Open Access Article
Open Access ArticleEffects of different ways of using visualizations on high school students’ electrochemistry conceptual understanding and motivation towards chemistry learning
Chia-Yin
Lin
a and
Hsin-Kai
Wu
 *ab
*ab
aGraduate Institute of Science Education, National Taiwan Normal University, Taipei, Taiwan. E-mail: hkwu@ntnu.edu.tw
bFaculty of Education, University of Johannesburg, Johannesburg, South Africa
First published on 4th June 2021
Abstract
The purpose of this study is to examine the effects of different ways to use visualizations on high school students’ electrochemistry conceptual understanding and motivation towards chemistry learning. Expanding upon a model-based learning approach (Khan, 2007), we adopted a VGEM sequence (View, Generate, Evaluate, and Modify) to create three instructional conditions. All conditions involved the viewing, evaluating, and modifying phases, whereas there were variations in the generating phase: (1) finishing worksheets (V group), (2) generating drawings (VD group), and (3) generating animations (VA group). Three intact classes with 109 eleventh graders from a public high school were randomly assigned to the three groups. A test of conceptual understanding was used as the pretest, posttest, and delayed posttest to assess respectively initial understanding, changes, and retention of understanding up to 6 weeks later. A questionnaire to measure students’ motivation to learn chemistry was administered before and after the instruction. Statistical results of the within-group comparisons revealed that all three instructional conditions could support students to develop a significantly better conceptual understanding of electrochemistry and that in the three groups, students’ understanding was retained after 6 weeks. Regarding the overall motivation before and after the instruction, only the VA group showed motivational benefits for chemistry learning. Furthermore, the between-group comparisons indicated no significant differences between the means of the three groups in the posttest and delayed posttest, and suggested that the three groups developed and retained a similar level of conceptual understanding after the instruction. Similarly, different uses of visualizations made no difference to students’ chemistry learning motivation. This study advances the understanding of how to develop effective instructional activities with visualizations for chemistry learning, and suggests possible conceptual and motivational benefits of viewing and generating visualizations.
Introduction
Over the past decades, a significant body of literature has emerged on the benefits of visualizations to enhance chemistry learning (e.g., Wu et al., 2001; Tasker and Dalton, 2006; Chang and Linn, 2013; Akaygun, 2016; Kelly et al., 2017). Visualizations, including graphics, drawings, animations, and simulations, can serve instructional functions such as attracting students’ attention to a specific area of the content (McElhaney et al., 2015), depicting concrete and abstract details or procedures (Tasker and Dalton, 2006), and showing the functioning of dynamic systems or the change of scientific phenomena over time (Kozma et al., 2000). Viewing and interpreting visualizations could help students realize the dynamic and interactive nature of chemistry, make connections between the macro and sub-micro levels, avoid reinforcing misconceptions, and promote understandings of chemistry concepts (Wu and Shah, 2004; Tasker and Dalton, 2006; Zhang and Linn, 2013).Although visualizations make unobservable chemistry processes visible, some visualizations can be cognitively demanding and challenging to students (Chiu et al., 2013; Berney and Bétrancourt, 2016). For example, previous studies have suggested that when viewing and making sense of animations, students could suffer from information overload because of the large amount and the transient nature of the information to be processed (Cook, 2006; Wu et al., 2013). Additionally, by briefly inspecting visualizations, students may overestimate their understanding of visualizations and gain deceptive clarity about the content presented because visualizations “can be so memorable that students become convinced they understand complex processes when they can recall only superficial features of what they have seen” (Linn et al., 2010, p. 241). To help students avoid or overcome these difficulties, some considerations should be taken into account when visualizations are integrated into chemistry teaching and learning.
The first consideration is regarding the modality of visualizations (Ainsworth, 2006). While the content of a static visualization such as a diagram, a drawing, or a picture does not change over time, a dynamic visualization including an animation and a video alters its content during the presentation. In the long debate of dynamic versus static visualizations, the question of whether one modality is more effective than another in teaching and learning has been explored, and mixed conclusions have been reached (Tversky et al., 2002; Hegarty et al., 2003; Hegarty, 2004; Mayer et al., 2005; McElhaney et al., 2015; Berney and Bétrancourt, 2016; Castro-Alonso et al., 2016). Some studies have shown advantages of dynamic over static graphics (e.g., Höffler and Leutner, 2007) because dynamic visualizations demonstrate the temporal and interactive nature of phenomena and “may compensate for a student's insufficient aptitude or skill to imagine motions” (Höffler and Leutner, 2007, p. 723). On the other hand, it has also been found that under some conditions, static visualizations could be more useful than dynamic ones (Tversky et al., 2002; Hegarty, 2004; Mayer et al., 2005). Static displays could minimize extraneous cognitive processing, require a lower cognitive load, and focus students’ attention on the important content (Tversky et al., 2002; Hegarty, 2004; Mayer et al., 2005). Additionally, research has suggested that factors including the topics to be taught, expected learning outcomes (e.g., conceptual understanding or process skills), and individual characteristics (e.g., age and spatial ability) could mediate or influence the effectiveness of static and dynamic representations (Hegarty et al., 2003; Wu et al., 2013; McElhaney et al., 2015). To contribute to reconciling the mixed conclusions, therefore, this study designed different instructional conditions to examine the effects of drawings and animations on students’ learning of electrochemistry.
A further consideration is how to support students to overcome superficial understanding and deceptive clarity. Because these difficulties are common when students passively view visualizations that present unseen processes (Linn et al., 2010), it is important to increase students’ engagement with and processing of the content in the visualizations (Stieff, 2017; Guo et al., 2020). One approach to enhancing the interactivity between learners and visualizations is through creating them on their own (Wu and Krajcik, 2006; Wu and Puntambekar, 2012). Instead of having students passively observe visualizations provided by teachers and learning materials, recent studies have encouraged students to construct their own visual displays (e.g., Ainsworth et al., 2011; Tytler et al., 2013; Yaseen, 2018; Yaseen and Aubusson, 2020). Research in chemistry education shows that the process of generating visualizations could help students make connections between the macro and sub-micro levels, externalize students’ understandings, increase their engagement, and improve their representational skills (Davidowitz et al., 2010; Hoban et al., 2011; Akaygun, 2016; Berg et al., 2019). However, Chang et al. (2010) argued that viewing animations could be as effective as creating animations “if such animations are not too complex for the students and if this is combined with activities that engage students in active learning” (p. 75). Thus, comparative studies are still needed to understand under what conditions creating animations may or may not be more effective than viewing them. Additionally, although previous studies have indicated that creating visuals can be effective in terms of improving learning, relatively little research has compared the effects of constructing static and dynamic visualizations on chemistry learning and motivation. Such comparison is meaningful because creating animations usually requires more instructional and technical resources such as animating software and electronic devices. If generating drawings and animations can enable students to achieve similar results, teachers could make more flexible instructional decisions depending on available resources and technology capabilities.
Another approach to active learning with visualizations is providing appropriate guidance, and scaffolding students’ interactions with visualizations (Chang and Linn, 2013). In addition to viewing experts’ visualizations and creating their own animations, students should be allowed to evaluate, compare, and discuss each other's visualizations (Chang et al., 2010; Kelly et al., 2017). These activities support students to ask questions, clarify their ideas, and contrast their conceptions with those of other students (Chang and Linn, 2013; Matuk et al., 2019). To engage students in active learning with visualizations, this study expanded upon a model-based learning approach (Khan, 2007) and adopted a View, Generate, Evaluate, and Modify (VGEM) sequence to enhance the learning benefits of drawings and animations.
Taking the aforementioned issues into consideration, in this study, we employed the VGEM sequence to design three instructional conditions to promote students’ learning of electrochemistry. All the conditions involved the viewing, evaluating, and modifying phases, but there were variations in the generating phase. In the first condition, students generated drawings (VD group); the second condition required students to use a mobile application, Alchemie Animator (https://www.alchem.ie/animator) to construct animations (VA group); and the third condition asked students to answer questions about animations they had just viewed (V group), so all three conditions took a similar amount of instructional time. As suggested by the literature, each of these conditions has the potential to support students’ chemistry learning.
The purpose of this study was to examine the effects of different ways of using visualizations on high school students’ conceptual understanding of electrochemistry and motivation towards chemistry learning. By contrasting the performances of the VD and VA groups, this study contributes to the understanding of whether creating visualizations with different modalities influences students’ chemistry learning. Also, the comparison of the VA and V groups offers evidence of the impact of creating versus viewing animations. Furthermore, the comparisons among the three conditions help determine which condition could provide the most support for students. The research questions that guide this study are as follows. (1) Which instructional condition supports students to develop a better conceptual understanding of electrochemistry? (2) Which instructional condition increases students’ motivation towards chemistry learning? (3) Are there significant differences in students’ conceptual understanding and motivation between the three groups after the students engage in activities with visualizations?
Learning electrochemistry with animations
Electrochemistry has been identified as one of the most difficult topics in secondary school chemistry (Garnett et al., 1995; Acar and Tarhan, 2007; Schmidt et al., 2007). The topic covers areas of electric circuits, electrochemical cells, and electrolysis, and involves complex concepts such as electron flows in aqueous solutions, oxidation–reduction reactions, and electrical neutrality (Garnett and Treagust, 1992a, 1992b; Sanger and Greenbowe, 1997). Two reasons are suggested that may contribute to students’ learning difficulties in electrochemistry. First, most of the processes in electrochemistry are invisible to students (Yang et al., 2003). Students are not able to directly observe the flow of electrons, oxidation–reduction reactions in cells, or the movement of ions in aqueous solutions. This invisible and dynamic nature may cause students’ alternative conceptions about electric circuits (Schmidt et al., 2007) and their difficulties in identifying components of electrochemical and electrolytic cells, such as the anode and cathode (Garnett and Treagust, 1992a, 1992b). Secondly, understanding and visualizing the processes in electrochemistry require connections between the macroscopic, submicroscopic, and symbolic levels (De Jong and Treagust, 2002; Osman and Lee, 2013). For example, when given a diagram of an electrochemical cell, students may first notice the electrodes, salt bridge, and the color of solutions at the macroscopic level. In order to explain how the cell works, students need to visualize the movement of ions in solutions at the submicroscopic level. Furthermore, to represent the chemical processes that happen in the salt bridge, electrodes, and solutions, students are required to use chemical formulas and symbols to complete chemical equations of half-reactions. Without conceptual and representational connections between the three levels, students could have problems understanding the concepts of electrochemistry (Wu and Shah, 2004).Previous research has developed instructional interventions to help students overcome their learning difficulties in electrochemistry, including conceptual change instruction, multimedia modules (Osman and Lee, 2013), cooperative learning strategies (Acar and Tarhan, 2007; Doymus et al., 2010), and computer-based visualizations (Yang et al., 2003; Doymus et al., 2010). Among them, computer-based visualizations have been found useful for addressing the above-mentioned two sources of learning difficulties.
Sanger and Greenbowe (1997) used computer animations of electron flows in solution as a lecture tool to enhance students’ conceptual understanding at the submicroscopic level. They found that the animations helped students visualize chemical reactions and decrease their alternative conceptions of electron flows. Doymus et al. (2010) also showed the benefits of using animations to enhance first-year undergraduate students’ understanding of electrochemistry. Compared to the control group taught by a lecture-based traditional method and the group using jigsaw cooperative learning strategies, the group using computer-animated presentations performed significantly better in the posttests of scientific reasoning and the particulate nature of matter. Doymus et al. argued that by showing the movement of particles, animations led to better conceptual understanding at the submicroscopic level.
In addition to students’ conceptual understanding, motivation to learn electrochemistry was examined in Osman and Lee (2013). Motivation has been viewed as an important goal for chemistry learning (Barak et al., 2011; Vaino et al., 2012) and refers to students’ internal state that instigates, directs, and sustains a goal-oriented activity (Pintrich and Schunk, 1996; Glynn et al., 2009). Studies of learning technologies have suggested that animations have a positive influence on students’ motivation to learn science (e.g., Rosen, 2009; Barak et al., 2011) because using animations may help students perceive that they are capable of learning science, increase their interests, and encourage them to be willing to invest effort in science learning (Rieber, 1991). However, Osman and Lee (2013) found that while secondary students who engaged in an interactive multimedia module with animations outperformed their counterparts who received the traditional teaching method in a concept test of electrochemistry, the two groups showed no difference in motivation to learn electrochemistry that included three dimensions of adhered value, expectancy components, and affective components.
Instead of making comparisons between the use of animations and the traditional teaching method, Yang et al. (2003) included visualizations with different modalities, and examined the effects of instructor-guided animations and static diagrams on undergraduate students’ chemistry knowledge and content understanding of electrochemistry. They found that, compared to static diagrams, animations could result in better conceptual understanding, and more so for students who had higher spatial ability. A possible explanation is that animations may support students to develop more accurate cognitive representations of invisible chemical processes, and high spatial ability students may be more capable of doing so.
However, animations are not always beneficial to the learning of electrochemistry. In their follow-up study, Sanger and Greenbowe (2000) employed a 2 × 2 research design to investigate the effects of two independent variables (i.e., the use of computer animations and the conceptual change instruction) on undergraduate engineering students’ learning of electron flows in aqueous solutions. The results of ANOVAs showed no main effect of animations on algorithmic, visual, or verbal conceptual questions. Although an interactional effect between the use of computer animations and conceptual change instruction was found, students who received only the conceptual change instruction had significantly higher scores than the students who received both the conceptual change and animation instruction. The results suggested that when both methods were presented, “animations may prove distracting when the questions do not require students to visualize” (p. 534). Additionally, the undergraduate students participating in their study may be capable of forming mental models by themselves (Hegarty et al., 2003) so the animations may not offer learning benefits.
Taken together, studies on learning electrochemistry with animations suggested that, compared to the traditional textbook-based teaching method, using animations could be beneficial to learning electrochemistry, and could address students’ learning difficulties. Yet, Sanger and Greenbowe (2000) implied that more teaching methods should be considered, and animations are not always effective in chemistry learning. Some issues thus need to be explored further. First, one explanation for the ineffectiveness of animations is learners’ prior knowledge and cognitive capabilities (Sanger and Greenbowe, 2000). Undergraduate students may be able to develop an adequate understanding of chemistry without the support of dynamic visualizations, whereas secondary school students may still need them, as shown in Osman and Lee (2013). As the participants of most studies on using animations for electrochemistry learning were undergraduate students, more studies involving secondary school students are needed. Secondly, the above-reviewed studies on electrochemistry learning allowed students to view animations only, which may lead to passive learning and superficial understanding (Linn et al., 2010). This may explain why animations did not add further learning benefits for the students in Sanger and Greenbowe's (2000) study. Recent studies have suggested the importance of increasing students’ interactivity and engagement with dynamic visualizations (McElhaney et al., 2015). In addition to viewing, other ways of using animations should be investigated. However, so far none of the studies on learning electrochemistry have compared the influence of viewing and creating animations. Therefore, by having 11th graders as participants and investigating the effects of viewing and generating animations, this study aimed at exploring the unresolved issues.
Student-generated animations
Previous research has revealed the benefits of student-generated visualizations (Ainsworth et al., 2011; Wu and Puntambekar, 2012; Tippett, 2016; Stieff, 2017). Constructing visualizations allows students to externalize their ideas and compare their conceptions to those of others (Davidowitz et al., 2010; Yaseen, 2018). Teachers can also use student-generated visualizations to evaluate the students’ conceptual understanding (Zhang and Linn, 2013). Among a variety of visualizations, student-generated animations have received increasing research attention since 2005 (Farrokhnia et al., 2020). Compared to paper-based or physical visualizations, in the past, computer-based animations were relatively difficult for learners to make because creating animations required high graphing and technical skills, and the authoring software was expensive and designed for professional users. Now, with the advances in drawing technologies, creating animations has become possible in classrooms and is as easy to making static drawings.In Hoban et al.'s case study (2011), three preservice teachers integrated research notes, storyboards, models, and photographs into a narrated animation. They found that by creating these visualizations, the participants interpreted information, transforming their science knowledge, and built links between science knowledge and their experiences of the real world. Also, Berg et al. (2019) had primary school teachers create their own animations to explain their observations during practical work. Results of the qualitative analysis showed that the process of creating animations engaged these teachers in reasoning between experiential, macroscopic, and submicroscopic levels (Taber, 2013). As learning electrochemistry also requires connections between levels, these studies suggest that creating animations could be a promising learning method.
For secondary school students who have relatively low conceptual knowledge and representational skills, well-designed instructional activities and teacher guidance are crucial when they generate animations. Yaseen (2018) collected multiple sources of data to investigate how 11th graders developed their chemistry understanding by generating animations with the assistance of their teachers and peers. She found that while the process of creating animations allowed students to discuss their conceptions and to represent the dynamic aspects of their understanding, teachers’ scaffolding and peer evaluations supported students to accurately represent their ideas. Yaseen and Aubusson (2020) further confirmed the positive influence of animation-based activities, i.e., creating, presenting, and critiquing animations, on high school students’ understanding of states of matter. Additionally, to examine the impact of designing and evaluating animations on seventh graders’ understanding of the particulate nature of matter, Chang et al. (2010) investigated the effects of three treatments: (T1) design, interpret, and evaluate animations, (T2) only design and interpret animations, or (T3) only view and interpret teacher-made animations. While the T1 group outperformed the T2 group in the total test score, content knowledge, and the abilities to construct, interpret, and evaluate visualizations, the T3 group performed significantly better than the T2 group in the total score, content knowledge, and two abilities (i.e., interpreting and evaluating). The results indicated that creating animations without evaluation was not effective, and that viewing animations could be more useful than designing them.
In summary, while designing and creating animations has the potential to promote students’ chemistry learning, the instructional conditions should be taken into consideration. Learning activities such as evaluating each other's animations and teacher support could affect students’ development of conceptual understanding. However, as indicated by Chang et al. (2010), other combinations of the animation-based activities are waiting to be explored, such as a comparison of combining the activities of creating, interpreting, and evaluating, and combining the activities of viewing, interpreting, and evaluating. Such a comparison could reveal “whether the effect of the viewing approach is also augmented by the evaluation activity” (p. 89). Additionally, Harrison and Treagust (2000) found that the process of generating paper-based and physical visualizations could have learning and motivational benefits. Creating visualizations could increase students’ sense of ownership and enable them to take an active role in learning; this in turn may facilitate students’ motivation (Pintrich and Schunk, 1996; Thurlow et al., 2004). Yet, there is relatively little research on the motivational effects of creating animations on chemistry learning (Picard et al., 2004). To address these unexplored issues, this study included the combinations of the animation-based activities suggested by Chang et al. (2010) to design the VA and V groups, and examined students’ motivation towards chemistry learning before and after the activities.
Furthermore, although generating animations provides high interactivity between students and visualizations, it may lead students to pay too much attention to the technical details of their animations without exploring the conceptual aspect of the chemical phenomena (Moreno and Valdez, 2005). One way to avoid the distracting details and reduce students’ cognitive load of designing and generating animations while keeping a high level of interactivity is to have students construct less complicated visualizations such as drawings and diagrams (Cooper et al., 2017). Can drawing or sketching be as effective as creating animations for chemistry learning? Below we review studies in chemistry education that involved constructing both drawings and animations.
Creating static versus dynamic visualizations
There has been a substantial amount of research on the effects of presenting dynamic versus static visualizations to students (see review studies Höffler and Leutner, 2007; McElhaney et al., 2015), but relatively few studies have involved students creating both dynamic and static visualizations for chemistry learning. One of these studies is that of Akaygun (2016), in which 10th and 11th graders created animations of an oxygen atom, and before and after generating the animations they drew a storyboard to show the atomic structure. The results showed that, compared to their initial drawings, students’ final drawings were significantly more refined and accurate, and included significantly more dynamic features. By analyzing students’ drawings, Akaygun (2016) concluded that generating animations could have a positive impact on students’ chemistry learning. Additionally, Wilkerson-Jerde et al. (2014) explored the modeling activities engaged in by five sixth-grade girls when they generated drawings, animations, and computational simulations of molecular diffusion. They found that generating different types of visualizations sustained the students’ engagement and allowed them to organize their science knowledge and experiences. However, although Akaygun (2016) and Wilkerson-Jerde et al. (2014) analyzed students’ visualizations and described related activities, they did not investigate the impact of drawing on learning.Another study of student-generated static and dynamic visualizations was done by Chang et al. (2014). In their study, 30 seventh graders who had completed a 10-week inquiry-based chemistry unit were encouraged to create visualizations to represent their understanding of a chemical reaction. A drawing tool, Chemation, was provided to the students which allowed them to create either dynamic or static visuals. Among the 30 participants, 19 constructed adequate dynamic molecular visualizations of the chemical reaction (the dynamic group), while 11 created static visuals (the static group). Three types of connections were identified and were used to score the visualizations and verbal explanations generated by the two groups of students: (1) drawing on existing knowledge, (2) linking to observable phenomena, and (3) reconstructing chemistry concepts. They found that overall the static group received significantly lower scores and that the dynamic group outperformed the static group on making the latter two types of connections. Chang et al. suggested that the lack of a dynamic view of chemical reactions may be associated with students’ difficulties in making connections between the phenomenon and molecular visualizations. Although Chang et al. (2014) involved students’ construction of drawings and animations, the dynamic and static groups were identified during interviews when students voluntarily generated either type of visualization. Creating visualizations was not part of the instructional intervention, and students’ chemistry knowledge levels in the two groups were not controlled. It is possible that students with a low knowledge level tended to create static visualizations because animations were more complicated and required more conceptual details.
Williamson et al. (2013) conducted a comparative study and examined the effects of constructing animations versus storyboards on college students’ mental rotation ability, equilibrium content knowledge, and attitudes. While one class was assigned to create two animations, the other class was told to create a storyboard that contained at least 10 pictures. Williamson et al. (2013) found that the animation group showed more positive attitudes towards visualization construction, but there was no difference between the two groups in terms of their mental rotation abilities and content knowledge. The findings suggested that creating animations could result in positive motivational effects and echoed the results of Harrison and Treagust (2000), but regarding the cognitive effects, more comparative studies and evidence are needed.
Furthermore, a recent review study of student-generated animations by Farrokhnia et al. (2020) indicated a need for quantitative studies and recommended experimental designs, so the effectiveness of student-generated animations in terms of both cognitive and non-cognitive learning outcomes can be examined and compared to other forms of instruction. Therefore, this study used a quasi-experimental design of three groups and included an instructional condition (VD group) that required students to create drawings, and compared the differences between creating static versus dynamic visualizations.
Methods
The purpose of this study was to examine the effects of different ways of using visualizations on high school students’ conceptual understanding of electrochemistry and their motivation towards chemistry learning. To achieve this purpose, a quasi-experimental design was employed that allowed us to establish the relationship between the instructional conditions and students’ learning outcomes (Creswell, 1994). In this section, we first describe the participants and the design of the three instructional conditions. We then introduce the instruments used to measure the students’ conceptual understanding and motivation. Finally, the process of data analysis is presented.Participants
This study took place at a public senior high school (grades 10–12) in northern Taiwan. To estimate the minimum sample size for between-group analyses of variance, G*Power 3 was used (Faul et al., 2007). Given the effect size of 0.4 with 80% power in a one-way ANCOVA (3 groups, α = 0.05, and 1 covariate), the suggested total sample size was 64. In Taiwan, the class size in high schools was between 30 and 35. Thus, three intact 11th grade classes with a total of 109 students were recruited for the study and randomly assigned to the three conditions with different uses of visualizations. The three classes were taught by the first author who had 5 years of teaching experience, a bachelor of science degree with a major in chemistry, and was pursuing a master's degree in science education. She had taught in the participating high school for a semester and was familiar with the school settings and students’ performances. This study followed the ethical considerations in Taber (2014). All students were informed of the research purposes, and participation was voluntary and anonymous. They could withdraw from the research at any stage of the research and their performances in the study would not affect their grades. All students agreed to use their data for research purposes.The numbers of students of the three groups were 35 (VD), 37 (VA), and 37 (V), and a total of 109 students (66 females and 43 males) participated in the study. Electrochemistry was not a new topic to the students; according to the curriculum guidelines in Taiwan (Ministry of Education, 2018), students should be introduced to the concepts of electrolytes, electrochemical cells, and electrolysis during grades 8 and 9. Yet, before they participated in this study, their understanding of electrochemistry was developed based on observable phenomena and hands-on experiments. For example, in one of the science experiments in grade 9, they built an electrochemical cell and observed that the cell could light up a bulb. In this study, the learning activities provided opportunities for them to develop in-depth conceptual understanding of electrochemistry at the submicroscopic level.
Regarding their prior learning experience with animations, the participating students had watched science videos and animations before, but they were not familiar with chemistry animations at the submicroscopic and symbolic levels. Additionally, animations were usually used by teachers for class demonstration and the students had very little experience of viewing and creating animations collaboratively with peers. Thus, in this study, guiding activities in the first class period were designed to support students’ learning with molecular animations (see details in the next section). Furthermore, not all students completed the instruction and finished the tests. At the end of the study, the numbers of students who received the instruction and took all three tests (i.e., the pretest, posttest, and delayed-posttest) were 30, 32, and 33 in the VD, VA, and V groups, respectively.
Design of the three instructional conditions
To provide appropriate guidance and to scaffold students’ interactions with visualizations, this study adopted the GEM (Generate, Evaluate, and Modify) approach (Khan, 2007) that was designed to support students’ engagement with model construction and revision in chemistry. The approach was developed based on model-based learning (Buckley, 2000; Lehrer and Schauble, 2000; Nersessian, 2002), which argues that students could construct scientific knowledge from building, critiquing, and revising their mental and expressed models (Penner, 2000). When engaging in these modeling processes, students apply their current knowledge to build models, experience cognitive conflicts between their models and critiques (or other students’ models), establish connections between existing understanding and new knowledge, and reconstruct their mental models. According to theories of conceptual change (Posner et al., 1982) and constructivist learning theories (von Glasersfeld, 1989), these processes are essential for students to promote meaningful understanding of science.The GEM approach was applicable to this study because the drawings and animations created by students are the external representations of their mental models (Akaygun, 2016), so creating visualizations can also be viewed as a process of building models. Additionally, Khan (2007) indicated that students’ sustained involvement in the GEM cycle could enable them to achieve important process and content goals in chemistry. The approach could be promising for integrating visualization in chemistry learning. Furthermore, previous studies have suggested the importance of having students view teacher-made or experts’ visualizations before evaluation (Chang et al., 2010; Yaseen, 2018). We thus expanded upon the GEM approach and used a sequence of View, Generate, Evaluate, and Modify (VGEM) to design the three instructional conditions of this study. Table 1 shows the design and procedures of the three groups.
| Class period | VGEM stage | VD (view and drawing) | VA (viewing and animating) | V (viewing) |
|---|---|---|---|---|
| 1 | Pretests of conceptual understanding and motivation | |||
| View an animation of the particulate nature matter | ||||
| Use a blank storyboard template to practice drawing a chemical process | Learn how to use Alchemie Animator and practice creating an animation | View an animation of methane combustion | ||
| 2 | V1 | Introduce concepts of electric circuits and electrochemical cells, and view an animation of a Zn–Cu cell | ||
| G1 | Generate drawings of a Ni–Ag cell | Generate animations of a Ni–Ag cell | View an animation of electrochemical cells and answer questions on worksheets | |
| E1 | Evaluate another student pair's drawings | Evaluate another student pair's animations | Evaluate the animations generated by the VA group | |
| M1 | Provide suggestions on how to modify and improve the visualizations | |||
| 3 | V2 | Introduce concepts of electrolysis and electroplating and view two animations | ||
| G2 | Generate drawings of electroplating of an iron spoon with silver | Generate animations of electroplating of an iron spoon with silver | View another animation of electroplating and answer questions on worksheets | |
| E2 | Evaluate another student pair's drawings | Evaluate another student pair's animations | Evaluate the animations generated by the VA group | |
| M2 | Provide suggestions on how to modify and improve the visualizations | |||
| 4 | Posttests of conceptual understanding and motivation | |||
| 6 weeks later | Delayed-posttest of conceptual understanding | |||
During the first class period, students first took the pretests of conceptual understanding and motivation individually. After the pretests were finished, each class was given 18 to 20 tablets. Students then worked in pairs and watched an animation of the particulate nature of matter provided by a textbook company (https://youtu.be/obQgnrIN1ws). The teacher handed out the worksheets and guided students to pay attention to different features in the animations, such as dots and arrows, and helped them understand how chemistry is represented in visualizations. By watching the animation and discussing in pairs, students could learn how chemical processes and reactions can be represented at the submicroscopic and symbolic levels, which in turn may support their viewing, interpreting, and creating of visualizations in later lessons. After watching the first animation, student pairs in the VD group were provided with a storyboard that contained six blank frames and were asked to draw at least three frames to represent the animation they just watched. The VA group was introduced to an animation creating application, Alchemie Animator. The teacher demonstrated how to use the application to add atoms to the screen, build molecules, generate frames, and create an animation. Student pairs in the VD group worked collaboratively on tablets to learn how to use the application, and recreated the animation of the particulate nature of matter. The V group viewed another animation of methane combustion (https://youtu.be/tZCbj0UT5hI), which was a chemical reaction they learned previously and represented at the submicroscopic level. By answering the questions on the worksheets, the V group learned to visualize a chemical reaction and to interpret a molecular animation.
During the second and third class periods, the students engaged in two cycles of VGEM (Table 1) and continued working in pairs. Learning collaboratively in pairs allowed students to externalize, share, and negotiate their ideas, and the social interactions between peers may create more opportunities for learning (Blumenfeld et al., 1996). As found in Sampson and Clark (2009), although compared to students who worked alone, student triads may not produce better artifacts initially, students from the collaborative condition demonstrated superior performance on the mastery and transfer problems later on their own. Sampson and Clark (2009) concluded that “collaboration was beneficial for individual learning but not for initial performance on the task” (p. 448). Given that students were tested individually in the posttest and delayed-posttest, collaborative learning may be useful for their later individual performances and was employed in this study.
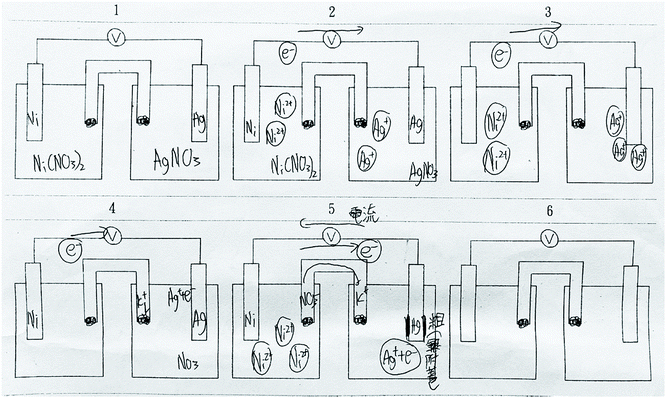
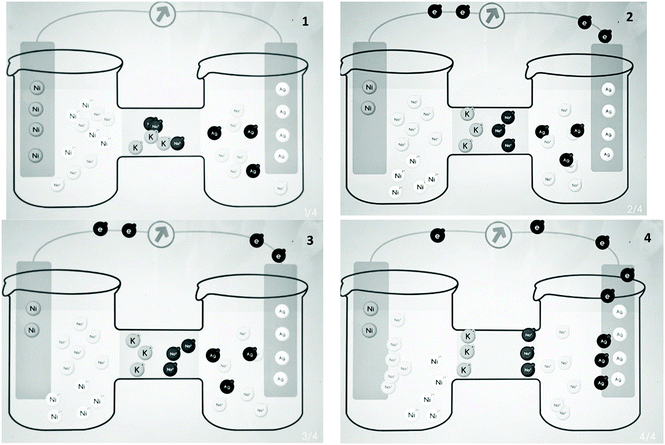
In the V1 phase, the teacher introduced and explained concepts of electrochemical cells with the use of an animation of a Zn–Cu cell (https://youtu.be/Q6bqTrEekZ8). In the G1 phase, the VD and VA groups were asked to generate drawings and animations of a Ni–Ag cell. Students in the VD group were given a blank storyboard and created at least three drawings to show how the electrochemical cell produces electricity. Fig. 1 displays the drawings created by a student pair. The VA group used Alchemie Animator to make an animation of the chemical processes in the Ni–Ag cell. Fig. 2 presents screenshots of an animation created by a student pair. A video of a student-generated animation of the Ni–Ag cell can be found at https://youtu.be/2ZshdYBCkEU. In the G1 phase, the V group did not create any visualization but watched an animation (https://youtu.be/V5TqMuHaDuY) of electrochemical cells and completed questions on a worksheet. In the worksheet, students in the V group had to describe the flows of electrons and ions in the solutions, identify the anode and cathode, and explain the purpose of the salt bridge. In the E1 phase, students in the VD and VA groups evaluated the visualizations created by another student pair, while the V group evaluated the animations generated by the VA group. A worksheet was provided to all the three groups to support their evaluation process. Students were asked to consider the following aspects and write down their comments on the worksheet: whether the chemical elements in the visualization (e.g., ions, electrons, and the numbers of anions and cations) are scientifically accurate, whether the materials and apparatus are properly set up and shown, whether the content of the visualization is understandable, and whether the flow of the animation (or the drawings) is smooth. Finally, in the M1 phase, on the basis of their evaluations, students in all groups provided suggestions on how to modify and improve their visualizations.
In the third class period, the V2 phase started with an introduction of electrolysis and electroplating. The teacher explained the concepts to the three groups and used two animations (electrolysis of copper sulphate using copper electrodes: https://youtu.be/uin83YfZTBI; electroplating of a metal key with copper: https://youtu.be/LbpwocW8Rgw) to illustrate her explanations. After the teacher's introduction, in the G2 phase, the VD and VA groups generated drawings and animations, respectively, to explain the process of electroplating of an iron spoon with silver. The V group watched another animation of electroplating (https://youtu.be/abMcjHau_Fc) and answered a worksheet. After generating or watching visualizations, the three groups moved to the E2 and M2 phases during which they engaged in the evaluating and modifying activities as they did in the E1 and M1 phases.
After experiencing two cycles of VGEM, all participants took the posttests of conceptual understanding and motivation. Overall the instruction and tests took four class periods to complete, with each class period lasting 50 minutes. Six weeks later, a delayed-posttest was administered to assess the students’ retention of conceptual understanding.
Assessment of conceptual understanding
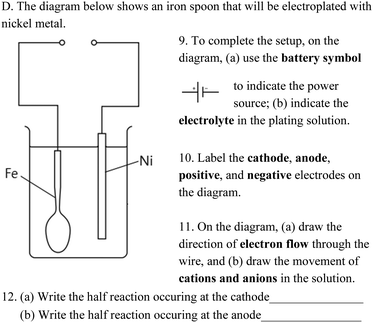
A concept test was designed to assess students’ conceptual understanding of electrochemistry. The test consisted of eight multiple-choice items and seven open-ended questions that were adapted from Sanger and Greenbowe (1997), Sanger and Greenbowe (2000), and Acar and Tarhan (2007). A group of specialists, including one university professor and three chemistry teachers, reviewed the test items to ensure that the content and format of the test items were in alignment with the major concept areas and representation levels of electrochemistry (Table 2). Among the 15 items, four concept areas were covered: (1) electric circuits in conductors and electrolytes, (2) components of electrochemical devices, (3) chemical processes occurring at the anode and cathode, and (4) oxidation–reduction equations. Additionally, six items involved only one representation level and were labeled “one-level” (e.g., items 3, 4, and 11 in Table 2), while the other nine items required understanding across two levels (e.g., items 6, 7, and 8) and were thus viewed as “across-level” items. The assessment items 9 to 12 are shown in Fig. 3. For example, item 9 was categorized into concept area 2 and “across-level” because it involved using the diagram of an electrochemical device (the macro level) and symbols to answer the questions of battery setup and electrolyte (the symbolic level). Item 12 was classified into concept area 4 and “one-level” as students were required to determine the half-reactions and use symbols to represent oxidation–reduction equations.| Concept area | Item | Representation level |
|---|---|---|
| (1) Electric circuits in conductors and electrolytes. | (One-level) 3, 4, 11a, 14a | Submicro |
| (Across-level) 6, 7, 8 | Macro and submicro | |
| (2) Components of electrochemical devices | (Across-level) 9a, 9b, 10, 13 | Macro and symbolic |
| (3) Chemical processes occurring at the anode and cathode | (One-level) 11b, 14b | Submicro |
| (Across-level) 1, 2 | Macro and submicro | |
| (4) Oxidation–reduction equations | (One-level) 12a, 12b, 15 | Symbolic |
| (Across-level) 5 | Submicro and symbolic |
A pilot was conducted with 59 twelfth graders who had learned the topic, and the test items and questions were revised based on the test results. The reliability of the final version of the test (Cronbach's alpha) was 0.82. The test was administered as the pretest, posttest, and delayed posttest to assess, respectively, initial understanding, changes, and retention of conceptual understanding up to 6 weeks later.
Questionnaire of student motivation towards chemistry learning
A self-report questionnaire was used to measure students’ motivation for chemistry learning before and after the instruction with visualizations (Table 1). The questionnaire was adapted from Tuan et al. (2005). There were several reasons to use Tuan et al.'s questionnaire in this study. First, the questionnaire was theory-based and covered important constructs of motivation. Secondly, the validity and reliability of the questionnaire have been established and examined by empirical studies (e.g., Yen et al., 2010; Acar Sesen and Tarhan, 2011). Thirdly, the questionnaire was developed for secondary science learning, so it fitted the context of the study, and it is appropriate for high school students. Finally, the Chinese version of the questionnaire was validated and available so we did not have to translate the items for students and validate the translated questionnaire.In this study, four scales of motivation towards chemistry learning were included: self-efficacy, active learning strategy, achievement goal, and learning environment stimulation. Examples of items include: “I am sure that I can do well on chemistry tests” (self-efficacy), “When I do not understand a chemistry concept, I find relevant resources that will help me” (active learning strategy), “During a chemistry course, I feel most fulfilled when I am able to solve a difficult problem” (achievement goal), and “I am willing to participate in this chemistry course because the content is exciting and changeable” (learning environment stimulation). The questionnaire consisted of 24 items using a 5-point Likert scale that ranged from strongly agree to strongly disagree. In this study, the internal consistency reliability of the questionnaire, computed using Cronbach's alpha, was 0.87. The reliabilities of the four scales ranged from 0.70 to 0.83, suggesting that all scales had an acceptable internal consistency.
Data analysis
To answer the first and second research questions, we used paired samples t tests to examine whether students’ conceptual understanding and motivation to learn chemistry improved significantly within the groups. In addition to the comparisons of students’ overall performances between the pretest, posttest, and delayed posttest, item analyses were also conducted. As shown in Table 2, the items of the concept test were grouped into four concept areas and two types of representation level (i.e., one-level and across-level). Statistical analyses between the concept areas and representation types were conducted. Also, the items of the motivation questionnaire were categorized into four scales. Students’ mean scores of the different types of items before and after the instruction were analyzed and compared. Effect sizes were calculated using Cohen's d to measure the magnitude of the treatment effect (Cohen, 1988).Regarding the third research question, we investigated the differences between the three groups’ conceptual understanding and motivation on the posttest and delayed posttest. To control for initial differences in the pretest scores of the three groups, analyses of covariance were performed, and students’ pretest scores were the covariate. We first employed one-way analyses of covariance (ANCOVAs) to compare the three groups’ overall performances on the two instruments. Multivariate analyses of covariance (MANCOVAs) were then used to examine differences between the three groups on the concept areas, representation types, and four scales of motivation.
Results
Within-group differences in students’ conceptual understanding
To examine whether students in the three groups improved their conceptual understanding after interacting with visualizations, paired-samples t tests were used for the comparisons of the pretest, posttest, and delayed posttest scores of each group. Descriptive statistics of the concept tests and the results of the t tests are presented in Table 3. First, as can be seen in Table 3, statistically significant differences were found between the overall means of the pretest and posttest in the VD, VA, and V groups (VD group: t(29) = 6.06, p < 0.001, ES = 1.41; VA group: t(31) = 7.07, p < 0.001, ES = 1.34; V group: t(32) = 5.68, p < 0.001, ES = 1.12). The effect sizes (Cohen's d) were all higher than 0.8, suggesting that, overall, the magnitude of the treatment effects in the three groups was large. Secondly, the items were classified based on the concept areas and representation levels (i.e., across-level and one-level items). Comparisons of the pretest and posttest regarding different concepts and representation types were made. Table 3 shows that all three groups had significant improvement in their posttest scores on the four concept areas as well as the representation levels.| n | Pretest | Posttest | Pretest–Posttest | Delayed posttest | Pretest-delayed posttest | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | t | p | ES | M | SD | t | p | ES | ||
| Concept | |||||||||||||
| VD group | 30 | ||||||||||||
| Overall | 30 | 4.75 | 2.94 | 12.42 | 7.09 | 6.06 | <0.001 | 1.41 | 10.02 | 5.47 | 4.53 | <0.001 | 1.20 |
| Concept 1 | 30 | 2.70 | 2.50 | 6.00 | 3.86 | 4.26 | <0.001 | 1.01 | 5.46 | 3.78 | 3.22 | 0.003 | 0.86 |
| Concept 2 | 30 | 0.21 | 0.31 | 2.03 | 1.21 | 9.15 | <0.001 | 2.06 | 0.71 | 0.75 | 3.47 | 0.002 | 0.87 |
| Concept 3 | 30 | 1.33 | 1.60 | 3.58 | 2.98 | 4.20 | <0.001 | 0.94 | 4.07 | 3.08 | 4.11 | <0.001 | 1.11 |
| Concept 4 | 30 | 0.56 | 0.89 | 2.30 | 1.66 | 6.11 | <0.001 | 1.30 | 1.46 | 1.88 | 2.34 | 0.026 | 0.61 |
| Across-level | 30 | 2.75 | 2.20 | 7.83 | 3.76 | 6.65 | <0.001 | 1.64 | 6.88 | 3.99 | 4.60 | <0.001 | 1.28 |
| One-level | 30 | 2.00 | 2.14 | 4.58 | 3.78 | 3.92 | <0.001 | 0.83 | 3.13 | 2.44 | 1.87 | 0.072 | 0.49 |
| VA group | |||||||||||||
| Overall | 32 | 6.03 | 4.26 | 12.63 | 5.50 | 7.07 | <0.001 | 1.34 | 10.52 | 6.11 | 4.39 | <0.001 | 0.85 |
| Concept 1 | 32 | 3.31 | 2.85 | 6.68 | 3.33 | 5.16 | <0.001 | 1.09 | 5.96 | 4.01 | 4.04 | <0.001 | 0.76 |
| Concept 2 | 32 | 0.43 | 0.56 | 1.51 | 1.10 | 5.23 | <0.001 | 1.24 | 0.90 | 0.78 | 3.26 | 0.003 | 0.69 |
| Concept 3 | 32 | 1.81 | 1.76 | 3.85 | 2.57 | 4.38 | <0.001 | 0.92 | 3.78 | 3.12 | 3.43 | 0.002 | 0.77 |
| Concept 4 | 32 | 0.65 | 1.07 | 1.81 | 1.78 | 3.60 | 0.001 | 0.79 | 1.93 | 1.86 | 3.73 | 0.001 | 0.84 |
| Across-level | 32 | 3.84 | 2.62 | 7.82 | 3.28 | 7.17 | <0.001 | 1.34 | 6.70 | 4.12 | 3.98 | <0.001 | 0.82 |
| One-level | 32 | 2.18 | 2.07 | 4.79 | 2.84 | 5.38 | <0.001 | 1.05 | 3.81 | 2.70 | 3.48 | 0.002 | 0.67 |
| V group | |||||||||||||
| Overall | 33 | 5.15 | 3.96 | 11.02 | 6.30 | 5.68 | <0.001 | 1.12 | 8.46 | 6.50 | 2.91 | 0.006 | 0.62 |
| Concept 1 | 33 | 2.66 | 2.48 | 5.54 | 3.55 | 4.35 | <0.001 | 0.94 | 4.60 | 4.22 | 2.34 | 0.025 | 0.56 |
| Concept 2 | 33 | 0.50 | 0.79 | 1.68 | 1.01 | 6.69 | <0.001 | 1.29 | 0.69 | 0.79 | 1.32 | 0.20 | 0.24 |
| Concept 3 | 33 | 1.43 | 1.62 | 3.21 | 2.48 | 4.08 | <0.001 | 0.84 | 3.30 | 3.17 | 3.29 | 0.002 | 0.74 |
| Concept 4 | 33 | 0.78 | 1.21 | 1.87 | 1.49 | 4.27 | <0.001 | 0.80 | 1.57 | 1.78 | 2.42 | 0.021 | 0.51 |
| Across-level | 33 | 3.65 | 2.82 | 7.59 | 4.08 | 5.43 | <0.001 | 1.12 | 5.48 | 4.49 | 2.39 | 0.023 | 0.48 |
| One-level | 33 | 1.50 | 1.89 | 3.42 | 2.94 | 3.37 | 0.002 | 0.77 | 2.97 | 3.09 | 2.36 | 0.024 | 0.57 |
| Motivation | |||||||||||||
| VD group | |||||||||||||
| Overall | 30 | 90.50 | 13.35 | 94.00 | 13.95 | 2.05 | 0.050 | 0.26 | |||||
| Self-efficacy | 30 | 21.97 | 5.11 | 23.20 | 5.05 | 1.69 | 0.10 | 0.24 | |||||
| Active learning | 30 | 30.13 | 5.07 | 30.40 | 4.57 | 0.37 | 0.71 | 0.055 | |||||
| Achievement goal | 30 | 18.17 | 3.44 | 18.97 | 3.81 | 1.70 | 0.098 | 0.22 | |||||
| Learning environment | 30 | 20.23 | 3.82 | 21.43 | 4.40 | 1.71 | 0.097 | 0.29 | |||||
| VA group | |||||||||||||
| Overall | 32 | 88.72 | 9.35 | 92.16 | 10.01 | 2.33 | 0.026 | 0.36 | |||||
| Self-efficacy | 32 | 21.09 | 5.55 | 22.44 | 4.71 | 2.50 | 0.018 | 0.26 | |||||
| Active learning | 32 | 29.28 | 4.09 | 30.09 | 4.36 | 1.31 | 0.199 | 0.19 | |||||
| Achievement goal | 32 | 17.31 | 2.82 | 17.91 | 3.32 | 1.13 | 0.267 | 0.19 | |||||
| Learning environment | 32 | 21.03 | 2.81 | 21.72 | 2.84 | 1.11 | 0.272 | 0.24 | |||||
| V group | |||||||||||||
| Overall | 33 | 88.45 | 11.93 | 90.79 | 13.30 | 1.57 | 0.126 | 0.19 | |||||
| Self-efficacy | 33 | 20.58 | 4.09 | 21.76 | 4.80 | 1.56 | 0.126 | 0.26 | |||||
| Active learning | 33 | 29.64 | 5.11 | 29.36 | 5.18 | 0.41 | 0.684 | 0.054 | |||||
| Achievement goal | 33 | 17.70 | 3.14 | 17.48 | 3.09 | 0.42 | 0.673 | 0.070 | |||||
| Learning environment | 33 | 20.55 | 3.18 | 22.18 | 3.27 | 4.08 | <0.001 | 0.50 | |||||
Additionally, the retention effects were examined. First, there were significant differences between the overall means of the pretest and delayed posttest in the three groups (VD group: t(29) = 4.53, p < 0.001, ES = 1.20; VA group: t(31) = 4.39, p < 0.001, ES = 0.85; V group: t(32) = 2.91, p = 0.006, ES = 0.62). The calculated effect sizes ranged from 0.62 to 1.20, which indicated that while the VD and VA conditions showed large positive effects on students’ conceptual understanding, the V condition had a medium retention effect 6 weeks after the instruction ended. Secondly, regarding the concept areas, all the groups retained their understanding of the four areas (p < 0.05), except for the V group in concept area 2 (components of electrochemical devices, p = 0.20). Thirdly, the VA and V groups still had significant increases in their delayed posttest scores on both the across-level and one-level items (p < 0.05), whereas the VD group showed the retention effect only on the across-level items (Table 3).
Within-group differences in students’ motivation
To investigate whether the three instructional conditions increased the students’ motivation to learn chemistry, we first compared the differences between the total scores of the pre- and post-motivation questionnaire. Table 3 shows that the VA group demonstrated significantly higher motivation in the posttest (VA group: t(31) = 4.39, p = 0.026), although the effect size indicated a small effect (ES = 0.36). On the other hand, motivation of students in the VD and V groups remained at a similar level, and no significant difference was found after the instruction (VD group: t(29) = 2.05, p = 0.050; V group: t(32) = 1.57, p = 0.13). The results revealed that, regarding the overall motivation for chemistry learning, among the three ways of using visualizations, only creating animations showed motivational benefits for chemistry learning.Also, we compared students’ motivation towards chemistry learning in the four scales before and after the instruction (Table 3). Significant differences were found in the self-efficacy scale of the VA group (t(31) = 2.50, p = 0.018) and the learning environment scale of the V group (t(32) = 4.08, p < 0.001). The results implied that creating animations could increase students’ chemistry learning self-efficacy, and that viewing animations could stimulate positive perceptions of the curriculum, the teacher, and peers.
Comparisons of the effects of different uses of visualizations on conceptual understanding
ANCOVA tests were employed to compare the three groups’ overall performances on the concept test. The analyses indicated no significant differences between the means of the three groups in the posttest and delayed posttest (posttest: F(2,91) = 0.64, p = 0.53; delayed posttest: F(2,91) = 0.91, p = 0.41). The results suggested that students in the three instructional conditions developed and retained a similar level of conceptual understanding after the instruction.Regarding students’ performances on the four concept areas, MANCOVAs were conducted to establish whether the different uses of visualizations resulted in significant differences in students’ performances on items of electric circuits, components of electrochemical devices, chemical processes occurring at the anode and cathode, and oxidation–reduction equations. The MANCOVA of the posttest revealed a statistically significant difference between the three groups on the combined dependent variables after controlling for the pretest scores (F(4,86) = 3.12, p = 0.019 < 0.05, η2 = 0.13).
To further investigate the impact of the instructional conditions on the individual concept areas, univariate F-tests using an alpha level of 0.05 were performed. Among the four dependent variables, while no significance was shown between the three groups for the concept areas 1, 3, and 4 (concept area 1: F(2,88) = 0.87, p = 0.42; concept area 3: F(2,88) = 0.42, p = 0.65; concept area 4: F(2,88) = 2.06, p = 0.13), a significant difference between groups was found for concept area 2 (F(2,88) = 3.76, p = 0.027 < 0.05). The partial eta squared (η2 = 0.079) suggested a small to medium effect. Pairwise comparison followed by the univariate F-tests indicated that a significant difference existed between the VD and VA groups’ understanding of concept area 2 (components of electrochemical devices). The VD group performed significantly better than the VA group (mean difference = 0.71, p = 0.009), while no significant difference was observed between the V and VD groups, and between the V and VA groups. However, the MANCOVA of the delayed posttest did not show any significance (F(4,86) = 1.12, p = 0.35, η2 = 0.05). These results indicated that although the VD group outperformed the other two groups in concept area 2 during the posttest, their advantage was not retained after 6 weeks.
Furthermore, we compared the between-group differences in students’ scores on the one-level and across-level items. The MANCOVA of the posttest showed no significant difference between the three groups on the two types of items after controlling for the pretest scores (F(2,90) = 1.84, p = 0.16, η2 = 0.039). Similarly, no significance was found in the MANCOVA of the delayed posttest (F(2,90) = 1.24, p = 0.29, η2 = 0.027). Thus, in terms of students’ performances on items of different representation levels during the posttest and delayed posttest, the statistical results were not in favor of any of the instructional conditions.
Comparisons of the effects of different uses of visualizations on motivation
To compare students’ overall motivation for chemistry learning between the three groups, an ANCOVA was conducted. The results found no significant difference among the three groups (F(2,91) = 0.30, p = 0.74, η2 = 0.007). MANCOVA was further used to examine the between-group differences in the four scales of motivation. Similar to what we found for overall motivation, comparisons of the three groups showed no differences in self-efficacy, active learning strategy, achievement goal, or learning environment stimulation (F(4,86) = 2.12, p = 0.085, η2 = 0.090). The results indicated that different uses of visualizations made no difference in students’ motivation towards chemistry learning.Discussion and implications
Within-group differences in students’ conceptual understanding
The first research question of the study was to investigate which instructional condition could enhance students’ conceptual understanding of electrochemistry. The statistical analyses of the within-group differences showed that with the implementation of the VGEM approach, all three instructional conditions could support students to develop significantly better conceptual understanding of electrochemistry in the posttest and delayed posttest. Also, the comparative results between the pretest and delayed posttest indicated that in the three groups, students’ understanding was retained after 6 weeks. These results suggest the value and necessity of applying an appropriate instructional method with the use of visualizations (Chang and Linn, 2013; Stieff, 2017; Farrokhnia et al., 2020; Yaseen and Aubusson, 2020). This study expanded the use of the GEM cycle (Khan, 2007) to the activities with visualizations. The implication of the within group results is that the View, Generate, Evaluate, and Modify sequence could be useful for teachers and curriculum designers to enhance students’ chemistry learning with drawings and animations.Yet, compared to the other two groups, the viewing only group had a relatively small delayed effect on conceptual understanding. This might infer that the cognitive benefit of creating visualizations could last longer. For teachers and educators who consider the delayed effect, allowing students to generate their own visualizations may be a better instructional decision.
Comparisons of the effects of different uses of visualizations on conceptual understanding
The comparative results of students’ conceptual understanding between the groups did not fully support an argument that any of the conditions were more effective than one another. As suggested by previous studies (e.g., Moreno and Valdez, 2005), there could be a trade-off between interactivity and cognitive load when students engage in activities with visualizations. Compared to viewing visualizations, generating animations or drawings allows students to engage in active learning with visualizations, but these generating activities could impose higher cognitive load and require students’ attention to the use of the drawing tools and technical details. This trade-off may explain why no overall significant difference was found between the means of the three groups in the posttest and delayed posttest. This result also echoed findings from Chang et al. (2010) that viewing animations could be as effective as creating animations in terms of developing students’ conceptual understanding. By making the comparison between combining the activities of creating, interpreting, and evaluating, and combining the activities of viewing, interpreting, and evaluating, this study suggests that the effect of the viewing approach could also be augmented by a useful VGEM approach. The results also imply that chemistry teachers who have limited technical resources could consider the use of drawings and storyboards with the VGEM approach to provide effective instruction.On the other hand, the item analyses showed that the VD group outperformed the other two groups in concept area 2 during the posttest, although their advantage was not retained after 6 weeks. Concept area 2 involved identifying components of electrochemical cells, and the test questions required students to recall what the anode, cathode, negative, and positive terminals are, and label them on the diagrams. For the VD group, labeling components of a cell was part of their drawing task (Fig. 1). Familiarity with the test questions might be a reason why the VD group received higher scores on these questions in the posttest. Yet, in contrast to other cognitive tasks such as recognition, predicting, explanation, and analysis, recalling factual knowledge could show worse retention (Semb and Ellis, 1994). This may explain why the VD group did not maintain their advantage in concept area 2 during the delayed posttest. Taken together, although the results regarding students’ conceptual understanding do not allow us to make a compelling argument for creating one type of visualization over another, this study provides initial evidence for whether creating visualizations with different modalities influences students’ chemistry learning. Additionally, for the long-term conceptual learning of electrochemistry, this study suggests that teachers could make a more flexible instructional decision depending on the resources available because creating either drawings or animations may have similar effects on students’ learning.
Effects on students’ motivation towards chemistry learning
Another issue addressed in this study is whether the three conditions increase students’ motivation for chemistry learning. The results of the between-group and within-group comparisons show two different perspectives on the issue. Similar to the findings in Osman and Lee (2013) that students in the multimedia animation group did not have significantly higher motivation in electrochemistry than the students receiving the traditional instruction, the comparative results between groups suggest that viewing, drawing, and creating animations made no difference to students’ motivation for chemistry learning. The result implies that if increasing students’ motivation is a major concern to teachers, teachers may need to employ teaching methods other than different uses of visualizations.Yet, results of the within-group differences showed that, regarding the overall motivation, among the three ways of using visualizations, only creating animations increased students’ motivation for chemistry learning. This result was inconsistent with what was found in Harrison and Treagust (2000) whose study showed the motivational benefits of generating paper-based visualizations. In this study, creating drawings did not enhance the overall or the four scales of motivation towards chemistry learning. Furthermore, generating animations significantly increased students’ self-efficacy in the post-questionnaire. Self-efficacy refers to the individual's perception of their ability in accomplishing learning tasks (Bandura, 1977). One main source of self-efficacy is mastery experiences that individuals gain when they take on a new challenge and succeed (Britner and Pajares, 2006). Because creating animations could be challenging and involve an understanding of chemistry concepts, students may be given a sense of achievement after completing their animation, which may enrich their mastery experiences about learning chemistry.
This study also found that viewing animations without creating drawings and animations could stimulate positive perceptions of the learning environment. A learning environment comprises teachers’ teaching strategies, learning activities, and classroom interactions. Previous studies have suggested that redesigning a course and changes to teaching strategies could affect students’ perceptions of the learning environment (Nijhuis et al., 2005). By viewing and evaluating animations created by others, students in the V group experienced activities and interactions which differed from those in their previous chemistry classes. The changes in the learning environment might in turn have shaped their perceptions of the curriculum, the teacher, and their peers. However, the changes in learning experiences cannot explain why the significant within-group differences on the same scale were not observed in the other two groups. The inconsistent results about students’ motivation suggest that more studies on how students’ motivation for chemistry learning evolves with the use of visualizations are still needed.
Limitations
Although this study advances understanding of the effects of different ways of using visualizations on high school students’ conceptual understanding in electrochemistry and motivation towards chemistry learning, the results of this study were subject to the following limitations. The first limitation is regarding the research design and the three instructional conditions. This study employed the VGEM approach to support students’ learning with visualizations and manipulated the generalization phase to examine differences between the three conditions. The one-way quasi-experimental design did not allow us to make conclusions about the effects from other controlled phases, such as evaluating and modifying visualizations, even though there may also be instructional benefits from these phases. Future studies may include different research and instructional designs, and consider examining the effects of other phases in the VGEM approach to chemistry learning.A second limitation is related to the data sources and analyses. This study generated results based on the quantitative data collected from an assessment and a questionnaire, but did not include qualitative analyses of students’ drawings and animations. The qualitative findings may provide more detailed information about how students’ conceptual understanding evolves, and offer insight into students’ collaborative learning process. Future comparative research may use a mixed-methods design, collect qualitative data from individual students and student groups, draw upon different analytical perspectives, and build a more comprehensive picture of how students learn from different uses of visualizations.
Thirdly, the assessment of conceptual understanding was paper-based with still pictures and may not able to fully evaluate students’ understanding about the dynamic nature of electrochemistry. Although the test items required students to draw the electron flow and the movement of ions, the dynamic nature of the reactions could not be fully represented by the assessment or students’ drawings. Performance assessments with the use of computer-based drawing tool could be considered by future research.
Finally, the motivation towards chemistry learning examined in this study was limited to four scales (i.e., self-efficacy, active learning strategy, achievement goal, and learning environment stimulation). Other important affective or motivational constructs in science education, e.g., interests and engagement, were not considered. While the results of this study found no significant differences between the groups in the four scales, future studies that examine more affective or motivational constructs would add more evidence regarding whether viewing and creating visualizations have differential impacts on these constructs.
Conclusion
Drawing upon recent studies on the use of visualizations for science learning, this study designed three instructional conditions and examined the effects of the three conditions on high school students’ conceptual understanding of electrochemistry and motivation for chemistry learning. The research design of the study and the choice of participants respond to the need for more comparative quantitative studies of secondary school students in terms of both cognitive and motivational learning outcomes suggested by Farrokhnia et al. (2020). The study found that, with the implementation of an appropriate instruction sequence approach, different ways of using visualizations (i.e., viewing, drawing, and animating) could significantly increase students’ conceptual understanding of electrochemistry. Particularly, creating animations showed motivational benefits for chemistry learning. The results of this study advance the understanding of how to develop effective instructional activities with visualizations for chemistry learning, and reveal possible conceptual and motivational benefits of viewing and generating visualizations.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the Ministry of Science and Technology in Taiwan under MOST 107-2511-H-003-012-MY3, MOST 109-2511-H-003-015-MY3, and the “Institute for Research Excellence in Learning Sciences” of National Taiwan Normal University from The Featured Areas Research Centre Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan.References
- Acar B. and Tarhan L., (2007), Effect of cooperative learning strategies on students' understanding of concepts in electrochemistry, Int. J. Sci. Math. Educ., 5, 349–373.
- Acar Sesen B. and Tarhan L., (2011), Inquiry-based Laboratory Activities in Electrochemistry: high School Students’ Achievements and Attitudes, Res. Sci. Educ., 43, 413–435.
- Ainsworth S., (2006), DeFT: a conceptual framework for considering learning with multiple representations, Learn. Instr., 16, 183–198.
- Ainsworth S., Prain V. and Tytler R., (2011), Drawing to learn in science, Science, 333, 1096–1097.
- Akaygun S., (2016), Is the oxygen atom static or dynamic? The effect of generating animations on students' mental models of atomic structure, Chem. Educ. Res. Pract., 17, 788–807.
- Bandura A., (1977), Self-efficacy: toward a unifying theory of behavioral change, Psychol. Rev., 84, 191–215.
- Barak M., Ashkar T. and Dori Y. J., (2011), Learning science via animated movies: its effect on students’ thinking and motivation, Comput. Educ., 56, 839–846.
- Berg A., Orraryd D., Pettersson A. J. and Hultén M., (2019), Representational challenges in animated chemistry: self-generated animations as a means to encourage students’ reflections on sub-micro processes in laboratory exercises, Chem. Educ. Res. Pract., 20, 710–737.
- Berney S. and Bétrancourt M., (2016), Does animation enhance learning? A meta-analysis, Comput. Educ., 101, 150–167.
- Blumenfeld P. C., Marx R. W., Soloway E. and Krajcik J., (1996), Learning with peers: from small group cooperation to collaborative communities, Educ. Res., 25, 37–40.
- Britner S. L. and Pajares F., (2006), Sources of science self-efficacy beliefs of middle school students, J. Res. Sci. Teach., 43, 485–499.
- Buckley B. C., (2000), Interactive multimedia and model-based learning in biology, Int. J. Sci. Educ., 22, 895–935.
- Castro-Alonso J. C., Ayres P. and Paas F., (2016), Comparing apples and oranges? A critical look at research on learning from statics versus animations, Comput. Educ., 102, 234–243.
- Chang H.-Y. and Linn M. C., (2013), Scaffolding learning from molecular visualizations, J. Res. Sci. Teach., 50, 858–886.
- Chang H.-Y., Quintana C. and Krajcik J. S., (2010), The impact of designing and evaluating molecular animations on how well middle school students understand the particulate nature of matter, Sci. Educ., 94, 73–94.
- Chang H.-Y., Quintana C. and Krajcik J., (2014), Using drawing technology to assess students’ visualizations of chemical reaction processes, J. Sci. Educ. Technol., 23, 355–369.
- Chiu J. L., Chen J. K. and Linn M. C., (2013), in Azevedo R. and Aleven V. (ed.), International Handbook of Metacognition and Learning Technologies, New York: Springer, pp. 517–531.
- Cohen J., (1988), Statistical power analysis for the behavioral sciences, Lawrence Erlbaum Associates.
- Cook M. P., (2006), Visual representations in science education: the influence of prior knowledge and cognitive load theory on instructional design principles, Sci. Educ., 90, 1073–1091.
- Cooper M. M., Stieff M. and DeSutter D., (2017), Sketching the invisible to predict the visible: from drawing to modeling in chemistry, Top. Cogn. Sci., 9, 902–920.
- Creswell J. W., (1994), Research design: qualitative and quantitative approaches, Thousand Oaks, CA: Sage.
- Davidowitz B., Chittleborough G. and Murray E., (2010), Student-generated submicro diagrams: a useful tool for teaching and learning chemical equations and stoichiometry, Chem. Educ. Res. Pract., 11, 154–164.
- De Jong O. and Treagust D., (2002), in Gilbert J. K., De Jong O., Justi R., Treagust D. F. and Van Driel J. H. (ed.), Chemical education: towards research-based practice, Dordrecht, Netherlands: Kluwer, pp. 317–337.
- Doymus K., Karacop A. and Simsek U., (2010), Effects of jigsaw and animation techniques on students’ understanding of concepts and subjects in electrochemistry, Educ. Technol. Res. Dev., 58, 671–691.
- Farrokhnia M., Meulenbroeks R. F. G. and van Joolingen W. R., (2020), Student-generated stop-motion animation in science classes: a systematic literature review, J. Sci. Educ. Technol., 29, 797–812.
- Faul F., Erdfelder E., Lang A.-G. and Buchner A., (2007), G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences, Behav. Res. Methods, 39, 175–191.
- Garnett P. J. and Treagust D. F., (1992a), Conceptual difficulties experienced by senior high school students of electrochemistry: electric circuits and oxidation-reduction equations, J. Res. Sci. Teach., 29, 121–142.
- Garnett P. J. and Treagust D. F., (1992b), Conceptual difficulties experienced by senior high school students of electrochemistry: electrochemical (galvanic) and electrolytic cells, J. Res. Sci. Teach., 29, 1079–1099.
- Garnett P. J., Garnett P. J. and Hackling M. W., (1995), Students' alternative conceptions in chemistry: a review of research and implications for teaching and learning, Stud. Sci. Educ., 25, 69–96.
- Glynn S. M., Taasoobshirazi G. and Brickman P., (2009), Science motivation questionnaire: construct validation with nonscience majors, J. Res. Sci. Teach., 46, 127–146.
- Guo D., McTigue E. M., Matthews S. D. and Zimmer W., (2020), The impact of visual displays on learning across the disciplines: a systematic review, Educ. Psychol. Rev., 32, 627–656.
- Harrison A. G. and Treagust D. F., (2000), Learning about atoms, molecules, and chemical bonds: a case study of multiple-model use in Grade 11 chemistry, Sci. Educ., 84, 352–381.
- Hegarty M., (2004), Dynamic visualizations and learning: getting to the difficult questions, Learn. Instr., 14, 343–351.
- Hegarty M., Kriz S. and Cate C., (2003), The roles of mental animations and external animations in understanding mechanical systems, Cogn. Instr., 21, 209–249.
- Hoban G., Loughran J. and Nielsen W., (2011), Slowmation: preservice elementary teachers representing science knowledge through creating multimodal digital animations, J. Res. Sci. Teach., 48, 985–1009.
- Höffler T. N. and Leutner D., (2007), Instructional animation versus static pictures: a meta-analysis, Learn. Instr., 17, 722–738.
- Kelly R. M., Akaygun S., Hansen S. J. R. and Villalta-Cerdas A., (2017), The effect that comparing molecular animations of varying accuracy has on students’ submicroscopic explanations, Chem. Educ. Res. Pract., 18, 582–600.
- Khan S., (2007), Model-based inquiries in chemistry, Sci. Educ., 91, 877–905.
- Kozma R., Chin E., Russell J. and Marx N., (2000), The roles of representations and tools in the chemistry laboratory and their implications for chemistry instruction, J. Learn. Sci., 9, 105–143.
- Lehrer R. and Schauble L., (2000), Developing model-based reasoning in mathematics and science, J. Appl. Dev. Psychol., 21, 39–48.
- Linn M. C., Chang H.-Y., Chiu J. L., Zhang Z. H. and McElhaney K., (2010), in Benjamin A. S. (ed.), Successful remembering and successful forgetting: a festschrift in honor of Robert A. Bjork, New York, NY: Routledge, pp. 239–262.
- Matuk C., Zhang J., Uk I. and Linn M. C., (2019), Qualitative graphing in an authentic inquiry context: how construction and critique help middle school students to reason about cancer, J. Res. Sci. Teach., 56, 905–936.
- Mayer R. E., Hegarty M., Mayer S. and Campbell J., (2005), When static media promote active learning: annotated illustrations versus narrated animations in multimedia instruction, J. Exp. Psychol. Appl., 11, 256–265.
- McElhaney K. W., Chang H.-Y., Chiu J. L. and Linn M. C., (2015), Evidence for effective uses of dynamic visualisations in science curriculum materials, Stud. Sci. Educ., 51, 49–85.
- Ministry of Education, (2018), Curriculum guidelines of 12-year basic education: natural sciences, Ministry of Education.
- Moreno R. and Valdez A., (2005), Cognitive load and learning effects of having students organize pictures and words in multimedia environments: the role of student interactivity and feedback, Educ. Technol. Res. Dev., 53, 35–45.
- Nersessian N. J., (2002), in Carruthers P., Stich S. and Siegal M. (ed.), The cognitive basis of science, Cambridge, UK: Cambridge University Press, pp. 133–153.
- Nijhuis J. F., Segers M. S. and Gijselaers W. H., (2005), Influence of redesigning a learning environment on student perceptions and learning strategies, Learn. Environ. Res., 8, 67–93.
- Osman K. and Lee T. T., (2013), Impact of interactive multimedia module with pedagogical agents on students’ understanding and motivation in the learning of electrochemistry, Int. J. Sci. Math. Educ., 12, 395–421.
- Penner D. E., (2000), Cognition, computers, and synthetic science: building knowledge and meaning through modeling, Rev. Educ. Res., 25, 1–35.
- Picard R. W., Papert S., Bender W., Blumberg B., Breazeal C., Cavallo D., Machover T., Resnick M., Roy D. and Strohecker C., (2004), Affective learning-a manifesto, BT Technol. J., 22, 253–269.
- Pintrich P. R. and Schunk D. H., (1996), Motivation in education: theory, research, and applications, Prentice-Hall.
- Posner G. J., Strike K. A., Hewson P. W. and Gertzog W. A., (1982), Accommodation of a scientific conception: toward a theory of conceptual change, Sci. Educ., 66, 211–227.
- Rieber L. P., (1991), Animation, incidental learning, and continuing motivation, J. Educ. Psychol., 83, 318–328.
- Rosen Y., (2009), The effects of an animation-based on-line learning environment on transfer of knowledge and on motivation for science and technology learning, J. Educ. Comput. Res., 40, 451–467.
- Sampson V. and Clark D., (2009), The impact of collaboration on the outcomes of scientific argumentation, Sci. Educ., 93, 448–484.
- Sanger M. J. and Greenbowe T. J., (1997), Students' misconceptions in electrochemistry regarding current flow in electrolyte solutions and the salt bridge, J. Chem. Educ., 74, 819–823.
- Sanger M. J. and Greenbowe T. J., (2000), Addressing student misconceptions concerning electron flow in aqueous solutions with instruction including computer animations and conceptual change strategies, Int. J. Sci. Educ., 22, 521–537.
- Schmidt H.-J., Marohn A. and Harrison A. G., (2007), Factors that prevent learning in electrochemistry, J. Res. Sci. Teach., 44, 258–283.
- Semb G. B. and Ellis J. A., (1994), Knowledge taught in school: what is remembered? Rev. Educ. Res., 64, 253–286.
- Stieff M., (2017), in Lowe R. and Ploetzner R. (ed.), Learning from dynamic visualization, Cham: Springer, pp. 333–356.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14, 156–168.
- Taber K. S., (2014), Ethical considerations of chemistry education research involving ‘human subjects’, Chem. Educ. Res. Pract., 15, 109–113.
- Tasker R. and Dalton R., (2006), Research into practice: visualisation of the molecular world using animations, Chem. Educ. Res. Pract., 7, 141–159.
- Thurlow C., Lengel L. and Tomic A., (2004), Computer mediated communication: social interaction and the Internet, Sage.
- Tippett C. D., (2016), What recent research on diagrams suggests about learning with rather than learning from visual representations in science, Int. J. Sci. Educ., 38, 725–746.
- Tuan H. L., Chin C. C. and Shieh S. H., (2005), The development of a questionnaire to measure students' motivation towards science learning, Int. J. Sci. Educ., 27, 639–654.
- Tversky B., Morrison J. B. and Betrancourt M., (2002), Animation: can it facilitate? Int. J. Hum. Comput. Stud., 57, 247–262.
- Tytler R., Prain V., Hubber P. and Waldrip B., (2013), Constructing representations to learn in science, Rotterdam: Sense.
- Vaino K., Holbrook J. and Rannikmäe M., (2012), Stimulating students' intrinsic motivation for learning chemistry through the use of context-based learning modules, Chem. Educ. Res. Pract., 13, 410–419.
- von Glasersfeld E., (1989), in Husen T. and Postlethwaite T. N. (ed.), The International Encyclopedia of Education, New York, NY: Pergamon Press, pp. 162–163.
- Wilkerson-Jerde M. H., Gravel B. E. and Macrander C. A., (2014), Exploring shifts in middle school learners’ modeling activity while generating drawings, animations, and computational simulations of molecular diffusion, J. Sci. Educ. Technol., 24, 396–415.
- Williamson V. M., Watkins J. T. and Williamson III K. C., (2013), in Suits J. P. and Sanger M. J. (ed.), Pedagogic roles of animations and simulations in chemistry courses, Washington, DC: ACS Publications, pp. 293–311.
- Wu H.-K. and Krajcik J. S., (2006), Inscriptional practices in two inquiry-based classrooms: a case study of seventh graders' use of data tables and graphs, J. Res. Sci. Teach., 43, 63–95.
- Wu H.-K. and Puntambekar S., (2012), Pedagogical affordances of multiple external representations in scientific processes, J. Sci. Educ. Technol., 21, 754–767.
- Wu H.-K. and Shah P., (2004), Exploring visuospatial thinking in chemistry learning, Sci. Educ., 88, 465–492.
- Wu H.-K., Krajcik J. S. and Soloway E., (2001), Promoting understanding of chemical representations: students' use of a visualization tool in the classroom, J. Res. Sci. Teach., 38, 821–842.
- Wu H.-K., Lin Y. F. and Hsu Y. S., (2013), Effects of representation sequences and spatial ability on students' scientific understandings about the mechanism of breathing, Instr. Sci., 41, 555–573.
- Yang E.-M., Andre T., Greenbowe T. J. and Tibell L., (2003), Spatial ability and the impact of visualization/animation on learning electrochemistry, Int. J. Sci. Educ., 25, 329–349.
- Yaseen Z., (2018), Using student-generated animations: the challenge of dynamic chemical models in states of matter and the invisibility of the particles, Chem. Educ. Res. Pract., 19, 1166–1185.
- Yaseen Z. and Aubusson P., (2020), Exploring student-generated animations, combined with a representational pedagogy, as a tool for learning in chemistry, Res. Sci. Educ., 50, 529–548.
- Yen H.-C., Tuan H.-L. and Liao C.-H., (2010), Investigating the influence of motivation on students’ conceptual learning outcomes in web-based vs. classroom-based science teaching contexts, Res. Sci. Educ., 41, 211–224.
- Zhang Z. H. and Linn M. C., (2013), Learning from chemical visualizations: comparing generation and selection, Int. J. Sci. Educ., 35, 2174–2197.
| This journal is © The Royal Society of Chemistry 2021 |