such as eye-tracking, drawings and visual problem-solving in order to understand students’ metavisual skills. She found some visual problem-solving patterns and many misconceptions in chemistry, for example with respect to equations and stoichiometric coefficients. In addition, it was observed that undergraduates arrived at university with different levels of knowledge in chemistry. In general, the undergraduates had low levels of development with respect to visual skills which are very important both for understanding and building representations in chemistry in order to achieve success in introductory chemistry. Hansen concluded that it is necessary for teachers to provide opportunities for students to interact with visualizations in classroom to improve chemistry education.
Van der Westhuizen (2015) conducted research with first-year students who often perform poorly in chemistry. Having chosen the theme of stoichiometry, she proposed a series of activities to try to integrate visualization in classes, as well as to develop metacognition and critical thinking in order for students to improve their understanding of stoichiometry. The findings from this study revealed that teaching based on this perspective, with more active student participation in the learning process, led to an improvement in the understanding of the topic of stoichiometry. Van der Westhuizen recommended that the strategy involving visualization, metacognition and critical thinking should be used to address other topics in chemistry.
Finally, Gilbert et al. (2010), in a study with 32 high school students, used a modeling-based teaching strategy to research whether students would be able to develop metavisual skills when studying the topic of ionic bonding. Their findings indicate that modeling activities enabled the student to develop their internal mental models as well as to express them as external representations which allow misconceptions to be reviewed. The authors conclude that “… some students need more experience in: drawing upon existing models and using them as analogies, and in the construction of new representations” (Gilbert et al., 2010, p. 51).
Thus, research has shown that students have difficulty understanding concepts related to stoichiometry (Van der Westhuizen, 2015), ions in solution, as well as the formation of a precipitate (Kelly et al., 2010). There has been little integration of visual activities in chemistry classes (Hansen, 2014, Van der Westhuizen, 2015) despite the suggestion by Gilbert et al. (2010) that students need to use existing models to build their own models. The current study aims to build on the research reported and seeks to answer the following guiding question: To what extent can a metavisual strategy (comparing models) contribute to self-regulation and the revision of an explanatory model regarding a chemical reaction between ions?
Theoretical framework
In order to evaluate the implementation of an especially designed metavisual strategy it is necessary to describe metavisualization, which provides the theoretical framework for this study. Metavisualization can be understood as being metacognition related, specifically, to visualizations (Gilbert, 2005), such as images, drawings, graphs, figures, diagrams, etc. Also, according to Gilbert (2005), metavisualization involves navigating between evaluation and interpretation of images. We start this description with a brief discussion of metacognition, which can be understood through two main aspects that are related.The first refers to knowledge about what is known, knowledge about knowledge (Flavell, 1981) or knowledge of cognition (Schraw, 1998). Basically, we can assume it to be what each person knows about his or her own cognition or about more general aspects of cognition. It includes knowing oneself as an apprentice, recognizing one's limits and possibilities, how to solve problems and when and why to use all this knowledge (Schraw, 1998).
The second aspect of metacognition refers to the regulatory aspect, which is the regulation of cognition (Schraw, 1998), which is the focus of this research. Gunstone (1994) refers to self-regulation and considers a metacognitive learner as “… one who undertakes the tasks of monitoring, integrating and extending their own learning… Correspondingly, there are good learning behaviours” (p. 135). Although there are many definitions of metacognition, Efklides (2006, p. 4) says, “it is generally accepted that metacognition is a model of cognition, which acts at a meta-level and is related to the object-world (i.e., cognition)”. This relationship (meta-level and object-world) involves the regulatory aspect of metacognition, in which, for example, the student can monitor, perceive some incorrect chemical concept (object-world) and self-regulate (meta-level) his or her learning. This self-regulation is also referred to as a metacognitive ability by some researchers (Schraw et al., 2012). Associated with the regulation component, we have to consider the fundamental character of ‘consciousness’ that is embraced by metacognition (Girash, 2014). According to him, consciousness is associated with metacognitive strategies, as it becomes necessary for the proposed activity to intentionally involve metacognitive aspects of regulation (Efklides, 2006). Schraw (1998, p. 114) summarizes the regulation of cognition as “a set of activities that help students control their learning”.
Thus, students should be guided to consider all these aspects when learning chemistry, since chemistry is based on explanatory models that are abstract. Harrison and Treagust (1996) have pointed out that these models are necessary for an understanding of the behavior of particles and students may have difficulty separating the model from reality. Models can be represented by diagrams that are widely used in learning chemistry. According to Hansen (2014), a molecular diagram can consist of a representation that will portray, for example, a system and/or even specific aspects of an object. She also notes that diagrams can assist in the communication of scientific concepts and, precisely for this reason, diagrams need to be properly understood.
Since these models reach the student through external representations, for instance a visual diagram (visualizations), it is necessary that all these aspects of metacognition should be considered, specifically for students to be provided with opportunities to revise their conceptions around the visualizations, which we call metavisualization. Davidowitz and Chittleborough (2009) emphasized the importance of metavisualization as being necessary for students to interpret and move between the levels of chemistry, submicro, macro and symbolic, were the symbolic level is one of the most common formats of representation in learning chemistry.
Locatelli et al. (2010) proposed a model that explains meta-visualization and its relationship to metacognition and visualization (Fig. 1). According to their model, metacognition involves regulation and active control by the individual who can change the internal representation (an image stored in memory), which may have been generated from an external visualization, for example a figure. Thus, visualizations can assist in the formation of mental models that are stored and can be changed by regulatory processes such as metacognition and/or metavisualization. Simply put, we could assume that this process involves revising internal representations. This idea, linking metacognition with visualization, is supported by Van der Westhuizen (2015) who added the notion of critical thinking to enhance the teaching of chemistry.
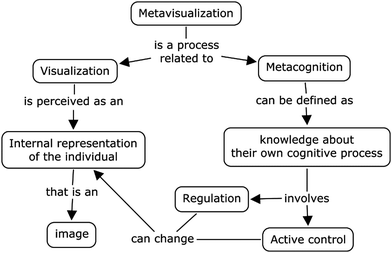 | ||
| Fig. 1 Metavisualization (Locatelli et al., 2010, p. 80). | ||
Hung et al. (2019) carried out research about the professional development of chemistry teachers, based on the model for metavisualization (Fig. 1) proposed by Locatelli et al. (2010) with a view to expanding the model and adding more elements. Among the main elements added by these authors, were “metavisualization knowledge and visualization skills” (p. 22) that “play a role in successful visualization” (Hung et al., 2019, p. 22), with which we agree, and which seem to give a broader view of the metavisual process. Hung et al. (2019) also found that enabling teachers to develop metavisual skills, including some metavisual strategies, was a way to lead them to reconstruct their own ideas and to help guide students to be fluent at interpreting multiple representations.
With regard to students being able to revise their ideas about the submicro level, which is the purpose of this research, Davidowitz and Chittleborough (2009) pointed out that metavisualization is important, given the characteristics of abstraction and difficulty of representation inherent at the submicro level. Additionally, Chang and Tzeng (2017) have also raised concerns regarding investigations of aspects of metavisualization, as this process is difficult to observe using only paper-and-pencil instruments, indicating the need to include several ways of visualizing the phenomenon. Finally, Hung et al. (2019) suggested further research concerning the concept of metavisualization as they pointed out “empirical and theoretical studies are needed to characterize metavisualization, which in turn can provide insights into its role in science teaching and learning”.
Akaygun, in her role as one of guest editors for a special edition of Chemistry Education Research and Practice focusing on visualizations and representations in chemistry education, noted: “At this stage, I think we need to switch our attention to how visualizations and representations could be more effectively used for conceptual learning so that we could guide teachers and instructors about the methods that they could adopt for their purpose.” (Kelly and Akaygun, 2019, p. 657). The present work is in line with their suggestion and focused on implementing and evaluating a metavisual strategy presented to undergraduate students and analyzing to what extent it contributed to revising their ideas about a chemical reaction. The strategy focused on the representational levels of chemistry since several studies in the literature revealed associated alternative conceptions for this reaction, especially at the submicro level.
There are several ways to represent a model, thus a drawing is an example of a visual model involving a 2D representation (Gilbert et al., 2010). Chiu and Linn (2012) pointed out that connections can be made with respect to scientific concepts with activities promoting self-monitoring, in which students can actively propose explanations to the scientific phenomena, for instance by using drawings.
To carry out the research, a drawing of an explanatory model at the submicro level, namely a chemical diagram constructed by the first researcher, was presented to students as suggested in a study by Davidowitz and Chittleborough (2009). These researchers noted that “chemical diagrams are used to represent chemical information, to help describe an idea, provide an explanation, present a visual image, to make predictions, deductions, motivate and form hypotheses” (p. 169). The authors recommended the use of submicro drawings in the teaching and learning of chemistry so that students can build mental models suitable for the chemical phenomenon under study. Davidowitz and Chittleborough (2009) also state that these chemical diagrams can be 2D or 3D, and as used in this research the students used clay models (3D), which according to Gilbert et al. (2010) represent a concrete mode. The students constructed the models in 3D (clay model) and compared them with a 2D model (drawing) presented by the researcher.
Methodology
The research is guided by a qualitative approach, given that “purposeful sampling, collection of open-ended data, analysis of text or pictures, representation of information in figures and tables, and personal interpretation of the findings all inform qualitative methods” (Creswell, 2014, p. 23).Characterization of research and research subjects
Six (6) students – two males (ages 27 and 47) and four females (ages 20, 24, 27 and 27) participated in the investigative activity. All six were taking the course, Chemistry Teaching Practices II, which is a compulsory subject of the chemistry degree, but which can be taken by any student of this Brazilian public university. Of the research subjects, only one had never taken any courses in pedagogy and all of them had taken a course in general chemistry. The course ran from September to December 2019, with a workload of 3 classes per week, totaling 36 hours. In order to provide an in-depth analysis, a single group, students Raul and Ana, was chosen for analysis, characterizing the present research as a case study as mentioned above. Raul and Ana, aged 27 and 24 respectively, were undergraduate students in chemistry and both intend to be chemistry teachers.The choice of this group was due to the availability of students to participate, voluntarily, in the research and because they had delivered their records for analysis. Permission to carry out the research was granted by Research Ethics Committee from the Federal University of ABC, and the students also signed a consent form agreeing to participate in the research.
Research design
The present study considered a pre-existing coding scheme to evaluate clay models constructed by students. The clay models and subsequent revisions made by the students were compared with a submicro drawing constructed by the first researcher, considering both differences and similarities between models. We decided to use a case study approach (Stake, 1994), to answer the research question above as this allowed for greater insight into students’ actions and thoughts. This approach allows for a detailed examination of a single case while enabling readers to relate to their own contexts. Moreover, the purpose of the research was to gain an in-depth view of how the implementation of a metavisual strategy contributes to allowing students to revise an explanatory model. According to Cohen et al. (2007), case studies strive to capture the close-up reality and thick description of participants’ lived experiences in a particular situation, which inevitably blends a description of events with an analysis of them.An investigative activity was presented to six undergraduate students for them to build and reconstruct the concept of chemical transformation, more specifically involving ions in aqueous solution.
Investigative activity
The option for a didactic approach involving an investigative class (IC) was due to the objectives to be achieved, which involve some important steps within an IC. The National Research Council, NRC (2000) addresses some fundamental aspects to which are considered essential features of an investigative class namely:“1. Learner engages in scientifically oriented questions
2. Learner gives priority to evidence in responding to questions
3. Learner formulates explanations from evidence
4. Learner connects explanations to scientific knowledge
5. Learner communicates and justifies explanations.” (p. 29)
These aspects of an IC were chosen as they apply to this research and have been cited by researchers from different countries, e.g. Israel and Turkey as being a strategy to scaffold inquiry-based learning in chemistry laboratories and classrooms respectively (Barnea et al., 2010, Kadioglu-Akbulut and Uzuntiryaki-Kondakci, 2020).
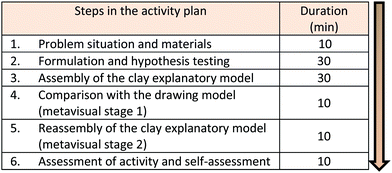
Based on the guidelines above, a simple investigative activity was proposed, based on a topic drawn from everyday life with the objective of engaging the students in the process. They were presented with a problem situation which would have to be solved using the materials available. This activity represents the macro level of the chemical phenomenon to be studied by them (Fig. 2).
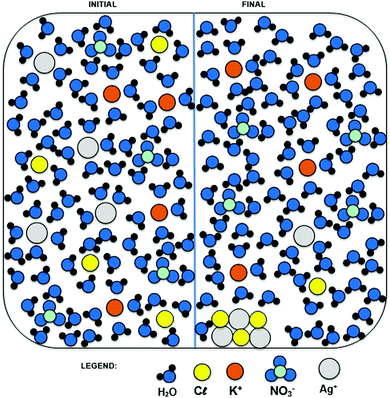
Fig. 3 shows the activity plan designed by the authors, as well as the approximate duration of each stage in minutes. This plan was presented to the students before the start of the activity. After providing the students with the problem situation (1), they were instructed to formulate a work plan to test their hypothesis of how they would solve the problem using evidence. For this, they took a small portion of soil from each of the two pots, placed them in different test tubes, added water and tested the mixtures with an aqueous solution of silver nitrate using a few drops in each case. This inquiry was based on their hypothesis that the content of the pot containing the potassium chloride would yield a precipitate in the presence of aqueous silver nitrate solution (2). After this phase, they were invited to construct an explanatory model at the submicro level using the modeling clay, considering the initial and final stages of the chemical reaction (3). The students were then presented with an example of a submicro representation depicting the formation of a precipitate of AgCl from aqueous solutions of AgNO3 and KCl (Fig. 4).
The submicro representation, Fig. 4, is an example of a diagram depicting the reaction and students were told that other diagrams exist to represent this phenomenon. Therefore, even if the students’ models and Fig. 4 were different it would not necessarily mean that there was an error, as there are several ways of representing a chemical reaction. However, the models made by students can uncover evidence of alternative conceptions, and the process of revising the models is the key to students’ awareness about their misconceptions. Thus, the activity provides an opportunity for students to revise their conceptions.
The next step in the process required students to compare the clay model that they had constructed with the submicro diagram presented to them, identifying similarities and differences. This is the first metavisual stage (4). Fig. 4 was given to the students to use for stages 4–6 of the activity. Based on this reflection, the students reassembled the clay models which would reveal any reconstruction of ideas. This is the second metavisual stage (5). Finally, students carried out an assessment of the activity as well as a self-assessment (6). This aspect of the task was designed to allow them to verbalize their feelings during the activity by answering the question; What did you think about this activity? It should be noted that throughout the process, students were instructed to express verbally whatever they were thinking, so that their dialogues could be analyzed later. In analyzing this research, all dialogues were captured in audio format, although only those referring to stages 4 and 5 were used as data to create categories since they were considered as intentional moments of reflection regarding explanatory models, encompassing the metavisual stages.
We chose a relatively simple model for Fig. 4 since Rosenthal and Sanger (2012) pointed out in their study that complex visualizations could compromise students’ understanding. It is worth noting that submicro diagrams seldom include lines to indicate electrostatic interactions; these are implied from the orientation of the water molecules around the ions, as shown in Fig. 4. It was also considered that chemical diagrams of the submicro level can help students to develop their mental models of the phenomenon in question (Davidowitz and Chittleborough, 2009). These diagrams constitute an excellent educational tool, depending on whether students can properly understand the diagram and how well they make the necessary connections (Davidowitz and Chittleborough, 2009). In her study, Hansen (2014) notes the importance of providing opportunities for the student to verbalize and reflect on visual representations in chemistry, for example, using a diagram like the one proposed in this research (Fig. 4).
Thus, considering available materials and in order to identify the potassium chloride in the sample, students were expected to propose the reaction of the chloride ions with silver ions, forming the precipitate as shown in Fig. 4.
Data collection and analysis
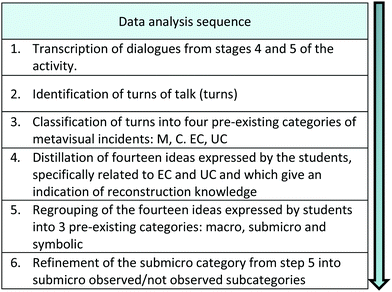
Data were collected during the second class of the Chemistry Teaching Practices II course which consisted of 18 classes. The class lasted for 120 minutes, with 100 minutes dedicated to the activity itself. Audio recordings were made to be able to transcribe the discussions, observations and photos were taken of the systems made with the modeling clay to allow a better understanding of what was being investigated. The data analysis sequence used in this study is shown in Fig. 5.Firstly, the transcripts of the discussions were read several times to identify and number the turns of talk, henceforth referred to as turns. A turn began when an individual took the lead in a conversation, and ended when another person took over (Hogan, 1999).
We used the codes for metavisual incidents described in Table 1 to classify each turn identified in the transcripts from steps 4 and 5. A doctoral student coded the turns according to the pre-existing categories of metavisual incidents M (monitoring), C (confirmation), PC (positive change) and NC (negative change) proposed by Locatelli and Arroio (2014), together with a conceptual description in the names of two of them by Paz and Locatelli (2019). Thus, EC (effective change) instead of PC and UC (under construction) (change instead of NC) were used, as shown in Table 1. The codes assigned by the doctoral student were checked by the first researcher and there was an 80% match. After discussion, the coding process was repeated and the agreement between the coders was 98%.
| Metavisual incidents, 2014 codes | Description | Metavisual incidents, 2019 codes |
|---|---|---|
| Monitoring (M) | Monitoring of any idea or reasoning by posing a question. | Monitoring (M) |
| Confirmation (C) | Confirmation of some idea or reasoning. | Confirmation (C) |
| Positive change (PC) | Change in the idea or reasoning, in the scientifically accepted sense. | Effective change (EC) |
| Negative change (NC) | Change in the idea or reasoning, but moving away from the scientifically accepted meaning. | Under construction change (UC) |
Metavisual incidents are a manifestation of metavisualization by students (Locatelli and Arroio, 2014). We used the terms “effective change” and “under construction change” (Paz and Locatelli, 2019) shown in Table 1 in order to make it clear that any change, in principle, is part of the process of construction and reconstruction of the reasoning.
More specifically, the “change” codes were necessary to analyze the moments that students intentionally revise their learning and whether or not they are capable of self-regulation, revealing possibilities (when the change is ‘effective’) and limitations (if the change is ‘under construction’).
Using inductive analysis, a method similar to the grounded theory formulated by Glaser and Strauss (1967), the turns of talk classified as “change” (EC and UC) were grouped into 14 ideas, I1–I14, which were expressed by the students as they worked through the activity.
Then we distributed these 14 ideas into the three representational levels described by Johnstone (1993). Finally, we refined the submicro level into two subcategories, observed/not observed submicro level, for the drawing shown to students (Fig. 4) as students discussed some characteristics, such as the crystalline structure of the precipitate, which was not shown in Fig. 4.
Results
A total of 121 turns of talk were identified and classified using the codes in Table 1. Of the 121 turns, 79 (65%) were classified as metavisual incidents and 42 (35%) as non metavisual incidents (NMI).The majority of metavisual incidents were coded as monitoring (57%), followed by confirmation (25%). Change incidents corresponded to 18% of the total comprising of 14% turns coded as EC (11) and 4% as UC (3). It is important to point out at this stage, that all changes, EC or UC, contribute to the construction of knowledge, since the conflict created when students were presented with an image of the reaction makes it possible for them to revise their conceptions (Zhang and Linn, 2011). This action provides them the opportunity to perfect their mental models about a particular scientific concept, as will be explained below.
Indications of reconstructions of knowledge: turns coded as EC and UC in the ideas expressed by students
In order to gain an indication of reconstructions of knowledge, the 14 ideas manifested by students were grouped into themes related to revising the production of models. There are 11 ideas coded as EC and 3 ideas as UC, totaling 14 moments of reconstructions of knowledge externalized by students, namely 14 ideas expressed by students, which we will deal with in sequence. We labelled these as I1 to I14 listed in the order in which they appear in the students’ dialogues. The 14 ideas are described below.The models built by the students, step 3 of the activity, is shown in Fig. 6. The legend was added by the researchers based on students’ description of the model captured in the transcript, which demonstrated that students knew which entity each sphere represented.
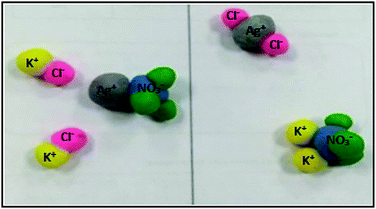 | ||
| Fig. 6 Representation using clay proposed by students: first explanatory model of the submicro level – left (initial) and right (final) – step 3 in the activity shown in Fig. 3 – the symbols were inserted by the authors, as the students identified the spheres by name during the activity. | ||
When the researcher presented the students with a possibility of a scientifically accepted answer, Fig. 4, at the beginning of step 5, they were surprised. This is the first idea (I1) expressed by the students which we classified as the first metavisual incident, as can be seen in the comment from Raul: “Look, we didn’t put the water in, look how cool” (turn 1, category EC). Raul expresses himself with excitement as he immediately noticed the presence of water in Fig. 4, which is in agreement with Ana who said: “Ahhh really” (category C). This shows an indication of revision of concepts by the students as part of a process of self-regulation, as now they recognize water as an important part of the process in the dissolution of ions.
Fig. 6 shows the absence of water in the students’ representation of the reaction. Water is very important in this process, as it makes possible the chemical reaction between silver and chloride ions. The finding with respect to the omission of water molecules in the representation of the reaction is in line with a similar observation by Nakiboglu and Nakiboglu (2019) in a study with a group of pre-service teachers.
The students noted another difference between the model and the figure which concerns the fact that the ions are dissociated in the water (I2). As Raul stated “we didn’t make it ionized, and actually everything is ionized” (turn 7, category EC) and confirmed by Ana: “we got it wrong!” (turn 8, category C). This was the second error noticed by the students, namely that they had constructed an ion pair, as depicted as a molecule (Fig. 6), which was also reported in other studies (Kelly et al., 2010; Rosenthal and Sanger, 2012) revealing an alternative conception for this type of chemical interaction between ions and which is related to the stoichiometry of the reaction and the products formed. Nakiboglu and Nakiboglu (2019) point out that a common alternative conception is considering ions as molecules, which can be seen in Fig. 6.
In the following excerpt, it seems that the students were still in doubt as to whether the nitrate ion is also dissociated, as we can see in the statements in Table 2, showing yet another idea (I3).
| Turn | Turns of talk | Category |
|---|---|---|
| 9 | “This green (spheres) here is what?” (Ana) | M |
| 10 | “It's nitrate.” (Raul, pointing at the nitrate ion) | C |
| 11 | “Ahh, everything everything… was all ionized, oh Jesus, hey” (Ana) | EC |
From the statements, they perceive that the nitrate ion has also dissociated. Rosenthal and Sanger (2012) also observed students’ difficulties specifically related to the nitrate ion, with evidence of difficulty in its representation.
The statements coded as I4 were not represented in Fig. 4 provided by the researchers, yet when the students noticed the silver chloride precipitate, they started to reflect on the crystalline structure of AgCl, at first commenting on the type of nomenclature, but still uncertain if it would be correct or not as seen in Table 3.
| Turn | Turns of talk | Category |
|---|---|---|
| 23 | “Yeah, right, we made a precipitate here, right … forming a crystalline solid, with the conformation … I don’t know what it is … do you remember?” (Raul) | M |
| 24 | “This one is cubic, right?” (Ana) | M |
| 25 | “Cubic face centered? I don’t know … I’ve forgotten …” (Raul) | M |
| 28 | “Yeah, I think it's body centered, isn’t it?” (Ana) | M |
| 29 | “It has a centered body, because there will be something here (pointing to the model).” (Raul) | EC |
In Table 3, we observe that 80% of the metavisual incidents are coded as monitoring. They are, however, important as in this case they were a trigger that prompted students’ memory with respect to concepts about crystalline structure. Raul raises the concept (turn 23) and Ana proposes an answer (turn 24). Raul proposes the correct answer for AgCl (turn 25) thus providing the correct construction of the concept. In the sequence, Ana shows doubt when speaking (turn 28), and Raul appears to change his mind about the type of crystalline structure (turn 29).
I5 deals with the need to add a legend to a diagram to identify all the components present in the system, since the students did not initially add one. They recognized later that they had forgotten that this is important feature, as shown in Table 4. Since there are several representations for a chemical phenomenon at the submicro level, the legend assumes an important function to guide the reader about what is being represented, and to enable a better understanding of the representation provided.
| Turn | Turns of talk | Category |
|---|---|---|
| 31 | “Ahh … it had a caption … but it would take too long …” (Ana) | EC |
| 32 | “Ah but I had to write, didn’t I? … I was thinking about it later” (Raul) | M |
Initially, I6 was considered as an example of a metavisual incident ‘under construction’ however, I6 was reclassified as EC as explained below. It occurred at the time that the students were revising their clay models for which Raul suggests drawing the water molecule, instead of making it with modeling clay, and decides at that moment to represent it by a single sphere, that is, a single sphere to represent a water molecule (I6), according to Table 5.
| Turn | Turns of talk | Category |
|---|---|---|
| 51 | “Well, let's draw the water here, we don’t have clay anymore” (Raul) | NMI |
| 52 | “Can’t we just take a color and represent the water? Like … make several spheres?” (Ana) | M |
| 53 | “Let's make a single sphere here … for the water … I think it's easier…” (Raul) | EC |
Depending on the representation, and what one wants to focus on, representing the water molecule by a sphere might be appropriate. In this case, however, it does not make sense, since the dissolution of the salts and the formation of the precipitate depends on the electrostatic forces that are established between the particles. This makes it necessary to show 3 spheres in the case of water, where there are different charge density regions. Thus, representing the water molecule as a single sphere in this case, could compromise students’ understanding of the concept. Therefore, this turn was initially coded as UC.
However, although Raul suggested representing the water molecule as a single sphere, in fact, in the final model the water molecule was represented by 3 spheres, as suggested by Ana, see Fig. 7. Almost all representations of the water molecules reflect the correct V-shape with many of them in the correct orientation with respect to the cations and anions. Drawing the water molecules in this way implies that the students are aware of the electrostatic interactions between the ions and the water molecules. This reconstruction of ideas led to a reclassification of I6 to EC.
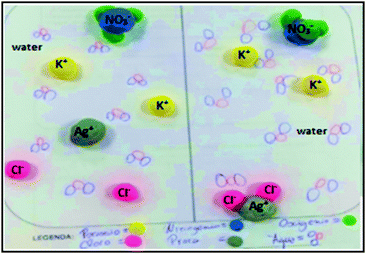 | ||
| Fig. 7 Revised representation of an explanatory model of the submicro level as proposed by the students; left (initial) and right (final). | ||
A little later in the discussion, students correctly conclude that there is no formation of a precipitate of potassium nitrate (I7), since the ions that do not react remain dissociated in the water, see also Fig. 7. However, they are mistaken, with respect to the incorrect formula of potassium nitrate (I8). Fig. 7 shows two yellow spheres associated with a blue sphere which is connected to 3 green ones as described in Table 6 implying the formula, K2NO3, for the salt.
| Turn | Turns of talk | Category |
|---|---|---|
| 63 | “The second compound forms a precipitate, but it has still ionized?” (Ana) | M |
| 64 | “The ionized one has … the nitrate …” (Raul) | EC |
| 65 | “The… K2NO3!” (Raul) | UC |
In Table 6, turns 63 and 64 illustrate I7 and turn 65 shows I8. In the sequence, the students correctly observe that the silver chloride is the precipitate (I9) and, recognizing the precipitate as the compound for which there are no dissociated ions present in solution, according to the dialogue in Table 7.
| Turn | Turns of talk | Category |
|---|---|---|
| 66 | “These are ionized (referring to the silver and chloride ions) … ionized.” (Ana) | NMI |
| 67 | “Yes… and no, wait… silver chloride is the precipitate one… own… yeah… it's the other way around! This one … the nitrate … is the ionized one, not the silver chloride…” (Raul) | EC |
According to dialogue, I9, Ana incorrectly suggests that the silver and chloride ions would be dissociated. In this sequence, Raul identifies the precipitate and that the ions that form it are not dissociated in water (turn 67), thus revising their ideas about the ions that form the precipitate.
Turn 69, I10, complements the previous reasoning, in which Raul recognizes the electrostatic interactions between which ions, however his reasoning is not correct: “This one is ionized (referring to nitrate and potassium ions), but I'll leave it close because it has electrostatic interaction” (Category UC). Although it is important to recognize the existence of electrostatic interaction between the particles, the ions are electrostatically attracted to water, with in turn will not remain close to each other, as suggested by the student, who mistakenly believes that ions would remain close each other in solution, which is why the UC category was applied.
In I11, students start by confirming correctly that the precipitate is silver chloride, but they are mistaken about the proportion between the silver and chloride ions, attributing the incorrect formula; AgCl2instead of AgCl, as shown in Table 8. In Turn 102 we can see the incorrect proportion in the formula of the salt formed is since the students assume a +2 charge for silver, which is not correct.
| Turn | Turns of talk | Category |
|---|---|---|
| 100 | “Ah really … well observed … there will form what, what is the solid? “ (Raul) | M |
| 101 | “Ah yes, the silver chloride …” (Raul) | C |
| 102 | “AgCl2…”.(Raul, stating the formula) | UC |
Regarding I12, students seem to be able to understand the chemical reaction that occurred and consider the electrostatic interactions between the particles as a decisive factor, as can be seen in the discussion recorded in Table 9.
| Turn | Turns of talk | Category |
|---|---|---|
| 105 | “Yes … there the two (referring to substances) were soluble (referring to the initial stage) and then formed something practically insoluble … this is because in fact…” (Raul) | C |
| 106 | “Ahh… because … it has a stronger interaction between the two ions (referring to silver and chloride ions) than between water!” (Ana) | EC |
| 107 | “Is it not!?” (Ana) | M |
| 108 | “Yeah!” (Raul) | C |
I13 refers to the perception of a term that students were using incorrectly, and which seems to have resurfaced, which is the use of the term dissociation of salt in water instead of ionization, as can be seen in Table 10.
| Turn | Turns of talk | Category |
|---|---|---|
| 110 | “Because, actually, ionization doesn’t exist here, does it?” (Raul) | M |
| 111 | “Both of them dissociate (Ana speaks, spelling the word) in water.” (Ana) | EC |
| 112 | “Yeah” (Raul) | C |
| 113 | “Did we necessarily have to use water?” (Ana) | M |
| 114 | “I think so, right, because the ionic salts dissociate (Raul smiles and speaks spelling the word as Ana did before) in water, right?” (Raul) | M |
As can be seen in Table 10, Ana provides the correct response about the concept of dissociation instead of ionization.
In conclusion, I14 begins with a question from Ana, in which the students discuss the presence of water required for the occurrence of the chemical reaction between ions to occur. She was referring to the application of the chemicals directly to the soil in the pot with contained only solid compounds (apparently without water); this discussion can be seen in Table 11.
| Turn | Turns of talk | Category |
|---|---|---|
| 115 | “If we put it directly on the soil, wouldn't it work?” (Ana) | M |
| 116 | “Probably on the contact surface?” (Raul) | M |
| 117 | “It was going to be white (precipitate in the pot) but what happened (formation of silver chloride) would not happen?” (Ana) | M |
| 118 | “Even if we didn't use water on the soil … we used water indirectly … because it's already in the solution.” (Raul) | EC |
In turn 118, Raul recognizes that there was water in the solutions in which the ions were dissolved, thus a chemical reaction would have occurred. Then, they conclude that, although the chemicals were placed directly to the soil, the aqueous solutions already provided the necessary water for the reaction between the ions.
Representational levels involved in the demonstrated reconstruction of knowledge: EC and UC
The 14 ideas which emerged from the student's discussions, provide evidence of the metavisual incidents, knowledge reconstructions (EC), as well as limitations where students were unable, at that moment, to redirect their ideas (UC). These ideas expressed by students (I1 to I14) are grouped according to the level of knowledge construction, Table 12.| Idea | Description |
|---|---|
| EC – Effective change – knowledge construction | |
| I1 | Presence of water |
| I2 | Ions are dissociated in the water |
| I3 | Nitrate ion is also dissociated |
| I4 | Crystalline structure of AgCl |
| I5 | Legend for drawing |
| I6 | Single sphere to represent water molecule |
| I7 | Ions that do not react remain dissociated in the water |
| I9 | Recognition of the precipitate as the compound which has no dissociated ions present in solution |
| I12 | Electrostatic interactions between the particles as a decisive factor |
| I13 | The use of the term dissociation of salt in water instead of ionization |
| I14 | Presence of water required for the occurrence of the chemical reaction between ions to occur |
| UC – Under construction change – conceptual misunderstanding | |
| I8 | Incorrect formula of potassium nitrate; K2NO3 instead of KNO3 |
| I10 | Ions would remain close to each other in solution |
| I11 | Incorrect formula; AgCl2, instead of AgCl |
We then collapsed the ideas into 3 categories associated with Johnstone's (1993) levels of representation of matter to summarize the difficulties associated with the levels, see Table 13. The submicro level was refined further by subdivision into two further subcategories to differentiate what the student may or may not have observed in the drawing, Fig. 4. This is important, since an image can not only trigger what is visible, but also implicit concepts that do not appear directly in the presented design.
Assessment of the activity and self-assessment by the students
Although the focus of this paper was on metavisualization, we give a brief account of students’ responses to the question posed in step 6 of the investigative plan described above. Students were asked to respond to the question: What did you think of this activity? Only Raul provided a response, in which, he said that he found the activity excellent, justifying his response by saying: “I really liked it, because the activity addresses the submicro level and at the same time serves as an assessment. During the “correction” of the activity, it was very interesting to notice my own mistakes and understand that this can occur with future students” (Raul). This response provides evidence of self-regulation of the student's perception (Schraw, 1998), with the perception of the error in the construction of knowledge, which was one of the objectives of the proposed activity and the reason for posing this question.Discussion
It is worth highlighting that the most important aspect of this activity was the analysis of the process of reconstruction of knowledge which occurred during the activity. Thus, we compared the first model proposed by students based on their prior knowledge (Fig. 6) with their final drawing (Fig. 7), which is a manifestation of the 14 ideas they expressed. These ideas provide an indication of revision and possible self-regulation of their thought processes as they worked through the activity.It is important to note that none of the 14 ideas expressed by students based on levels of representation in Table 13 was classified as macro. This was to be expected, since the design using the explanatory model was formulated with a view to understanding the submicro level by comparing two models made based at the submicro level, namely that of the student and that provided by the researcher.
The difficulties expressed by I8 and I11 concerning the symbolic level were not corrected by the students. The two formulae that students reported namely K2NO3 and AgCl2 were not perceived as incorrect during the activity. Rosenthal and Sanger (2012) and Kelly et al. (2010) also reported difficulties related to chemical formulae depicted incorrectly. In particular, the charge incorrectly attributed to the silver, ion Ag2+, for which the correct notation would be Ag+, was also reported by Rosenthal and Sanger (2012). Possible explanations relate to the diagram (Fig. 4) as according to Davidowitz and Chittleborough (2009), some aspects of the representations may not be understood or may have been unfamiliar to students.
The idea, I6, namely the depiction of the water molecule as a single sphere was initially classified as UC, based on turns 51–53, in which Raul says that he will represent the water with a single sphere. In turn 53 he suggests drawing a single sphere to represent water molecule, however Raul redraws the water molecule comprising 3 spheres, showing 2 elements (depicted in blue and red) and the V-shape as expected for water molecules (Fig. 7). While he does not provide a verbal explanation for choosing this representation, we believe that the drawing provides evidence of a metavisual incident classified as effective change (EC).
The only idea at submicro level that was not corrected by the students was I10 which expressed the students’ belief that ions would remain close to each other in solution. They failed to consider the fact that despite the electrostatic attraction between the ions, the interactions between water and ions are stronger, wherein ions will be solvated by water molecules. According to Yamabe et al.'s (2000) explanation for the mechanism of dissolution of sodium and chloride ions in aqueous solution, the orientation of water molecules constitutes a fundamental factor for the separation of ions, which is why this orientation was considered in Fig. 4.
The other features of the submicro levels, I1 to I5, I7 to I9 and I12 to I14, were understood by the students, which suggests that visualization of particles at the submicro level are important in the development of mental models by students (Davidowitz and Chittleborough, 2009). Thus, a metavisual activity that provides the opportunity for demonstrating internal representations with a view to revise them (Gilbert et al., 2010) may be a valuable teaching tool. Our findings are aligned with Rapp and Kurb (2008) who found that students' mental models can be revised from the interaction of a visualization, for example a chemical diagram, with the student's prior knowledge.
This does not mean that a generalization can be made in this case, since the activities of only two students were analyzed, however, this study provides evidence to suggest that revision and reconstruction of knowledge may be possible. This is relevant, especially if there is mediation by the teacher during the activity, which in turn may validate the results shown here regarding to reformulating students’ mental models.
Additionally, it is recommended special attention should be paid to the submicro aspects of a reaction that are not observed in Fig. 4 (I4, I10, I13 and I14), which are based on the assumption that students have some prior knowledge in chemistry so that they can advance and achieve construction of knowledge. This is the reason why we created the subcategory, Submicro not observed in the students’ model, differentiating it from Submicro observed in the students’ model (Table 13) as Cheng and Gilbert (2009) warn that students may not be able to reconcile their prior knowledge with the new information, confirming the importance of subsequent mediation by the teacher.
Specifically with regard to the submicro level, the data provides evidence that the proposed activity may be fruitful in the sense of promoting revision and reconstruction of ideas, given that 12 of the ideas expressed by students were classified as being at the at the submicro level, 11 of which were understood by the students. It is worth mentioning that many researchers consider the submicro level difficult to understand (Davidowitz and Chittleborough, 2009; Kelly et al., 2010).
We draw attention to a possible difficulty, namely the use of a submicro diagram that is not very familiar to the students which can constitute an obstacle in the completion of the task. For example, Kelly (2017) observed in similar work, that the students identify more easily with simpler images which are linked to aspects of the macro level. In this research, this difficulty was not observed, possibly because the students were already more experienced in terms of being familiar with the language of chemistry and different ways of representing chemical reactions. This is in line the findings by with Davidowitz et al. (2010) that the use of drawings with representations at the submicro level can help students to better understand the entire process involved in understanding a chemical reaction, leading to deeper learning related to construction of concepts in chemistry.
In line with the Locatelli et al. (2010) model of metavisualization, Fig. 1, the metavisual strategy used in this study made it possible for the students to reconstruct an alternative conception of the reaction between ions. Through a combination of visualization and metacognition, the students could take active control and regulate their learning as well as reflecting and reconstructing their ideas. Wada et al. (2015, p. 92) found that “metavisualization is promoted through social interaction involving observation sharing ones’ and others’ metacognitions and outcomes”. Our findings are in line with Wada et al. (2015) who recommend that for the student to become competent at using metavisualization he or she needs to bring his or her own hypotheses, to interact with other students and visualizations, for example, Fig. 4.
We end the discussion by saying that the present article contributes to the improvement in teaching chemistry in the direction pointed out by Chittleborough and Treagust (2008) about working metavisual skills through chemical diagrams with students to make chemical concepts explicit. In the current study there are indications of the occurrence of reconstruction of chemical knowledge by students.
Implications for practice
Zhang and Linn (2011) pointed out that images are not self-explanatory to students as often the teacher may consider it, which also was observed in this present research. This could be an obstacle to learning; thus, the teacher needs to be aware that images have limitations and consider these in the teaching–learning process, as some details could not be perceived by students. In addition, Kelly (2017) pointed out that there is an inherent limitation to any figure, no matter how well diagrams were conceptualized or depicted.The fact that aspects of the submicro level could be reviewed by students using the metavisual strategy, signals the importance of the teacher in considering using a similar approach in his or her chemistry classes with this objective of reviewing issues at the submicro level. Since the submicro level is not observable, students have difficulty accepting its existence (Chittleborough and Treagust, 2008), which reinforces the contribution of this research to classroom practise. The opportunity to review aspects of the submicro level with students using this metavisual strategy, as suggested by Blank (2000), provides specific moments of metacognitive thinking during the activity.
An important implication for chemistry education arising from this study shows how metavisual strategies experienced by pre-service teachers can contribute to improving their professional development. This could lead to an improvement in the conduct of their classes and may lead their future students to being able to build coherent scientific models regarding to chemistry. This is in line with Akagun's suggestion for teachers and instructors to use visualizations and representations as methods that they could adopt to effect conceptual learning in their classrooms (Kelly and Akaygun, 2019, p. 657).
While we did not focus on issues related to gender in this study, it is important for the teacher to be aware, as the interactions in gender diverse groups can be different, discussions should be mediated to ensure participation by all members of a group.
Conclusions
This research intended to provide an answer to the question: to what extent can the metavisual strategy used in this study contribute to revising and possible self-regulation of difficulties expressed by students related to representational levels? From some alternative conceptions documented in the literature related to chemical reactions between ions, and demonstrated by the students who participated in this study, we observed that the metavisual strategy used offered opportunities for possible self-regulation, especially with regard to the submicro level, which is considered difficult for students due to the fact that it cannot be observed directly. There is evidence of revision of representations of aspects of the reaction at the submicro level, since almost all difficulties experienced by students, as represented by ideas in Table 12, were reviewed and reformulated. Our findings suggest that viewing a representation of the submicro level and being able to revise it based on students’ prior knowledge, and interaction with their peers, can work together to contribute to forming adequate mental models necessary for understanding chemistry. For the difficulties demonstrated, specifically at the symbolic level, these were not revised, which may have been caused by the lack of understanding, unfamiliarity or by not paying sufficient attention to the legend associated with the representation which contained the formulae of the ions.A limitation inherent to this research refers to it being characterized as a case study and, therefore, the data found are valid only for the sample considered, in the context of that research. Thus, it is not possible to generalize the findings. However, they provide evidence that the strategy may be useful to enhance the self-regulation of chemical concepts concerning the chemical reaction between ions, especially at the submicro level. Further studies are recommended that focus on metavisual strategies for a better understanding of the submicro level, considering a larger number of students so that the understanding of the submicro level is better clarified.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
To the São Paulo Research Foundation (FAPESP, 2018/26142-0) for the financing of the research project, to the undergraduate students who voluntarily participated in this research, to Giovanni Paz for the validation of the data, and to Eileen Murray for helpful comments on the preparation of this manuscript.References
- Barnea N., Dori Y. J., Hofstein A., (2010), Development and implementation of inquiry-based and computerized-based laboratories: reforming high school chemistry in Israel, Chem. Educ. Res. Pract., 11, 218–228 10.1039/C005471M.
- Blank L. M., (2000), A metacognitive learning cycle: A better warranty for student understanding? Sci. Educ., 4 (1), 486–506.
- Chang H. Y. and Tzeng S. F., (2017), Investigating Taiwanese students’ visualization competence of matter at the particulate level, Int. J. Sci. Math. Educ., 16, 1207–1226.
- Cheng M. and Gilbert J. K., (2009), Towards a better utilization of diagrams in research into the use of representative levels in chemical education, in Gilbert J. K. and Treagust D. F. (ed.), Multiple representations in Chemical Education, Springer Science + Business Media B.V., vol. 4, pp. 55–73.
- Chiu J. L. and Linn M. C., (2012), The Role of Self-monitoring in Learning Chemistry with Dynamic Visualizations, in Zohar A. and Dori Y. (ed.), Metacognition in Science Education: Trends in Current Research, Dordrecht: Springer, vol. 40, pp. 133–163.
- Chittleborough G. and Treagust D., (2008), Correct interpretation of chemical diagrams requires transforming from one level of representation to another, Res. Sci. Educ., 38(4), 463–482, DOI:10.1007/s11165-007-9059-4.
- Cohen L., Manion L. and Morrison K., (2007), Research Methods in Education, 6th edn, London: Routledge.
- Creswell J. W., (2014), Research design: qualitative, quantitative and mixed methods approaches, 4th edn, USA: Sage.
- Davidowitz B. and Chittleborough G., (2009), Linking the macroscopic and submicroscopic levels: diagrams, in Gilbert J. K. and Treagust D. F. (ed.), Multiple Representations in Chemical Education, Springer Science + Business Media B.V., vol. 4, pp. 169–191.
- Davidowitz B., Chittleborough G. and Murray E., (2010), Student-generated submicro diagrams: a useful tool for teaching and learning chemical equations and stoichiometry, Chem. Educ. Res. Pract., 11(3), 154–164 10.1039/C005464J.
- Driver R., (1989), Students' conceptions and the learning of science, Int. J. Sci. Educ., 11, special issue, 481–490.
- Efklides A., (2006), Metacognition and affect: What can metacognitive experiences tell us about the learning process? Educ. Res. Rev., 1(1), 3–14.
- Flavell J. H., (1976), Metacognitive aspects of problem solving, in Resnick L. B. (ed.), The nature of intelligence, Hillsdale, N.Y.: Erlbaum, pp. 231–235.
- Flavell J. H., (1981), Cognitive monitoring, in Dickson W. P. (ed.), Children's oral communication skills, New York: Academic Press, pp. 35–60.
- Glaser B. G. and Strauss A. L., (1967), Discovery of grounded theory: strategies for qualitative research, Chicago: Aldine.
- Gilbert J. K., (2005), Visualization: A Metacognitive Skill in Science and Science Education, in Gilbert J. K. (ed.), Visualization in Science Education, Netherlands: Springer, pp. 9–27.
- Gilbert J. K. and Treagust D. F., (2009), Introduction: macro, submicro and symbolic representations and the relationship between them: key models in chemical education, in Gilbert J. K. and Treagust D. F. (ed.), Multiple Representations in Chemical Education, Springer Science + Business Media B.V, vol. 4, pp. 1–8.
- Gilbert J. K., Justi R. and Queiroz A. S., (2010), The use of a model of modelling to develop visualization during the learning of ionic bonding, in Taşar M.F. and Çakmakcı G. (ed.), Contemporary Science Education Research: International Perspectives, Ankara, Turkey: Pegem Akademi, pp. 43–51.
- Girash J., (2014), Metacognition and instruction, in Benassi V. A., Overson C. E. and Hakala C. M. (ed.), Applying science of learning in education: Infusing psychological science into the curriculum, Society for the Teaching of Psychology, pp. 152–168.
- Gunstone R. F., (1994), The Importance of specific science content in the enhancement of metacognition, in Fensham P. J., Gunstone R. F. and White R. T. (ed.), The content of science: A constructivist approach to its teaching and learning, London: The Falmer Press, pp. 131–146.
- Hansen S. J. R., (2014), Multimodal Study of Visual Problem Solving in Chemistry with Multiple Representations, PhD thesis, Columbia University, United States of America, Available: https://academiccommons.columbia.edu/doi/10.7916/D83B5X9H.
- Harrison A. G. and Treagust D. F., (1996), Secondary students’ mental models of atoms and molecules: Implications for teaching chemistry, Sci. Educ., 80(5), 509–534.
- Hogan K., (1999), Relating students’ personal frameworks for science learning to their cognition in collaborative contexts, Sci. Educ., 83(1), 1–32.
- Hung J. Y., Chang H. Y. and Hung J. F., (2019), An Experienced Science Teacher's Metavisualization in the Case of the Complex System of Carbon Cycling, Res. Sci. Educ., 1–29 DOI:10.1007/s11165-018-9804-x.
- Johnstone A. H., (1993), The development of chemistry teaching: a changing response to changing demand, J. Chem. Educ., 70(9), 701–705.
- Kadioglu-Akbulut C. and Uzuntiryaki-Kondakci E., (2020), Implementations of self-regulatory instruction to promote students’ achievement and learning strategies in the high school chemistry classroom, Chem. Educ. Res. Pract., 1–18.
- Kelly R. M., (2017), Learning from contrasting molecular animations with a metacognitive monitor activity, Educ. Quim., 28(3), 181–194, DOI:10.1016/j.eq.2017.02.003.
- Kelly R. and Akaygun S., (2019), Visualizations and representations in chemistry education, Chem. Educ. Res. Pract., 20, 657–658, 10.1039/c9rp90009h.
- Kelly R. M., Barrera J. H. and Mohamed S. C., (2010), An analysis of undergraduate general chemistry students’ misconceptions of the submicroscopic level of precipitation reactions, J. Chem. Educ., 87(1), 113–118.
- Kuhn D. and Dean D., (2004), Metacognition: A bridge between cognitive psychology and educational practice, Theory Into Pract., 43(4), 268–273. DOI:10.1207/s15430421tip4304_4.
- Lai E. R., (2011), Metacognition: a literature review, Pearson Assessments. Available: http://www.shorturl.at/evIJS.
- Locatelli S. W. and Arroio A., (2014), The monitoring of an introductory class on geometrical isomerism by metavisual incidents, J. Sci. Educ., 15(2), 62–67.
- Locatelli S. W., Ferreira C. and Arroio A., (2010), Metavisualization: an important skill in the learning chemistry, Problems of Education in the 21st Century, 24, 75–83. Available: http://www.scientiasocialis.lt/pec/node/441.
- Nakiboglu C and Nakiboglu N., (2019), Exploring prospective chemistry teachers’ perceptions of precipitation, conception of precipitation reactions and visualization of the sub-microscopic level of precipitation reactions, Chem. Educ. Res. Pract., 20, 873–889.
- National Research Council, (2000), Inquiry and the National Science Education Standards: A Guide for Teaching and Learning, Washington, DC: The National Academies Press DOI:10.17226/9596.
- Paz G. S.B. and Locatelli S. W., (2019), Metacognitive incidents manifested by students of youth and adult education in an investigative activity, Proceedings of 3rd International Baltic Symposium on Science and Technology Education, BalticSTE2019, Lithuania, Available: https://scientiasocialis.lt/files/BalticSTE2019_Proceedings.pdf.
- Rapp D. and Kurby C., (2008), The ‘ins’ and ‘outs’ of learning: internal representations and external visualizations, in Gilbert J. K., Reiner M. and Nakleh M. (ed.), Visualization: Theory and Practice in Science Education, Netherlands: Springer, pp. 29–52.
- Rosenthal D. and Sanger M., (2012), Student misinterpretations and misconceptions based on their explanations of two computer animations of varying complexity depicting the same oxidation–reduction reaction, Chem. Educ. Res. Pract., 13, 471–483 10.1039/C2RP20048A.
- Schraw G., (1998), Promoting general metacognitive awareness, Instr. Sci., 26, 113–125 DOI:10.1023/A:1003044231033.
- Schraw G., Olafson L., Weibel M. and Sewing D., (2012), Metacognitive knowledge and field-based science learning in an outdoor environmental education program, in Zohar A. and Dori Y. J. (ed.), Metacognition in science education: Trends in current research. New York, NY: Springer, pp. 57–78.
- Stake R. E., (1994), Case studies, in Denzin N. K. and Lincoln Y. S. (ed.), Handbook of qualitative research, Thousand Oaks, CA: Sage Publications, pp. 236–247.
- Van der Westhuizen L., (2015), The development of the conceptual understanding of first-year chemistry university students in stoichiometry using thinking skills, visualization and metacognitive strategies, MSc Dissertation, South Africa: Potchefstroom Campus of the North-West University, Available: https://repository.nwu.ac.za/bitstream/handle/10394/15680/Van_der_Westhuizen_L_2015.pdf?sequence=1&isAllowed=y.
- Wada I., Miyamura R., Sawada K. and Morimoto S., (2015), Analysis of effects of social interaction on metavisualization in science learning, J. Res. Sci. Educ., 56(1), 75–92 DOI:10.11639/sjst.sp14010.
- Yamabe S., Kouno H. and Matsumura K., (2000), A Mechanism of the Ion Separation of the NaCl Microcrystal via the Association of Water Clusters. J. Phys. Chem., 104, 10242–10252.
- Zhang Z. H. and Linn M. C., (2011), Can generating representations enhance learning with dynamic visualizations? J. Res. Sci. Teach., 48(10), 1177–1198.
| This journal is © The Royal Society of Chemistry 2021 |